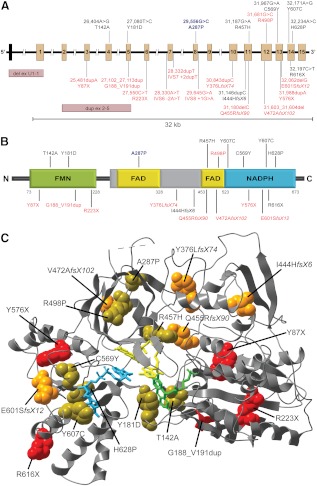
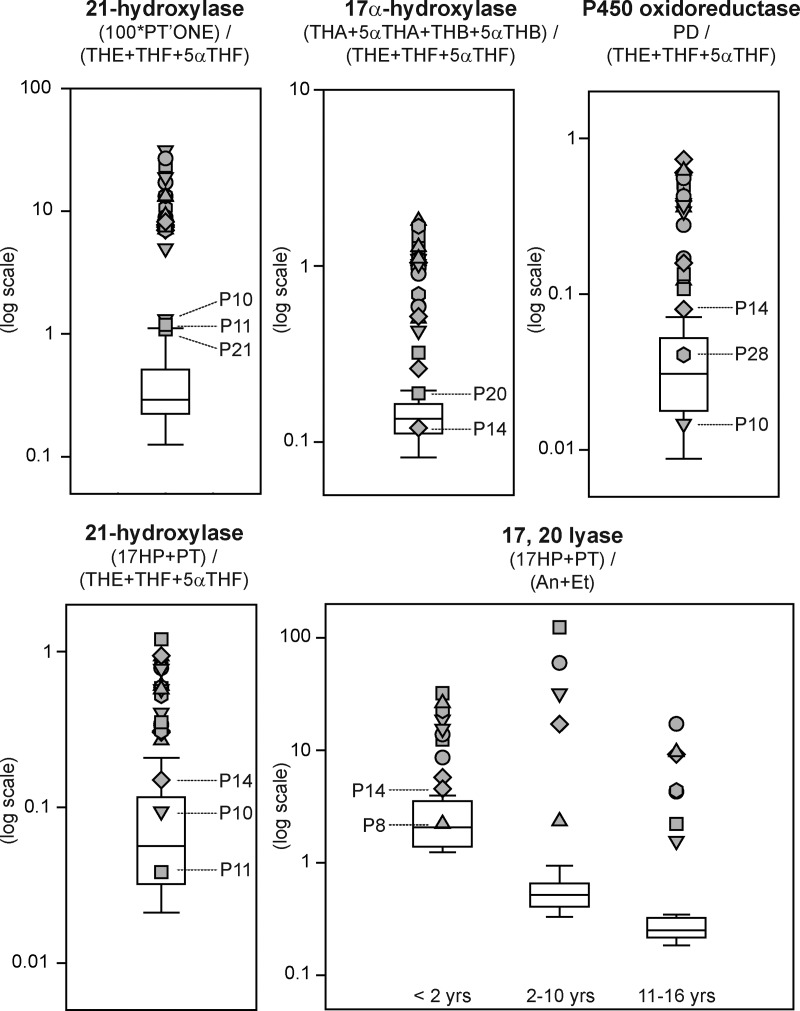
Nils Krone
Nils Krone
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Nicole Reisch
Nicole Reisch
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Jan Idkowiak
Jan Idkowiak
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Vivek Dhir
Vivek Dhir
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Hannah E Ivison
Hannah E Ivison
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Beverly A Hughes
Beverly A Hughes
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Ian T Rose
Ian T Rose
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Donna M O'Neil
Donna M O'Neil
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Raymon Vijzelaar
Raymon Vijzelaar
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Matthew J Smith
Matthew J Smith
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Fiona MacDonald
Fiona MacDonald
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Trevor R Cole
Trevor R Cole
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Nicolai Adolphs
Nicolai Adolphs
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
John S Barton
John S Barton
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Edward M Blair
Edward M Blair
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Stephen R Braddock
Stephen R Braddock
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Felicity Collins
Felicity Collins
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Deborah L Cragun
Deborah L Cragun
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Mehul T Dattani
Mehul T Dattani
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Ruth Day
Ruth Day
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Shelley Dougan
Shelley Dougan
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Miriam Feist
Miriam Feist
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Michael E Gottschalk
Michael E Gottschalk
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
John W Gregory
John W Gregory
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Michaela Haim
Michaela Haim
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Rachel Harrison
Rachel Harrison
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Ann Haskins Olney
Ann Haskins Olney
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Berthold P Hauffa
Berthold P Hauffa
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Peter C Hindmarsh
Peter C Hindmarsh
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Robert J Hopkin
Robert J Hopkin
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Petr E Jira
Petr E Jira
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Marlies Kempers
Marlies Kempers
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Michiel N Kerstens
Michiel N Kerstens
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Mohamed M Khalifa
Mohamed M Khalifa
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Birgit Köhler
Birgit Köhler
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Dominique Maiter
Dominique Maiter
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Shelly Nielsen
Shelly Nielsen
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Stephen M O'Riordan
Stephen M O'Riordan
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Christian L Roth
Christian L Roth
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Kate P Shane
Kate P Shane
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Martin Silink
Martin Silink
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Nike M M L Stikkelbroeck
Nike M M L Stikkelbroeck
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Elizabeth Sweeney
Elizabeth Sweeney
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Maria Szarras-Czapnik
Maria Szarras-Czapnik
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
John R Waterson
John R Waterson
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Lori Williamson
Lori Williamson
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Michaela F Hartmann
Michaela F Hartmann
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Norman F Taylor
Norman F Taylor
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Stefan A Wudy
Stefan A Wudy
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Ewa M Malunowicz
Ewa M Malunowicz
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Cedric H L Shackleton
Cedric H L Shackleton
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,
Wiebke Arlt
Wiebke Arlt
1Centre for Endocrinology, Diabetes, and Metabolism (N.K., N.R., J.I., V.D., H.E.I., B.A.H., I.T.R., D.M.O., C.H.L.S., W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom; MRC-Holland bv (R.V.), 1057-DN Amsterdam, The Netherlands; Clinical Genetics Unit (M.J.S., F.M., T.R.C.), Birmingham Women's Hospital, Birmingham B15 2TG, United Kingdom; Department of Craniofacial Reconstructive Surgery (N.A.) and Institute of Experimental Paediatric Endocrinology (B.K.), Charité, Universitätsmedizin Berlin, 12200 Berlin, Germany; Department of Paediatrics (J.S.B.), Royal Gwent Hospital, Newport NP20 2UB, United Kingdom; Department of Clinical Genetics (E.M.B.), Oxford Radcliffe Hospitals National Health Service Trust, Oxford OX3 9DU, United Kingdom; Division of Medical Genetics (S.R.B.), Department of Pediatrics, Saint Louis University School of Medicine, St. Louis, Missouri 63104; Departments of Clinical Genetics (F.C.) and Paediatric Endocrinology (M.S.), Children's Hospital at Westmead, Sydney 2145, Australia; Division of Human Genetics (D.L.C., R.J.H.), Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio 45229; Developmental Endocrinology Research Group (M.T.D., S.M.O., P.C.H.), UCL Institute of Child Health, University College London, London WC1E 6BT, United Kingdom; Department of Clinical Genetics (R.D., E.S.), Royal Liverpool Children's Hospital, Liverpool L12 2AP, United Kingdom; Division of Genetics and Molecular Medicine (S.D.), Kingston General Hospital, Kingston, Ontario, Canada K7L 2V7; Altonaer Kinderkrankenhaus (M.F.), 22763 Hamburg, Germany; Division of Endocrinology (M.E.G.), Department of Pediatrics, University of California, San Diego, School of Medicine, San Diego, California 92093; Department of Child Health (J.W.G.), Cardiff University, Cardiff CF10 3US, United Kingdom; Division of Neonatology (M.H.), University Children's Hospital, Graz 8036, Austria; Department of Clinical Genetics (R.H.), City Hospital, Nottingham University Hospitals NHS Trust, Nottingham NG5 1PB, United Kingdom; Munroe-Meyer Institute (A.H.O., S.N.), University of Nebraska Medical Center, Omaha, Nebraska 68198; Division of Paediatric Endocrinology and Diabetes (B.P.H.), University Children's Hospital Essen, 45122 Essen, Germany; Jeroen Bosch Ziekenhuis Hospital (P.E.J.), 5223 GZ Hertogenbosch, The Netherlands; Departments of Clinical Genetics (M.K.) and Endocrinology (N.M.M.L.S.), Radboud University Nijmegen Medical Centre, 6500 HC Nijmegen, The Netherlands; Department of Endocrinology (M.N.K.), University Medical Centre Groningen, 9700 RB Groningen, The Netherlands; Division of Medical Genetics and Metabolism (M.M.K.), Department of Pediatrics, Akron Children's Hospital, Akron, Ohio 44308; Department of Endocrinology (D.M.), University Hospital Saint Luc, B-1200 Brussels, Belgium; Division of Endocrinology and Diabetes (C.L.R.), Seattle Children's Research Institute, University of Washington, Seattle, Washington 98108; Division of Human Genetics (K.P.S.), Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio 43210; Departments of Endocrinology and Diabetology (M.S.-C.) and Biochemistry and Experimental Medicine (E.M.M.), The Children's Memorial Health Institute, 04-730 Warsaw, Poland; Children's Hospital and Research Center (J.R.W.), Oakland, California 94609; Department of Pediatrics (L.W.), University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104; Steroid Research Unit (M.F.H., S.A.W.), Centre of Child and Adolescent Medicine, Justus Liebig University, 35385 Giessen, Germany; and Department of Clinical Biochemistry (N.F.T.), King's College Hospital, University of London, SE5 9RS London, United Kingdom
1,✉