Abstract
Human respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection in infants. In human infants, plasmacytoid dendritic cells (pDC) are recruited to the nasal compartment during infection and initiate host defense through the secretion of type I IFN, IL-12 and IL-6. However, RSV-infected pDCs are refractory to TLR7-mediated activation. Here, we used the rodent-specific pathogen, pneumonia virus of mice (PVM), to determine the contribution of pDC and TLR7-signaling to the development of the innate inflammatory and early adaptive immune response. In wild-type (WT) but not TLR7- or myeloid differentiation protein 88 (MyD88)-deficient mice, PVM inoculation led to a marked infiltration of pDCs and increased expression of type I, II and III IFNs. The delayed induction of IFNs in the absence of TLR7 or MyD88 was associated with a diminished innate inflammatory response and augmented virus recovery from lung tissue. In the absence of TLR7, PVM-specific CD8+ T cell cytokine production was abrogated. The adoptive transfer of TLR7-sufficient but not TLR7-deficient pDC to TLR7-gene-deleted mice recapitulated the antiviral responses observed in WT mice and promoted virus clearance. In summary, TLR7-mediated signaling by pDC is required for appropriate innate responses to acute pneumovirus infection. It is conceivable that as-yet-unidentified defects in the TLR7 signaling pathway may be associated with elevated levels of RSV-associated morbidity and mortality among otherwise healthy human infants.
Introduction
RSV is an enveloped, negative-sense, single-stranded RNA (ssRNA) virus of the family Paramyxoviridae, genus Pneumovirus. RSV infects approximately two-thirds of all infants in the first year of life and is the leading cause of hospitalisation for respiratory tract illnesses (1, 2). While most RSV infections are self-limited, severe RSV bronchiolitis is characterised by pulmonary granulocytic infiltrates, and occlusion of the bronchioles can develop as a result of edema, sloughing of necrotic epithelia from small airways, and increased secretion of mucus (3). Recently, the importance of innate pattern recognition receptors (PRRs) in sensing signature motifs of invading pathogens and in initiating the appropriate innate and adaptive immune response has been realised (4, 5). Dendritic cells located within the airway mucosa sample foreign molecules and sense viral nucleic acids through the activation of toll-like receptors (TLRs), the retinoic acid-inducible gene (RIG)-I-like helicase receptor (RLR) and/or nucleotide-binding domain-like receptor (NLR) systems (6–10). Plasmacytoid dendritic cells (pDC) were originally described as IFN-producing cells that preferentially utilise TLR7 and TLR9 to recognise RNA and DNA viruses respectively. In so doing these cells initiate an anti-viral state and protective immunity through the release of preformed type I IFNs (11–13). Although significant increases in pDC numbers have been detected in nasal wash samples obtained from infants hospitalised with acute RSV infection (14), functional studies performed in vitro have revealed that clinical isolates of RSV can infect human pDC and abolish TLR7-mediated production of type I IFN (14, 15). This finding suggests that RSV-induced attenuation of the innate immune response could be among the factors leading to the development of bronchiolitis and/or incomplete immunity. Despite this, the role of TLR7 in the generation of host defense against pneumovirus infection remains to be determined.
TLR7 is expressed in the endosome and can therefore detect virions following engulfment by endocytosis (i.e. prior to cellular infection) (16, 17). Interestingly, Lee and colleagues demonstrated that actively-replicating, infectious vesicular stomatitis virus promoted a more substantial type I IFN response via TLR7 than was achieved with inactivated, non-replicating virus (18). Moreover, human RSV induces the release of type I IFN from human pDC in a replication dependent manner (19). It is clear from these findings that the nature of the infectious pathogen and its ability to replicate in vivo may have substantial impact on the findings obtained. As such, we elected not to use hRSV, which replicates poorly in mice. Our study utilized pneumonia virus of mice (PVM), a rodent-specific pneumovirus pathogen which undergoes robust replication in response to a minimal virion inoculation and models the more severe forms of infantile RSV disease in inbred strains of mice (20, 21). Our study examined the unique contributions of pDC, of the pathogen sensing receptor, TLR7, and its cognate intracellular adaptor molecule MyD88, in early innate immune recognition and the development of host defense to pneumovirus infection in vivo.
Materials and Methods
Animals and PVM inoculation
All mice were backcrossed to BALB/c for 10 generations and housed at the University of Newcastle specific pathogen free facility. All experiments were approved by the University of Newcastle AnimalCare and Ethics Committee. Stocks of PVM (J3666 strain) were maintained as described previously (22). Isofluorane-anaesthetised mice were inoculated intranasally with 5 plaque forming units (PFU) of PVM in a volume of 10 μl at 7 days of age. Vehicle (DMEM containing 10% fetal calf serum) inoculated mice served as controls.
Flow cytometry
Leukocytes in the left lung lobe were enumerated by flow cytometry as described previously (23). Briefly, lung cells were mashed through a cell strainer and red blood cells lysed with ammonium chloride. Cells were seeded into a U bottom 96 well plate at 106/well, and pre-incubated with anti-FcγRIII/II (‘Fc-block’) in PBS/2% FCS medium prior to a 20 minute incubation with one or more of the following fluorochrome-labelled antibodies (BD Biosciences unless otherwise stated): FITC- and PE-B220 (RA3-6B2); Per-CP-conjugated Gr-1 (RB6-8C5); FITC-conjugated CD3 (145-2C11); APC-conjugated CD4 (RM4-5); PerCP-conjugated CD8a (clone 53-6.7); APC-conjugated Sca-1 (e-Bioscience); FITC-conjugated CD11c (clone HL3); PE-conjugated CD49b (clone DX5); Per-CP-conjugated CD11b (clone M1/70); APC-conjugated Siglec-H (e-Bioscience, clone eBio440c). After three washes in PBS/2% FCS medium cells were resuspended in PBS/formalin (0.5% v/v) and analysed using a BD CANTO within 3 days.
Quantification of lung gene and protein expression
mRNA was quantified as described previously (24). Briefly, the left lung lobe was harvested into RNA later (Ambion, TX) and left at 4°C overnight prior to storage at −80°C. The remaining lung lobes, with the exception of the large left lobe (used to measure inflammatory cells) were harvested into 1 ml RIPA buffer (Sigma) and stored at −80°C. RNA was isolated and cDNA transcribed using random primers and Moloney murine leukemia virus reverse transcriptase. The real-time polymerase chain reaction was performed with an ABI 7700 Sequence Detector System (Applied Biosystems, Scoresby, Australia). Expression of a housekeeping gene (HPRT) was also determined against plasmid controls to generate a standard curve. Gene expression was normalized againstthe expression of the housekeeping gene, which was not altered bythe treatment, and expressed as fold change compared to vehicle-inoculated wild type mice. Primer sequences used were as follows:
IFN-α4 (fwd) 5′ – ACCAACAGATCCAGAAGGCTCAAG-3′,
(rse) 5′ – AGTCTTCCTGGGTCAGAGGAGGTT- 3′;
IFN-β (fwd) 5′ – AGAGTTACACTGCCTTTGCCATCC-3′,
(rse) 5′ – CCACGTCAATCTTTCCTCTTGCTT-3′;
IFN-λ2 (IL-28B) (fwd) 5′ – TTGAGAAGGACATGAGGTGCAGTT- 3′,
(rse) 5′ – CTCTGCTGTGGCCTGAAGCTGT- 3′;
IFN-γ (fwd) 5′ – TCTTGAAAGACAATCAGGCCATCA- 3′,
(rse) GAATCAGCAGCGACTCCTTTTCC- 3′;
IFN regulatory factor (IRF)7 (fwd) 5′ – CTTAGCCGGGAGCTTGGATCTACT- 3′,
(rse) 5′ – CCCTTGTACATGATGGTCACATCC- 3′,
CCL3 (fwd) 5′ – CCTCTGTCACCTGCTCAACA- 3′, (rse) 5′ –
GATGAATTGGCGTGGAATC- 3′
RIG-I (fwd) 5′ – ACAAACCACAACCTGTTCCTGACA- 3′, (rse) 5′ –
TGGCGCAGAATATCTTTGCTTTCT- 3′
Mx-1 (fwd) 5′ – GACTCTCATTGACCTGCCTGGAAT- 3′, (rse)
5′ – TTACGAAGGCAGTTTGGACCATCT- 3′, NOD2 (fwd) 5′ –
GCCAGTACGAGTGTGAGGAGATCA- 3′, (rse) 5′ –
CAGCTCCAAGATGTTCTCCGTGTA- 3′, NLRP3 (fwd) 5′ –
GATTGACTTCAATGGCGAGGAGAA- 3′, (rse) 5′ –
AACCTGCTTCTCACATGTCGTCTG- 3′, PVM SH (fwd) 5′ –
GCCTGCATCAACACAGTGTGT- 3′, (rse) 5′ – GCCTGATGTGGCAGTGCTT- 3′, HPRT
(fwd) 5′ – AGGCCAGACTTTGTTGGATTTGAA- 3′,
(rse) 5′ – CAACTTGCGCTCATCTTAGGCTTT- 3′.
For analysis of cytokine/chemokine protein expression, the right lung lobes were collected and the tissue homogenised in RIPA buffer using a tissue tearer (Diantree Scientific, Tasmania, Australia). After 10 minute incubation on ice, samples were centrifuged at 13000 rpm for 10 mins at 4°C to remove cellular debris. CCL3 (R & D Systems, Gymea, Australia), IL-12p40 (e-Bioscience), IL-6 (e-Bioscience), TNF–α (e-Bioscience) or IFN–γ (BD Biosciences) were measured by ELISA according to the manufacturer’s instructions.
Immunohistochemistry
Lung biopsies from WT and TLR7-gene-deleted mice were processed and immunohistochemistry performed as described previously (25). In brief, sections were permeabilised with saponin/PBS (0.1% w/v) buffer for 30 minutes, and treated with 10% normal goat serum to reduce non-specific binding prior to overnight incubation with rabbit anti-TLR7 (Abcam; 10 μg/ml) or rabbit anti-phosphorylated IRF7 (Cell Signalling Technology, 1/50). After three wash steps, alkaline phosphatise-conjugated goat anti-rabbit (Sigma) was incubated at 1/200 dilution. Positive cells stained red after development with Fast Red (Sigma). Cells were counter stained with Harris’ Haematoxylin (Dako) and mounted in glycergel (Dako).
In vivo depletion of pDC
1.6 mg/kg body weight of functional grade anti-CD317 antibody (BST-2, PDCA-1; e-Bioscience) was injected into the peritoneum on day 5 and day 6 of life.
Generation of pDC from bone marrow
pDC were derived from bone marrow cells of WT and TLR7-gene-deleted mice cultured in 100 ng/mL Flt3-L (a generous gift from Amgen Inc., CA) as described previously (26). On day 3 and 6 of culture, half the media was replenished with Flt3-L-supplemented RPMI.
In vitro infection of pDC with PVM and intracellular IFN–α staining
After a 10 day culture with Flt3-L, un-fractionated bone marrow cells were removed from culture flasks, washed, and seeded into 96 well plates at 106/well and incubated at 37°C for 2 hrs with PVM at a multiplicity of infection of 0.1 before the addition of brefeldin A (5 mg/ml; Sigma). Cells were cultured for an additional 5 hours prior to treatment with anti-FcγRIII/II (‘Fc-block’), then labelled with fluorescence-labelled antibodies against B220, CD11c, and Siglec-H. Following fixation and permeabilisation cells were incubated with rat anti-IFN-α at 10 μg/ml (HyCult biotechnology, HM1001) for 30 mins at 4°C (27). After three wash steps, cells were incubated with a FITC-conjugated rabbit anti-rat antibody (1:500 dilution; Sigma).
Adoptive transfer of purified pDC
On day 10 of culture, bone marrow cells from WT and TLR7-gene-deleted mice were counted and incubated for 30 min on ice with anti-B220-DM beads (BD Biosciences) at a ratio of 2 × 105 cells/μl of beads. pDC were positively selected using an iMagnet (BD Biosciences) and found to be >95% pure based on siglec-H and CD11clow expression analysed by flow cytometry. pDC were resuspended at 25 × 106 cells/ml and 0.5 × 106 pDC administered both intranasally and via injection to the peritoneum of TLR7-gene-deleted mice. All mice were inoculated intranasally with 5 PFU PVM 2 h later.
Draining lymph node cell culture and measurement of T cell cytokine production
Draining lymph nodes were removed and cells dissociated by applying gentle pressure with a syringe plunger over a cell strainer. Red blood cells were depleted by ammonium chloride lysis. Cells were resuspended in complete Hanks’ buffered saline solution (HEPES, sodium pyruvate, L-glutamine, 10% v/v FCS, PSN, β-mercapto-ethanol) seeded into a flat-bottomed 96 well plate at 0.3 × 106/well and stimulated with P261–270 peptide (28)(CYLTDRARI, Biomolecular Resource Facility, Australian National University) at 20μg/mL or diluent (complete RPMI). After a three day incubation, cell-free supernatants were collected and stored at −80°C prior to IFN-γ (BD Biosciences) and TNF (e-Bioscience) detection by ELISA.
Statistical analysis
Data presented are the means ± s.e.m. Data sets were analysed by Student t test except for the time courses which were analysed by ANOVA and Bonferroni Post Hoc Test, and Figure 5A where the Mann-Whitney test was employed. The software package GraphPadPrism 3.01 (GraphPad Software, San Diego, CA) was used for alldata analysis and preparation of graphs.
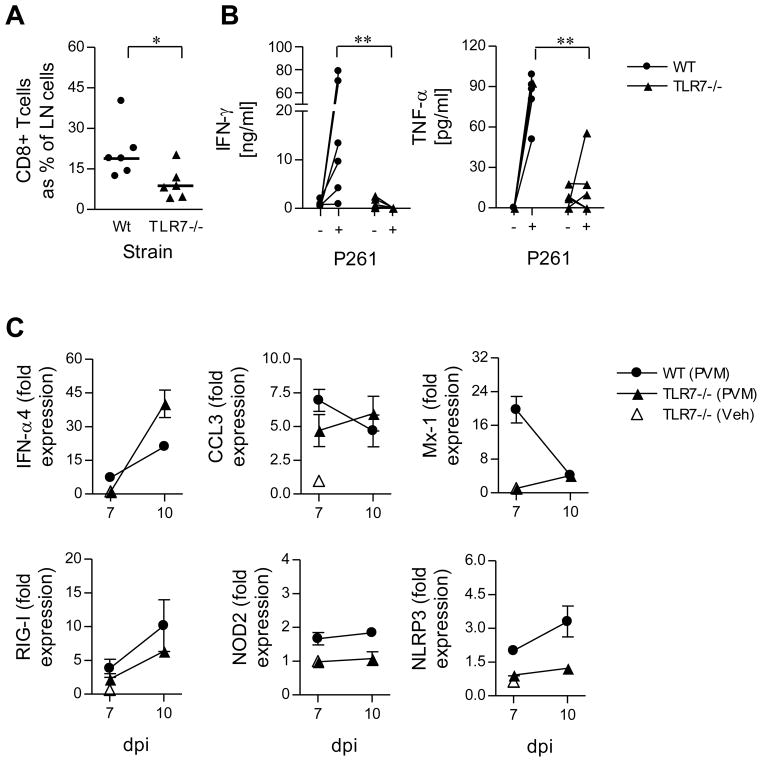
Figure 5. PVM-specific CD8+ T cell responses and the innate IFN response are delayed in the absence of TLR7.
WT (circles) and TLR7-deficient (triangles) mice were inoculated with PVM (closed) or vehicle (open) and sacrificed 10 days later. (A) The percent of LN cells that were CD3+CD8+ T cells at 10 days post infection (DPI). (B) Mediastinal lymph nodes were harvested, seeded at 0.3 × 106/well and stimulated with the PVM-specific peptide (P261) or media alone. After a 3 day culture, cell-free supernatants were quantified for IFN–γ and TNF–α protein expression. (C) IFNα4, CCL3, Mx-1, RIG-I, NOD2 and NLRP3 gene expression in whole lung extracts was measured by qRT-PCR. Data are mean +/− s.e.m, 5–6 mice in each group. Data in (B) were compared by a two-tailed Mann-Whitney and in (A) and (C) by unpaired t test. * p<0.05, ** p<0.01.
Results
The absence of TLR7 and MyD88 delays the onset of clinical symptoms and viral clearance
Virus detection may occur via a number of innate sensors including TLRs, RLRs and NLRs. To determine whether the TLR7 pathway is required for the clearance of the PVM virus pathogen, WT, TLR7-gene-deleted and MyD88-gene-deleted mice were inoculated at 7 days of age and virus recovery was measured by qPCR detection assay targeting the PVM SH gene as described previously (29). Virus titers were elevated at 5 and 7 days post infection (dpi) in both TLR7- and MyD88-gene-deleted mice as compared to WT mice (Fig. 1A). To assess the impact of this finding on morbidity, mice were weighed daily. PVM-infected WT mice exhibited blunted weight gain from as early as 2 dpi, and diverged significantly from vehicle-inoculated WT mice beginning at 4 dpi (Fig. 1B). In contrast, PVM infection had no impact on weight-gain among the 7-day old TLR7- and MyD88-gene-deleted mice until 7 dpi, when a sudden weight loss (11% of maximum body weight) was observed over the course of 24 hours (Fig. 1C and D). Of note, the organisation of the airway epithelium in the TLR7- and MyD88-gene-deleted mice was disturbed as evidenced by epithelial cell sloughing and denudation of the basement membrane. The presentation of clinical symptoms followed a similar pattern to weight loss, with WT mice presenting earlier but less severe symptoms, including reduced movement, piloeraction, than mice deficient in either TLR7 or MyD88 (data not shown). However, in some experiments, a few of the TLR7- and MyD88-deficient mice died between the ages of 5 and 10 dpi.
Figure 1. The absence of TLR7-MyD88 signaling delays weight loss, clinical symptoms and virus recovery.
Open symbols/bars represent vehicle-inoculated mice; closed symbols/bars, PVM-inoculated mice. WT mice are represented by circles; TLR7−/− mice by triangles; and MyD88−/− mice by diamonds. (A) Virus recovery (copies of PVM SH gene per 107 copies HPRT) in the lungs of WT, TLR7−/− and MyD88−/− mice was measured by qRT-PCR at 1, 3, 5 and 7 days post inoculation (dpi). (B) WT, (C) TLR7−/− and (D) MyD88−/−mice were weighed at 7 days of age immediately prior to inoculation (day 0) with vehicle or PVM. Mice were weighed every 24 hours thereafter until euthanasia, and weight expressed relative to day 0. Data are mean +/− s.e.m, 4–13 mice in each group and * p<0.05, ** p<0.01, *** p<0.001.
PVM-induced lung inflammation requires the expression of TLR7 and MyD88
The failure to mount an inflammatory response is often associated with the absence of weight loss (30). To compare the cellular inflammatory responses in lung tissue of PVM-infected WT, TLR7 and MyD88 gene-deleted mice, mechanically dispersed lung cells were labelled with fluorochrome-conjugated antibodies and phenotyped by flow cytometry. In WT mice, infiltrates of neutrophils and NK cells were elevated at 5 dpi and increased further at 7 dpi (Fig. 2A and B). In contrast, in the absence of TLR7 and MyD88, no NK cells and only few neutrophils (in TLR7−/− mice only) were detected at these time points. The NK cell and neutrophil chemoattractant, CCL3 can be expressed by the respiratory epithelium in response to pneumovirus infection in adult WT mice (31), although it is noteworthy that pDC are also a rich source of CCL3 (32, 33). In neonatal mice, we observed elevated CCL3 transcripts within 1 day of PVM inoculation in WT but not TLR7- or MyD88-gene-deleted mice (data not shown). Immunoreactive CCL3 was detected in whole lung homogenates at 5 and 7 dpi in WT but not TLR7- or MyD88-gene-deleted mice, consistent with the observed pattern of neutrophil and NK cell recruitment (Fig. 2C).
Figure 2. PVM inoculation of WT but not TLR7- or MyD88-deficient neonatal (7 day old) BALB/c mice induces an innate inflammatory response.
Open symbols represent vehicle-inoculated mice; closed symbols, PVM-inoculated mice. WT mice are represented by circles; TLR7−/− mice by triangles; and MyD88−/− mice by diamonds. The left lung lobe was mechanically dispersed and (A) FSclowGr-1highCD11b+ CD11c− neutrophils and (B) CD3-CD49b+ natural killer (NK) cells identified by flow cytometry. (C) CCL3 protein expression in right lung lobe homogenates was measured by ELISA. Data are mean +/-− s.e.m, 4–16 mice in each group and * p<0.05, ** p<0.01, *** p<0.001. * WT compared to TLR7-deficient mice, # WT compared to MyD88-deficient mice.
pDC recruitment and IFN response is absent in TLR7- and MyD88-deficient mice
Through its cognate receptor CCR5, CCL3 can promote the migration of pDC from the blood to sites of infection (34). In WT mice, inoculation with PVM led to a rapid infiltration of pDC (Siglec-H+, CD11clo, B220+) into the lung within 24 hours (Fig. 3A and supplementary Fig. 1). pDC remained elevated until 5 dpi and waned thereafter. By contrast, elevation of pDC numbers was not detected in lungs of TLR7- or MyD88-deficient mice. At 5 dpi, greater than 80% of WT pDC from PVM-infected mice were positive for MHC class II, an increase from 40% at baseline; no such response was observed in the absence of TLR7 or MyD88 (Fig. 3B). In contrast to pDC, classical DCs (CD11chigh CD11bhigh MHC class IIhigh) were not elevated in WT mice following inoculation with PVM. Of note, however, classical DC were greater in TLR7-deficient mice at 7 dpi (data not shown). pDC constitutively express type I IFNs and IFN regulatory factor (IRF)7 (35), an important transcription factor activated downstream of TLR and RLR signaling. We detected augmented levels of IFN-α4 and IFN-β transcripts in the lung in WT but not TLR7- or MyD88-deficient mice at 5 dpi (Fig. 3C; all data normalised against WT vehicle controls). Likewise, IRF7, was up-regulated in WT but not TLR7- or MyD88-deficient mice (Fig. 3C). Similar findings were obtained for the type III interferon, IFN-λ2 (IL-28A, Fig. 3C), and the IFN stimulated gene Mx-1 (data not shown). Collectively these data suggest that the early induction of IFNs and IFN-stimulatory genes in response to inoculation with PVM was dependent on the TLR7-MyD88 signaling pathway.
Figure 3. pDC recruitment and PVM-induced IFN and pro-inflammatory cytokine production requires TLR7-MyD88.
Open symbols/bars represent vehicle-inoculated mice; closed symbols/bars, PVM-inoculated mice. WT mice are represented by circles; TLR7−/− mice by triangles; and MyD88−/− mice by diamonds. The key to symbols is shown in (A) and is applicable to panels (C) and (D). (A) The left lung lobe was mechanically dispersed and pDC (Siglec-H+, CD11clo, B220+) expressed as a percent of lung cells. pDC from vehicle-inoculated TLR7- and MyD88-deficient mice are shown at day 1 only for clarity. (B) Percentage of MHC class II-positive pDC in the lung at 5 days post inoculation (DPI) in response to vehicle or PVM. (C) qRT-PCR analysis of IFN–α4, IFN–β, IFN-λ2, and IRF-7 mRNA expression in whole lung from wild-type, TLR7−/− and MyD88−/− mice (normalised to lungs from vehicle-inoculated WT mice) at 5 dpi. (D) Right lung homogenates were analysed at 1, 3, 5 and 7 days post inoculation (DPI) for IL-12p40, IL-6 and TNF–α protein expression by ELISA. Data are represented as mean +/− s.e.m, 4–8 mice in each group, or shown as individual mice, and * p<0.05, ** p<0.01, *** p<0.001. * WT compared to TLR7-deficient mice, # WT compared to MyD88-deficient mice.
Innate cytokine responses are attenuated in the absence of TLR7 or MyD88
In addition to IFNs, activated pDCs are a rich source of pro-inflammatory cytokines such as IL-12, IL-6 and TNF, which promote the development of cell-mediated and humoral immunity (27, 36). IL-12p40 was detected in lung homogenates of PVM-infected WT mice at 3 dpi (Fig. 3D), peaked at 5 dpi then waned but remained elevated at 7 dpi. In contrast, IL-12p40 expression remained at constant low levels throughout in PVM-infected TLR7- and MyD88-gene-deleted mice. Similarly, IL-6 and TNF expression peaked at 5 dpi and returned to baseline levels by 7 dpi, and were likewise dependent on TLR7-MyD88 signaling (Fig. 3D). Further studies will be required to confirm the cellular source of these cytokines.
T cell activation and the IFN–γ response requires TLR7 and MyD88
In adult mice, PVM infection is associated with pulmonary T cell activation (37). To determine whether the T cells in the lungs of neonatal mice were activated in response to PVM, we measured surface expression of Sca-1, an antigen which responds positively to stimulation with IFN-α (38). At 7 dpi, the fraction of Sca-1+CD4+ T cells detected from WT mice inoculated with PVM had increased as compared to those from mice inoculated with vehicle control (Fig. 4A). In contrast, the fraction of CD4+Sca-1+ T cells was unaltered following PVM-inoculation of TLR7- or MyD88-gene-deleted mice (Fig. 4A). Analysis of CD8+ T cells revealed an identical profile; augmented expression of Sca-1 in response to PVM was likewise dependent on TLR7 and MyD88 (Fig. 4B). IFN-γ is produced by activated NK cells, T helper 1 (Th1) cells and cytotoxic CD8+ T cells. In response to infection, augmented expression of transcripts encoding IFN–γ were observed at 5 dpi (Fig. 4C). Further increases were observed at by 7 dpi, most likely as a result of the increasing numbers of NK cells (Fig. 2B). In contrast, IFN–γ transcripts and protein expression was significantly lower in both TLR7-and MyD88-deficient as compared to WT mice (Fig. 4C and D). However, of note, the concentration of immunoreactive IFN–γ in infected mice was significantly greater than vehicle-inoculated mice in both TLR7- and MyD88-deficient mice at 7 dpi (Fig. 4D). Moreover, there was a significant increase in the expression of IFN–γ protein in the TLR7-but not MyD88-deficient mice at 7 dpi, indicating that another receptor family that operates via MyD88 (conceivably an IL-1 family member such as IL-18) may also contribute to the induction of IFN–γ. Using tetramer technology, Claassen et al have previously demonstrated that 11% of CD8+ T cells in the lungs of PVM-infected adult mice are specific for the P261 peptide (28). To address whether the absence of TLR7 affected the production of IFN–γ by PVM-specific CD8+ T cells, mediastinal lymph node cells were isolated at 10 dpi and stimulated with the P261 peptide. Although the degree of lymph node hyperplasia did not differ between WT and TLR7-gene-deleted mice (data not shown), CD8+ T cells as a fraction of mediastinal lymph nodes cells were significantly reduced in the absence of TLR7 (Fig. 5A). Correspondingly, in WT mice, P261 peptide-stimulation induced the production of both IFN–γ and TNF–α, but this response was significantly attenuated in the absence of TLR7 (Fig. 5B). Intriguingly, the fraction of lung CD8+ T cells positive for Sca-1 was now ~40% in both the WT and TLR7-deficient mice, even in the absence of pDC recruitment in the TLR7-deficient mice (data not shown). Together with the elevated neutrophilia (Fig. 2C) and IFN–γ expression (Fig. 4D) at 7 dpi, these data suggest that a delayed type I and type II IFN response occurs in the absence of TLR7. Therefore, we examined the lungs of TLR7-sufficient and –deficient mice at 7 and 10 dpi for IFN-α4, Mx-1, CCL3 and transcripts of other PRR know to participate in anti-viral immunity, namely RIG-1, NOD2 and NLRP3. Consistent with our findings at 5 dpi (see Fig. 3C), IFN-α4 expression remained low at 7 dpi in the absence of TLR7. However, there was a dramatic burst of IFN-α transcription at 10 dpi and this superseded the levels observed in WT mice (Fig. 5C). Consistent with the late IFN–α response, TLR7-deficient mice presented with a delayed increase in the expression of the IFN-stimulatory genes CCL3 and Mx-1 (Fig. 5C). These data suggested that in the absence of TLR7 another PRR can be activated to induce an IFN response albeit with markedly slower kinetics. Of the PRRs, all three were elevated in WT mice, while only RIG-I showed a greater than 2-fold increase in the absence of TLR7 (Fig. 5C).
Figure 4. Activation of the TLR7-MyD88 pathway is essential for T cell infiltration, activation and production of IFN–γ.
Open symbols/bars represent vehicle-inoculated; closed symbols/bars, PVM-inoculated. WT mice are represented by circles; TLR7−/− by triangles; and MyD88−/− by diamonds. (A–B) The percent of (A) CD4+ and (B) CD8+ T cells expressing stem cell antigen-1 (Sca-1, also known as LY6A, a surrogate marker of IFN-activation), in the lungs of wild-type, TLR7−/− and MyD88−/− mice were quantitated by flow cytometry at 7 days post inoculation (DPI). (C and D) IFN–γ gene and protein expression was measured in whole lung by (C) qRT-PCR and (D) ELISA at 1, 3, 5 and 7 dpi. Data are mean +/− s.e.m, 5 mice in each group and * p<0.05, ** p<0.01, *** p<0.001. Data in A and B, were compared by unpaired t test and in C and D by ANOVA and Bonferroni Post Hoc Test. * p<0.05, *** p<0.001. * WT compared to TLR7-deficient mice, # WT compared to MyD88-deficient mice.
TLR7-sufficient but not -deficient pDC recapitulate host defense and decrease viral load
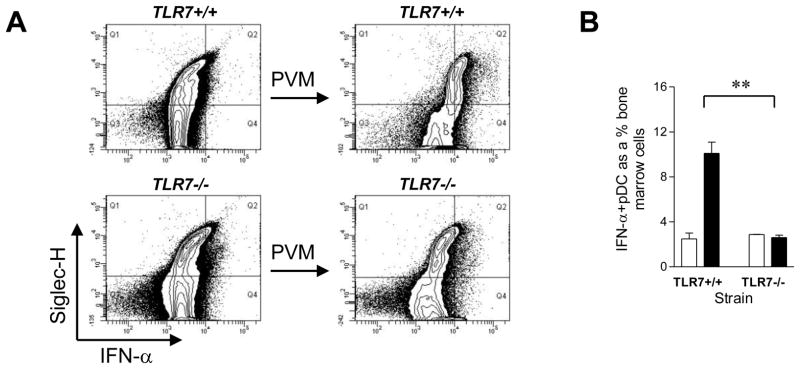
In order to elucidate further the requirement for TLR7 in pDC recognition of PVM, we cultured FLT3L bone marrow-derived TLR7-sufficient (TLR7 +/+) and TLR7-deficient (TLR7−/−) pDC in vitro. Cultured cells were challenged with PVM and stained for IFN–α production by intracellular cytokine staining by flow cytometry. Challenge with PVM augmented the expression of IFN–α in TLR7-sufficient (Fig. 6A, upper panels) but not TLR7-deficient (lower panels) pDC (B220+Siglec-H+ cells), as summarised in Fig. 6B.
Figure 6. PVM-induced upregulation of IFN–α expression by BM-derived pDC requires TLR7.
(A) Bone marrow cells were harvested and cultured in Flt3L to generate a pDC-rich cell population (~20%). After a 10 day culture, cells were cultured overnight with PVM at an MOI of 1 in the presence of brefeldin A. IFN–α was detected by intracellular cytokine staining and localised to Siglec-H+, B220+ cells. (B) The percentage of Siglec+IFN–α+ cells as a fraction of total Flt3L-treated WT and TLR7−/− bone marrow cells cultured with or without PVM. Data are mean +/− s.e.m, 3–4 mice in each group and ** p<0.01.
TLR7-sufficient but not -deficient pDC recapitulate host defense and decrease viral load
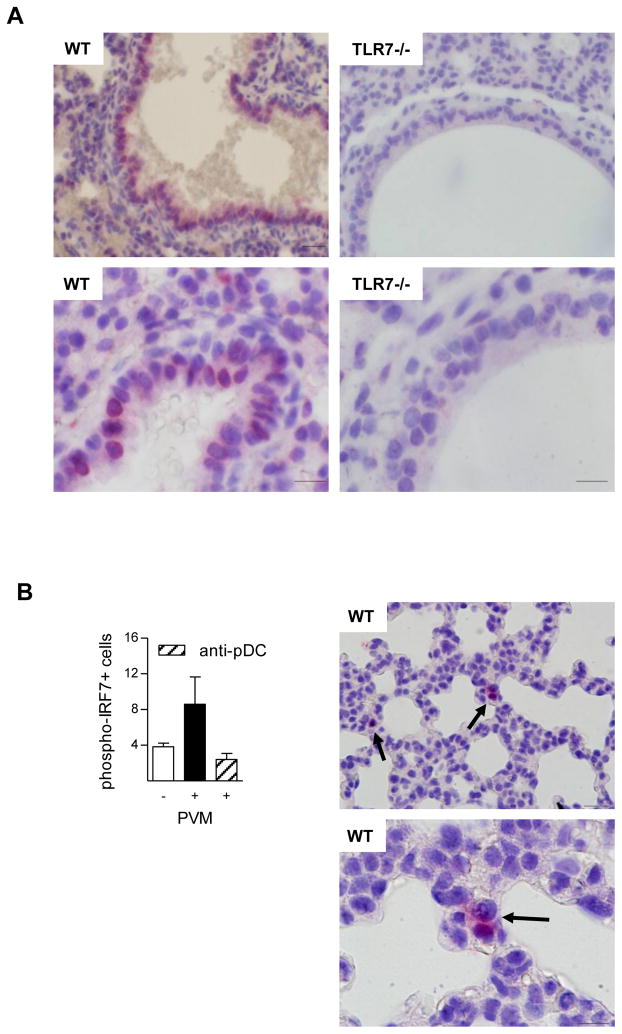
Although PVM-induced IFN-α expression in pDC was TLR7-dependent, it remained possible that non-pDC may contribute to the TLR7-dependent antiviral responses observed in vivo. To determine the cellular distribution of TLR7 expression in the lungs of naive neonatal mice we employed immunohistochemistry. In addition to leukocytes residing or circulating through the tissue, TLR7 immunoreactivity was present in the majority of airway epithelial cells (Fig. 7A, left panels). In contrast, no imunoreactivity was seen in both TLR7-deficient mice (Fig. 7A, right panels) and isotype –matched controls. Uniquely, pDC constitutively express IRF7 whereas IRF7 is transcriptionally regulated by type I IFNs in non-haematopoietic cells (35, 39). Thus, engagement of TLR7 in the absence of IRF7 would not prevent IFN-I production. Since IRF7 is phosphorylated in response to TLR7 stimulation, we used a phospho-IRF7 specific antibodyand performed immunohistochemistryon tissue biopsies obtained at 1 dpi. Critically, we observed that all of the immunoreactive cells were in the parenchymal tissue, consistent with the localisation of pDC in the lung shown by others (40). In contrast, airway epithelial cells of both large and small airways were negative for phospho-IRF7 at 1 dpi (Fig. 7B). Critically, when we depleted pDC with anti-CD317 prior to inoculation, the number of phospho-IRF7-positive cells at 1 dpi was markedly reduced.
Figure 7. TLR7 but not phosphorylated IRF7 is widely distributed.
(A) Lung biopsies from naive neonatal WT (left panels) and TLR7-deficient (right panels) mice were probed for TLR7. Scale bar equals 20μm, top panels (×400 magnification); and 10μm, (×1000 magnification) lower panels. (B) Enumeration of phosphorylated IRF7-immunoreactive cells in WT mice at 1 dpi following inoculation with vehicle or PVM +/− anti-pDC antibody, and photomicrograph demonstrating phosphorylated IRF7-immunoreactive cells in PVM-inoculated WT mice. Scale bar equals 20 μm, top panel (×400 magnification); and 10 μm, (×1000 magnification) lower panel.
To further address whether the phenotype of TLR7-deficient mice was primarily due to the absence of TLR7 on pDC, we generated highly pure pDC populations from FLT3L-cultured bone marrow of TLR7-sufficient and -deficient mice and adoptively transferred these cells via the intranasal and intraperitoneal routes to TLR7-deficient mice 2 hr prior to inoculation with PVM. The transfer of TLR7-sufficient but not TLR7-deficient pDC led to the development of an innate inflammatory response at 7 dpi, characterised by recruitment of neutrophils and NK cells and expression of the chemokine CCL3 (Fig. 8A–C) to an extent that was comparable to that observed in WT mice (Fig. 2A–C). Production of TNF (Fig. 8D) was also observed following transfer of TLR7-sufficient pDC, although interestingly, this had no impact on expression of IL-6 (data not shown). Similarly, TLR7-sufficient but not TLR7-deficient pDC resulted in augmented Sca-1 expression on both CD4+ and CD8+ T cells (Fig. 8E), suggesting that the activation of TLR7 on pDC is also necessary for the production of type I IFN. Consistent with our earlier findings, the induction of the cellular inflammatory response in the TLR7-deficient mice as a result of the reconstitution with TLR7-sufficient pDC led to stunted growth as measured by body weight, while transfer of TLR7-deficient pDC led to a similar pattern of weight gain to that observed in TLR7-deficient mice (Fig. 8F vs Fig. 1B and C). Likewise, the induction of an early inflammatory response following the transfer of TLR7-sufficient pDC was associated with significantly diminished virus recovery when compared to mice that received TLR7-deficient pDC (Fig. 8G).
Figure 8. Reconstitution of TLR7−/− mice with TLR7+/+ but not TLR7−/− BM-derived pDC recapitulates host defense and accelerates viral clearance.
Purified Flt3L-expanded pDC from TLR7+/+ and TLR7−/− bone marrow cells were adoptively transferred to TLR7−/−recipients. Mice were inoculated with PVM 2 h later and endpoints measured at 7 dpi (A and B). The left lung lobe was mechanically dispersed and (A) neutrophils (FSclowGr-1highCD11b+ CD11c−) and NK cells (CD3−CD49b+) identified by flow cytometry. (C and D) CCL3 and TNF-α protein expression in right lung lobe homogenates was measured by ELISA. (E) Representative histogram of lung CD3+CD4+Sca-1+ T cells following transfer of TLR7−/−(top panel) and TLR7+/+ (bottom panel) pDC to TLR7−/− mice. Graphs represent the percent CD3+CD4+ and CD3+CD8+ T cells expressing Sca-1 as detected by flow cytometry. (F) Recipient TLR7−/− mice were weighed at 7 days of age immediately prior to inoculation with PVM (day 0). Mice were weighed every 24 hours thereafter until euthanasia, and weight expressed relative to day 0. (G) Virus recovery in the lungs was measured by qRT-PCR.
Discussion
RSV is a primary aetiologic agent of bronchiolitis in infants and is associated with significant morbidity in this population. In this study, we employed the natural rodent pneumovirus pathogen, PVM, to model the more severe forms of RSV disease in mice and to investigate the role of TLR7 in promoting host defense. Using neonatal mice to reflect the more typical age of initial RSV infection in humans, we demonstrated that the absence of TLR7 or its intracellular adaptor protein MyD88, led to a diminished innate immune response and to significantly augmented virus recovery from lung tissue of infected mice. The adoptive transfer of TLR7-sufficient but not TLR7-deficient pDC restored the innate inflammatory response and resulted in diminished virus recovery, suggesting that TLR7-mediated recognition of PVM by pDC constitutes a critical pathway in the initiation of a timely and appropriate immune response to acute pneumovirus infection in the neonatal period.
Elevated numbers of pDC have been detected in nasal washings of infants with severe RSV infections (14). Consistent with this, we observed recruitment of pDC into the lungs of PVM-infected WT mice as early as 24 hours after inoculation. This response was not observed in TLR7- or MyD88-gene-deleted mice, which suggests that the TLR7-MyD88 pathway is engaged early on during the course of infection to mediate the early recruitment of pDC. Viral recognition may occur via the activation of resident leukocytes know to express TLR7 (e.g. pDC, conventional DC, monocytes, B cells (17)), or following detection by airway epithelial cells, which we identify here for the first time as being highly immunoreactive for TLR7. Based on the literature, pDC are uniquely placed to perform this task since they constitutively express high levels of the IRF7 (particularly in contrast to non-haematopoietic cells)(35, 39, 41), a necessary transcription factor for TLR7-induced transcription of type I IFN. Thus, non-pDCs may be largely incompetent and unable to execute TLR7-mediated signals in the initial phase of the host response. In an attempt to discern the relative contribution of haematopoietic cells verses non-heamatopoietic cells to TLR7-mediated recognition in the immediate phase of infection, we obtained lung tissue sections at 1 dpi and probed for phosphorylated IRF7 by immunohistochemistry. Immunoreactive cells were found in the parenchyma only, and not in the airway epithelium, indicating that the TLR7-IRF7 cascade is activated primarily in leukocytes at this time. Since the depletion of pDC reduced the number of phospho-IRF7 immunoreactive cells to baseline levels, it is likely that pDC are amongst the first cells to respond to the virus, even though pDC constitute only around 0.5% of all lung cells. We speculate that the up-regulation of IRF7 transcripts 5 dpi enables the epithelium to generate type I IFN in response to TLR7 engagement. However, since the activation of TLR7 can induce a non-IRF7-dependent signalling cascade, we cannot exclude the possibility that the epithelium is an active participant in the initial phase of infection also. Future studies will need to address the specific contribution of the epithelium to viral recognition and the prevention of viral dissemination.
In an illuminating study, Colonna and colleagues generated pDC-diptheria toxin receptor knock-in mice to inducibly deplete pDC. The findings from this study and those in which antibody-mediated depletion of pDC have been performed, suggest that pDC provide the immediate source of type I IFN (42, 43). Consistent with this paradigm, we observed that the transfer of TLR7-sufficient but not TLR7-deficient pDC enabled the recapitulation of the innate response at the same magnitude as that observed in infected WT mice. Future studies using the pDC-diptheria toxin receptor knock-in mice or bone marrow chimeras (in adult mice) will further clarify the contribution of pDC, however, based on our findings we postulate that in response to PVM, pDC mediate the immediate antiviral innate response.
CCL3 has been detected in airway secretions from RSV-infected infants (44) and CCL3/CCR1 is crucial for neutrophil recruitment in response to PVM (45). Here we detected production of CCL3 that was temporally associated with the infiltration of neutrophils and NK cells to the lung of WT mice; expression of CCL3, as well as neutrophil and NK cell recruitment were eliminated in both TLR7 and MyD88 gene-deleted mice. Although we did not specifically examine the cellular source of the CCL3, others have demonstrated that pDC, but not myeloid DC, can generate an array of chemokines including CCL3 (32, 33, 46). In addition, pDC may indirectly induce the expression of CCL3 through the release of IFN-α (47), although we have shown previously that PVM-induced up-regulation of CCL3 occurs in the absence of IFNαβ-receptors (22). In stark contrast to WT mice where blunted weight growth was observed as early as 4 dpi, in both TLR7- and MyD88-deficient mice the absence of an inflammatory response led to normal weight gain. However, the absence of an early TLR7/MyD88-mediated innate inflammatory (NK cell and neutrophil) response and CTL activation led to increased virus recovery at 5 and 7 dpi. Of note, both the TLR7- and MyD88-deficient, but not WT mice, exhibited >10% loss in body weight but at 7 dpi. The adoptive transfer of TLR7-sufficient (but not deficient) pDC to TLR7-deficient mice led to the restoration of CCL3 (and TNF–α) production and associated neutrophilia to recapitulate the attenuated weight growth observed in infected WT mice. In contrast, mice that received TLR7-deficient pDC presented a late fall in body weight. Our data suggest that TLR7-mediated activation of pDC promotes the early innate inflammatory response which underlies the early and mild pathophysiologic symptoms of disease. The delayed and heightened morbidity that was evident in the absence of TLR7 may suggest that pDC are protective although longer term studies will be needed to validate this.
In some studies, some of the TLR7-deficient mice MyD88-deficient mice, died at 4–7 dpi in the absence of any clinical symptoms, and prior to the onset of cellular inflammation. For ethical reasons we were unable to perform a definitive LD50 study, however, these findings consolidate the notion that the absence of TLR7 is not beneficial (as might be inferred from the lack of clinical symptoms at 4 dpi). Intriguingly, emerging clinical data suggest that the absence of NK cells may be related to increasing severity of disease (3, 48–50). Analysis of lung tissue from infants with fatal cases of RSV has revealed a near absence of NK cells and CTLs (49), while the bronchial epithelia of these subjects was highly immunoreactive for RSV antigen, indicative of virus replication in situ. Future studies will need to address whether alterations in TLR7 expression or function (or related signaling molecules) predispose toward virus-associated bronchiolitis. In addition, it remains to be determined whether the delayed presentation of symptoms observed in the gene-deleted mice occurred as a result of a late, albeit weak innate response in these mice, or whether it was related to increased virus replication and associated cellular damage. However, we did observe that the organisation of the airway epithelium of TLR7-deficient mice was significantly perturbed, with evidence of denudation of the basement membrane and epithelial cell sloughing, similar to that seen in clinical autopsy specimens (3). We speculate that while the absence of the innate inflammatory response protects against the early presentation of morbidities, the resultant failure to control the virus may either lead to mortality through epithelial dysfunction and airway obstruction by oedema, or cause a delayed but heightened inflammatory response that causes significant morbidity.
Although the focus of this study was the effect of TLR7-deficiency on the innate inflammatory response, we also demonstrated that T cell activation and production of effector cytokines were attenuated in the absence of TLR7, supporting the notion that activation of the innate immune response is critical for the induction of T cell priming. A limitation of this experiment was the absence of a tetramer based strategy to enumerate absolute numbers of P261-specific T cells, however it is noteworthy that in the absence of TLR7, the fraction of CD8+ T cells in the MLN was reduced by 50%, while the production of both IFN-γ and TNF was reduced by >95% and >80% respectively. Consistent with our findings, influenza-specific IFN-γ production from memory CD4+ but not CD8+ T cells was significantly reduced in mice lacking TLR7 (51). In contrast, lymphocytoid choriomeningitis virus-specific CTL responses were not affected by the absence of TLR7, although in this latter study the CTL response was found to be MyD88-dependent (52). This discrepancy in host response may relate to the host’s repertoire of PRR that can recognise any given pathogen as well as the ability of the pathogen to evade detection or subvert immune function (51, 53–55). In our study, it is possible that the attenuated P261 PVM-specific CTL response in TLR7-deficient mice marked a delayed adaptive response as a consequence of the delayed innate response. Alternatively, the ‘secondary’ PRR system engaged in the absence of TLR7-mediated recognition may be less effective in inducing a robust adaptive response. As such, it will be interesting to determine whether the TLR7-MyD88 pathway is necessary for the development of a protective antibody and memory T cell responses to PVM, as has been shown for influenza virus and RSV (51, 56).
The nature of the alternative PRR system remains unknown. The elevated IFN–γ protein and the up-regulation of RIG-I gene expression in TLR7- but not MyD88-deficient mice implicates a role for another MyD88-dependent toll-interleukin-1 receptor family member, such as TLR4 (57) or the IL-1β receptor complex. Of note, influenza virus has recently been shown to activate the NLRP3 inflammasome, which catalyses the formation of biologically active IL-1β through the activation of caspase-1 (7, 8). In addition to NLRP3, both NOD2 and RIG-I can induce the activation of caspase-1and have been reported to recognise RSV-derived ssRNA (10, 56). We observed an increase in RIG-I but not NOD2 or NLPR3 gene expression from 5 to 10 dpi in TLR7-deficient mice. Other paramyxoviruses (such as RSV, measles, mumps and Sendai virus) are recognised by RIG-I and we speculate that the activation of RIG-I (as opposed to MDA-5) may underlie the late IFN response observed in this study. It should also be noted that RIG-I and IRF7 were up-regulated in WT mice, consistent with the notion that the early release of type I IFN by pDC bolsters the RLR system to support viral recognition by non-specialised cells (6, 58). Taken together, the novel findings presented here suggest a hierarchy amongst PRR systems in the recognition of PVM; whereby activation of the TLR7-MyD88 axis in pDC not only establishes the early anti-viral state, but also primes other innate sensors such as the RLR and NLR systems. Thus, the strategic targeting of pDCs by RSV (15) and/or genetic polymorphisms associated with key genes in the TLR7-signaling pathway (e.g. IRF7) may have significant consequences for the induction of host defense and underlie susceptibility to RSV-induced bronchiolitis and pneumonia.
It is conceivable that the failure to clear the virus increases its dissemination and infectivity of additional cell types that may preferentially employ the alterative PRR. Indeed, our observation that the airway epithelium was damaged in TLR7-deficient but not TLR7-sufficient mice lends support to this proposition. It is also possible that this PRR is activated as a result of the greater viral burden that occurs in the absence of TLR7. In preliminary experiments we have observed that inoculation with higher doses of PVM induces markedly higher concentrations of IL-1β in TLR7-deficient as compared to WT mice (data not shown), suggesting that viral detection may be ‘dose-dependent’. Our observations have significant ramifications for studies in mice where sizable inocula (105 to 107 PFU hRSV per mouse) are used in order to recover virions at later time points. Other studies that have used gene-deleted mice found that the clearance of hRSV in mice was more dependent on the RLR than the TLR family (56, 59). However, pDC depleted mice are also characterised by higher viral titres (43, 60). Since pDC are proposed to primarily sense viruses via TLR7 and TLR9 it is difficult to reconcile these findings. Since the TLR7-MyD88 pathway is employed by pDC, while the RLR system (and possibly TLR7 pathway) is used by epithelial cells, we speculate that inoculation with high doses of RSV triggers an inflammatory response that is predominantly mediated via activation of RLRs. Our findings emphasise the need to distinguish between virus replication and simple antigen recognition when investigating the molecular and cellular processes that underlie host-pathogen interactions. Thus, as PVM is recognised, infects and replicates within the host the natural sequelae of events can be followed, and as a consequence the temporal hierarchy of innate receptor-mediated recognition and cellular activation can be better delineated.
In summary, our study has focused on a host-specific pneumovirus that initiates severe respiratory disease at physiological inocula in a neonatal mouse model to investigate the mechanisms that underlie the immediate innate responses to pneumovirus infection in human infants. Collectively, our findings underscore a critical role for TLR7 and MyD88 in the early recognition and development of an immediate innate inflammatory response necessary to limit pneumovirus load.
Supplementary Material
Acknowledgments
The authors would like to thank Amgen Inc. for the generous gift of Flt3-L.
Abbreviations
- CTL
cytotoxic T lymphocyte
- IRF
IFN regulatory factor
- NLR
nucleotide-binding domain-like receptor
- pDC
plasmacytoid dendritic cell
- PRR
pattern recognition receptor
- PVM
pneumonia virus of mice
- RLR
retinoic acid-inducible gene (RIG)-I-like helicase receptor
- RSV
respiratory syncytial virus
- ssRNA
single-stranded RNA
References
- 1.Glezen P, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 2.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 3.Aherne W, Bird T, Court SD, Gardner PS, McQuillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970;23:7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, Chen ZJ. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Ito T, Wang YH, Liu YJ. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin Immunopathol. 2005;26:221–229. doi: 10.1007/s00281-004-0180-4. [DOI] [PubMed] [Google Scholar]
- 14.Gill MA, Palucka AK, Barton T, Ghaffar F, Jafri H, Banchereau J, Ramilo O. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J Infect Dis. 2005;191:1105–1115. doi: 10.1086/428589. Epub 2005 Mar 1101. [DOI] [PubMed] [Google Scholar]
- 15.Schlender J, Hornung V, Finke S, Gunthner-Biller M, Marozin S, Brzozka K, Moghim S, Endres S, Hartmann G, Conzelmann KK. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 17.Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, Beninati C. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol. 2009;10:587–594. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- 18.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 19.Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg HF, Bonville CA, Easton AJ, Domachowske JB. The pneumonia virus of mice infection model for severe respiratory syncytial virus infection: identifying novel targets for therapeutic intervention. Pharmacol Ther. 2005;105:1–6. doi: 10.1016/j.pharmthera.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg HF, Domachowske JB. Pneumonia virus of mice: severe respiratory infection in a natural host. Immunol Lett. 2008;118:6–12. doi: 10.1016/j.imlet.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garvey TL, Dyer KD, Ellis JA, Bonville CA, Foster B, Prussin C, Easton AJ, Domachowske JB, Rosenberg HF. Inflammatory responses to pneumovirus infection in IFN-alpha beta R gene-deleted mice. J Immunol. 2005;175:4735–4744. doi: 10.4049/jimmunol.175.7.4735. [DOI] [PubMed] [Google Scholar]
- 23.Phipps S, Lam CE, Kaiko GE, Foo SY, Collison A, Mattes J, Barry J, Davidson S, Oreo K, Smith L, Mansell A, Matthaei KI, Foster PS. Toll/IL-1 signaling is critical for house dust mite-specific helper T cell type 2 and type 17 [corrected] responses. Am J Respir Crit Care Med. 2009;179:883–893. doi: 10.1164/rccm.200806-974OC. [DOI] [PubMed] [Google Scholar]
- 24.Phipps S, Hansbro N, Lam CE, Foo SY, Matthaei KI, Foster PS. Allergic sensitization is enhanced in early life through toll-like receptor 7 activation. Clin Exp Allergy. 2009;39:1920–1928. doi: 10.1111/j.1365-2222.2009.03335.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaiko GE, Phipps S, Angkasekwinai P, Dong C, Foster PS. NK cell deficiency predisposes to viral-induced Th2-type allergic inflammation via epithelial-derived IL-25. J Immunol. 2010;185:4681–4690. doi: 10.4049/jimmunol.1001758. [DOI] [PubMed] [Google Scholar]
- 26.Bjorck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–3526. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- 27.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. Epub 2004 Feb 1519. [DOI] [PubMed] [Google Scholar]
- 28.Claassen EA, van Bleek GM, Rychnavska ZS, de Groot RJ, Hensen EJ, Tijhaar EJ, van Eden W, van der Most RG. Identification of a CD4 T cell epitope in the pneumonia virus of mice glycoprotein and characterization of its role in protective immunity. Virology. 2007;13:13. doi: 10.1016/j.virol.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Percopo CM, Qiu Z, Phipps S, Foster PS, Domachowske JB, Rosenberg HF. Pulmonary eosinophils and their role in immunopathologic responses to formalin-inactivated pneumonia virus of mice. J Immunol. 2009;183:604–612. doi: 10.4049/jimmunol.0802270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. Epub 2004 Aug 2015. [DOI] [PubMed] [Google Scholar]
- 31.Bonville CA, Bennett NJ, Koehnlein M, Haines DM, Ellis JA, DelVecchio AM, Rosenberg HF, Domachowske JB. Respiratory dysfunction and proinflammatory chemokines in the pneumonia virus of mice (PVM) model of viral bronchiolitis. Virology. 2006;349:87–95. doi: 10.1016/j.virol.2006.02.017. Epub 2006 Mar 2024. [DOI] [PubMed] [Google Scholar]
- 32.Penna G, Vulcano M, Roncari A, Facchetti F, Sozzani S, Adorini L. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J Immunol. 2002;169:6673–6676. doi: 10.4049/jimmunol.169.12.6673. [DOI] [PubMed] [Google Scholar]
- 33.Krug A, Uppaluri R, Facchetti F, Dorner BG, Sheehan KC, Schreiber RD, Cella M, Colonna M. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J Immunol. 2002;169:6079–6083. doi: 10.4049/jimmunol.169.11.6079. [DOI] [PubMed] [Google Scholar]
- 34.Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, Narumi S, Morikawa S, Ezaki T, Lu B, Gerard C, Ishikawa S, Matsushima K. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol. 2004;16:915–928. doi: 10.1093/intimm/dxh093. Epub 2004 May 2024. [DOI] [PubMed] [Google Scholar]
- 35.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. Epub 2005 Mar 2030. [DOI] [PubMed] [Google Scholar]
- 36.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 37.Claassen EA, van der Kant PA, Rychnavska ZS, van Bleek GM, Easton AJ, van der Most RG. Activation and inactivation of antiviral CD8 T cell responses during murine pneumovirus infection. J Immunol. 2005;175:6597–6604. doi: 10.4049/jimmunol.175.10.6597. [DOI] [PubMed] [Google Scholar]
- 38.Khan KD, Shuai K, Lindwall G, Maher SE, Darnell JE, Jr, Bothwell AL. Induction of the Ly-6A/E gene by interferon alpha/beta and gamma requires a DNA element to which a tyrosine-phosphorylated 91-kDa protein binds. Proc Natl Acad Sci U S A. 1993;90:6806–6810. doi: 10.1073/pnas.90.14.6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 40.De Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barchet W, Cella M, Odermatt B, Asselin-Paturel C, Colonna M, Kalinke U. Virus-induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J Exp Med. 2002;195:507–516. doi: 10.1084/jem.20011666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid Dendritic Cell Ablation Impacts Early Interferon Responses and Antiviral NK and CD8(+) T Cell Accrual. Immunity. 2010;33(6):955–66. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med. 2006;203:1153–1159. doi: 10.1084/jem.20052359. Epub 2006 May 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garofalo RP, Patti J, Hintz KA, Hill V, Ogra PL, Welliver RC. Macrophage inflammatory protein-1alpha (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J Infect Dis. 2001;184:393–399. doi: 10.1086/322788. [DOI] [PubMed] [Google Scholar]
- 45.Domachowske JB, Bonville CA, Gao JL, Murphy PM, Easton AJ, Rosenberg HF. The chemokine macrophage-inflammatory protein-1 alpha and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. J Immunol. 2000;165:2677–2682. doi: 10.4049/jimmunol.165.5.2677. [DOI] [PubMed] [Google Scholar]
- 46.Del Corno M, Gauzzi MC, Penna G, Belardelli F, Adorini L, Gessani S. Human immunodeficiency virus type 1 gp120 and other activation stimuli are highly effective in triggering alpha interferon and CC chemokine production in circulating plasmacytoid but not myeloid dendritic cells. J Virol. 2005;79:12597–12601. doi: 10.1128/JVI.79.19.12597-12601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salazar-Mather TP, Lewis CA, Biron CA. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein 1alpha delivery to the liver. J Clin Invest. 2002;110:321–330. doi: 10.1172/JCI15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welliver TP, Reed JL, Welliver RC., Sr Respiratory syncytial virus and influenza virus infections: observations from tissues of fatal infant cases. Pediatr Infect Dis J. 2008;27:S92–96. doi: 10.1097/INF.0b013e318168b706. [DOI] [PubMed] [Google Scholar]
- 49.Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, McKinney L, Reed JL, Welliver RC., Sr Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Weerd W, Twilhaar WN, Kimpen JL. T cell subset analysis in peripheral blood of children with RSV bronchiolitis. Scand J Infect Dis. 1998;30:77–80. doi: 10.1080/003655498750002349. [DOI] [PubMed] [Google Scholar]
- 51.Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, Kawai T, Akira S. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 52.Jung A, Kato H, Kumagai Y, Kumar H, Kawai T, Takeuchi O, Akira S. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J Virol. 2008;82:196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barchet W, Krug A, Cella M, Newby C, Fischer JA, Dzionek A, Pekosz A, Colonna M. Dendritic cells respond to influenza virus through TLR7- and PKR-independent pathways. Eur J Immunol. 2005;35:236–242. doi: 10.1002/eji.200425583. [DOI] [PubMed] [Google Scholar]
- 54.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, Barrat FJ, Coffman RL, Staprans SI, Feinberg MB. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 55.Heinze B, Frey S, Mordstein M, Schmitt-Graff A, Ehl S, Buchholz UJ, Collins PL, Staheli P, Krempl CD. Both Nonstructural Proteins 1 and 2 of Pneumonia Virus of Mice are Inhibitors of the Interferon Type I and III Response In Vivo. J Virol. 2011;9:9. doi: 10.1128/JVI.01365-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhoj VG, Sun Q, Bhoj EJ, Somers C, Chen X, Torres JP, Mejias A, Gomez AM, Jafri H, Ramilo O, Chen ZJ. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2008;105:14046–14051. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 58.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 59.Rudd BD, Schaller MA, Smit JJ, Kunkel SL, Neupane R, Kelley L, Berlin AA, Lukacs NW. MyD88-Mediated Instructive Signals in Dendritic Cells Regulate Pulmonary Immune Responses during Respiratory Virus Infection. J Immunol. 2007;178:5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J Immunol. 2006;177:6263–6270. doi: 10.4049/jimmunol.177.9.6263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.