Abstract
Inflammatory breast cancer (IBC) is the most aggressive form of breast cancer, and here, we examined in vitro the interactions between the human IBC cell line SUM149 and U937 human naive monocytes. We found an altered morphology, enhanced invasiveness and proteolytic activity of SUM149 cells when cultured with U937 cells or in U937-conditioned media (U937-CM). Increases in expression and activity of the cysteine protease cathepsin B and expression of caveolin-1 were also detected in SUM149 cells grown in U937-CM, thus suggesting a contribution of these proteins to the augmented invasion through and proteolysis of the extracellular matrix by the IBC cells.
Keywords: cathepsin B, caveolin-1, co-culture, extracellular matrix, proteolysis
Inflammatory breast cancer (IBC) is the most lethal form of primary breast cancer and disproportionately targets younger women. Although IBC accounts for up to 5% of all breast cancer cases in the United States, its incidence is significantly higher among African-American women (10.1%) and women of Northern African countries (1–10%) (Omar et al., 2003; Labidi et al., 2008). Surveillance Epidemiology and End Results (SEER) analysis revealed that the incidence of IBC increased approximately 2.5-fold between 1990 and 1997, whereas the incidence of non-inflammatory locally advanced breast cancer (LABC) decreased by approximately 2.5-fold (Hance et al., 2005). Despite multi-modal treatment protocols utilizing neoadjuvant chemotherapy, mastectomy followed by adjuvant chemotherapy and accelerated hyperfractionated radiation, the 3-year survival rate for patients with IBC is merely 40% as compared to 85% for non-IBC patients (Lerebours et al., 2005). Dismal survival rates for IBC patients are attributed to the rapid progression, significant lymph node involvement and distant metastasis of the disease at the time of diagnosis. Moreover, a palpable tumor mass is not usually present in IBC, thus making diagnosis challenging using conventional mammography or breast ultrasound (Cariati et al., 2005; Hance et al., 2005).
Clinically, IBC is a form of LABC that is of ductal cellular origin and has distinct features that include rapid onset, erythema, edema of the breast and a ‘peau d’orange’ appearance of the skin. IBC tumors are highly proliferative, angiogenic and invasive, particularly into dermal lymphatic vessels where they form tumor emboli (Van Laere et al., 2006). Lymphatic obstruction by these tumor emboli underlies the inflammatory nature of the disease (Giordano and Hortobagyi, 2003; Lerebours et al., 2005). While there are recent efforts to study IBC, the molecular and genetic nature of this disease remains poorly understood. A number of prognostic markers for IBC, including HER2/neu amplification/overexpression and lack of estrogen receptor, have been identified (Delarue et al., 1981). Overexpression of genes, such as epidermal growth factor receptor (egfr), rhoC and ecadherin, and the loss of expression of genes, including libc (lost in inflammatory breast cancer) and wisp3 (Wnt-1-inducible secreted protein 3), highly correlate with IBC tumorigenesis (van Golen et al., 1999; Kleer et al., 2005). Unfortunately, these markers do not distinguish IBC from non-IBC and fail to explain their distinct biology (Bieche et al., 2004).
Along with IBC cells, investigators are now profiling stromal cells associated with IBC. For example, fibro-blasts associated with breast cancer can induce ‘genetic alterations’ related to poor prognosis of the disease (Radisky and Radisky, 2007). As well, monocytes that infiltrate into the breast tissue proliferate and differentiate into tumor-associated macrophages, a cell type for which a strong association has been made with poor prognosis of breast cancer (Leek et al., 1996). Advances in intravital imaging technology have provided new insights into how migration and invasion of breast cancer cells are regulated by elements of the local microenvironment, including the presence of macrophages (Condeelis and Pollard, 2006). For example, toll-like receptors, which are highly expressed by myelomonocytic cells, including circulating monocytes, macrophages and dendritic cells, in response to microbial or viral infections (Nagai et al., 2006), are also overexpressed in IBC patient tissue (Van Laere et al., 2005). These receptors are also upstream activators of NF-κB transcription, and in breast cancer NF-κB promotes invasion by increasing cell motility and migration (Huber et al., 2004). Thus, these data support our hypothesis that inflammatory cells, such as monocytes enhance IBC tumorigenesis, malignancy and lymphovascular invasion (Schoppmann et al., 2006).
Previously, we investigated the interactions between non-IBC cell lines (MDA-MB-231 and BT20) and naive or tumor-educated U937 human monocytes (Sameni et al., 2003; Mohamed and Sloane, unpublished data). In these studies, breast cancer cells were either co-cultured with naive or tumor-educated monocytes or treated with the conditioned media of these cells. These interactions result in increased proteolytic activity, extracellular matrix (ECM) degradation and invasiveness of the breast tumor cells. Moreover, using cytokine antibody arrays, we identified the major cytokines and proteases potentially involved in the cross-talk between these breast tumor cells and human monocytes (Mohamed and Sloane, unpublished data). Here, we studied the response of the human IBC cell line SUM149 to U937 human monocytes. Using confocal microscopy, we assessed the morphology of SUM149 cells grown on a reconstituted basement membrane (rBM) and overlaid with 2% rBM, a method adapted from one used by Brugge and colleagues to analyze morphogenesis and oncogenesis of breast epithelial cells (Debnath et al., 2003). We found that SUM149 cells grown alone on rBM for 24 h formed spheroid-like structures (Figure 1A). When these cells were grown in co-culture with U937 (Figure 1B) or in media conditioned by U937 cells (U937-CM; Figure 1C), we observed in both cases a morphological change from spheroidal to branching-like structures. In addition, U937-CM enhanced the invasion of SUM149 cells through rBM-coated filters as compared to control media (Ham’s F-12 media plus 5% fetal bovine serum, FBS) (Figure 2). Our data suggest that secretions from U937 cells stimulate morphological changes in SUM149 cells and promote their invasion.
Figure 1. U937 cells affect the morphogenesis of IBC cells.

SUM149 cells (provided by Dr. Stephen P. Ethier, Barbara Ann Karmanos Cancer Institute, Wayne State University, Detroit, USA) were grown for 24 h in rBM (Cultrex, Trevigen, Gaithersburg, MD, USA) overlay cultures (adapted from Debnath et al., 2003) in complete media [Ham’s F12 media with 5% FBS (Sigma, St. Louis, MO, USA)] (A), in co-culture (ratio 3:1) with U937-cells (American Type Culture Collection, Manassas, VA, USA) pre-labeled with CellTracker Orange (Invitrogen, Carlsbad, CA, USA) (red) (B), or in U937-CM (serum-free Ham’s F12 media conditioned by U937 cells for 24 h) (C). Confocal microscopy was performed in the Microscopy and Imaging Resources Laboratory (MIRL) with a Zeiss LSM 510 META NLO microscope (Carl Zeiss, Thornwood, NY, USA). Images are representative of at least three experiments. Bars, 100 μm.
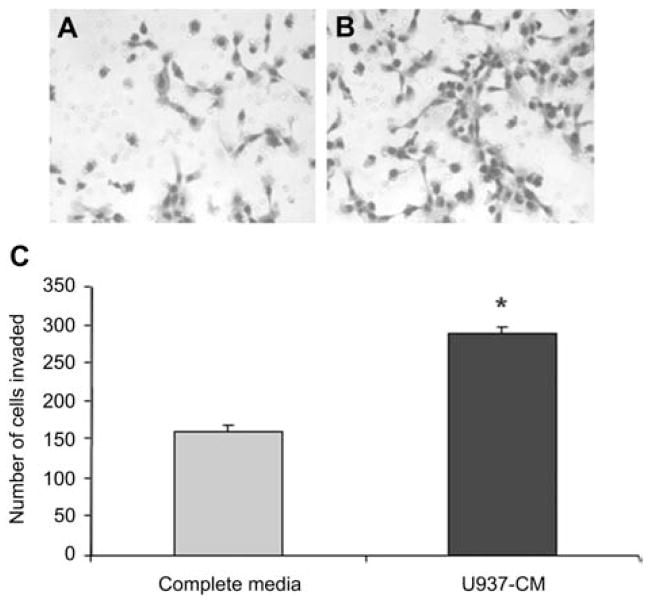
Figure 2. Conditioned medium from U937 cells enhances the invasion of IBC cells.
Equal numbers of SUM149 cells were seeded and incubated for 24 h on rBM-coated filters within invasion chambers (BD Bioscience, San Jose, CA, USA) (Cavallo-Medved et al., 2005). The stimulants for invasion were either complete media (Ham’s F12 media with 5% FBS) (A) or U937-CM (B). Images of three representative fields were taken at 100× magnification. SUM149 cells that had invaded through the filters were stained using Diff-Quik Stain Set (Dade Behring, Newark, DE, USA), counted in three random microscopic fields and expressed as number of cells that had invaded (C). Results from three independent experiments are expressed as mean±standard deviation (SD). *Represents a p-value <0.05, as determined by Student’s t-test. Confocal microscopy was performed in MIRL with a Zeiss LSM 510 META NLO microscope (Carl Zeiss, Thornwood, NY, USA).
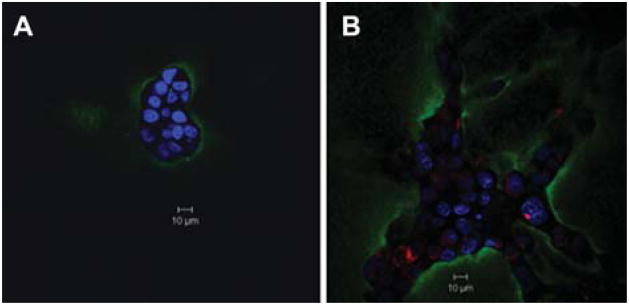
Since invasion of tumor cells is linked to degradation of ECM proteins, we examined the effects of monocytes on degradation of the ECM protein type IV collagen by SUM149 cells. In a live cell proteolysis assay that uses a dye-quenched fluorescent (DQ)-collagen IV substrate, the most abundant collagen type found in basement membrane, mixed with rBM (Sameni et al., 2003), we found increased DQ-collagen IV degradation (observed as green fluorescence) when SUM149 cells were co-cultured with U937 cells as compared to when they were grown alone (Figure 3). The increase in DQ-collagen IV degradation correlates with the branched morphology of SUM149 cells induced by co-culture with U937 cells and the enhanced invasiveness of SUM149 cells in response to U937-CM.
Figure 3. Functional imaging of proteolysis by SUM149 cells grown alone or in co-culture with U937 cells.
In a live cell proteolysis assay, SUM149 cells were grown on rBM (Cultrex) mixed with 25 μg/ml of quenched fluorescent (DQ)-collagen IV substrate (Invitrogen Life Technologies, Carlsbad, CA, USA) and overlaid with 2% rBM for 24 h either alone (A) or in co-culture (ratio 3:1) with U937 cells pre-labeled with CellTracker Orange (red) (B) (Sameni et al., 2003). Degradation of DQ-collagen IV (green fluorescence) by live SUM149 cells alone and in co-culture with U937 cells was observed with a Zeiss LSM 510 META NLO microscope (Carl Zeiss) using a 40× water immersion objective. Images represent a single confocal slice at the equatorial plane. Nuclei were stained with DAPI (blue) (Invitrogen Life Technologies). Results are representative of at least three independent experiments. Bars, 10 μm.
As a first step in identifying which enzymes are involved in ECM degradation, we examined the expression and activity of a lysosomal cysteine protease, cathepsin B, in SUM149 cells. Cathepsin B is involved in various steps of tumor progression, including digestion of adhesion molecules, degradation of extracellular matrix, motility, angiogenesis, invasion and metastasis (Ren and Sloane, 1996; Mohamed and Sloane, 2006). Moreover, cathepsin B has been suggested to be a biological marker for breast tumors (Wulfkuhle et al., 2002) and has been implicated in breast tumor cell invasion (Lah et al., 2000; Premzl et al., 2003). In a recent study, a nanoparticulate delivery system composed of poly(D,L-lactide-coglycolide) nanoparticles, anti-cytokeratin monoclonal IgG and cystatin was used to penetrate breast cancer cells and inhibit intracellular cathepsin B activity, respectively, thereby reducing the invasiveness of these tumor cells in vitro (Obermajer et al., 2007). Here, in immunoblotting and fluorogenic enzymatic assays (Linebaugh et al., 1999), we detected increases in cathepsin B expression and activity in SUM149 cells grown in U937-CM (Figure 4). In these experiments, SUM149 cells were grown on plastic to examine the direct effects of U937-CM (i.e., in the absence of rBM) on these cells. CA074, a highly selective cathepsin B inhibitor (Montaser et al., 2002), was used to verify cathepsin B activity detected in the enzymatic assay (Figure 4B). Moreover, both immunoblotting and enzymatic assays verified the absence of cathepsin B in U937-CM as compared to conditioned media of SUM149 cells (SUM149-CM) used as a positive control (Figure 4C). This indicated that the increase in cathepsin B expression and activity detected in SUM149 cells grown in U937-CM was not a result of uptake of cathepsin B secreted from U937 cells (Figure 4C). Thus, the upregulation in cathepsin B expression and activity in SUM149 cells would be consistent with this enzyme contributing to the increases in both ECM degradation and invasion by these cells. Furthermore, we analyzed subcellular distribution of cathepsin B in SUM149 grown in coverslips in the absence and presence of U937-CM (Figure 4D and E, respectively). Increased expression of cathepsin B, localized in vesicles in the perinuclear region, was detected in SUM149 grown in U937-CM (Figure 4E). Analyses of other proteases that may contribute to increases in ECM degradation and invasion are ongoing.
Figure 4. Conditioned media from U937 cells increases the levels and activity of cathepsin B in SUM149 cells.

To specifically see the effect of U937 secretions on the expression of cathepsin B in SUM149 without the influence of rBM, SUM149 cells were grown on plastic for 48 h in complete media alone (Ham’s F12 media with 5% FBS) (lane 1) or with U937-CM (lane 2), washed several times with phosphate buffered saline and incubated overnight in serum-free medium. Cells were solubilized in lysis buffer [250 mM sucrose, 25 mM MES pH 7.5, 1 mM EDTA, 0.1% Triton X-100 (Sigma)] and cell lysates were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with polyclonal antibodies against cathepsin B (Moin et al., 1992) and β-actin (Sigma) as a loading control. Cathepsin B forms observed are the proenzyme (43/46 kDa), an intermediate form (38 kDa), single chain mature enzyme (31 kDa) and the heavy chain of double chain mature enzyme (25/26 kDa heavy chain) (A). Immunoblots are representative of at least three independent experiments. Cathepsin B activity in cell lysates was determined using a fluorogenic enzymatic assay that employs 100 μM Z-Arg-Arg-NHMec (Bachem, Torrance, CA, USA), which under the conditions of this assay is a selective substrate for cathepsin B (Linebaugh et al., 1999), in the presence and absence of 10 μM CA074 (Peptide Institute, Louisville, KY, USA), a highly selective cathepsin B inhibitor (Linebaugh et al., 1999; Montaser et al., 2002). Cathepsin B activity in the cell lysates of SUM149 cells grown in complete media (white bar) and in U937-CM (black bars) is expressed as pmol/min/μg DNA (B). Pepsin (Roche, Indianapolis, IN, USA)-activated cathepsin B activity was also measured in the conditioned media of both SUM149 (positive control) and U937 cells using 100 μM Z-Arg-Arg substrate (Linebaugh et al., 1999) and expressed as pmol/min/μg DNA (C). The insert panel shows SUM149 (lane 1) and U937 (lane 2) conditioned media subjected to SDS-PAGE and immunoblotting using polyclonal cathepsin B antibody. Graphs are representative of at least three independent experiments and presented as mean±S.D. *Indicates a p-value <0.05, as determined by Student’s t-test. Intracellular immunostaining for cathepsin B (green) was performed on SUM149 cells grown on glass coverslips in complete media (Ham’s F12 medium with 5% FBS) (D) or in U937-CM (E). Cells were incubated in the presence of saponin (Sigma) with a primary polyclonal antibody against cathepsin B followed by FITC-conjugated affinity-purified donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA, USA) plus 5% normal donkey serum (Cavallo-Medved et al., 2005). Confocal microscopy was performed in MIRL with a Zeiss LSM 510 META NLO microscope (Carl Zeiss) using a 63× lens under oil immersion. On glass coverslips, IBC cells form a monolayer, thus differing in morphology from those illustrated in Figure 1 where they are grown in a three-dimensional rBM culture. These images are representative of three experiments. Nuclei were stained with DAPI (blue). Bars, 10 μm.
Caveolin-1, the structural protein of lipid raft caveolae, was initially reported to act as a breast tumor suppressor (Bouras et al., 2004); however, recent studies have classified caveolin-1 and -2 as biomarkers for the basal-like phenotype in the breast carcinoma subgroup (Sagara et al., 2004; Pinilla et al., 2006) that includes IBC (Perou et al., 2000). Upregulation of caveolin-1 has been observed in other malignancies, such as colon, prostate and pancreatic cancers (Yang et al., 1998; Fine et al., 2001; Suzuoki et al., 2002), particularly in the metastatic stage of the disease. In IBC cell lines and patient tumor tissues, overexpression of caveolin-1 and -2 correlates with increased expression of RhoC, implicating these proteins in the aggressive phenotype of IBC (Van den Eynden et al., 2006). We have previously suggested that caveolin-1 and caveolae contribute to tumor invasion by compartmentalizing several cell surface proteases involved in ECM degradation (Cavallo-Medved et al., 2005), and our ongoing studies using patient samples will elucidate the contribution of caveolae-associated proteases in the metastatic phenotype of IBC. We have identified cell surface cathepsin B associated with caveolae of human, colorectal carcinoma cells (Cavallo-Medved et al., 2003). Moreover, we found that downregulation of caveolin-1 alters trafficking of cathepsin B to caveolae and decreases secretion of this enzyme, thereby reducing ECM degradation by and invasion of these cells (Cavallo-Medved et al., 2005). Here, we found that in SUM149 cells grown in U937-CM, caveolin-1 expression was significantly increased compared to control SUM149 cells (Figure 5). These results correlate with the overexpression of cathepsin B in SUM149 cells under these conditions and may suggest an increase in the association of cathepsin B with caveolae.
Figure 5. Conditioned medium of U937 cells increases the expression of caveolin in SUM149 cells.

Cell lysates of SUM149 cells grown on plastic for 48 h in complete media alone (Ham’s F12 medium with 5% FBS) (lane 1) or with U937-CM (lane 2) were subjected to 12% SDS-PAGE and immunoblotted with polyclonal antibodies against caveolin (BD Biosciences, San Jose, CA, USA) and β-actin (loading control). Results are representative of at least three independent experiments.
Our study supports the hypothesis that interaction of monocytes with IBC cells stimulates the invasiveness of IBC cells. We speculate that this is the result of enhanced cell surface activity of the cysteine protease cathepsin B in IBC cells. The increased invasion and proteolysis in the IBC cells are accompanied by: (i) increased expression and activity of cathepsin B, and (ii) increased expression of caveolin-1, caveolae being a site for localization of cathepsin B on the tumor cell surface.
Acknowledgments
We acknowledge with special gratitude the contributions of J. Dosescu, M.B. Olive, D. Rudy and K. Moin to these studies. We also thank Dr. Stephen P. Ethier, Barbara Ann Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA, for kindly providing us with the SUM149 cell line. The authors were supported by an Avon Foundation American Association for Cancer Research International Scholar Award in Breast Cancer Research (M.M.M.), an Avon grant #02-2007-049 (M.M.M., D.C.M., B.F.S.) and National Institutes of Health (NIH) grant CA 56586 (B.F.S.). Microscopy and Imaging Resources Laboratory is supported in part by National Institutes of Health Center Grants P30ES06639 and P30CA22453 and a Roadmap Grant U54RR020843.
References
- Bieche I, Lerebours F, Tozlu S, Espie M, Marty M, Lidereau R. Molecular profiling of inflammatory breast cancer: identification of a poor-prognosis gene expression signature. Clin Cancer Res. 2004;10:6789–6795. doi: 10.1158/1078-0432.CCR-04-0306. [DOI] [PubMed] [Google Scholar]
- Bouras T, Lisanti MP, Pestell RG. Caveolin-1 in breast cancer. Cancer Biol Ther. 2004;3:931–941. doi: 10.4161/cbt.3.10.1147. [DOI] [PubMed] [Google Scholar]
- Cariati M, Bennett-Britton TM, Pinder SE, Purushotham AD. ‘Inflammatroy’ breast cancer. Surg Oncol. 2005;14:133–143. doi: 10.1016/j.suronc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Cavallo-Medved D, Dosescu J, Linebaugh BE, Sameni M, Rudy D, Sloane BF. Mutant K-ras regulates cathepsin B localization on the surface of human colorectal carcinoma cells. Neoplasia. 2003;5:507–519. doi: 10.1016/s1476-5586(03)80035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo-Medved D, Mai J, Dosescu J, Sameni M, Sloane BF. Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J Cell Sci. 2005;118:1493–1503. doi: 10.1242/jcs.02278. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Delarue JC, May-Levin F, Mouriesse H, Contesso G, Sancho-Garnier H. Oestrogen and progesterone cytosolic receptors in clinically inflammatory tumours of the human breast. Br J Cancer. 1981;44:911–916. doi: 10.1038/bjc.1981.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine SW, Lisanti MP, Galbiati F, Li M. Elevated expression of caveolin-1 in adenocarcinoma of the colon. Am J Clin Pathol. 2001;115:719–724. doi: 10.1309/YL54-CCU7-4V0P-FDUT. [DOI] [PubMed] [Google Scholar]
- Giordano SH, Hortobagyi GN. Inflammatory breast cancer: clinical progress and the main problems that must be addressed. Breast Cancer Res. 2003;5:284–288. doi: 10.1186/bcr608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97:966–975. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleer CG, Griffith KA, Sabel MS, Gallagher G, van Golen KL, Wu ZF, Merajver SD. RhoC-GTPase is a novel tissue biomarker associated with biologically aggressive carcinomas of the breast. Breast Cancer Res Treat. 2005;93:101–110. doi: 10.1007/s10549-005-4170-6. [DOI] [PubMed] [Google Scholar]
- Labidi SI, Mrad K, Mezlini A, Ouarda MA, Combes JD, Ben Abdallah M, Ben Romdhane K, Viens P, Ben Ayed F. Inflammatory breast cancer in Tunisia in the era of multimodality therapy. Ann Oncol. 2008;19:473–480. doi: 10.1093/annonc/mdm480. [DOI] [PubMed] [Google Scholar]
- Lah TT, Kalman E, Najjar D, Gorodetsky E, Brennan P, Somers R, Daskal I. Cells producing cathepsins D, B, and L in human breast carcinoma and their association with prognosis. Hum Pathol. 2000;31:149–160. doi: 10.1016/s0046-8177(00)80214-2. [DOI] [PubMed] [Google Scholar]
- Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- Lerebours F, Bieche I, Lidereau R. Update on inflammatory breast cancer. Breast Cancer Res. 2005;7:52–58. doi: 10.1186/bcr997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linebaugh BE, Sameni M, Day NA, Sloane BF, Keppler D. Exocytosis of active cathepsin B enzyme activity at pH 7.0, inhibition and molecular mass. Eur J Biochem. 1999;264:100–109. doi: 10.1046/j.1432-1327.1999.00582.x. [DOI] [PubMed] [Google Scholar]
- Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- Moin K, Day NA, Sameni M, Hasnain S, Hirama T, Sloane BF. Human tumour cathepsin B. Comparison with normal liver cathepsin B. Biochem J. 1992;285:427–434. doi: 10.1042/bj2850427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaser M, Lalmanach G, Mach L. CA-074, but not its methyl ester CA-074Me, is a selective inhibitor of cathepsin B within living cells. Biol Chem. 2002;383:1305–1308. doi: 10.1515/BC.2002.147. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermajer N, Kocbek P, Repnik U, Kuznik A, Cegnar M, Kristl J, Kos J. Immunonanoparticles, an effective tool to impair harmful proteolysis in invasive breast tumor cells. FEBS J. 2007;274:4416–4427. doi: 10.1111/j.1742-4658.2007.05971.x. [DOI] [PubMed] [Google Scholar]
- Omar S, Khaled H, Gaafar R, Zekry AR, Eissa S, el-Khatib O. Breast cancer in Egypt: a review of disease presentation and detection strategies. East Mediterr Health J. 2003;9:448–463. [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Pinilla SM, Honrado E, Hardisson D, Benitez J, Palacios J. Caveolin-1 expression is associated with a basal-like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res Treat. 2006;99:85–90. doi: 10.1007/s10549-006-9184-1. [DOI] [PubMed] [Google Scholar]
- Premzl A, Zavasnik-Bergant V, Turk V, Kos J. Intracellular and extracellular cathepsin B facilitate invasion of MCF-10A neoT cells through reconstituted extracellular matrix in vitro. Exp Cell Res. 2003;283:206–214. doi: 10.1016/s0014-4827(02)00055-1. [DOI] [PubMed] [Google Scholar]
- Radisky ES, Radisky DC. Stromal induction of breast cancer: inflammation and invasion. Rev Endocr Metab Disord. 2007;8:279–287. doi: 10.1007/s11154-007-9037-1. [DOI] [PubMed] [Google Scholar]
- Ren WP, Sloane BF. Cathepsins D and B in breast cancer. Cancer Treat Res. 1996;83:325–352. doi: 10.1007/978-1-4613-1259-8_16. [DOI] [PubMed] [Google Scholar]
- Sagara Y, Mimori K, Yoshinaga K, Tanaka F, Nishida K, Ohno S, Inoue H, Mori M. Clinical significance of Caveolin-1, Caveolin-2 and HER2/neu mRNA expression in human breast cancer. Br J Cancer. 2004;91:959–965. doi: 10.1038/sj.bjc.6602029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameni M, Dosescu J, Moin K, Sloane BF. Functional imaging of proteolysis: stromal and inflammatory cells increase tumor proteolysis. Mol Imaging. 2003;2:159–175. doi: 10.1162/15353500200303136. [DOI] [PubMed] [Google Scholar]
- Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, Gnant M, Horvat R, Jakesz R, Birner P. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery. 2006;139:839–846. doi: 10.1016/j.surg.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Suzuoki M, Miyamoto M, Kato K, Hiraoka K, Oshikiri T, Nakakubo Y, Fukunaga A, Shichinohe T, Shinohara T, Itoh T, et al. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer. 2002;87:1140–1144. doi: 10.1038/sj.bjc.6600619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eynden GG, Van Laere SJ, Van der Auwera I, Merajver SD, Van Marck EA, van Dam P, Vermeulen PB, Dirix LY, van Golen KL. Overexpression of caveolin-1 and -2 in cell lines and in human samples of inflammatory breast cancer. Breast Cancer Res Treat. 2006;95:219–228. doi: 10.1007/s10549-005-9002-1. [DOI] [PubMed] [Google Scholar]
- van Golen KL, Davies S, Wu ZF, Wang Y, Bucana CD, Root H, Chandrasekharappa S, Strawerman M, Ethier SP, Merajver SD. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatroy breast cancer phenotype. Clin Cancer Res. 1999;5:2511–2519. [PubMed] [Google Scholar]
- Van Laere S, Van der Auwera I, Van den Eynden GG, Fox SB, Bianchi F, Harris AL, van Dam P, Van Marck EA, Vermeulen PB, Dirix LY. Distinct molecular signature of inflammatory breast cancer by cDNA microarray analysis. Breast Cancer Res Treat. 2005;93:237–246. doi: 10.1007/s10549-005-5157-z. [DOI] [PubMed] [Google Scholar]
- Van Laere SJ, Van den Eynden GG, Van der Auwera I, Vandenberghe M, van Dam P, Van Marck EA, van Golen KL, Vermeulen PB, Dirix LY. Identification of cell-of-origin breast tumor subtypes in inflammatory breast cancer by gene expression profiling. Breast Cancer Res Treat. 2006;95:243–255. doi: 10.1007/s10549-005-9015-9. [DOI] [PubMed] [Google Scholar]
- Wulfkuhle JD, Sgroi DC, Krutzsch H, McLean K, Mc-Garvey K, Knowlton M, Chen S, Shu H, Sahin A, Kurek R, et al. Proteomics of human breast ductal carcinoma in situ. Cancer Res. 2002;62:6740–6749. [PubMed] [Google Scholar]
- Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, Nasu Y, Bangma CH, Kattan MW, Scardino PT, Thompson TC. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998;4:1873–1880. [PubMed] [Google Scholar]