Abstract
Aims
To develop an immunosensor for ultrasensitive detection of the NANOG protein. NANOG regulates pluripotency in stem cells and some cancer cells. This article reports the first electrochemical immunosensor for ultrasensitive detection and absolute quantification of the NANOG protein. The sensor features dense capture antibody-coated gold nanoparticle layers on a pyrolytic graphite underlayer.
Materials & methods
Two separate multilabel detection strategies were used to achieve moderate and ultra-high sensitivity.
Results
Good sensitivity was achieved for NANOG over the concentration range 0.1–160 pg/ml. The moderate sensitivity approach gave a detection limit of 25 pg/ml, while the ultrasensitive method achieved a 250-fold lower detection limit of 0.1 pg/ml. Amounts of NANOG detected in human embryonic stem cell lysates correlated well with qualitative western blots and mRNA expression.
Conclusion
The electrochemical gold nanoparticle immunosensor is suitable for measuring NANOG protein expression in stem and carcinoma cell tissue lysates at very low levels.
Keywords: cancer biomarker, electrochemical immunosensor, gold nanoparticle, magnetic particle, NANOG, pluripotency, stem cell
Accurate, sensitive detection of proteins is a central need for progress in biomedical science. Proteins that are upregulated or downregulated correlated with changes in cell differentiation or at the onset of disease hold significant value in diagnostics and research [1–3]. Development of devices and protocols to measure these proteins sensitively, cheaply and accurately is currently the subject of intense research activity, especially with regard to cancer biomarkers [2,4–7].
NANOG is a key transcription factor first found to be expressed specifically in mouse embryonic stem cells (ESCs). The protein NANOG in mouse and human stem cells is a critical gatekeeper for maintaining pluripotency [8–11], that is, the ability of a single cell to differentiate into any of the three germ layers (endoderm, mesoderm or ectoderm). When NANOG is downregulated in human embryonic stem cells, they differentiate [10]. Also, NANOG is overexpressed in germ cell, embryonal, breast and testicular carcinomas, and is thus a potentially valuable diagnostic biomarker for undifferentiated germ cell tumors [10,12–14]. A low level of NANOG transcriptional expression has also been detected in adult murine tissues [15]. Based on human-expressed sequence tag profiles, NANOG mRNA transcripts are expressed in embryonic tissue and two adult tissues, testis and blood. The NANOG expression in testis [16,17] may be due to the constant high turnover and formation of sperm, which are derived from a pool of adult stem cells present in this tissue. Kim et al. reported the expression of NANOG in bone marrow and cord blood cells [18], which may be correlated with the existence of circulating adult stem cells [19,20].
Given the critical general role of NANOG in regulating cell pluripotency and germ cell cancer development, accurate and precise methods for its detection should be a high priority. NANOG transcription can be analyzed by quantitative PCR techniques at the RNA level [21]. For correlating transcript expression to detecting NANOG protein, semiquantitative western blotting [22] and immunohistochemical approaches [23] are often employed. However, none of these techniques provide absolute levels of NANOG present in cells. Relative quantitation of NANOG can be obtained by a Taqman protein assay utilizing PCR amplification of an oligonucleotide label [24]. NANOG quantification can also be done by recently introduced human NANOG ELISA kits by Antigenix America (NY, USA) and CUSABIO Biotech Co. (DE, USA) with detection limits (DLs) of approximately 2 pg/ml. In this article, we report the first electrochemical immunosensor for the detection of NANOG protein and an assay protocol to quantify absolute levels of NANOG in cell lysates down to 0.1 pg/ml.
The new immunosensor described herein builds on our recently developed nanostructured electrochemical sensors for cancer biomarker proteins featuring single-wall nanotube forests or gold nanoparticle (AuNP) platforms coupled with multilabel enzyme-antibody particles. Chemically functionalized nanostructured surfaces provide high densities of accessible, attached capture antibodies to help enhance sensitivity [25]. These approaches have achieved sub-pg/ml detection of cancer biomarker proteins such as prostate-specific antigen (PSA) [26,27] and IL-6 [28,29] in serum. These sensors also detected PSA accurately in cancer patient serum and tissue lysates [26,27]. We used an array of four nanotube forest sensors for simultaneous accurate measurement of prostate cancer biomarkers PSA, prostate-specific membrane antigen, platelet factor-4 and IL-6 in cancer patient serum [30].
Antibody-loaded magnetic nanoparticles have been used previously in immunoassay protocols for offline analyte capture [31–34], a strategy that can greatly decrease nonspecific binding of interfering biomolecules. Using this approach with clustered magnetic particle (MP) labeling, we measured PSA in serum at a DL of 10 fg/ml in a surface plasmon resonance immunoassay [33]. Using MPs massively labeled with enzymes, we detected IL-8 in diluted serum and cancer cell conditioned media with an ultralow DL of 1.0 fg/ml [35].
In this paper, we report the application of our immunosensor strategies to develop a new sensor for quantitative and sensitive measurements of NANOG in cell lysates. We combined enzyme-labeled secondary antibody (Ab2) protocols with the AuNP sensor platform in sandwich immunoassays. Briefly, pyrolytic graphite sensor disk electrodes are coated with dense films of 5-nm AuNPs decorated with antibodies that capture NANOG protein very efficiently from a liquid sample. Two separate multilabel detection strategies were used to achieve sensitivity over a broad range of concentrations. In a moderate sensitivity approach, Ab2-biotin-streptavidin- horseradish peroxidase (HRP) bioconjugates (Figure 1A) were used to bind to NANOG captured on the sensor surface to provide 14–16 labels per antigen [28]. In a second, ultrasensitive approach (Figure 1B), we used streptavidin-coated magnetic beads conjugated with biotinylated Ab2 and biotinylated-HRP (400,000 HRPs per particle) in the detection step. The sensor detected NANOG with DL of 100 fg/ml (3 fM). Sensor validation was confirmed by successful measurements of NANOG in various cell lysates with good correlation to western blots and relative RNA expression levels.
Figure 1. Alternative strategies for electrochemical immunosensors featuring a gold nanoparticles sensor platform with attached antibodies that capture the protein analyte.
(A) Immunosensor after incubating with biotinylated secondary antibody and streptavidin-horseradish peroxidase (HRP) complex, providing 14–16 HRP labels. (B) Immunosensor after incubating with streptavidin-coated magnetic particles conjugated with biotinylated secondary antibody and biotinylated HRP (400,000 labels/particle). Amperometric signals are developed by injecting hydrogen peroxide at −0.3 V with hydroquinone mediator into the buffer.
AuNP: Gold nanoparticles; PDDA: Poly(diallyldimethylammonium chloride).
BSA: Bovine serum albumin; PG: Pyrolytic graphite.
Materials & methods
Chemicals & materials
Monoclonal (mouse) primary NANOG antibody (Ab1 ab76586), biotinylated rabbit polyclonal NANOG antibody (Ab2 ab84231), NANOG protein (ab50053) and streptavidin protein (HRP ab7403) were obtained from Abcam (Cambridge, UK). Tissue lysate samples were obtained from Protein Biotechnologies (CA, USA). l-gluthathione reduced (99% HAuCl4 × 3H2O [99.9%]), sodium borohydride (99%) poly(diallydimethylammonium chloride) (MW 20%) and bovine serum albumin (BSA) were from Sigma-Aldrich (MO, USA). Streptavidin coupled MPs (Dynabeads® MyOne Streptavidin T1), and biotinylated HRP were from Invitrogen (CA, USA). 1-(3-(dimethylamino) propyl)-3-ethylcarbodiimide hydrochloride and N-hydroxysulfosuccinimide, from Sigma-Aldrich, were dissolved in water immediately before use. Hydrogen peroxide (H2O2; 30%) was from Fisher (NJ, USA). Tween-20 was from Sigma-Aldrich. Immunoreagents were dissolved in pH 7.0 phosphate-buffered saline (PBS 0.01 M in phosphate, 0.14 M NaCl, 2.7 mM KCl) unless otherwise noted.
Instrumentation
A CHI 660 electrochemical workstation (CH Instruments, TX, USA) was used for amperometry at ambient temperature (22 ± 2°C). Rotating disk amperometry was done at −0.3 V versus standard calomel reference electrode with electrodes rotating at 3000 rpm.
Preparation of MP-Ab2-HRP conjugates
Supplier (Invitrogen) protocol was followed to conjugate the biotinylated-Ab2 and biotinylated-HRP with MPs. A total of 20 µl of MPs (100 mg/ml) were washed twice with 0.1% BSA in PBS buffer (pH 7.4) and resuspended to the same volume. Then 50 µl of biotinylated antibodies (Ab2 5 µg/ml) and 30 µl of biotinylated HRP (2.5 mg/ml) were added to the MPs and incubated at room temperature for 30 min using a slow tilt rotator. MP-Ab2-HRP conjugates were separated magnetically and washed with 0.1% BSA in PBS four-times and later reconstituted in PBS (pH 7.4), and stored at 4°C till further use.
The number of HRP labels per MP was calculated from the measured enzyme activity using 2,2’-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) assay [36] as reactant to be 4 (± 0.9) × 105 per bead, estimated on the total number of magnetic beads in 1 ml, 9 × 109.
Fabrication of the immunosensor
The AuNP sensor platform was fabricated using layer-by-layer alternate electrostatic adsorption [37,38]. Abraded disks (A = 0.14 cm2) of pyrolytic graphite (GE Ceramics, DE, USA) embedded in heat-shrinkable tubing and mounted on steel rods were used as the base electrodes onto which the immunosensors were fabricated. Initially, a 0.5-nm layer of cationic poly(dimethyldiallylammonium) chloride was adsorbed from a 20-µl drop placed onto the (0.14 cm2) pyrolytic graphite disk electrode, followed by washing with water. Next, a negatively charged layer of glutathione-decorated 5 nm AuNPs was deposited from a dispersion onto the poly (diallyldimethylammonium chloride)-coated electrode [27]. The AuNP layer caused a 29% increase in surface area over the underlying pyrolytic graphite surface. Surface area was estimated from cyclic voltammetry using the Randles–Sevcik equation [39] and slope of peak currents of soluble 1 mM ferrocyanide in 0.1 M KCl versus square root of scan rate.
Antibodies for NANOG were anchored to the AuNP-glutathione layer by placing 30 µl of freshly prepared 400 mM ethylcarbodiimide hydrochloride and 100 mM N-hydroxysulfosuccinimide in water onto AuNP electrodes for 10 min, rinsing with water and then incubating the activated electrode surface for 2.5 h with 20 µl of a 3.3 pmol solution (25 µg/ml) of primary NANOG antibody in pH 7.0 PBS. Unbound antibody was removed by rinsing thoroughly with 0.05% Tween-20 in PBS and then PBS for 2 min each. To block the free antibody binding sites on the sensor surface, the immunosensors were incubated with 10 µl of 2% BSA and 0.05% Tween-20 in PBS for 45 min at 37°C, followed by washing with 0.05% Tween-20 in PBS and then PBS for 2 min each.
Sensor operation
For the actual NANOG measurements, the immunosensors were incubated with 10 µl of NANOG standard solutions in pH 7.0 PBS buffer or cell/tissue lysates for 1 h at 37°C. After the washing steps, the sensors were exposed to 10 µl of 1 µg/ml biotinylated-Ab2 conjugates in buffer containing 0.1% BSA in pH 7.4 PBS buffer for 1 h, followed by washing with 0.05% Tween-20 in PBS and PBS buffer for 2 min each.
Using the moderate sensitivity protocol (Figure 1A), the sensors were next incubated with 10 µl of streptavidin-HRP for 30 min, followed by thorough washing with 0.05% Tween-20 in PBS and then PBS for 2 min each. For the high-sensitivity protocol (Figure 1B), the sensors with captured NANOG from standards or cell/tissue lysates were incubated with multilabel MP conjugates (conjugated with biotinylated-Ab2 and biotinylated-HRP) for 1 h. Finally, the completed immunosensors were placed in an electrochemical cell containing 1 mM hydroquinone in PBS buffer. Rotating-disk electrode amperometry was carried out by controlling the potential at −0.3 V versus saturated calomel electrode injecting H2O2 to obtain 0.1 mM concentration and measuring the amperometric reduction current. When using MP bioconjugates, where the concentration of hydroquinone was 0.25 mM, injected H2O2 was adjusted to reach a 0.05 mM concentration.
Sodium dodecyl sulfate gel electrophoresis and immunoblotting
Total cell lysates were obtained from human embryonic stem cells (hESCs) following manufacturer’s instructions (Ambion, TX, USA). In total, 7 µg of proteins or various concentrations of pure NANOG protein were run on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and transferred onto nitrocellulose membrane (Bio-Rad). Membranes were washed with 1X Tris-buffered saline containing 0.05% Tween-20, blocked in 5% milk in 1X Tris-buffered saline containing 0.05% Tween-20 and incubated with respective primary antibodies at conditions recommended by the manufacturers (Abcam NANOG monoclonal antibody, ab76586, 1:500 and β-actin, Cell Signaling 1:1000). After washing with 1X Tris-buffered saline containing 0.05% Tween-20, membranes were incubated with respective HRP-conjugated Ab2. Signals were detected with LumiGLO chemiluminescent reagent (Cell Signaling Technology, MA, USA).
Results
Sensor characteristics & detection limits
Nearly complete coverage of AuNPs on the sensor surface and attachment of a high density of capture antibodies have been confirmed previously for this sensor design by atomic force microscopy [27]. Using sensors with antibodies attached, a drop of NANOG standard solution or cell lysate is placed onto the sensor, which is then incubated and washed (see ‘Material & methods’ section). Next, a HRP-labeled Ab2 or HRP-antibody-MP is used to bind to the captured NANOG to enable the detection step. At this point, the sensor is washed to minimize nonspecific binding and placed into the measuring cell containing buffer, then rotated while a potential of −0.3 V versus saturated calomel electrode is applied. Injection of H2O2 into the buffer solution activates the HRP labels to ferryloxy species that then get reduced by a hydroquinone mediator also present in the solution. The role of hydroquinone is to shuttle electrons from the underlying sensor surface to the HRP labels to complete the catalytic cycle and provide an amperometric steady-state current proportional to the amount of NANOG in the sample. Entire assays from placement of the sample on sensor to signal measurement takes approximately 135 min for high sensitivity and 155 min for moderate sensitivity.
Responses for the immunosensors to NANOG protein in the pg/ml range using the moderate sensitivity Ab2-biotin-strepatavidin-HRP labels (Figure 1A) show a fast rise to the steady-state currents in Figure 2A upon injection of H2O2. The control with no NANOG gives a finite steady-state current due to a small amount of residual nonspecific binding of the labeled Ab2 plus residual direct reduction of the H2O2. Steady-state responses increased from 25 to 150 pg/ml NANOG (Figure 2B) with a sensitivity as the slope of the calibration graph of 4.6 nA-ml/cm2/pg NANOG. The DL as the blank response plus three-times the average noise was 25 pg/ml NANOG.
Figure 2. Immunosensor data using the moderate sensitivity protocol.
(A) Amperometric responses of gold nanoparticle immunosensor with attached capture antibody incubated with NANOG protein standards (pg/ml) in 10 µl buffer for 1 h after treating with 20 µl of 2% bovine serum albumin for 45 min, followed by the addition of 10 µl biotinylated enzyme-labeled secondary antibody in 0.1% bovine serum albumin and 0.05% Tween-20 for 45 min, and later incubating with streptavidin–horseradish peroxidase for 30 min. The current profiles were recorded upon injection of H2O2 (0.1 mM), into a buffer solution containing the sensor with captured NANOG and detection antibodies, and 1 mM hydroquinone, with electrode potential at −0.3 V and a rotation speed of 3000 rpm. (B) Immunoarray calibration plot showing the influence of NANOG concentration on the steady-state sensor current.
Standardization results for the high sensitivity AuNP immunosensors featuring massively labeled streptavidin-MP-biotin Ab2-biotin-HRP bioconjugates (Figure 1B) are shown in Figure 3. Good quality, low signal/noise traces with well defined steady-state signals were obtained. Calibration graphs are shown in a linear-log format that covers the range 0.1–20 pg ml−1 as well as a plot of the lowest concentration range. Despite the nonlinearity of these calibrations, sensitivity of the sensor is still applicable for quantitation of NANOG in this range. Using the streptavidin-MP-biotin Ab2-biotin-HRP bioconjugates, the DL of 100 fg/ml was 250-fold better than that measured with the Ab2-biotin-streptavidin-HRP system, mainly due to significant signal amplification brought about by the 400,000 HRP labels on each magnetic bead. Sensitivity of the immunosensor in the lower concentration range was 171 nA-ml/cm2/pg NANOG, 175-fold better than the moderate sensitivity approach. Sensitivity was estimated by dividing the slope of the current versus NANOG (from a linear fit in low concentration range [Chikkaveeraiah B, Soldà A, Rusling J, Unpublished Data]) by area of the working electrode (0.14 cm2). Standard deviations show that reproducibility was a little worse than that in the higher concentration range but still acceptable for such low levels. Even a small amount of nonspecific binding of the MP bioconjugates in the amplification strategy causes a significant blank signal as the signal is due to approximately 400,000 HRP labels on the MP, as seen for the zero NANOG blank (Figure 3A).
Figure 3. Immunoarray data using the high-sensitivity protocol.
(A) Amperometric responses of gold nanoparticle immunosensor with attached capture antibody incubated with NANOG protein standards (pg/ml) in 10 µl buffer for 1 h after treating with 20 µl of 0.1% bovine serum albumin (20 mM phosphate buffered saline; pH 7.4) for 45 min, followed by addition of 10 µl magnetic particles bioconjugates and incubation for 1 h. The current profiles were recorded upon injection of H2O2 (0.05 mM), into a buffer solution containing 0.25 mM hydroquinone, while keeping the electrode potential at −0.3 V and rotation speed at 3000 rpm. (B) Linear-log immunoarray calibration plot showing the influence of the NANOG concentration on the steady-state current signals. (C) Influence of very low NANOG concentrations on immunosensor steady-state signals.
Quantitative NANOG detection in cell lysates
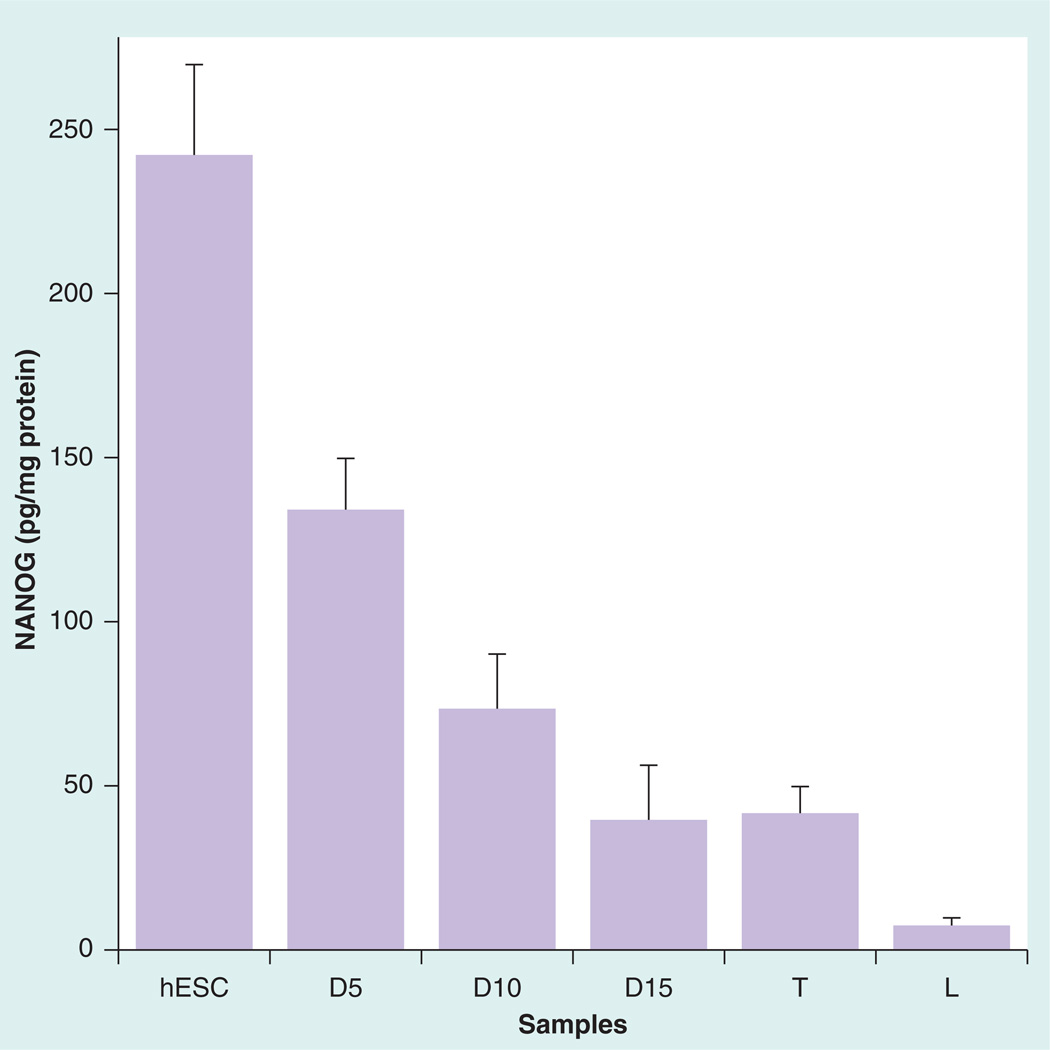
We evaluated the sensor for determining levels of NANOG protein in cell lysates of testis tissue [17], with liver tissue as a negative control and hESCs as positive controls. We found that NANOG protein expression normalized to total cellular protein was six-times higher in hESCs compared with the testis tissue (Figure 4) and the negative control was very low in NANOG.
Figure 4. Immunosensor assay results normalized for total protein for human embryonic stem cells, testis and liver tissue lysate samples.
Labels at the bottom of the graph denote differentiated hESCs samples tested at different times.
D5: Day 5; D10: Day 10; D15: Day15; hESC: Human embryonic stem cells; L: Liver tissue; T: Testis tissue.
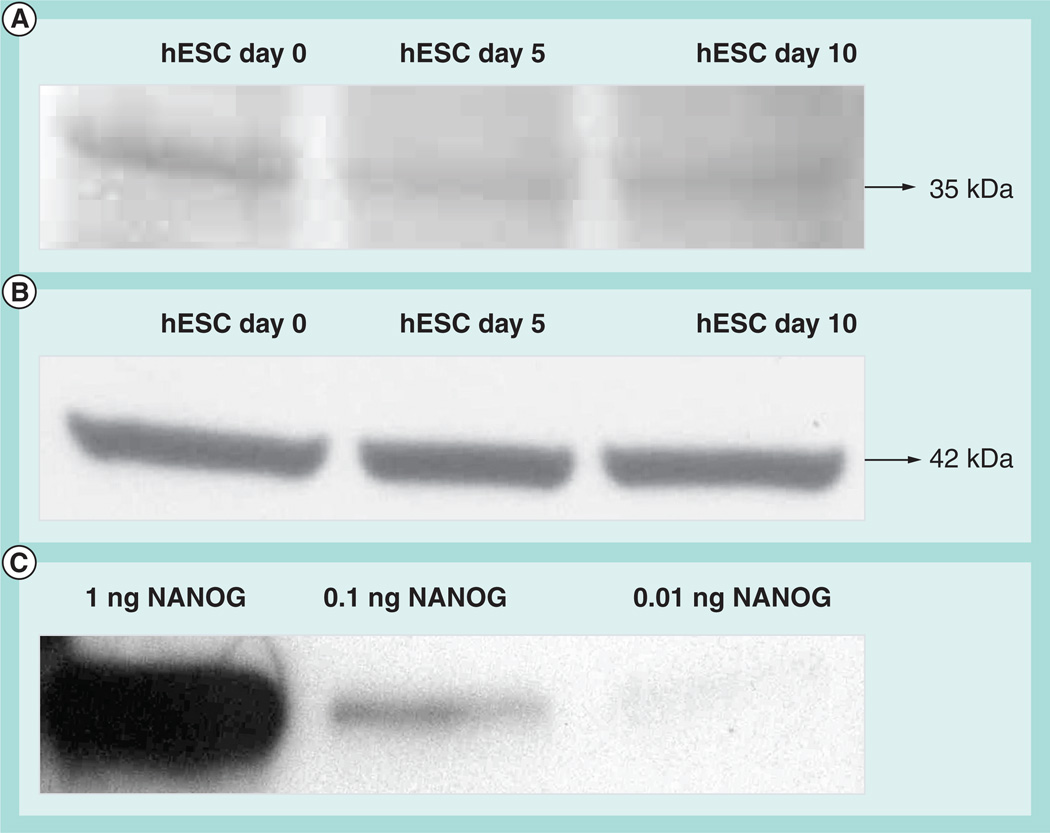
Because NANOG expression is known to be downregulated under differentiating conditions [8,10,16], we also examined its expression in differentiating hESCs at different times. The spontaneous method of differentiation was used, which involves culturing the hESCs in the stem cell medium devoid of FGF-2 [40,41]. Our data, as expected, showed that NANOG expression decreased significantly with increasing differentiation time. We confirmed this by sodium dodecyl sulfate-gel electrophoresis and immunoblotting with NANOG-specific antibody that showed a similar trend in decreased intensity with increasing differentiation of the band corresponding to NANOG (Figure 5). However, the differences found by the immunosensor are much more apparent than the western blot analyses, with its low sensitivity being apparent in the relative similarity of the undifferentiated and differentiated samples in Figure 5, compared with more precisely determined differences seen in the immunosensor responses in Figure 4.
Figure 5. Western blot analyses of cell lysates of human embryonic stem cells at differentiation time points in days.
(A) NANOG protein expression and (B) β-actin protein expression. Total protein (7 µg) was run on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and transferred onto nitrocellulose membranes and were initially probed with NANOG monoclonal antibody (ab76586; 1:500). This was followed by labeling of the nitrocellulose membrane with β-actin antibody to show equal loading of the protein samples. (C) Western blot analysis of pure NANOG protein (ab50053) probed with same NANOG antibody (ab76586).
hESC: Human embryonic stem cells.
The regulation of NANOG expression can be detected at the transcriptional level by quantifying the NANOG transcripts with real-time PCR. Hence, we correlated our NANOG protein expression data with the relative mRNA expression level in the differentiating hESCs. The NANOG transcript expression showed a 21% decrease at D5 compared with control hESCs followed by a 52% decrease at D10. This shows qualitative correlation with immunosensor results (Figure 4) of 40% decrease in actual amounts of NANOG at D5 and 70% decrease in NANOG at D10. Thus, the cellular machinery responsible for NANOG transcripts expression followed a similar trend of decline with the directly observed NANOG levels measured by the immunosensor.
Discussion
The results document the fabrication, characterization and practical evaluation of the first nanostructured immunosensor for direct quantitative measurement of NANOG. This achievement is highly significant due to the key role of this protein in regulating stem and carcinoma cell differentiation. Using the new immunosensor and simple, inexpensive protocols, absolute amounts of NANOG were determined in cell lysates for the first time. The two protocols employed, covering moderate and high sensitivity, provide viable NANOG detection from approximately 0.1 to 150 pg/ml, with higher concentrations becoming accessible by simple sample dilution. The highest sensitivity is enabled by combining the nanostructured sensor platform with high antibody loadings on MPs with 400,000 HRP labels, which leads to an excellent limit of detection of 100 fg/ml.
Proof-of-concept data were presented for applicability of the new immunosensor to quantitative detection of NANOG in cell lysates. Results for lysates of hESC, testes tissue and control liver tissue gave results consistent with expected levels of NANOG, as well as with western blot results. Similarly, decreased NANOG measured in differentiating hESC tissue was consistent with independently measured relative mRNA expression levels.
Conclusion & future perspective
We have developed and validated the first electrochemical immunosensor for the quantitative ultrasensitive detection of NANOG at sub-pg/ml (fM) levels. Good accuracy of the immunosensor was supported by good correlations between NANOG levels obtained in tissue lysates of hESC and testes cells with those estimated by semiquantitative western blot analysis and with mRNA expression levels. Good selectivity was confirmed by results from the same lysates, which contain many potentially interfering proteins. The main advantage of these immunosensors is ease of fabrication, low cost, ultrasensitivity, speed and viable quantification of NANOG.
At present there is no other method to measure absolute amounts of NANOG in cell lysates at such low levels without using PCR amplification, so the new immunosensor fulfills an important niche for direct quantitative NANOG detection. This sensor is expected to be a valuable tool in stem cell research and future cancer diagnostics. In addition, the technology is amenable to incorporation in microfludic systems [34] that could detect NANOG and other biomarker proteins simultaneously. Such a device could provide semi-automated, multiplexed analyses of stem and carcinoma cell lysates, possibly with higher sensitivity and shorter analysis times, and expand the value of the applications described above.
Executive summary.
-
■
A new nanostructured electrochemical immunosensor was developed for the pluripotency gatekeeper protein NANOG that provides absolute quantitative detection in cell lysates.
-
■
NANOG detection can be achieved in the 0.1–150 pg/ml range, with higher levels accessible by sample dilution.
-
■
Ultrasensitive sensor response is achieved by combining a nanostructured surface with a massively labeled detection particle.
-
■
The NANOG immunosensor provides a valuable quantitative tool for stem cell research and future cancer diagnostics.
Acknowledgments
This work was supported financially by the National Institutes Health (NIH) through grants ES013557 from National Institute of Environmental Health Sciences/NIH and EB014586 from National Institutes Biomedical Imaging and Bioengineering/NIH to JF Rusling, the University of Connecticut and by Connecticut Stem Cell Research Fund Grant 08-SAC-UCHC-033 to D Choudhary. A Soldà thanks the Fondazione Cariparo Foundation for financially supporting her participation in this project.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Wulfkuhle JD, Liotta LA, Petricoin EF. Proteomic applications for the early detection of cancer. Nat. Rev. Cancer. 2003;3:267–275. doi: 10.1038/nrc1043. [DOI] [PubMed] [Google Scholar]
- 2. Rusling JF, Kumar CV, Gutkind JS, Patel V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst. 2010;135:2496–2511. doi: 10.1039/c0an00204f. ▪ Comprehensive review of protein detection strategies related to cancer diagnostics.
- 3.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 4.Wang J. Electrochemical biosensors: towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006;21:1887–1892. doi: 10.1016/j.bios.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Kingsmore SF. Multiplexed protein measurement: technologies and applications of protein and antibody arrays. Nat. Rev. Drug Discov. 2006;5:310–321. doi: 10.1038/nrd2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giljohann DA, Mirkin CA. Drivers of biodiagnostic development. Nature. 2009;462:461–464. doi: 10.1038/nature08605. ▪ Brief review of new approaches to protein measurements related to medical diagnostics.
- 7.Tothill IE. Biosensors for cancer markers diagnosis. Semin. Cell Dev. Biol. 2009;20:55–62. doi: 10.1016/j.semcdb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Mitsui K, Tokuzawa Y, Itoh H, et al. The homeoprotein NANOG is required for maintainance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 9. Chambers I, Colby D, Robertson M, et al. Functional expression cloning of NANOG, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. ▪ Seminal paper describing the expression of NANOG in embryonic stem cells.
- 10.Hyslop L, Stojkovic M, Armstrong L, et al. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–1043. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Stojkovic P, Przyborski S, et al. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669–2676. doi: 10.1634/stemcells.2006-0377. [DOI] [PubMed] [Google Scholar]
- 12. Hoei-Hansen CE, Almstrup K, Nielsen JE, et al. Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology. 2005;47:48–56. doi: 10.1111/j.1365-2559.2005.02182.x. ▪ Seminal paper describing NANOG expression in human germ cell tumors.
- 13. Hart AH, Hartley L, Parker K, et al. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer. 2005;104:2092–2098. doi: 10.1002/cncr.21435. ▪ Seminal paper describing NANOG expression in human germ cell tumors.
- 14. Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104:2255–2265. doi: 10.1002/cncr.21432. ▪ Seminal paper describing NANOG expression in human germ cell tumors.
- 15. Hart AH, Hartley L, Ibrahim M, Robb L. Identification, cloning and expression analysis of the pluripotency promoting NANOG genes in mouse and human. Develop. Dynamics. 2004;230:187–198. doi: 10.1002/dvdy.20034. ▪ Seminal paper describing NANOG expression in human germ cell tumors.
- 16. Clark AT, Rodriguez RT, Bodnar MS, et al. Human STELLAR, NANOG, and GDF3 genes are expressed in pluripotent cells and map to chromosome 12p13, a hotspot for teratocarcinoma. Stem Cells. 2004;22:169–179. doi: 10.1634/stemcells.22-2-169. ▪ Seminal paper describing NANOG gene expression in human carcinomas.
- 17.Kuijk EW, de Gier J, Lopes SMC, et al. A distinct expression pattern in mammalian testes indicates a conserved role for NANOG in spermatogenesis. PLoS ONE. 2010;5:e10987. doi: 10.1371/journal.pone.0010987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JS, Kim J, Kim BS, et al. Identification and functional characterization of an alternative splice variant within the fourth exon of human NANOG. Exp. Mol. Med. 2005;37:601–607. doi: 10.1038/emm.2005.73. [DOI] [PubMed] [Google Scholar]
- 19.Beltrami AP, Cesselli D, Bergamin N, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 20.Cesselli D, Beltrami AP, Rigo S, et al. Multipotent progenitor cells are present in human peripheral blood. Circ. Res. 2009;104:1225–1234. doi: 10.1161/CIRCRESAHA.109.195859. [DOI] [PubMed] [Google Scholar]
- 21.Stahlberg A, Bengtsson M, Hemberg M, Semb H. Quantitative transcription factor analysis of undifferentiated single human embryonic stem cells. Clin. Chem. 2009;55:2162–2170. doi: 10.1373/clinchem.2009.131433. [DOI] [PubMed] [Google Scholar]
- 22.Eberle I, Pless B, Braun M, Dingermann T, Marschalek R. Transcriptional properties of human NANOG1 and NANOG2 in acute leukemic cells. Nucleic Acids Res. 2010;38:5384–5395. doi: 10.1093/nar/gkq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeter CR, Badeaux M, Choy G, et al. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells. 2009;27:993–1005. doi: 10.1002/stem.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Applied Biosystems Applications Note. Novel Protein Assays Using TaqMan® Chemistry for the Detection and Relative Quantification of Protein Markers in Embryonic Stem Cells. CA, USA: 2010 Life Technologies Corporation; 2010. [Google Scholar]
- 25.Malhotra R, Papadimitrakopoulos F, Rusling JF. Sequential layer analysis of protein immunosensors based on single wall carbon nanotube forests. Langmuir. 2010;26:15050–15056. doi: 10.1021/la102306z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu X, Munge B, Patel V, et al. Carbon nanotube amplification strategies for highly sensitive immunodetection of cancer biomarkers. J. Am. Chem. Soc. 2006;128:11199–11205. doi: 10.1021/ja062117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani V, Chikkaveeraiah BV, Patel V, Gutkind JS, Rusling JF. Ultrasensitive immunosensor for cancer biomarker proteins using gold nanoparticles film electrodes and multienzyme-particle amplification. ACS Nano. 2009;3:585–594. doi: 10.1021/nn800863w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malhotra R, Patel V, Vaque JP, Gutkind JS, Rusling JF. Ultrasensitive electrochemical immunosensor for oral cancer biomarker IL-6 using carbon nanotube forest electrodes and multilabel amplification. Anal. Chem. 2010;82:3118–3123. doi: 10.1021/ac902802b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munge BS, Krause CE, Malhotra R, Patel V, Gutkind JS, Rusling JF. Electrochemical immunosensors for interleukin-6. Comparison of carbon nanotube forest and gold nanoparticle platforms. Electrochem. Comm. 2009;11:1009–1012. doi: 10.1016/j.elecom.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chikkaveeraiah BV, Bhirde A, Malhotra R, Patel V, Gutkind JS, Rusling JF. Single-wall carbon nanotube forest arrays for immunoelectrochemical measurement of four protein biomarkers for prostate cancer. Anal. Chem. 2009;81:9129–9134. doi: 10.1021/ac9018022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soelberg SD, Stevens RC, Limaye AP, Furlong CE. Surface plasmon resonance detection using antibody-linked magnetic nanoparticles for analyte capture, purification, concentration and signal amplification. Anal. Chem. 2009;81:2357–2363. doi: 10.1021/ac900007c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao L, Zhuang J, Nie L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotech. 2007;2:577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan S, Mani V, Wasalathanthri D, Kumar CV, Rusling JF. Attomolar detection of a cancer biomarker protein in serum by surface plasmon resonance using superparamagnetic particle labels. Angew. Chem. Int. Ed. 2010;122:1–5. doi: 10.1002/anie.201005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chikkaveeraiah BV, Mani V, Patel V, Gutkind JS, Rusling JF. Microfluidic electrochemical immunoarray for ultrasensitive deteciton of two cancer biomarker proteins in serum. Biosens. Bioelectron. 2011;26:4477–4483. doi: 10.1016/j.bios.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munge BS, Coffey AL, Doucette JM, et al. Nanostuctured immunosensor for attomolar detection of cancer biomarker interleukin-8 using massively labeled superparamgentic particles. Angew. Chem. Int. Ed. 2011;50:7915–1918. doi: 10.1002/anie.201102941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pütter J. In: Methods of Enzymatic Analysis. Becker R, Bergmeyer HU, editors. FL, USA: Verlag Chemie; 1983. pp. 286–293. [Google Scholar]
- 37.Rusling JF. Electroactive and enzyme-active protein-polyion films assembled layer by layer. In: Lvov Y, Mohwald H, editors. Protein Architecture: Interfacing Molecular Assemblies and Immobilization Biotechnology. NY, USA: Marcel Dekker; 2000. pp. 337–354. [Google Scholar]
- 38.Lvov YM. Thin film nanofabrication by alternate adsorption of polyions, nanoparticles, and proteins. In: Nalwa HS, editor. Handbook of Surfaces and Interfaces of Material: Nanostructured Materials, Micelles, and Colloids. CA, USA: Academic Press; 2001. [Google Scholar]
- 39.Bard AJ, Faulkner LR. Electrochemical Methodsm (2nd Edition) NY, USA: John Wiley & Sons; 2001. p. 231. [Google Scholar]
- 40.Vugler A, Carr AJ, Lawrence J, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp. Neurol. 2008;214:347–361. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–2434. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]