Abstract
Previous studies have shown that continuous exposure throughout gestation until the juvenile period to environmentally-relevant doses of trichloroethylene (TCE) in the drinking water of MRL+/+ mice promoted adverse behavior associated with glutathione depletion in the cerebellum indicating increased sensitivity to oxidative stress. The purpose of this study was to extend our findings and further characterize the impact of TCE exposure on redox homeostasis and biomarkers of oxidative stress in the hippocampus, a brain region prone to oxidative stress. Instead of a continuous exposure, the mice were exposed to water only or two environmentally relevant doses of TCE in the drinking water postnatally from birth until 6 weeks of age. Biomarkers of plasma metabolites in the transsulfuration pathway and the transmethylation pathway of the methionine cycle were also examined. Gene expression of neurotrophins was examined to investigate a possible relationship between oxidative stress, redox imbalance and neurotrophic factor expression with TCE exposure. Our results show that hippocampi isolated from male mice exposed to TCE showed altered glutathione redox homeostasis indicating a more oxidized state. Also observed was a significant, dose dependent increase in glutathione precursors. Plasma from the TCE treated mice showed alterations in metabolites in the transsulfuration and transmethylation pathways indicating redox imbalance and altered methylation capacity. 3-Nitrotyrosine, a biomarker of protein oxidative stress, was also significantly higher in plasma and hippocampus of TCE-exposed mice compared to controls. In contrast, expression of key neurotrophic factors in the hippocampus (BDNF, NGF, and NT-3) was significantly reduced compared to controls. Our results demonstrate that low-level postnatal and early life TCE exposure modulates neurotrophin gene expression in the mouse hippocampus and may provide a mechanism for TCE-mediated neurotoxicity.
Keywords: trichloroethylene, glutathione, hippocampus, neurotrophins, oxidative stress
1. Introduction
Trichloroethylene (TCE) is a volatile organic chemical used primarily as an industrial solvent. In 2008, the EPA's Toxic Release Inventory data base reported that 3.6 million pounds of TCE was released in the United States. Although its use has declined, TCE is still a persistent soil and water contaminant. Consequently, the potential for human exposure is likely and represents a significant public health concern. One of the predominant non-cancer health effects associated with exposure to TCE is neurotoxicity (Bale et al., 2011; Chiu et al., 2006). Acute exposure to concentrations of TCE associated with occupational exposure produces effects in the central nervous system (CNS) including deficits in sensory, cognitive, and motor function (Rasmussen et al., 1993). Although less is known about the impact of non-occupational TCE exposure in the CNS, epidemiologic studies have shown that environmental TCE exposure (e.g., consumption of contaminated well water) was associated with higher mean scores for depression, lower intelligence scores, memory recall, and various mood disorders (Reif et al., 2003; Kilburn et al., 1993). As far as developmental TCE exposure, children of mothers with occupational exposure to TCE had poorer visual acuity and lower composite neurobehavioral scores (Laslo-Baker et al., 2004; Till et al., 2001). Environmental TCE exposure has also been linked to autism and Parkinson's Disease (Gash et al., 2008; Windham et al., 2006). The fact that TCE can be detected in the blood of approximately 10% of adults and children in the US underscores an urgent need to determine underlying mechanisms of TCE toxicity (Adgate et al., 2004; Ashley et al., 1994; Sexton et al., 2005).
The perinatal brain is particularly vulnerable to environmental toxicants compared to the adult brain (Bayer et al., 1993; Dietert et al., 2008). While the neurobehavioral and neurotoxic effects of TCE have been studied extensively with regard to adult-only exposure, the mechanisms whereby TCE exerts its effects in the brain are not fully understood. In addition, there is limited information with regard to developmental TCE neurotoxicity and which region of the brain may be more prone to its effects. Evidence suggests that the hippocampal region may represent a sensitive functional target for TCE's effects. The first 12 days of life in rodents is approximately comparable to the third trimester of human gestation (Clancy et al., 2007; Dobbing, 1971; Quinn, 2005). During this period, the hippocampus undergoes a dramatic increase in size and a corresponding change in excitatory neurotransmission that underlies the maturation of synaptic plasticity by the end of the second postnatal week (Dumas, 2005). Hippocampal neurogenesis continues to occur throughout adulthood albeit at a lesser degree as compared to early development. Studies have documented selective hippocampal damage in rodents exposed developmentally to low-level TCE orally via the drinking water (i.e., 16-32 mg/kg/day). Both combined prenatal and neonatal (Isaacson et al., 1989) and neonatal-only exposure (Isaacson et al., 1990) was associated with a decrease in myelinated fibers in CA1 region of the hippocampus at weaning age. In contrast, there was no reported effect of TCE treatment in other brain regions. Other studies have shown changes in neuronal plasticity in hippocampus in vitro and in vivo with TCE exposure (Ohta et al., 2001; Altmann et al., 2002). Collectively both human and experimental research supports the hypothesis that TCE may impact the hippocampus, a dynamic and sensitive region of the brain that is involved in learning and memory.
It is not clear how TCE promotes hippocampal toxicity during developmental periods. One mechanism may involve the hippocampal region's inherent sensitivity to oxidative stress due to its higher rate of oxygen consumption and reactive oxygen species (ROS) generation compared to other brain regions such as the cerebellum (Uysal et al., 2012; Sato et al., 2010; Funke et al., 2011; Shankar, 2010). To compensate for this vulnerability, the hippocampus has developed mechanisms to combat oxidative stress. The tripeptide glutathione (γ-L-glutamyl-L-cysteinylglycine) functions as the major intracellular antioxidant against oxidative stress and plays an important role in the detoxification of ROS in the brain (Biswas et al., 2006; Filomeni et al., 2002; Bobyn et al., 2002; Jain et al., 1991; Anderson et al., 1989; Martensson et al., 1991). A decrease in the active reduced form of glutathione (GSH) and increase in the inactive oxidized disulfide form (GSSG) is a strong indicator of oxidative stress leaving the cell vulnerable to oxidative damage from pro-oxidant environmental exposures. Alterations in glutathione redox potential have been shown to modulate the fate of oligodendrocyte precursor cells (Noble et al., 2005), fetal cortical neurons,(Maffi et al., 2008) and maturing neurons (McLean et al., 2005) suggesting that altered brain redox status and increased oxidative stress could hinder neural development and promote developmental pathology.
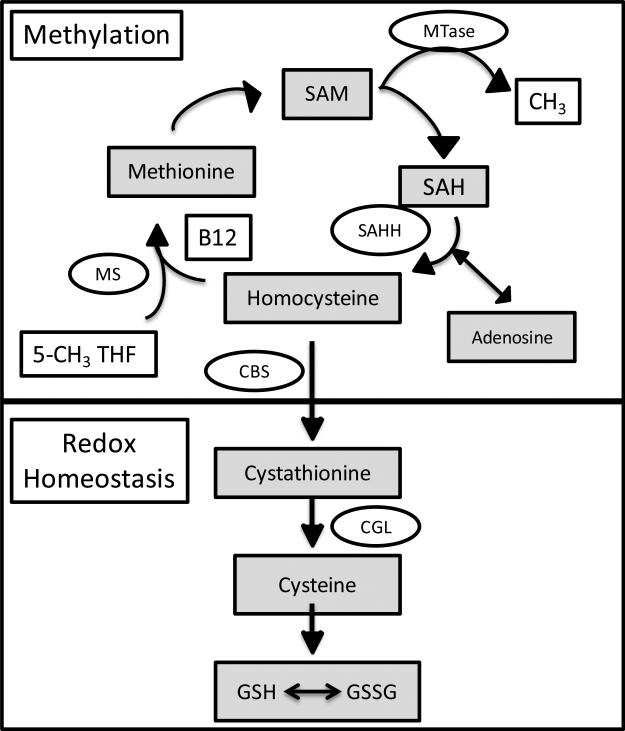
One crucial pathway that intersects with the transsulfuration pathway leading to glutathione synthesis is the methionine cycle [(i.e., transmethylation) [for review see (Selhub, 2002)]. The methionine cycle is important for over 100 essential cellular methylation reactions including DNA, RNA, protein, phospholipid and neurotransmitter methylation. As shown in Fig. 1, this pathway involves the regeneration of methionine from homocysteine via the B12-dependent transfer of a methyl group from 5-methyl-tetrahydrofolate (5-CH3 THF) by way of the methionine synthase (MS) reaction. Methionine is then converted to S-adenosylmethionine (SAM), the major methyl donor that participates in multiple cellular methylation reactions. Through the transfer of its methyl group, SAM is demethylated and converted to S-adenosylhomocysteine (SAH). The hydrolysis of SAH to homocysteine and adenosine completes the methionine cycle. Homocysteine can regenerate methionine for an additional methylation cycle by acquiring a new methyl group from 5-CH3 THF or can be irreversibly removed from the methionine cycle by cystathionine beta synthase (CBS) which initiates the transsulfuration pathway for the synthesis of cysteine and glutathione. From a functional standpoint, deficits in transmethylation metabolites can alter DNA methylation and ultimately impact cell differentiation, gene expression, and chromatin structure, as well as impact glutathione pathway components leading to oxidative stress (Castro et al., 2003; Caudill et al., 2001; Feil, 2006; Yi et al., 2000).
Fig. 1.
Folate-dependent methionine transmethylation and transsulfuration pathways involved in redox potential and cellular methylation.
Epigenetic mechanisms including DNA methylation are increasingly being recognized as functionally important regulators of gene expression, including neurotrophic factor gene expression, in the brain. Changes in these mechanisms can lead to adverse behavior.(Lubin et al., 2011; Numata et al., 2012; Chestnut et al., 2011; Wolstenholme et al., 2011) Neurotrophic factors, including Brain Derived Neurotrophic Factor (BDNF), Nerve Growth Factor (NGF), and Neurotrophin-3 (NT-3) are classically recognized as important mediators of neural growth and plasticity. BDNF, NGF, and NT-3, act by binding to their high affinity receptors TrkA, B, and C, respectively, and activate signaling pathways. Neurotrophin-receptor complexes are internalized and transported to cell bodies where they promote neuronal survival and differentiation (Reichardt, 2006). Increasing evidence suggests that neurotrophic factors can regulate glutathione redox status and maintain control of inflammation in the brain (Kapczinski et al., 2008; Wu et al., 2004; Sable et al., 2011). It has been shown that developmental exposure to toluene,(Win-Shwe et al., 2010) lead,(Chao et al., 2007) and infectious agents (Pang et al., 2010) increased biomarkers of oxidative stress and neuroinflammation that were associated with decreased expression of neurotrophins in regions of the brain including the hippocampus. Along this line, NGF has been shown to upregulate glutathione synthesis in mice,(Arsenijevic et al., 2007) and antioxidant therapy increased the expression of BDNF in hippocampus (Xu et al., 2011; Moriya et al., 2011; Liu et al., 2009). In disorders of the CNS such as autism, oxidative stress appears inextricably linked to the loss of neurotrophic support (Sajdel-Sulkowska et al., 2009; Sajdel-Sulkowska et al., 2011a). Collectively, these results suggest that normal functioning of the hippocampus involves a positive feedback loop between anti-oxidant processes and neurotrophic support in response to pro-oxidant exposures.
Our laboratory reported that continuous low-level TCE exposure from gestational day 0 through the juvenile period [postnatal day (PND) 42] promoted peripheral T cell activation in male, but not female mice (Blossom et al., 2007a; Blossom et al., 2008). Also observed was a significant depletion of glutathione in the cerebella of TCE treated, but not unexposed, male mice. The purpose of the current study was to determine whether low-level early life TCE exposure affected the glutathione redox potential, related methyl metabolism, and oxidative stress in the hippocampus of mice in association with a decrease in neurotrophic gene expression. The results obtained confirm an inverse association between oxidative stress and neurotrophin expression that supports our hypothesis that TCE exposure may affect glutathione and methyl metabolism via an epigenetic modulation of neurotrophin-related genes in the hippocampus.
2. Materials and Methods
2.1. Mice
Our laboratory has used the MRL+/+ strain (also known as MRL/MpJ mice) to characterize the ability of TCE to promote T cell hyperactivity and to accelerate an autoimmune response (Blossom and Doss, 2007a; Gilbert et al., 2006; Griffin et al., 2000). MRL+/+ mice are autoimmune-prone and develop a relatively mild lupus-like disease late in life. MRL+/+ mice have been used as controls for MRL/lpr mice that develop a fulminate, early-life lupus due to a well defined genetic mutation that is absent in the MRL+/+ strain. In addition to autoimmune manifestations, MRL/lpr mice, but not MRL+/+ mice, develop several behavioral deficits and neuropathological changes with age and are considered to be a model of idiopathic neurological lupus (Ballok, 2007; Ballok, 2007; Ballok et al., 2004; Sakic et al., 2005; Ma et al., 2006). However, recently the MRL+/+ strain has been identified as a novel model to study hippocampal neurogenesis. MRL+/+ mice apparently exhibit an enhanced response to pharmacologic agents that target neuroplasticity in the hippocampus over the response observed in C57BL/6 mice (Balu et al., 2009; Hodes et al., 2010). Given the autoimmune prevalence, immune dysfunction observed in neurologic disorders like autism (Cabanlit et al., 2007; Comi et al., 1999; Molloy et al., 2006; Sweeten et al., 2003), MRL +/+ were used in the current study to study TCE-induced hippocampal neurotoxicity.
As in our previous study, only male, but not female, offspring were evaluated here. The decision to exclude female offspring was based on our published evidence showing male mice were more sensitive than female mice to TCE-mediated neurologic effects (Blossom and Doss, 2007a; Blossom, et.al, 2008). The reason for this disparity is not known, but inherent differences in glutathione related enzymes in males vs. females may account for this difference (Chen et al., 2011) Gender sensitivity to neurologic disorders have been described and are clearly evidenced in autism, for example, where there is a 4 to 1 male to female gender bias (Charles, 2006). Since the experimental endpoints included an examination of neurotrophic factors, the decision to use male mice only was made final based on evidence that estrogen and neurotrophin receptors can apparently co-localize leading to convergence or cross-coupling of their signaling pathways (Singh et al., 1999).
Eight-week-old MRL+/+ breeder pairs were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were acclimated to the animal facility for one week before breeding cages were established. During breeding the mice were housed in standard polycarbonate cages and provided with drinking water and food ad libitum. The pregnant mice were housed in separate cages and provided with nesting material (Ancare, Bellmore, NY) and checked twice daily for the presence of pups. The date was recorded on the day the pups were born (PND 0) and the dams were then randomly assigned to treatment groups (0, 0.01, and 0.1 mg/ml TCE). TCE (purity 99+% from Sigma, St. Louis, MO) was suspended in drinking water with 1% of the emulsifier Alkamuls EL-620 from Rhone-Poulenc (Cranbury, NJ). Mice not receiving TCE were given water with 1% Alkamuls. The dams (six per treatment group) received a freshly made solution of TCE in their drinking water every 2-3 days beginning at PND0. At PND21, the offspring were weaned and the male mice continued their exposure to water or TCE in the drinking water until PND42. All studies were approved by the Animal Care and Use Committee at the University of Arkansas for Medical Sciences.
2.2. Extraction and HPLC Quantification of Hippocampal and plasma 3-Ntrotyrosine levels, and glutathione and Cysteine Redox Status
At PND42, one randomly selected male mouse in from each of 6 litters per treatment group was selected and deeply anesthetized with Isoflurane inhalant (Fisher). The anesthetized mouse was subjected to retro-orbital bleeding and the blood placed in heparinized tubes and processed as described (Blossom and Doss, 2007a). Mice were then sacrificed and the hippocampus was collected and flash frozen in liquid nitrogen. Samples were stored at -80°C until extraction. The methodological details for metabolite detection by HPLC have been described previously (Melnyk et al., 1999). The analyses were performed using HPLC with a Shimadzu solvent delivery system (ESA model 580) and a reverse phase C18 column (3 um; 4.6 × 150 mm, MCM, Inc., Tokyo Japan) obtained from ESA, Inc. (Chemsford, MA). Hippocampus and plasma extracts were directly injected onto the column using Beckman Autosampler (model 507E). All metabolites were quantified using a model 5200A Coulochem II and CoulArray electrochemical detection system (ESA, Inc., Chelmsford, MA) equipped with a dual analytical cell (model 5010), a 4 channel analytical cell (model 6210) and a guard cell (model 5020). The concentrations of 3-Nitrotyrosine and metabolites in hippocampus and plasma were calculated from peak areas and standard calibration curves using HPLC software. Intracellular results are expressed as nanomoles per milligram of protein using the BCA Protein Assay Kit (Pierce, Rockford, IL). Plasma results are expressed as micromoles per liter. The percentage of oxidized glutathione is expressed in absolute glutathione equivalents and calculated as [2GSSG/(GSH + 2GSSG) (Melnyk et al., 2011)].
2.3. Quantitative Real-time PCR
Fluorescence-based quantitative real-time PCR (qRT-PCR) was conducted using RNA isolated (using RNEasy, Qiagen, Germantown, MD) from hippocampi isolated from one randomly selected male from each litter. For each sample, complementary DNA (cDNA) was synthesized with random hexamers and 0.5 μg of total RNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA). PCR primer sequences were designed using the National Center for Biotechnology Information gene databases and synthesized by IDT, Inc., Coralville, IA using PrimerQuest. All qRT-PCR reactions were carried out using iQ SYBR Green Supermix (Bio-Rad) on an Applied Biosystems Veriti Model thermocyler (American Life Technologies, Carlsbad, CA) according to manufacturer's protocols. No-template controls were included to detect primer dimer artifacts. DNA melting curve analysis was used as another control against primer-dimer formation and to ensure that contaminating DNA was not present in the RNA preparations. Ct values >36 were not reported because of implied low efficiency. The relative gene expression was calculated using the delta-delta Ct method based on normalization to the reference gene Pgk1, known to be a reliable control for mouse brain regions during development, maturation, and aging (Boda et al., 2009) The Ct value of this reference gene did not vary more than 5% among the treatment groups and was found to be superior to other reference genes (e.g., 18S ribosomal RNA) in terms of copy number and treatment-associated invariance in the hippocampus. The results were reported as fold change in hippocampus from treated compared with expression in hippocampus from control mice.
2.4. Statistical Analysis
Statistical analysis was performed using the data analysis software, GraphPad Prism 5.0 (LaJolla, CA). All data were expressed as mean ± standard deviation (SD). One way ANOVA with a Dunnett's Multiple Comparison Test was used to determine significant differences between control vs. low or high treatment groups. Treatment related differences were considered to be significant at p<0.05.
3. Results
3.1. Water consumption and physical characteristics
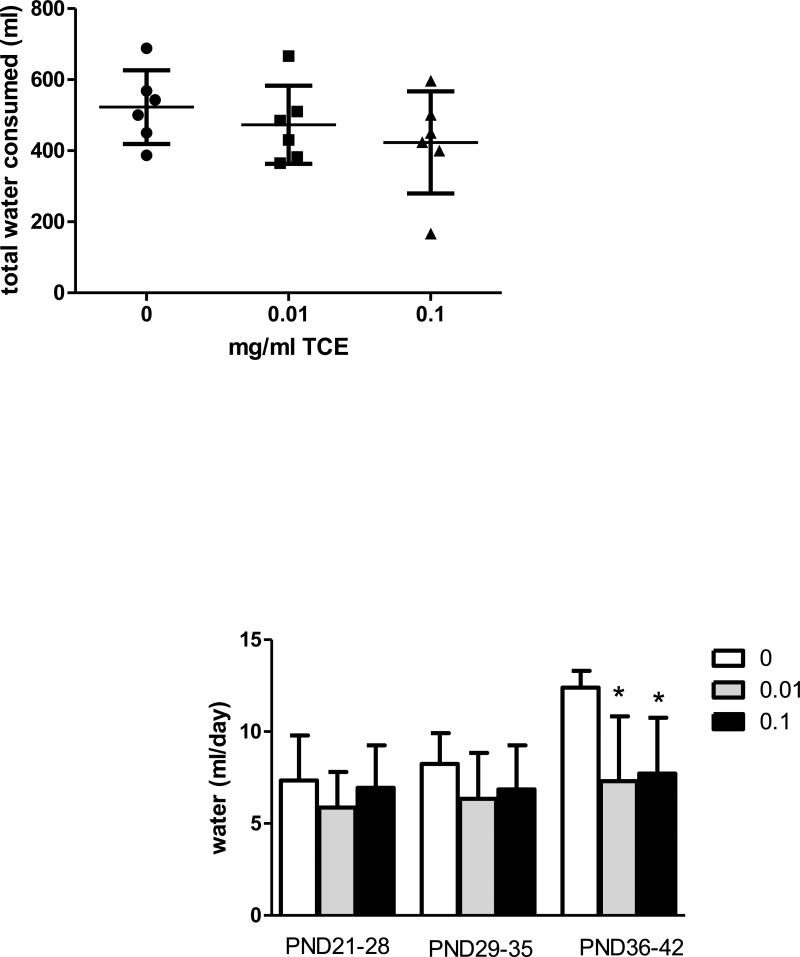
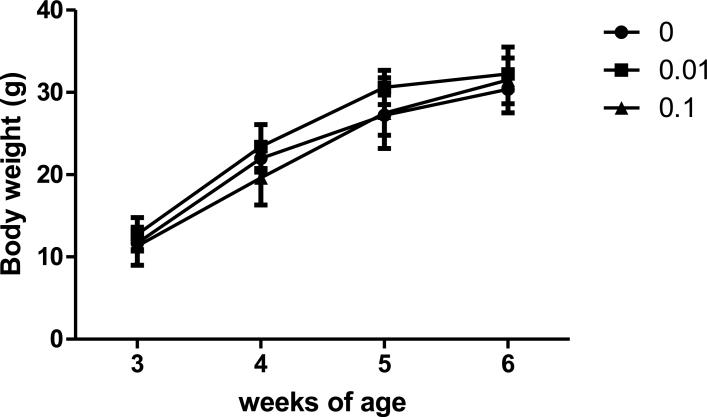
Water consumption was measured in the dams (PND1-20) and in male mice after weaning (PND21-42). There were no statistically significant differences in water consumption among the treatment groups in the dams Fig. 2A. In the offspring, a significant decrease in water consumption was observed in both TCE-treated groups compared to controls from PND36-PND42 (Fig. 2B). Because the TCE-treated mice exhibited normal growth and body weight as compared to controls (Fig. 3), and no signs of dehydration (i.e., piloerection, lethargy or recessed eyes) were evident it appeared that the decreased water consumption in offspring at PND36-42 did not induce a significant functional impact.
Fig.2. Effects of TCE on water consumption in dams and male offspring.
Water consumption was monitored over time. Figure 2A presents the total volume of water (ml) the dams (n=6) consumed from PND1-PND20; Figure 2B represents the volume of water (ml/day) the male offspring consumed from weaning until sacrifice (PND21-PND42). The data is presented as mean ± SD per mouse per day. *Statistically different (p<0.05) from the results obtained from control mice (0 TCE) at PND36-42.
Fig.3.
Effects of TCE on Body Weight. Body weight was measured each week in individual male offspring each week from weaning until sacrifice. The weights of the male pups in each litter were averaged. Data points represent the mean ± SD of weights in each litter (n=6 per treatment group).
3.1.1. Altered redox homeostasis in hippocampus of TCE exposed mice
The data presented in Table 1 shows the concentrations of glutathione (GSH), oxidized glutathione disulfide (GSSG), the glutathione redox ratio, and the percentage of oxidized glutathione equivalents in hippocampus of PND42 male mice. Compared to control mice, the GSH concentration and GSH/GSSG ratio in hippocampus of mice exposed to 0.1 mg/ml TCE was significantly decreased. Similarly, the percent oxidized glutathione was significantly increased by ~25% in the highest exposure group.
Table 1.
Comparison of transsulfuration metabolites in hippocampus between control and TCE-treated mice.
| Glutathione and Cysteine Redox Status in hippocampus (nmol/mg/protein) | 0 mg/ml TCE | 0.01 mg/ml TCE | 0.1 mg/ml TCE |
|---|---|---|---|
| GSH | 28.98 ± 4.25 | 27.95 ± 2.93 | 25.15 ± 1.81* |
| GSSG | 0.35 ± 0.11 | 0.39 ± 0.09 | 0.41 ± 0.03 |
| GSH/GSSG ratio | 86.97 ± 20.64 | 75.02 ± 23.33 | 62.60 ± 6.68* |
| Oxidized GSH (%) | 2.34 ± 0.58 | 2.77 ± 0.72 | 3.11 ± 0.30* |
| Cysteine | 5.18 ± 0.87 | 5.57 ± 0.34 | 5.09 ± 0.73 |
| Cystine | 5.34 ± 1.47 | 7.54 ± 0.94** | 7.71 ± 1.58* |
| Cysteine/Cystine ratio | 1.03 ± 0.33 | 0.75 ± 0.12* | 0.69 ± 0.19* |
Data is presented as mean ± SD.
p≤0.05 is statistically significant
p<0.001.
Also shown are concentrations of cysteine, cystine, and the cysteine/cystine redox ratio. There was a significant dose dependent increase in cystine, the oxidized form of cysteine, with TCE exposure. Similarly, the cysteine/cystine redox ratio was significantly decreased in a dose dependent manner (~28% and ~34%; 0.01 and 0.1 mg/ml TCE, respectively). Collectively these data reveal a more oxidized environment in the hippocampus of TCE-treated mice.
3.1.2. Increased hippocampal CysGly in TCE-exposed mice
CysGly is increasingly being recognized one of the major precursors of neuronal glutathione. Therefore, concentrations of this dipeptide were measured in the hippocampus. As shown in Fig. 4, CysGly levels significantly increased with TCE treatment in a dose dependent manner; ~59% and ~74% increase with 0.01 and 0.1 mg/ml TCE, respectively over that of controls.
Fig. 4.
CysGly levels are elevated in hippocampus of TCE-exposed mice. Metabolites in the transsulfuration pathway in hippocampus were measured by HPLC and electrochemical detection as described in the methods. Presented is nmol/mg protein of hippocampal CysGly levels in control and TCE-exposed male offspring at PND42 (n=6 per treatment group). *Statistically different from the results obtained from control mice (p <0.001).
3.1.3. TCE exposure alters transmethylation and transsulfuration metabolites in plasma
Studies have shown abnormal plasma levels of metabolites in the transmethylation and transsulfuration pathways in humans with neurologic disorders, but it has not been possible to examine a corresponding association with abnormal redox status in the brain. Thus, plasma levels of transmethylation and transsulfuration metabolites were evaluated in order to see if the reduced anti-oxidant detoxification status observed in TCE-exposed hippocampus correlated with an abnormal peripheral (i.e., plasma) metabolic profile. As shown in Table 2, the highest concentration of TCE was associated with a significant decrease in methionine levels. Mean levels of SAM were decreased by ~39%. In contrast, SAH, a potent product inhibitor of cellular methyltransferase enzymes was significantly increased by ~38%. The dramatic decrease in the SAM/SAH ratio (~42% and ~60%; at 0.01 0.1 mg/ml TCE, respectively), suggests that TCE promoted metabolic deficits in cellular methylation capacity. A corresponding TCE-mediated significant increase in homocysteine, the hydrolysis product of SAH, was also found in mice treated with the highest dose of TCE. The combined levels of precursor methionine + homocysteine were lower in TCE treated mice compared to controls indicating that the total intermediates dedicated to methylation capacity are lower in TCE-treated mice than in controls. Similarly, SAM + SAH levels were lower in TCE-treated mice than in controls.
Table 2.
Comparison of plasma metabolite concentration in the transmethylation pathway between control and TCE-treated mice.
| Plasma Transmethylation Metabolites | 0 mg/ml TCE | 0.01 mg/ml TCE | 0.1 mg/ml TCE |
|---|---|---|---|
| Methionine (μM) | 39.4 ± 5.36 | 34.1 ± 7.94 | 29.2 ± 6.02* |
| SAM (nM) | 155 ± 36.02 | 120.9 ± 14.9 | 94.82 ± 22.47** |
| SAH (nM) | 26.32 ± 5.26 | 34.3 ± 6.39 | 41.91 ± 10.95* |
| SAM/SAH ratio | 6.20 ± 2.4 | 3.61 ± 0.68* | 2.51 ± 1.31** |
| SAM + SAH | 181.4 ± 34.26 | 155.2 ± 16.76 | 136.7 ± 15.41* |
| Homocysteine (μM) | 1.47 ± 0.22 | 1.54 ± 0.16 | 2.05 ± 0.56* |
| Methionine + homocysteine | 40.87 ± 5.41 | 35.64 ± 7.04 | 31.38 ± 5.65* |
Data is presented as mean ± SD.
p≤0.05 is statistically significant.
p<0.001.
Plasma metabolites in the transsulfuration pathway are shown in Table 3. Both total GSH, a measure of combined protein-bound and free GSH after reduction of disulfide bonds, and free GSH which reflects unbound GSH remaining after protein precipitation were measured. Both total and free GSH were significantly lower in mice treated with TCE compared with controls. Correspondingly, both total and free GSH/GSSG ratios were also significantly lower (high TCE compared to controls). The levels of cystine were significantly increased by ~59% and ~76%; 0.01 mg/ml and 0.1 mg/ml TCE, respectively compared to controls. Total cysteine and the cysteine/cystine redox ratio were significantly decreased in TCE-treated groups. Taken together these data reflect impaired redox and methylation status in the plasma and reflect significant alterations in the transsulfuration pathway metabolites in the hippocampus.
Table 3.
Comparison of plasma metabolite concentration in the transsulfuration pathway between control and TCE-treated mice.
| Plasma Transsulfuration Metabolites | 0 mg/ml TCE | 0.01 mg/ml TCE | 0.1 mg/ml TCE |
|---|---|---|---|
| Free GSH | 4.97 ± 0.92 | 3.41 ± 0.78* | 2.32 ± 1.26** |
| GSSG | 0.79 ± 0.25 | 0.78 ± 0.28 | 0.94 ± 0.42 |
| Free GSH/GSSG ratio | 6.71 ± 1.75 | 4.73 ± 1.82 | 2.51 ± 0.57** |
| Total GSH | 13.96 ± 1.84 | 10.92 ± 1.49* | 9.46 ± 1.58** |
| Total GSH/GSSG ratio | 18.96 ± 4.84 | 15.19 ± 5.03 | 11.48 ± 4.31* |
| Cystine | 2.28 ± 1.10 | 5.50 ± 1.81* | 9.15 ± 3.15** |
| Free Cysteine | 1.95 ± 0.78 | 2.32 ± 0.68 | 3.22 ± 0.96* |
| Free Cysteine/Cystine ratio | 0.98 ± 0.18 | 0.43 ± 0.09** | 0.36 ± 0.03** |
| Total Cysteine | 121.3 ± 20.76 | 141 ± 27.01 | 116.20 ± 10.19 |
| Total Cysteine/Cystine ratio | 63.68 ± 32.64 | 27.96 ± 9.74** | 14.07 ± 0.237** |
Data is presented as mean ± SD.
p≤0.05 is statistically significant
P<0.001.
3.1.4. TCE exposure enhances a biomarker of protein reactive nitrogen species in the hippocampus and plasma
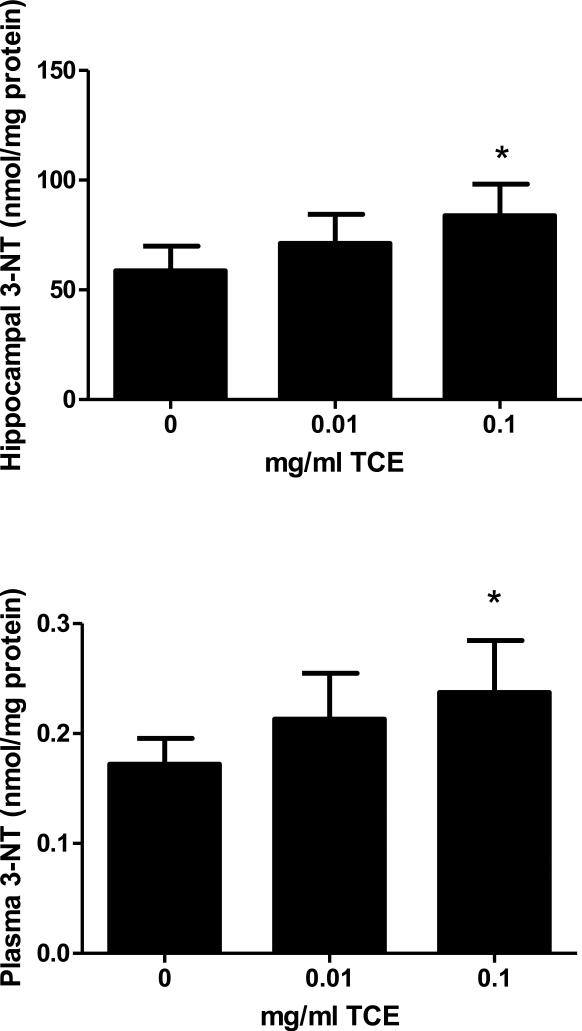
Tyrosine nitration is a common protein modification that occurs in disease associated with oxidative stress (Andreazza et al., 2009). To determine whether or not impaired intracellular anti-oxidant capacity and methylation potential was associated with protein oxidative damage, the level of 3-Nitrotyrosine was measured in the plasma and hippocampus. 3-Nitrotyrosine concentrations were increased in a dose-dependent manner in both hippocampus (Fig 5A) and plasma (Fig. 5B) indicating increased oxidative stress and protein damage in mice exposed to 0.1 mg/ml TCE.
Fig. 5.
Increased oxidative stress in TCE-exposed mice. 3-Nitrotyrosine, a biomarker of oxidative stress, was measured in the hippocampus (Figure 5A) and plasma (Figure 5B) by HPLC and electrochemical detection. Presented is nmol/mg protein of 3-Nitrotyrosine levels in control and TCE-exposed male offspring at PND42 (n=6 per treatment group). *Statistically different from the results obtained from control mice (p<0.05).
3.1.5. TCE exposure downregulates the expression of neurotrophic factors in hippocampus
Expression of three major neurotrophins, BDNF, NGF, and NT-3 were evaluated in hippocampus of control and TCE-exposed mice by real time qRT-PCR. There was a statistically significant dose-dependent downregulation in the expression of BDNF, NGF and NT-3 mRNA in hippocampus of TCE exposed mice as compared with controls (Fig. 6). These data suggest a mechanistic link between increased oxidative stress and decreased neurotrophin expression in the brain and may be functionally important in certain neurologic disorders.
Fig. 6.
TCE exposure decreases neurotrophic factor gene expression in hippocampus. qRT-PCR was performed using hippocampus samples from 6 mice in each treatment group collected at PND42. *Statistically different from results obtained from control mice for each neurotrophic factor (p<0.05).
4. Discussion
Our laboratory has previously shown that continuous TCE exposure (i.e., gestation until PND 42) altered glutathione redox status in the cerebellum (Blossom, et.al., 2008). In the present study, we switched our focus to the hippocampal region which is more prone to oxidative stress than other brain regions. The study employed two different doses of TCE and an exposure period of 42 days (postnatal day 1-42) that included a period of lactational exposure and direct exposure to TCE via the drinking water. Based on water intake, body weight, and measured TCE degradation in the water bottles from weaning (PND21) until sacrifice (PND42) the mice exposed to water or TCE present 0.01 or 0.1 mg/ml were directly exposed to an average of 2.0 and 28 mg/kg/day respectively. In humans the occupational exposure limit for TCE is 100 ppm or approximately 76 mg/kg/day. Thus, the concentrations of TCE used in the current study were considerably lower than the current 8-h exposure limit. Although the exposure levels used in this study are higher than the Agency for Toxic Substances and Disease Registry (ASTDR) Minimal Risk Levels (MRL) for oral exposure to TCE (0.2 mg/kg/day), the oral exposure to TCE represents only a fraction of the TCE absorbed via inhalation and dermal contact (Lee et al., 2002) Thus, since the mice were exposed to oral TCE it could be argued that the TCE exposure levels in this study are comparable to total human exposures. Water intake of the control and TCE-exposed dams was measured from PND1-PND20 (gestation and lactation). There was no significant difference in water consumption among the treatment groups. However, TCE levels in the dams were not calculated during this period. This decision was based on the concern that the handling (i.e., weighing) required to calculate mg/kg/day TCE during the lactational period would induce maternal stress and result in cannibalization of the pups or impact neurologic outcomes in the offspring as shown by others (Mueller et al., 2008; Lee et al., 2007; Ellenbroek et al., 1998).
The goal of this study was to examine how TCE exposure affected biomarkers of methylation metabolism, oxidative stress and glutathione redox capacity, as well as neurotrophic factors associated with neuroprotection in the hippocampus of juvenile male mice. Our results clearly show that TCE exposure dose-dependently alters glutathione redox status in the brain by decreasing GSH and the GSH/GSSG ratio and increasing the percentage of GSSG. Neurons are unable to complete the transsulfuration pathway to synthesize glutathione due to insufficient quantities of cysteine, the rate limiting precursor in glutathione synthesis (Bannai, 1984). Therefore, the extracellular redox pair, cysteine and cystine, as well as the important neuronal GSH precursor, CysGly were also evaluated in the current study. Levels of oxidized cystine were significantly elevated, and the cysteine/cystine redox couple was significantly decreased in the hippocampus of TCE-exposed mice indicating an oxidized state. Interestingly, the CysGly GSH precursor was significantly elevated in the hippocampus of TCE-exposed mice. In the brain, CysGly is generated from glutathione released from astrocytes in the extracellular space. Although the mechanism by which CysGly is utilized by neurons is not completely understood, it is apparently hydrolyzed to cysteine and glycine and taken up into neurons via sodium-dependent transport processes to complete synthesis of neuronal glutathione. An increase in CysGly as was shown in this study could indicate that CysGly is being generated in order to compensate for TCE-mediated glutathione depletion. Alternatively, TCE may somehow inhibit the uptake or impact the exchange systems responsible for cystine or CysGly transport into cells. This mechanism could lead to glutamate toxicity, glutathione depletion, and oxidative stress. Clearly any event that interferes with glutathione homeostasis in the brain including cystine or cysteine uptake, glutathione release or utilization of CysGly or cysteine by neurons would, therefore, likely decrease neuronal glutathione levels and increase their sensitivity to oxidative stress. Such a mechanism has been demonstrated with methylmercury's ability to disrupt astrocyte transport of cystine and cysteine (Shanker et al., 2001b; Shanker et al., 2001a; Allen et al., 2001). Further study is needed to determine whether TCE modulates this process in astrocytic and neuronal cultures.
Consistent with our observation of altered glutathione redox status, we also observed increased 3-Nitrotyrosine levels with TCE exposure. The reaction of superoxide anion and nitric oxide to produce peroxynitrite modifies protein tyrosine residues to generate 3-nitrotyrosine. Formation of reactive nitrogen species is presumed to play a major role in neuronal cell death and the presence of 3-nitrotyrosine is presumed to be a biomarker of this event (Nakazawa et al., 2000; Butterfield et al., 2008). This result provides additional functional evidence of a redox imbalance with TCE exposure, and also suggests that 3-nitrotyrosine levels in the plasma corresponded with brain and could potentially be an excellent candidate for a biomarker of brain oxidative stress observed in human disease. The significance of this finding is underscored by the presence of this biomarker in patients with neurologic disorders including Parkinson's disease,(Mythri et al., 2011) Alzheimer's disease (Butterfield et al., 2006) and autism (Sajdel-Sulkowska et al., 2011b).
Plasma biomarkers of methylation capacity were evaluated in the current study due to the interrelated transsulfuration and transmethylation pathways (see Fig 1). In an earlier study in our laboratory, a metabolomics analysis revealed a significant increase in cystathionine and decreased SAH in liver of adult mice chronically exposed to moderate levels of TCE (Gilbert et al., 2009). The ratio of SAM: SAH is frequently used as an indicator of cellular methylation capacity whereby a decrease in this ratio as shown in our results would predict reduced methylation potential. In contrast, the effect of TCE on methylation dependent expression of neurotrophins would suggest hypermethylation. The apparent contradiction of a generalized TCE-induced hypomethylation and possible hypermethylation of specific genes in the same tissues or cells has been described. Global hypomethylation accompanied by gene-specific CpG island promoter hypermethylation has been documented in several human conditions including cancer, atherosclerosis, and aging (Dunn, 2003; Turunen et al., 2009).
Epigenetic mechanisms are crucial for the functional expression of neurotrophic genes (Fuchikami et al., 2011; Pittenger, 2011; Branchi et al., 2011; Roth et al., 2011). Based on our transmethylation metabolites results in plasma, studies are planned to examine whether TCE alters the methyl metabolism and methylation status of neurotrophins in the hippocampus. Emerging evidence suggests that developmental exposure to toxicants such as polycyclic aromatic hydrocarbons and bisphenol A may promote neurodevelopmental disease via epigenetic mechanisms (Perera et al., 2011). Studies by others have shown that exposure to methylmercury (Onishchenko et al., 2008) and ethanol (Boehme et al., 2011; Heaton et al., 2011; Kulkarny et al., 2011) modulate hippocampal BDNF in a DNA methylation dependent manner.
The effects of TCE on DNA methylation are not well defined. Low- level TCE (10 ppb) suppressed the expression of the cardiac gene Serca2a in association with hypermethylation of its promoter region (Palbykin et al., 2011). In contrast, acute high dose TCE (1000 mg/kg/day) decreased methylation of the promoter regions for c-jun and c-myc in the liver and a corresponding increased expression of these genes was reversed by supplemental methionine (Tao et al., 1999; Tao et al., 2000). More recently, studies from our laboratory found that CD4+ T cells from adult mice exposed to TCE for a shorter time period (i.e., 12 weeks) demonstrated alterations in key factors associated with both DNA methylation and hypomethylation depending upon duration of exposure (Gilbert et al. in press). Thus the effects of TCE on DNA methylation appear to be tissue- and concentration-specific and therefore difficult to generalize.
It is currently not clear how TCE impacts transsulfuration and transmethylation pathways leading to oxidative stress. One potential mechanism may involve the activity of TCE's reactive metabolite, trichloroacetaldehyde hydrate (TCAH). TCE is metabolized primarily by the cytochrome P-450s isoform CYP2E1 to a trichloroethylene oxide intermediate, which spontaneously rearranges to TCAH. TCAH is a highly reactive aldehyde that has been proposed to spontaneously condense with the biogenic amine tryptamine to produce an alkaloid-type neurotoxin (Bringmann et al., 1990). Our lab has studied the ability of TCAH extensively to form adducts with T cells and promote their activation in vitro and in vivo (Blossom et al., 2004; Blossom et al., 2006a; Blossom et al., 2006b; Blossom et al., 2007b; Gilbert et al., 2004) The ability of reactive aldehydes (i.e., from ethanol metabolism) to inhibit methionine synthase activity and subsequently lower glutathione has been documented (Waly et al., 2011; Waly et al., 2004). Decreased methionine synthase activity would therefore result in an accumulation of SAH and inhibition of SAM, and a depletion of GSH similar to what is observed in our model. Therefore, it is reasonable to hypothesize that TCE, via its metabolite TCAH, acts in a similar manner.
In addition to a TCE-mediated alteration of DNA methylation, metabolic intermediates for methyl metabolism can also be lowered by drawing homocysteine (and indirectly methionine via SAM and SAH) through the transsulfuration pathway to make cysteine as demonstrated in liver (Mosharov et al., 2000), plasma (Melnyk, et.al., 2011) and in brain (Vitvitsky et al., 2006). Cysteine can then contribute to glutathione synthesis and to anti-oxidant defenses. Consistent with this possibility we found lower methionine + homocysteine levels and lower SAM + SAH levels in TCE-treated mice compared to controls. This result indicated that the transsulfuration pathway converting homocysteine to cysteine is more active in TCE-treated mice than in controls and may come at the expense of methyl metabolism and methylation capacity. The TCE-induced increase in plasma homocysteine may also reflect a deficit in folate levels. Although we did not test folate levels, TCE has been shown to promote a B12 and folate deficiency in rodents (Dow et al., 2000). Together this evidence suggests that TCE modulates DNA methylation and folate levels and future studies to examine this possibility are needed.
Our study demonstrates for the first time that the postnatal and early life period represents a sensitive window of TCE exposure by promoting a more oxidized state that is associated with decreased neurotrophin gene expression in the hippocampus. Further studies to evaluate the existence of a positive relation between alterations observed here and neurobehavior with particular relevance hippocampal function, including learning and memory, following early life postnatal TCE exposure are planned. Our results could enhance understanding of the mechanisms of neurotoxicity in susceptible human populations in order to prevent exposure and develop targeted treatments to improve neurologic function in neurodevelopmental diseases.
Acknowledgements
This work was supported by the National Institutes of Health R21ES017311-01A2 (SJB) and the Arkansas Biosciences Institute New Investigator Funds (#035425 to SJB). We wish to thank Cemeka Agugbuem, Meagan Kreps, Ashley Nelson, Oleksandra Pavliv, and Jenny Rau for their expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
We have no conflicting interests to declare.
References
- Adgate JL, Eberly LE, Stroebel C, Pellizzari ED, Sexton K. Personal, indoor, and outdoor VOC exposures in a probability sample of children. J Expo Anal Environ Epidemiol. 2004;14(Suppl 1):S4–S13. doi: 10.1038/sj.jea.7500353. [DOI] [PubMed] [Google Scholar]
- Allen JW, Shanker G, Aschner M. Methylmercury inhibits the in vitro uptake of the glutathione precursor, cystine, in astrocytes, but not in neurons. Brain Res. 2001;894:131–140. doi: 10.1016/s0006-8993(01)01988-6. [DOI] [PubMed] [Google Scholar]
- Altmann L, Welge P, Mensing T, Lilienthal H, Voss B, Wilhelm M. Chronic exposure to trichloroethylene affects neuronal plasticity in rat hippocampal slices. Environ Toxicol Pharmacol. 2002;12:157–167. doi: 10.1016/s1382-6689(02)00032-7. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Underwood M, Bridges RJ, Meister A. Glutathione metabolism at the blood-cerebrospinal fluid barrier. FASEB J. 1989;3:2527–2531. doi: 10.1096/fasebj.3.13.2572501. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Kapczinski F, Kauer-Sant'Anna M, Walz JC, Bond DJ, Goncalves CA, et al. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci. 2009;34:263–271. [PMC free article] [PubMed] [Google Scholar]
- Arsenijevic D, Hernadfalvi N, von Meyenburg C, Onteniente B, Richard D, Langhans W. Role for nerve growth factor in the in vivo regulation of glutathione in response to LPS in mice. Eur Cytokine Netw. 2007;18:93–101. doi: 10.1684/ecn.2007.0091. [DOI] [PubMed] [Google Scholar]
- Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Wooten JV. Blood concentrations of volatile organic compounds in a nonoccupationally exposed US population and in groups with suspected exposure. Clin Chem. 1994;40:1401–1404. [PubMed] [Google Scholar]
- Bale AS, Barone S, Jr, Scott CS, Cooper GS. A review of potential neurotoxic mechanisms among three chlorinated organic solvents. Toxicol Appl Pharmacol. 2011;255:113–126. doi: 10.1016/j.taap.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Ballok DA. Neuroimmunopathology in a murine model of neuropsychiatric lupus. Brain Res Rev. 2007;54:67–79. doi: 10.1016/j.brainresrev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballok DA, Earls AM, Krasnik C, Hoffman SA, Sakic B. Autoimmune-induced damage of the midbrain dopaminergic system in lupus-prone mice. J Neuroimmunol. 2004;152:83–97. doi: 10.1016/j.jneuroim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Balu DT, Hodes GE, Anderson BT, Lucki I. Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments. Neuropsychopharmacology. 2009;34:1764–1773. doi: 10.1038/npp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S. Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta. 1984;779:289–306. doi: 10.1016/0304-4157(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Doss JC. Trichloroethylene Alters Central and Peripheral Immune Function in Autoimmune-Prone MRL(+/+) Mice Following Continuous Developmental and Early Life Exposure. J Immunotoxicol. 2007a;4:129–141. doi: 10.1080/15476910701337035. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Doss JC, Gilbert KM. Ability of Trichloroethylene Metabolite to Promote Immune Pathology is Strain-Specific. J Immunotoxicol. 2006a;3:179–187. doi: 10.1080/15476910600978046. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Doss JC, Gilbert KM. Chronic exposure to a trichloroethylene metabolite in autoimmune-prone MRL+/+ mice promotes immune modulation and alopecia. Toxicol Sci. 2007b;95:401–411. doi: 10.1093/toxsci/kfl149. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Doss JC, Hennings LJ, Jernigan S, Melnyk S, James SJ. Developmental exposure to trichloroethylene promotes CD4+ T cell differentiation and hyperactivity in association with oxidative stress and neurobehavioral deficits in MRL+/+ mice. Toxicol Appl Pharmacol. 2008;231:344–353. doi: 10.1016/j.taap.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Gilbert KM. Exposure to a metabolite of the environmental toxicant, trichloroethylene, attenuates CD4+ T cell activation-induced cell death by metalloproteinase-dependent FasL shedding. Toxicol Sci. 2006b;92:103–114. doi: 10.1093/toxsci/kfj212. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Pumford NR, Gilbert KM. Activation and attenuation of apoptosis of CD4+ T cells following in vivo exposure to two common environmental toxicants, trichloroacetaldehyde hydrate and trichloroacetic acid. J Autoimmun. 2004;23:211–220. doi: 10.1016/j.jaut.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Bobyn PJ, Franklin JL, Wall CM, Thornhill JA, Juurlink BH, Paterson PG. The effects of dietary sulfur amino acid deficiency on rat brain glutathione concentration and neural damage in global hemispheric hypoxia-ischemia. Nutr Neurosci. 2002;5:407–416. doi: 10.1080/1028415021000055952. [DOI] [PubMed] [Google Scholar]
- Boda E, Pini A, Hoxha E, Parolisi R, Tempia F. Selection of reference genes for quantitative real-time RT-PCR studies in mouse brain. J Mol Neurosci. 2009;37:238–253. doi: 10.1007/s12031-008-9128-9. [DOI] [PubMed] [Google Scholar]
- Boehme F, Gil-Mohapel J, Cox A, Patten A, Giles E, Brocardo PS, et al. Voluntary exercise induces adult hippocampal neurogenesis and BDNF expression in a rodent model of fetal alcohol spectrum disorders. Eur J Neurosci. 2011;33:1799–1811. doi: 10.1111/j.1460-9568.2011.07676.x. [DOI] [PubMed] [Google Scholar]
- Branchi I, Karpova NN, D'Andrea I, Castren E, Alleva E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci Lett. 2011;495:168–172. doi: 10.1016/j.neulet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Bringmann G, Hille A. Endogenous alkaloids in man, VII: 1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline--a potential chloral-derived indol alkaloid in man. Arch Pharm (Weinheim) 1990;323:567–569. doi: 10.1002/ardp.19903230903. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Perluigi M, Sultana R. Oxidative stress in Alzheimer's disease brain: new insights from redox proteomics. Eur J Pharmacol. 2006;545:39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Sultana R. Identification of 3-nitrotyrosine-modified brain proteins by redox proteomics. Methods Enzymol. 2008;440:295–308. doi: 10.1016/S0076-6879(07)00819-1. [DOI] [PubMed] [Google Scholar]
- Cabanlit M, Wills S, Goines P, Ashwood P, Van de WJ. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- Castro R, Rivera I, Struys EA, Jansen EE, Ravasco P, Camilo ME, et al. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin Chem. 2003;49:1292–1296. doi: 10.1373/49.8.1292. [DOI] [PubMed] [Google Scholar]
- Caudill MA, Wang JC, Melnyk S, Pogribny IP, Jernigan S, Collins MD, et al. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J Nutr. 2001;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- Chao SL, Moss JM, Harry GJ. Lead-induced alterations of apoptosis and neurotrophic factor mRNA in the developing rat cortex, hippocampus, and cerebellum. J Biochem Mol Toxicol. 2007;21:265–272. doi: 10.1002/jbt.20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles JM. Autism spectrum disorders: an introduction and review of prevalence data. J S C Med Assoc. 2006;102:267–270. [PubMed] [Google Scholar]
- Chen Y, Ji LL, Liu TY, Wang ZT. Evaluation of gender-related differences in various oxidative stress enzymes in mice. Chin J Physiol. 2011;54:385–390. doi: 10.4077/CJP.2011.AMM080. [DOI] [PubMed] [Google Scholar]
- Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ. Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci. 2011;31:16619–16636. doi: 10.1523/JNEUROSCI.1639-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WA, Caldwell JC, Keshava N, Scott CS. Key scientific issues in the health risk assessment of trichloroethylene. Environ Health Perspect. 2006;114:1445–1449. doi: 10.1289/ehp.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol. 1999;14:388–394. doi: 10.1177/088307389901400608. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Dietert JM. Potential for early-life immune insult including developmental immunotoxicity in autism and autism spectrum disorders: focus on critical windows of immune vulnerability. J Toxicol Environ Health B Crit Rev. 2008;11:660–680. doi: 10.1080/10937400802370923. [DOI] [PubMed] [Google Scholar]
- Dobbing J. Undernutrition and the developing brain: the use of animal models ot elucidate the human problem. Psychiatr Neurol Neurochir. 1971;74:433–442. [PubMed] [Google Scholar]
- Dow JL, Green T. Trichloroethylene induced vitamin B(12) and folate deficiency leads to increased formic acid excretion in the rat. Toxicology. 2000;146:123–136. doi: 10.1016/s0300-483x(00)00156-6. [DOI] [PubMed] [Google Scholar]
- Dumas TC. Late postnatal maturation of excitatory synaptic transmission permits adult-like expression of hippocampal-dependent behaviors. Hippocampus. 2005;15:562–578. doi: 10.1002/hipo.20077. [DOI] [PubMed] [Google Scholar]
- Dunn BK. Hypomethylation: one side of a larger picture. Ann N Y Acad Sci. 2003;983:28–42. doi: 10.1111/j.1749-6632.2003.tb05960.x. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, van den Kroonenberg PT, Cools AR. The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr Res. 1998;30:251–260. doi: 10.1016/s0920-9964(97)00149-7. [DOI] [PubMed] [Google Scholar]
- Feil R. Environmental and nutritional effects on the epigenetic regulation of genes. Mutat Res. 2006;600:46–57. doi: 10.1016/j.mrfmmm.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Filomeni G, Rotilio G, Ciriolo MR. Cell signalling and the glutathione redox system. Biochem Pharmacol. 2002;64:1057–1064. doi: 10.1016/s0006-2952(02)01176-0. [DOI] [PubMed] [Google Scholar]
- Fuchikami M, Morinobu S, Segawa M, Okamoto Y, Yamawaki S, Ozaki N, et al. DNA methylation profiles of the brain-derived neurotrophic factor (BDNF) gene as a potent diagnostic biomarker in major depression. PLoS One. 2011;6:e23881. doi: 10.1371/journal.pone.0023881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke F, Gerich FJ, Muller M. Dynamic, semi-quantitative imaging of intracellular ROS levels and redox status in rat hippocampal neurons. Neuroimage. 2011;54:2590–2602. doi: 10.1016/j.neuroimage.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Gash DM, Rutland K, Hudson NL, Sullivan PG, Bing G, Cass WA, et al. Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Ann Neurol. 2008;63:184–192. doi: 10.1002/ana.21288. [DOI] [PubMed] [Google Scholar]
- Gilbert KM, Nelson AR, Cooney CA, Blossom SJ. Epigenetic alterations may regulate temporary reversal of CD4+ T cell activation caused by trichloroethylene exposure. Toxicological Sciences. 2012 doi: 10.1093/toxsci/kfs093. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert KM, Przybyla B, Pumford NR, Han T, Fuscoe J, Schnackenberg LK, et al. Delineating Liver Events in Trichloroethylene-Induced Autoimmune Hepatitis. Chem Res Toxicol. 2009 doi: 10.1021/tx800409r. [DOI] [PubMed] [Google Scholar]
- Gilbert KM, Pumford NR, Blossom SJ. Environmental Contaminant Trichloroethylene Promotes Autoimmune Disease and Inhibits T-cell Apoptosis in MRL(+/+) Mice. J Immunotoxicol. 2006;3:263–267. doi: 10.1080/15476910601023578. [DOI] [PubMed] [Google Scholar]
- Gilbert KM, Whitlow AB, Pumford NR. Environmental contaminant and disinfection by-product trichloroacetaldehyde stimulates T cells in vitro. Int Immunopharmacol. 2004;4:25–36. doi: 10.1016/j.intimp.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Blossom SJ, Jackson SK, Gilbert KM, Pumford NR. Trichloroethylene accelerates an autoimmune response by Th1 T cell activation in MRL +/+ mice. Immunopharmacology. 2000;46:123–137. doi: 10.1016/s0162-3109(99)00164-2. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Siler-Marsiglio K. Ethanol influences on Bax translocation, mitochondrial membrane potential, and reactive oxygen species generation are modulated by vitamin E and brain-derived neurotrophic factor. Alcohol Clin Exp Res. 2011;35:1122–1133. doi: 10.1111/j.1530-0277.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Hill-Smith TE, Lucki I. Fluoxetine treatment induces dose dependent alterations in depression associated behavior and neural plasticity in female mice. Neurosci Lett. 2010;484:12–16. doi: 10.1016/j.neulet.2010.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson LG, Spohler SA, Taylor DH. Trichloroethylene affects learning and decreases myelin in the rat hippocampus. Neurotoxicol Teratol. 1990;12:375–381. doi: 10.1016/0892-0362(90)90057-j. [DOI] [PubMed] [Google Scholar]
- Isaacson LG, Taylor DH. Maternal exposure to 1,1,2-trichloroethylene affects myelin in the hippocampal formation of the developing rat. Brain Res. 1989;488:403–407. doi: 10.1016/0006-8993(89)90739-7. [DOI] [PubMed] [Google Scholar]
- Jain A, Martensson J, Stole E, Auld PA, Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci U S A. 1991;88:1913–1917. doi: 10.1073/pnas.88.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapczinski F, Frey BN, Andreazza AC, Kauer-Sant'Anna M, Cunha AB, Post RM. Increased oxidative stress as a mechanism for decreased BDNF levels in acute manic episodes. Rev Bras Psiquiatr. 2008;30:243–245. doi: 10.1590/s1516-44462008000300011. [DOI] [PubMed] [Google Scholar]
- Kilburn KH, Warshaw RH. Effects on neurobehavioral performance of chronic exposure to chemically contaminated well water. Toxicol Ind Health. 1993;9:391–404. doi: 10.1177/074823379300900301. [DOI] [PubMed] [Google Scholar]
- Kulkarny VV, Wiest NE, Marquez CP, Nixon SC, Valenzuela CF, Perrone-Bizzozero NI. Opposite effects of acute ethanol exposure on GAP-43 and BDNF expression in the hippocampus versus the cerebellum of juvenile rats. Alcohol. 2011;45:461–471. doi: 10.1016/j.alcohol.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laslo-Baker D, Barrera M, Knittel-Keren D, Kozer E, Wolpin J, Khattak S, et al. Child neurodevelopmental outcome and maternal occupational exposure to solvents. Arch Pediatr Adolesc Med. 2004;158:956–961. doi: 10.1001/archpedi.158.10.956. [DOI] [PubMed] [Google Scholar]
- Lee LJ, Chan CC, Chung CW, Ma YC, Wang GS, Wang JD. Health risk assessment on residents exposed to chlorinated hydrocarbons contaminated in groundwater of a hazardous waste site. J Toxicol Environ Health A. 2002;65:219–235. doi: 10.1080/15287390252800828. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Jiang QH, Hao JL, Zhou LL. Protective effect of total flavones of Abelmoschus manihot L. Medic against poststroke depression injury in mice and its action mechanism. Anat Rec (Hoboken ) 2009;292:412–422. doi: 10.1002/ar.20864. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Gupta S, Parrish RR, Grissom NM, Davis RL. Epigenetic mechanisms: critical contributors to long-term memory formation. Neuroscientist. 2011;17:616–632. doi: 10.1177/1073858411386967. [DOI] [PubMed] [Google Scholar]
- Ma X, Foster J, Sakic B. Distribution and prevalence of leukocyte phenotypes in brains of lupus-prone mice. J Neuroimmunol. 2006;179:26–36. doi: 10.1016/j.jneuroim.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Maffi SK, Rathinam ML, Cherian PP, Pate W, Hamby-Mason R, Schenker S, et al. Glutathione content as a potential mediator of the vulnerability of cultured fetal cortical neurons to ethanol-induced apoptosis. J Neurosci Res. 2008;86:1064–1076. doi: 10.1002/jnr.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensson J, Jain A, Stole E, Frayer W, Auld PA, Meister A. Inhibition of glutathione synthesis in the newborn rat: a model for endogenously produced oxidative stress. Proc Natl Acad Sci U S A. 1991;88:9360–9364. doi: 10.1073/pnas.88.20.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CW, Mirochnitchenko O, Claus CP, Noble-Haeusslein LJ, Ferriero DM. Overexpression of glutathione peroxidase protects immature murine neurons from oxidative stress. Dev Neurosci. 2005;27:169–175. doi: 10.1159/000085989. [DOI] [PubMed] [Google Scholar]
- Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, et al. Metabolic Imbalance Associated with Methylation Dysregulation and Oxidative Damage in Children with Autism. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk S, Pogribna M, Pogribny I, Hine RJ, James SJ. A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J Nutr Biochem. 1999;10:490–497. doi: 10.1016/s0955-2863(99)00033-9. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Court, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Moriya J, Chen R, Yamakawa J, Sasaki K, Ishigaki Y, Takahashi T. Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol Pharm Bull. 2011;34:354–359. doi: 10.1248/bpb.34.354. [DOI] [PubMed] [Google Scholar]
- Mosharov E, Cranford MR, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000;39:13005–13011. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mythri RB, Harish G, Dubey SK, Misra K, Bharath MM. Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: implications for Parkinson's disease. Mol Cell Biochem. 2011;347:135–143. doi: 10.1007/s11010-010-0621-4. [DOI] [PubMed] [Google Scholar]
- Nakazawa H, Fukuyama N, Takizawa S, Tsuji C, Yoshitake M, Ishida H. Nitrotyrosine formation and its role in various pathological conditions. Free Radic Res. 2000;33:771–784. doi: 10.1080/10715760000301291. [DOI] [PubMed] [Google Scholar]
- Noble M, Mayer-Proschel M, Proschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal. 2005;7:1456–1467. doi: 10.1089/ars.2005.7.1456. [DOI] [PubMed] [Google Scholar]
- Numata S, Ye T, Hyde TM, Guitart-Navarro X, Tao R, Wininger M, et al. DNA methylation signatures in development and aging of the human prefrontal cortex. Am J Hum Genet. 2012;90:260–272. doi: 10.1016/j.ajhg.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Saito T, Saito K, Kurasaki M, Hosokawa T. Effect of trichloroethylene on spatiotemporal pattern of LTP in mouse hippocampal slices. Int J Neurosci. 2001;111:257–271. doi: 10.3109/00207450108994236. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Karpova N, Sabri F, Castren E, Ceccatelli S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J Neurochem. 2008;106:1378–1387. doi: 10.1111/j.1471-4159.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- Palbykin B, Borg J, Caldwell PT, Rowles J, Papoutsis AJ, Romagnolo DF, et al. Trichloroethylene induces methylation of the Serca2 promoter in H9c2 cells and embryonic heart. Cardiovasc Toxicol. 2011;11:204–214. doi: 10.1007/s12012-011-9113-3. [DOI] [PubMed] [Google Scholar]
- Pang Y, Campbell L, Zheng B, Fan L, Cai Z, Rhodes P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience. 2010;166:464–475. doi: 10.1016/j.neuroscience.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31:363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C. Epigenetic modification of the BDNF locus by early-life enrichment: towards a molecular correlate of resilience? Neurosci Lett. 2011;495:165–167. doi: 10.1016/j.neulet.2011.03.041. [DOI] [PubMed] [Google Scholar]
- Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Arlien-Soborg P, Sabroe S. Clinical neurological findings among metal degreasers exposed to chlorinated solvents. Acta Neurol Scand. 1993;87:200–204. doi: 10.1111/j.1600-0404.1993.tb04101.x. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif JS, Burch JB, Nuckols JR, Metzger L, Ellington D, Anger WK. Neurobehavioral effects of exposure to trichloroethylene through a municipal water supply. Environ Res. 2003;93:248–258. doi: 10.1016/s0013-9351(03)00131-2. [DOI] [PubMed] [Google Scholar]
- Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45:919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable P, Dangat K, Kale A, Joshi S. Altered brain neurotrophins at birth: consequence of imbalance in maternal folic acid and vitamin B metabolism. Neuroscience. 2011;190:127–134. doi: 10.1016/j.neuroscience.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, Xu M, Koibuchi N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum. 2009;8:366–372. doi: 10.1007/s12311-009-0105-9. [DOI] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, Xu M, McGinnis W, Koibuchi N. Brain region-specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD). Cerebellum. 2011;10:43–48. doi: 10.1007/s12311-010-0223-4. [DOI] [PubMed] [Google Scholar]
- Sakic B, Hanna SE, Millward JM. Behavioral heterogeneity in an animal model of neuropsychiatric lupus. Biol Psychiatry. 2005;57:679–687. doi: 10.1016/j.biopsych.2004.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takahashi T, Sumitani K, Takatsu H, Urano S. Glucocorticoid Generates ROS to Induce Oxidative Injury in the Hippocampus, Leading to Impairment of Cognitive Function of Rats. J Clin Biochem Nutr. 2010;47:224–232. doi: 10.3164/jcbn.10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6:39–42. [PubMed] [Google Scholar]
- Sexton K, Adgate JL, Church TR, Ashley DL, Needham LL, Ramachandran G, et al. Children's exposure to volatile organic compounds as determined by longitudinal measurements in blood. Environ Health Perspect. 2005;113:342–349. doi: 10.1289/ehp.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SK. Biology of aging brain. Indian J Pathol Microbiol. 2010;53:595–604. doi: 10.4103/0377-4929.71995. [DOI] [PubMed] [Google Scholar]
- Shanker G, Allen JW, Mutkus LA, Aschner M. Methylmercury inhibits cysteine uptake in cultured primary astrocytes, but not in neurons. Brain Res. 2001a;914:159–165. doi: 10.1016/s0006-8993(01)02791-3. [DOI] [PubMed] [Google Scholar]
- Shanker G, Aschner M. Identification and characterization of uptake systems for cystine and cysteine in cultured astrocytes and neurons: evidence for methylmercury-targeted disruption of astrocyte transport. J Neurosci Res. 2001b;66:998–1002. doi: 10.1002/jnr.10066. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr., Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ. Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics. 2003;112:e420. doi: 10.1542/peds.112.5.e420. [DOI] [PubMed] [Google Scholar]
- Tao L, Ge R, Xie M, Kramer PM, Pereira MA. Effect of trichloroethylene on DNA methylation and expression of early-intermediate protooncogenes in the liver of B6C3F1 mice. J Biochem Mol Toxicol. 1999;13:231–237. doi: 10.1002/(sici)1099-0461(1999)13:5<231::aid-jbt2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Tao L, Yang S, Xie M, Kramer PM, Pereira MA. Effect of trichloroethylene and its metabolites, dichloroacetic acid and trichloroacetic acid, on the methylation and expression of c-Jun and c-Myc protooncogenes in mouse liver: prevention by methionine. Toxicol Sci. 2000;54:399–407. doi: 10.1093/toxsci/54.2.399. [DOI] [PubMed] [Google Scholar]
- Till C, Koren G, Rovet JF. Prenatal exposure to organic solvents and child neurobehavioral performance. Neurotoxicol Teratol. 2001;23:235–245. doi: 10.1016/s0892-0362(01)00141-6. [DOI] [PubMed] [Google Scholar]
- Turunen MP, Aavik E, Yla-Herttuala S. Epigenetics and atherosclerosis. Biochim Biophys Acta. 2009;1790:886–891. doi: 10.1016/j.bbagen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Uysal N, Tugyan K, Aksu I, Ozbal S, Ozdemir D, Dayi A, et al. Age-related changes in apoptosis in rat hippocampus induced by oxidative stress. Biotech Histochem. 2012;87:98–104. doi: 10.3109/10520295.2011.556665. [DOI] [PubMed] [Google Scholar]
- Vitvitsky V, Thomas M, Ghorpade A, Gendelman HE, Banerjee R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem. 2006;281:35785–35793. doi: 10.1074/jbc.M602799200. [DOI] [PubMed] [Google Scholar]
- Waly M, Olteanu H, Banerjee R, Choi SW, Mason JB, Parker BS, et al. Activation of methionine synthase by insulin-like growth factor-1 and dopamine: a target for neurodevelopmental toxins and thimerosal. Mol Psychiatry. 2004;9:358–370. doi: 10.1038/sj.mp.4001476. [DOI] [PubMed] [Google Scholar]
- Waly MI, Kharbanda KK, Deth RC. Ethanol lowers glutathione in rat liver and brain and inhibits methionine synthase in a cobalamin-dependent manner. Alcohol Clin Exp Res. 2011;35:277–283. doi: 10.1111/j.1530-0277.2010.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win-Shwe TT, Tsukahara S, Yamamoto S, Fukushima A, Kunugita N, Arashidani K, et al. Up-regulation of neurotrophin-related gene expression in mouse hippocampus following low-level toluene exposure. Neurotoxicology. 2010;31:85–93. doi: 10.1016/j.neuro.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the san francisco bay area. Environ Health Perspect. 2006;114:1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Taylor JA, Shetty SR, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS One. 2011;6:e25448. doi: 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- Xu JX, Yang M, Deng KJ, Zhou H. Antioxidant activities of Dracocephalum tanguticum maxim extract and its up-regulation on the expression of neurotrophic factors in a rat model of permanent focal cerebral ischemia. Am J Chin Med. 2011;39:65–81. doi: 10.1142/S0192415X11008658. [DOI] [PubMed] [Google Scholar]
- Yi P, Melnyk S, Pogribna M, Pogribny IP, Hine RJ, James SJ. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J Biol Chem. 2000;275:29318–29323. doi: 10.1074/jbc.M002725200. [DOI] [PubMed] [Google Scholar]