Abstract
Introduction
Tumor-associated macrophages (TAMs) are divided into M1 and M2 macrophages. M1 macrophages inhibit tumor growth, whereas M2 macrophages promote tumor growth and metastasis. The aim of this study was to study the possible causes leading to formation of an M2 macrophage-dominant tumor microenvironment in non-small cell lung cancer.
Methods
Forty-eight archived lung tumor samples were examined for expression of interleukin-17 (IL-17) receptors IL-17RA and IL-17RC and the number of TAMs using immunohistochemical staining. Twenty fresh lung tumors and matched normal lung tissues were examined for expression of IL-17, cyclooxygenase-2, and prostaglandin E2, using enzyme-linked immunosorbent assay (ELISA) and Western blot analysis. Macrophage migration assays were performed using fresh lung tumor tissues and IL-17 as chemoattractants. Induction of M2 macrophage differentiation was analyzed using real-time quantitative polymerase chain reaction.
Results
TAMs expressed IL-17RA and IL-17RC. Lung tumors expressed higher levels of IL-17, cyclooxygenase-2, and prostaglandin E2, compared to normal lung tissues. Lung tumor tissues attracted migration of mouse RAW264.7 macrophages and primary peritoneal macrophages through IL-17, which was mediated by IL-17RA and IL-17RC. IL-17 did not induce either M1 or M2 macrophage differentiation. However, human lung cancer A549 cells strongly induced M2 macrophage differentiation of RAW264.7 macrophages when the two cell lines were co-cultured. The inductive factor secreted by A549 cells was identified to be prostaglandin E2.
Conclusions
IL-17 recruits macrophages and prostaglandin E2 induces M2 macrophage differentiation, hence the increased levels of IL-17 and prostaglandin E2 in lung cancer contribute to formation of an M2 macrophage-dominant tumor microenvironment.
Keywords: IL-17, PGE2, macrophage, COX-2, tumor microenvironment
Introduction
Lung cancer is the most common cause of cancer-related death worldwide. The American Cancer Society estimated that there were approximately 221,130 new cases and 156,940 deaths caused by lung cancer in the United States in 2011. Lung cancer is the second most common malignancy and the most common cause of cancer-related death in both American men and women.1 In China, the total estimated new lung cancer cases and deaths were 536, 407 and 475,768, respectively, in 2005.2
Tumor microenvironment plays an important role in lung cancer growth and metastasis. Tumor microenvironment includes tumor cells, fibroblasts, endothelial cells, macrophages, dendritic cells, and lymphocytes, as well as these cells’ products such as extracellular matrix, cytokines, chemokines, growth factors, enzymes, and cellular metabolites.3 Macrophages are critical in influencing tumor growth, angiogenesis, invasion, and metastasis, as they express growth factors, cytokines, chemokines, and enzymes.4 The tumor-associated macrophages (TAMs) are composed of heterogeneous populations with a spectrum of diverse biological properties.5 TAMs at the two ends of the spectrum are called M1 and M2 macrophages, respectively, mirroring the TH-1 and TH-2 nomenclature of T helper cells.5 Interferon-γ (IFN-γ), lipopolysaccharides (LPS), tumor necrosis factor α (TNFα), and granulocyte-monocyte colony-stimulating factor (GM-CSF) are known to induce monocytes to differentiate into M1 macrophages. M1 macrophages express high levels of inducible nitric oxide synthase (iNOS), TNFα, interleukin (IL)-1β, IL-6, IL-12, IL-18, IL-23, CXC ligand 10 (CXCL10), human leukocyte antigen (HLA)-DR, and reactive oxygen and nitrogen intermediates.5–9 IL-4, IL-10, IL-13, IL-21, activin A, immune complexes, and glucocorticoids induce monocyte differentiation into M2 macrophages.5 M2 macrophages express high levels of arginase I, IL-1 receptor antagonist (IL-1ra), IL-10, CC ligand 22 (CCL22), mannose receptor, galactose receptor, and CD163 antigen.5, 10 It is believed that M1 macrophages inhibit tumor growth by producing effector molecules such as reactive oxygen intermediates, reactive nitrogen intermediates, and TNFα, whereas M2 macrophages promote tumor growth and metastasis by secretion of growth factors, vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), and immunosuppressive cytokines/chemokines.6 The balance between M1 and M2 forms of macrophages determines the net anti- or pro-tumor effects of the TAM population.11
We have previously reported that the number of TAMs in the tumor islets is positively associated with survival time, whereas the number of TAMs in the tumor stroma is negatively associated with survival time in patients with non-small cell lung cancer (NSCLC).12 Approximately 70% of TAMs are M2 macrophages and the remaining 30% are M1 macrophages in NSCLC.13 It is not clear why there are more M2 macrophages than M1 macrophages in lung tumor microenvironment. In this study, we found that lung tumor tissues expressed significantly higher levels of IL-17 and PGE2 than normal lung tissues; IL-17 served as a chemoattractant to recruit macrophages into the lung tumors; and, PGE2 induced differentiation of M2 macrophages. These findings suggest that IL-17 and PGE2 are involved in creating an M2 macrophage-dominant tumor microenvironment in NSCLC.
Materials and Methods
Human tissue samples
The collection and use of human tissue samples were approved by the Institutional Review Board of West China Hospital, Sichuan University. The procedures were in accordance with the Ethical Principles for Medical Research Involving Human Subjects as formulated in the World Medical Association Declaration of Helsinki (revised in 2008). Forty-eight lung tumor specimens were obtained from the archives of formalin-fixed, paraffin-embedded tissue blocks in the Department of Thoracic Surgery, West China Hospital, Sichuan University. The tissues were collected from surgeries performed from August, 1999 to August, 2001. The patients were followed up until December, 2007, through outpatient visits and/or correspondences to family members. In addition, twenty fresh lung tumor samples and matched normal lung tissues were collected and used from December, 2010 to June, 2011. The normal lung tissues were sampled approximately 5 cm away from the edge of the lung tumors. Both the lung tumors and normal lung tissues were confirmed by pathological examination. Histological evaluation was based on the World Health Organization criteria.14 Tumor stage was evaluated according to the International Union against Cancer TNM classification system.15 Tumor grading criteria were published previously.12 The clinicopathological characteristics were summarized in Table 1. All lung cancer patients operated during the sampling period were included; the exclusion criteria are metastatic cancers, small cell cancers, chemotherapy or radiotherapy prior to surgery, and absence of follow-up (except the fresh samples). For the twenty pairs of fresh lung tumors and normal lung samples, no follow-up data were available at the time of this report.
TABLE 1.
Clinicopathological characteristics of patients with non-small cell lung cancer
| Variable | Archived Samples | Fresh Samples | ||
|---|---|---|---|---|
| N = 48 | 5-yr survival (%) | N=20 | ||
| Age years median (range) | 62 (37 – 80) | Overall = 33 | 58 (45–77) | |
| Gender (male : female) | 39: 9 | 14:6 | ||
| Tumor stage: | ||||
| I | 16 | 50 | 7 | |
| II | 9 | 22 | 3 | |
| III | 18 | 27 | 9 | |
| IV | 5 | 20 | 1 | |
| Histology | ||||
| Adenocarcinoma | 19 | 21 | 10 | |
| Squamous cell carcinoma | 29 | 41 | 10 | |
| Tumor grade | ||||
| Well differentiation | 1 | N/C | 0 | |
| Moderate differentiation | 26 | 42 | 9 | |
| Poor differentiation | 15 | 26 | 11 | |
| Not recorded | 6 | 16 | 0 | |
| Lymph node metastasis | ||||
| No | 31 | 45 | 10 | |
| Yes | 17 | 11 | 10 | |
N/C: not calculated; this patient lived for 4.5 years after surgery.
Immunohistochemical staining
Immunohistochemical staining (IHC) was performed as described previously.12 Rabbit anti-IL-17RC intracellular domain antibodies were described previously.16, 17 Rabbit anti-IL-17RA antibodies were purchased from Beijing Biosynthesis Biotechnology Co., Beijing, China. Mouse anti-human CD68 monoclonal antibodies (clone KP1) were obtained from Zhongshan Goldenbridge Biotechnology Co., Beijing, China. The tissue sections were stained with anti-IL-17RC (or anti-IL-17RA) and/or anti-CD68 antibodies, using the DouSP™ double-stain kit (Maxim-Bio, Fuzhou City, Fujian Province, China). Non-immune rabbit serum or mouse isotype IgG1 served as negative control. Development of red color was performed using streptavidin-peroxidase conjugate and 3-Amino-9-ethylcarbazole (AEC). Development of black-purple color was performed using streptavidin-alkaline-phosphatase conjugate and 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitro blue tetrazolium (NBT). Five representative high-power fields (× 400 magnification; area equals 0.44 mm2 per field) of tumor islets or stroma per tissue section were randomly selected using an Olympus BX51 microscope (Olympus, Tokyo, Japan). Tumor islet is a region in a tumor tissue section that contains a solid sheet of tumor cells side-by-side in a continuous manner (that is, a nest of tumor cells; also named tumor nest), giving an appearance of “island” extending into the “sea” (that is, the stroma). Tumor stroma is the space between individual sheets of tumor cells, which is often filled with fibroblasts and other types of cells. The number of macrophages that were double stained positive for IL-17RC and CD68 was counted by two investigators who were blinded in regard to the clinicopathological data. Macrophage density of each case was an average of the five high-power fields.
Measurement of IL-17 and PGE2 levels
Approximately 200 mg of fresh lung tumor or normal lung tissues were put into a Dounce homogenizer with 200 µl of radioimmunoprecipitation assay (RIPA) lysis buffer. After homogenization on ice, the protein extract was centrifuged at 14,000 g for 10 min at 4°C. The supernatant was stored at −80°C until measurement. To obtain conditioned media from cultured cell lines, human lung cancer cell lines (SPC-A-1, YTMLC-90, and A549) and mouse Lewis lung carcinoma cell line (LLC1) were cultured in serum-free DMEM for 24 h. The conditioned media were centrifuged at 14,000 g for 10 min at 4°C and the supernatants were stored at −80°C until usage. IL-17 levels in the tissue protein extracts were measured using a human IL-17 ELISA kit (Wuhan Boster Biological Technology, Wuhan, China) according to the manufacturer’s instructions. PGE2 levels in the tissue protein extracts and conditioned media were measured using a human PGE2 kit (Uscn Life Science Inc., Wuhan, China) according to the manufacturer’s instructions.
Isolation of mouse peritoneal macrophages
Animal protocol was approved by the Institutional Animal Care and Use Committee of Tulane University. IL-17RA knockout (KO) mice (C57BL/6 background) were provided by Amgen Inc., Seattle, WA.18 IL-17RC KO mice were generated in Dr. You’s laboratory at Tulane University using C57BL/6 derived embryonic stem (ES) cells purchased from the Texas Institute for Genomic Medicine, Houston, TX. A Bgeo trap sequence was inserted immediately upstream to the start codon of Il17rc gene to abolish Il17rc expression in the ES cells. The ES cells were injected into C57BL/6 albino mouse blastocysts. The resulting male chimeras were crossed to C57BL/6 females to generate mice heterozygous for the IL-17RC mutation (Il17rc+/−), which were subsequently intercrossed to generate IL-17RC KO mice (Il17rc−/−). Genotypes were confirmed by PCR using mouse tail DNA. About 1 ml of sterile 4% thioglycolate broth was injected into C57BL/6 wild-type (WT), IL-17RA KO, and IL-17RC KO mouse peritoneal cavities. Four days later, the mice were euthanized and 5 ml PBS was injected into the peritoneal cavities to collect the peritoneal macrophages as described.19
Cell migration assays
Transwell (Boyden chamber) assays were performed to exam migration of mouse RAW264.7 macrophages and peritoneal macrophages induced by tissues or recombinant mouse IL-17 (R&D Systems, Inc., Minneapolis, MN). Approximately 100 mg lung tumor or normal lung tissues were minced into 1-mm pieces and washed 3 times with PBS. Each sample was split equally into five lower chambers with 600 µl serum-free DMEM and cultured for 16 h in a 37°C, 5% CO2 humidified incubator. Then, 20,000 RAW264.7 cells in 200 µl serum-free DMEM were plated into each upper chamber for 2 h. The cells that migrated through the inserts were stained with 0.5% crystal violet and counted. To test if IL-17 in the tumor tissues was inducing macrophage migration, 5 µg/ml of mouse isotype control IgG or mouse anti-IL-17 neutralizing antibodies (R&D Systems) were added into the lower chamber 1 h prior to addition of RAW264.7 cells in the upper chamber. Similarly, 0.01 or 1 ng/ml rmIL-17 was added to serum-free DMEM in the lower chamber; 20,000 RAW264.7 cells or mouse peritoneal macrophages were loaded into the upper chamber for 2 h; and then, the migrated cells were counted.
Macrophage differentiation
RAW264.7 cells were treated without or with 50 ng/ml of recombinant mouse IL-4, IFN-γ, and/or IL-17 for 3 h. Real-time PCR was performed to determine the mRNA levels of M1 macrophage markers (iNOS, TNFα, and IL-18) and M2 macrophage markers (arginase I, IL-10, and IL-1ra) as described.17 In separate experiments, RAW264.7 cells were treated without or with 10, 100, and 500 nM of PGE2 (Sigma-Aldrich, St. Louis, MO) for 3 h, or with conditioned medium for 24 h; then, real-time PCR was performed. One million RAW264.7 cells were mixed and co-cultured with 1 million LNCaP (human prostate cancer cell line) or A549 cells in serum-free medium for 24 h, and real-time PCR was performed using the mouse-specific PCR primers (see sequences in Table 2), thus the human mRNAs from LNCaP and A549 cells in the co-culture system did not interfere with the real-time PCR analysis.
TABLE 2.
Primer sequences for real-time PCR
| Name | Forward | Reverse | Product (bp) |
|---|---|---|---|
| Gapdh | TaqMan® Rodent GAPDH Control Reagents (#4308313) from Applied Biosystems, Foster City, CA, sequences are proprietary, spanning exons 2 and 3 | 177 | |
| Nos2 (iNOS) | CAGCTGGGCTGTACAAACCTT | CATTGGAAGTGAAGCGTTTCG | 95 |
| Il18 | GACTCTTGCGTCAACTTCAAGG | CAGGCTGTCTTTTGTCAACGA | 169 |
| Tnf (TNFα) | CTACTCCCAGGTTCTCTTCAA | GCAGAGAGGAGGTTGACTTTC | 111 |
| Arg1 | TGGCTTGCGAGACGTAGAC | GCTCAGGTGAATCGGCCTTTT | 160 |
| Il10 | CTGGACAACATACTGCTAACCG | GGGCATCACTTCTACCAGGTAA | 108 |
| Ilrn (IL-1ra) | AAATCTGCTGGGGACCCTAC | TGAGCTGGTTGTTTCTCAGG | 164 |
Western blot
Protein extracts from lung tumors or normal lung tissues were analyzed with Western blot to determine levels of cyclooxygenase-2 (COX-2) and β-actin as described.17 The integrated density values of the protein bands were analyzed with Quantity One software (Version 4.6.5, Bio-Rad Laboratories, Hercules, CA). The ratios of COX-2/β-actin between lung tumors and normal lung tissues were compared. RAW264.7 cells were treated with control medium or conditioned medium from A549 cells and arginase I expression was determined by Western blot.
Characterization of cell lines
Human lung carcinoma cell line (A549), human prostate cancer cell line (LNCaP), mouse Lewis lung carcinoma cell line (LLC1), and mouse macrophage cell line (RAW264.7) were obtained from the American Type Culture Collection, Manassas, VA, from 2003 to 2010. These cell lines were frozen in liquid nitrogen within one month after receipt and were cultured for less than six months after resuscitation from frozen status. The methods of characterization can be found online from http://www.atcc.org. Human lung adenocarcinoma cell line SPC-A-1 and human lung squamous cell carcinoma cell line YTMLC-90 were originally characterized and reported in Chinese journals.20, 21 The two lung cancer cell lines were used widely by Chinese investigators.22, 23
Statistical analysis
Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS, version 13.0, SPSS Inc., Chicago, IL). For the 48 archived samples, the median number of macrophages was used as a cut-off point to dichotomize the data. The Kaplan–Meier survival curves were used to look for correlation between the macrophage number and patient’s survival time, or between the ratio of cells in the tumor islets versus stroma and patient’s survival time. Statistical significance was analyzed using the log-rank test. Data from the twenty fresh lung tumors and matched normal lung tissues were analyzed using paired Student’s t test. Student’s t test was used to analyze the remaining data. P < 0.05 was considered statistically significant.
Results
TAMs in human lung cancer express IL-17RC and IL-17RA
To investigate if human lung tumors express IL-17 receptor C (IL-17RC), immunohistochemical staining of IL-17RC was performed. Red blood cells and inflammatory cells were seen in the tumor sections, including macrophages with hemosiderin deposits within the cells (Fig. 1A). IL-17RC expression was not detectable in the tumor cells; however, macrophage-like cells were stained strongly positive for IL-17RC (Fig. 1B). To confirm that the IL-17RC-positive cells were macrophages, the tissue sections were double stained for IL-17RC (dark-purple in color) and macrophage marker CD68 (red in color). Indeed, the IL-17RC-positive cells were also stained positive for CD68 (Fig. 1C). Since IL-17RA is ubiquitously expressed in many cell types,24 the tumor sections were also double stained for IL-17RA and CD68. We found that CD68-positive macrophages and the tumor cells expressed IL-17RA (Fig. 1D).
Figure 1.
Tumor-associated macrophages expressed IL-17RC and IL-17RA. A, H&E staining of lung adenocarcinoma; open arrows, adenocarcinoma cells; arrows, macrophages with hemosiderin deposits; arrowheads, red blood cells. B, IHC of IL-17RC; open arrows, adenocarcinoma cells; arrows, macrophages. C, double staining of IL-17RC (dark-purple in color) and CD68 (red in color); open arrows, adenocarcinoma cells; arrows, macrophages; square boxes indicate macrophages in the tumor stroma and a circle indicates macrophages in the tumor islet. D, double staining of CD68 (dark-purple in color) and IL-17RA (red in color); open arrow, adenocarcinoma cells; arrows, macrophages; square boxes indicate macrophages in the tumor stroma and a circle indicates macrophages in the tumor islet. Original magnifications, 200× for photomicrographs and 400× for inserts. E to G, Kaplan-Meier survival curves demonstrate the number or ratio of macrophages in correlation to patient’s survival. Macrophage number represents an average number of IL-17RC/CD68 double stained cells in five high-power fields of each tumor specimen; a total of 48 specimens were separated into two groups by the median value, with one group having macrophage number or the ratio > median value and another group having macrophage number or the ratio < median value; statistical significance was analyzed using the log-rank test. The median number of macrophages was 23.4/mm2 in the tumor islets and 25.7/mm2 in the tumor stroma. The median ratio of macrophage number in the tumor islets divided by macrophage number in the tumor stroma was 0.9.
The number of macrophages is associated with patient’s survival
We counted the number of IL-17RC/CD68 double-positive macrophages in the tumor islets and stroma. The median number of macrophages was 23.4/mm2 (range 2.3 – 168.9/mm2) in the tumor islets and 25.7/mm2 (range 2.3 – 102.3/mm2) in the tumor stroma. The median ratio of macrophage number in the tumor islets divided by macrophage number in the tumor stroma was 0.9 (range 0.6 – 2.1). We found that the patients with above-the-median macrophage number in the tumor islets had a significantly better cumulative survival, compared to the patients with below-the-median macrophage number in the tumor islets (P = 0.034, Fig. 1E). In contrast, the patients with above-the-median macrophage number in the tumor stroma had a significantly worse cumulative survival, compared to the patients with below-the-median macrophage number in the tumor stroma (P = 0.042, Fig.1F). When the ratio of the macrophage number in the tumor islets versus the macrophage number in the tumor stroma was compared, the patients with above-the-median ratio (islets/stroma) had a significantly better cumulative survival, compared to the patients with below-the-median ratio (P = 0.000, Fig. 1G). These findings are consistent with our previous results.12
Lung tumors recruit macrophages through expressing high levels of IL-17
Since TAMs expressed both IL-17RA and IL-17RC receptors, we examined if IL-17 cytokine was also present in the lung tumors. We found that the mean IL-17 level was 25.3 pg/mg protein (95% confidence interval: 23.4 – 27.2 pg/mg protein) in normal lung tissues and 37.2 pg/mg protein (95% confidence interval: 33.9 – 40.5 pg/mg protein) in lung tumors. The IL-17 level in lung tumors increased by 47% compared to normal lung tissues, which was statistically significant (P = 0.010, Fig. 2A). It has been reported that IL-17 can recruit neutrophils into the rat airways,25 so we tested if lung tumors could attract migration of macrophages. We found that lung tumor tissues in ex vivo culture significantly stimulated migration of mouse RAW264.7 macrophages compared to normal lung tissues and serum-free medium (P = 0.023 or 0.017, Fig. 2B). The effects of lung tumor tissues on macrophage migration were inhibited by neutralizing IL-17 with anti-IL-17 antibodies (Fig. 2C). To further test if IL-17 is responsible for macrophage migration, recombinant mouse IL-17 was used instead of the lung tumor tissues in the migration assays. We found that IL-17 dose-dependently induced migration of RAW264.7 macrophages (Fig. 2D). Furthermore, we found that IL-17 induced migration of mouse peritoneal macrophages that were isolated from wild-type mice, but IL-17 failed to induce migration of mouse peritoneal macrophages that were isolated from IL-17RA KO or IL-17RC KO mice (Fig. 2E).
Figure 2.
Lung tumors recruited macrophages through expressing high levels of IL-17. A, IL-17 levels in twenty lung tumors and matched normal lung tissues. B, migration of RAW264.7 macrophages in Transwell assays; RAW264.7 macrophages were plated in the upper chamber and human tissues were placed in the lower chamber; results represent averages and standard errors (error bars) from twelve pairs of tissue samples. C, migration of RAW264.7 macrophages in Transwell assays; 5 µg/ml of mouse control IgG or anti-IL-17 neutralizing antibodies were added into the lower chamber 1 h prior to plating RAW264.7 macrophages in the upper chamber; results were from five pairs of tissue samples. D, migration of RAW264.7 macrophages in Transwell assays; recombinant mouse IL-17 was added in the lower chamber; results were from five independent experiments. E, migration of mouse peritoneal macrophages in Transwell assays; IL-17 was added in the lower chamber; results were representatives of three independent experiments.
Lung cancer cells induce M2 macrophage differentiation
To test if IL-17 can affect macrophage differentiation besides macrophage recruitment, we treated RAW264.7 macrophages with IL-4, INF-γ, and/or IL-17, as it has been shown that IL-4 and INF-γ play opposing roles in macrophage differentiation.26 We found that IL-4 induced expression of arginase I and IL-10 (markers of M2 macrophages), whereas INF-γ induced expression of iNOS (a marker of M1 macrophages) (Fig. 3A). In contrast, IL-17 did not induce any of the M1 or M2 macrophage markers (Fig. 3A). When IL-17 was combined with either IL-4 or INF-γ, IL-17 did not enhance the effects of IL-4 or INF-γ (Fig. 3B). To mimic macrophage differentiation after being recruited into the lung tumors, RAW264.7 macrophages were co-cultured with human lung cancer A549 cells and human prostate cancer LNCaP cells. We found that co-culture with A549 cells dramatically and significantly induced expression of arginase I and IL-10 by 4000 to 6000 fold in RAW264.7 macrophages, compared to co-culture with LNCaP cells (Fig. 3C). Expression of IL-1ra, iNOS, IL-18, and TNFα was only slightly induced by 2 to 6 fold (Fig. 3D). To test if any factors were secreted by A549 cells to induce M2 macrophage differentiation, the conditioned medium of A549 cells was used to treat RAW264.7 macrophages. We found that mRNA expression of arginase I and IL-10 was induced by the conditioned medium but not the control medium (Fig. 3E). In addition, when the conditioned medium was boiled for 10 min to denature any protein products, the boiled conditioned medium induced more arginase I and IL-10, compared to the conditioned medium (Fig. 3E). Furthermore, arginase I protein expression was also increased by 24-hour treatment with the conditioned medium and particularly the boiled conditioned medium (Fig. 3F).
Figure 3.
Lung cancer cells induced M2 macrophage differentiation. A to E, real-time PCR analysis of mRNA expression in RAW264.7 macrophages. A and B, RAW264.7 macrophages were treated without or with 50 ng/ml of IL-4, INF-γ, and/or IL-17 for 3 h. C and D, RAW264.7 macrophages were co-cultured with A549 or LNCaP cells for 24 h. E, RAW264.7 macrophages were treated with the culture medium (DMEM), conditioned medium from A549 cells (CM), boiled medium, or boiled CM for 24 h. F, Western blot analysis of arginase I expression in RAW264.7 macrophages treated with medium, boiled medium, CM, or boiled CM.
Lung tumors synthesize PGE2 to induce M2 macrophage differentiation
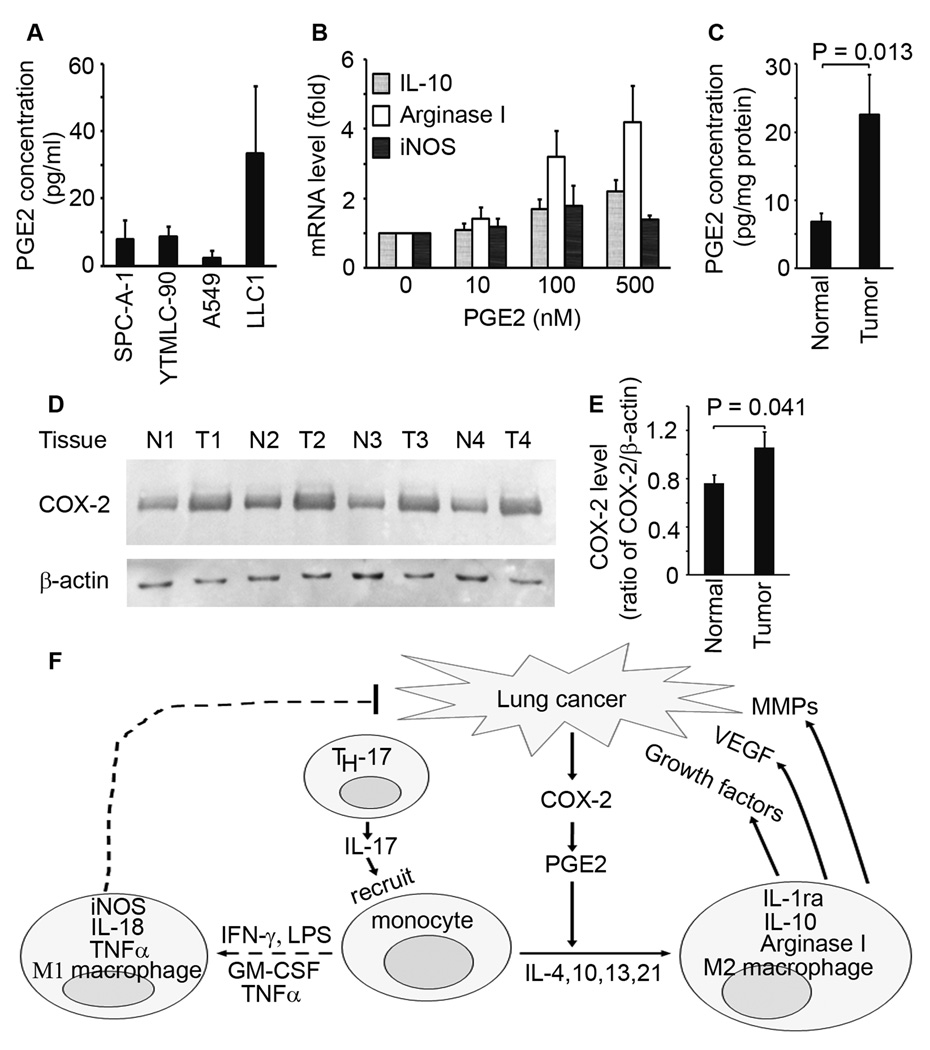
To investigate which factors are responsible for induction of arginase I expression, we searched the literature reports on proteins and metabolites that are produced by A549 cells. Since boiling did not impair the ability of the conditioned medium to induce arginase I, we speculated that the factor(s) must be heat-stable, thus ruling out all protein products such as chemokines/cytokines. It has been reported that prostaglandin E2 (PGE2), a heat-stable metabolite of arachidonic acid, can induce arginase activity in murine bone marrow-derived macrophages,27 and that murine Lewis lung carcinoma cells can produce PGE2 to induce arginase I expression in myeloid suppressor cells and peritoneal macrophages.28 Therefore, we measured PGE2 levels in the conditioned media from human and mouse lung cancer cell lines. We found that human lung cancer cell lines (SPC-A-1, YTMLC-90, and A549) and murine Lewis lung carcinoma cell line (LLC1) secreted PGE2 (Fig. 4A). We found that PGE2 dose-dependently induced arginase I and IL-10 expression in RAW264.7 macrophages (Fig. 4B). To determine if human lung tumors secrete PGE2, we measured PGE2 levels in twenty fresh lung tumor tissues. We found that human lung tumors had significantly higher levels of PGE2 compared to normal lung tissues (P = 0.013, Fig. 4C). Because cyclooxygnease-2 (COX-2) is a key enzyme in biosynthesis of prostaglandin H2 and subsequently PGE2, we measured COX-2 levels in twenty lung tumors. We found that lung tumors expressed significantly higher levels of COX-2 compared to normal lung tissues (Fig. 4D and 4E).
Figure 4.
Lung tumors synthesized PGE2 to induce M2 macrophage differentiation. A, PGE2 concentrations in the conditioned media from lung cancer cell lines; 1×105 cells were cultured in 5 ml of serum-free DMEM for 24 h and PGE2 levels in the media were determined by ELISA. B, real-time PCR analysis of mRNA expression in RAW264.7 macrophages treated without or with PGE2 for 3 h. C, PGE2 levels in twenty lung tumors and matched normal lung tissues were measured by ELISA. D, Western blot analysis of COX-2 expression in twenty lung tumors and matched normal lung tissues; β-actin was probed to normalize the amounts of loaded proteins; four representative pairs are shown; N, normal lung tissue; T, lung tumor. E, relative COX-2 levels are shown as ratios of COX-2/β-actin calculated by imaging analysis of protein bands on Western blots. F, schematic summary of macrophage recruitment and differentiation in lung cancer. TH-17 cells in lung tumors secrete IL-17 that recruits monocytes/macrophages into the tumor microenvironment; lung cancer cells express COX-2 and secrete PGE2; then, PGE2 and other cytokines (e.g., IL-4, IL-10, IL-13 and IL-21) induce monocytes/macrophages to differentiate into M2 macrophages that express IL-1ra, IL-10 and arginase I. IFN-γ, LPS, GM-CSF and TNFα induce monocytes/macrophages to differentiate into M1 macrophages that express iNOS, IL-18 and TNFα. M1 macrophages inhibit tumor growth, whereas M2 macrophages promote tumor growth and metastasis by secretion of growth factors, VEGF and MMPs. In lung cancer, the signals that induce M2 macrophage differentiation are more powerful than the signals that induce M1 macrophage differentiation, resulting in more M2 macrophages than M1 macrophages in the lung tumor microenvironment.
Discussion
The present study shows that tumor-associated macrophages in lung tumors express IL-17RC and IL-17RA, suggesting that macrophages may respond to IL-17 stimuli. Because IL-17RC staining alone or in combination with CD68 staining clearly identifies TAMs, IL-17RC appears to be a promising new marker for TAMs. Indeed, when we used IL-17RC/CD68 double staining to count the number of TAMs, we showed that the number of TAMs was correlated with patient’s survival. This finding is consistent with our previous study, in which CD68 staining alone was used to detect TAMs.12 In both studies, we found that the number of TAMs in the tumor islets is positively associated with prognosis, whereas the number of TAMs in the tumor stroma is negatively associated with prognosis. We speculate that this phenomenon is caused by a higher percentage of M2 macrophages in the tumor stroma than in the tumor islets. We have recently reported that approximately 70% to 85% of TAMs in the tumor stroma are M2 macrophages, whereas the percentages of M2 macrophages in the tumor islets vary from approximately 53% in the five-year survival group to 94% in the one-year survival group. 13 Since M2 macrophages promote tumor growth and metastasis, more M2 macrophages appear to correlate with poorer prognosis. It is worth pointing out that IL-17RC staining is unlikely to distinguish between M1 and M2 macrophages, as IL-17RC is co-expressed with CD68 in all TAMs.
Our data demonstrate that IL-17 is a chemoattractant for RAW264.7 macrophages and mouse peritoneal macrophages. IL-17’s chemotactic function is mediated through IL-17RA and IL-17RC as knockout of either receptor completely abolishes macrophage migration induced by IL-17. This is consistent with the literature report that a receptor heterodimer is preferred by IL-17 cytokines,29 thus, either IL-17RA KO or IL-17RC KO abolishes IL-17 signaling.18, 30 IL-17-induced macrophage migration is not due to increased chemokinesis, as macrophage migration was not increased when IL-17 was added to both the upper and lower chambers at the same concentration (data not shown). Because lung tumors express higher levels of IL-17 compared to normal lung tissues, we speculate that monocytes/macrophages may be recruited into the tumor microenvironment by IL-17. The source of IL-17 is likely from the IL-17-secreting T helper cells (TH-17 cells) in the tumor tissues rather than the tumor cells per se, as we did not detect any IL-17 protein secreted by the four lung cancer cell lines studied (data not shown) and other investigators found no IL-17 mRNA or protein expression in Sq-19 and A549 lung cancer cell lines.31 Our data show that lung tumor tissues can attract migration of RAW264.7 macrophages, which is mediated by IL-17 as anti-IL-17 neutralizing antibodies inhibit IL-17-mediated induction of macrophage migration. On the other hand, our data show that lung tumors express higher levels of COX-2 compared to normal lung tissues, leading to higher levels of PGE2 in the lung tumors. PGE2 induces expression of arginase I and IL-10, markers of M2 macrophages, in RAW264.7 macrophages. Taken together, our data suggest that, in the lung tumor microenvironment, monocytes/macrophages are recruited by chemoattractant IL-17 and subsequently PGE2 induces their differentiation into M2 macrophages (Fig. 4F). Thus, IL-17 and PGE2 contribute to creation of an M2 macrophage-dominant tumor microenvironment in lung cancer.13
It is worth pointing out that IL-17 and PGE2 are not the only factors that can affect the tumor microenvironment. Lung tumors express other chemokines and cytokines such as IL-8 and CSF1 that can recruit macrophages.32 IL-4, IL-10, TNFα, and INF-γ are also expressed in lung tumors.32 IL-4 and IL-10 can induce M2 macrophage differentiation and TNFα and INF-γ can induce M1 macrophage differentiation.5–9 Thus, monocytes/macrophages are exposed to complex signals to differentiate into either M1 or M2 macrophages (Fig. 4F). Based on our previous finding that 70% of TAMs are M2 macrophages,13 we speculate that the signals for M2 macrophage differentiation dominate the lung tumor microenvironment. This is supported by our data showing that M2 macrophage markers (arginase I and IL-10) are increased by 4000 to 6000 fold in RAW264.7 macrophages when the macrophages are co-cultured with human lung cancer cells, whereas M1 macrophage markers are induced by a maximum of 6 fold. Other investigators have also found that arginase I expression in mouse peritoneal macrophages is induced by co-culture with murine Lewis lung carcinoma cells.28 Of note, the conditioned medium of A549 cells is less effective in induction of arginase I and IL-10 in RAW264.7 macrophages than co-culture of A549 cells with RAW264.7 macrophages, suggesting that there may be some paracrine factors and/or direct intercellular interactions involved in macrophage differentiation in lung tumor microenvironment. Our data show that the boiled conditioned medium is more effective than the native conditioned medium, indicating that some counteracting heat-labile factors are deactivated during boiling, thus enhancing the effects of heat-stable factors such as PGE2. However, pure PGE2 appears to be less effective in induction of arginase I and IL-10 than the boiled conditioned medium, suggesting a possibility that other heat-stable factors may exist in the conditioned medium. It has been reported that PGE2 can induce differentiation of myeloid-derived suppressor cells from bone marrow stem cells33 and IL-10 secreted by myeloid-derived suppressor cells can skew macrophages toward an M2 phenotype.34 Thus, PGE2 may induce M2 macrophage differentiation through direct and indirect mechanisms in the in vivo tumor microenvironment.
In summary, the present study provides evidence that IL-17 and PGE2 contribute to formation of an M2 macrophage-dominant tumor microenvironment in lung cancer. Because M2 macrophages are believed to promote tumor growth and metastasis, IL-17 and PGE2 might be potential therapeutic targets in the treatment of lung cancer.
Acknowledgments
We thank Dr. Prescott L. Deininger (Director of Tulane Cancer Center) for his mentoring and support. This study used several Tulane Cancer Center Core Facilities. We thank Amgen Inc. for providing the IL-17RA knockout mice.
Financial support: This project was supported by the Centers of Biomedical Research Excellence (COBRE) grant from the National Center for Research Resources (P20RR020152) and the National Institute of General Medical Sciences (P20GM103518) from the National Institutes of Health, Department of Defense grants (W81XWH-05-1-0567 and W81XWH-10-1-0937), Developmental Fund of Tulane Cancer Center, Louisiana Cancer Research Consortium Fund, and Tulane University School of Medicine Research Pilot Fund (to Z.Y.); and by National Natural Science Foundation of China (NSFC 81172236), Key Science and Technology Program of Sichuan Province, China (2009SZ0152 and 2011SZ0111) (to L.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare no conflicts of interest.
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zhang S, Zou X. Evaluation on the incidence, mortality and tendency of lung cancer in China. Thoracic Cancer. 2010;1:35–40. doi: 10.1111/j.1759-7714.2010.00011.x. [DOI] [PubMed] [Google Scholar]
- 3.Witz IP, Levy-Nissenbaum O. The tumor microenvironment in the post-PAGET era. Cancer Lett. 2006;242:1–10. doi: 10.1016/j.canlet.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, et al. Tumour-derived microvesicles modulate biological activity of human monocytes. Immunol Lett. 2007;113:76–82. doi: 10.1016/j.imlet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 9.Martinez FO, Sica A, Mantovani A, et al. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 10.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Bottazzi B, Colotta F, et al. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 12.Dai F, Liu L, Che G, et al. The number and microlocalization of tumor-associated immune cells are associated with patient's survival time in non-small cell lung cancer. BMC Cancer. 2010;10:220. doi: 10.1186/1471-2407-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J, Liu L, Che G, et al. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. World Health Organization Classification of Tumours. [Google Scholar]
- 15.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 16.You Z, Dong Y, Kong X, et al. Differential expression of IL-17RC isoforms in androgen-dependent and androgen-independent prostate cancers. Neoplasia. 2007;9:464–470. doi: 10.1593/neo.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You Z, Shi XB, DuRaine G, et al. Interleukin-17 receptor-like gene is a novel antiapoptotic gene highly expressed in androgen-independent prostate cancer. Cancer Res. 2006;66:175–183. doi: 10.1158/0008-5472.CAN-05-1130. [DOI] [PubMed] [Google Scholar]
- 18.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu S, Su J, Wang E, et al. The establishment of human lung adenocarcinoma cell line SPC-A-1 and its biological characteristics. Science in China (Scientia Sinica) (Series B) (in Chinese) 1982;10:913–921. [Google Scholar]
- 21.Mao B, Zhang J, Ma Q, et al. The establishment of a human lung squamous cell carcinoma cell line YTMLC-90 from a miner of the Yunnan Tin Mine and study on its biological characteristics. Yunxi Keji (Yunxi Science and Technology) (in Chinese) 1994;21:50–53. 59. [Google Scholar]
- 22.Lu QJ, Huang CY, Yao SX, et al. Effects of fat-soluble extracts from vegetable powder and beta-carotene on proliferation and apoptosis of lung cancer cell YTMLC-90. Biomed Environ Sci. 2003;16:237–245. [PubMed] [Google Scholar]
- 23.Wang R, Wang ZX, Yang JS, et al. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14) Oncogene. 2011;30:2644–2658. doi: 10.1038/onc.2010.642. [DOI] [PubMed] [Google Scholar]
- 24.Yao Z, Spriggs MK, Derry JM, et al. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9:794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino H, Lotvall J, Skoogh BE, et al. Neutrophil recruitment by interleukin-17 into rat airways in vivo. Role of tachykinins. Am J Respir Crit Care Med. 1999;159:1423–1428. doi: 10.1164/ajrccm.159.5.9806008. [DOI] [PubMed] [Google Scholar]
- 26.Coccia EM, Stellacci E, Marziali G, et al. IFN-gamma and IL-4 differently regulate inducible NO synthase gene expression through IRF-1 modulation. Int Immunol. 2000;12:977–985. doi: 10.1093/intimm/12.7.977. [DOI] [PubMed] [Google Scholar]
- 27.Corraliza IM, Soler G, Eichmann K, et al. Arginase induction by suppressors of nitric oxide synthesis (IL-4, IL-10 and PGE2) in murine bone-marrow-derived macrophages. Biochem Biophys Res Commun. 1995;206:667–673. doi: 10.1006/bbrc.1995.1094. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez PC, Hernandez CP, Quiceno D, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ely LK, Fischer S, Garcia KC. Structural basis of receptor sharing by interleukin 17 cytokines. Nat Immunol. 2009;10:1245–1251. doi: 10.1038/ni.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Ota N, Peng I, et al. IL-17RC Is Required for IL-17A- and IL-17F-Dependent Signaling and the Pathogenesis of Experimental Autoimmune Encephalomyelitis. J Immunol. 2010;184:4307–4316. doi: 10.4049/jimmunol.0903614. [DOI] [PubMed] [Google Scholar]
- 31.Numasaki M, Watanabe M, Suzuki T, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 32.Seike M, Yanaihara N, Bowman ED, et al. Use of a cytokine gene expression signature in lung adenocarcinoma and the surrounding tissue as a prognostic classifier. J Natl Cancer Inst. 2007;99:1257–1269. doi: 10.1093/jnci/djm083. [DOI] [PubMed] [Google Scholar]
- 33.Sinha P, Clements VK, Fulton AM, et al. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 34.Sinha P, Clements VK, Bunt SK, et al. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]