Fig 4.
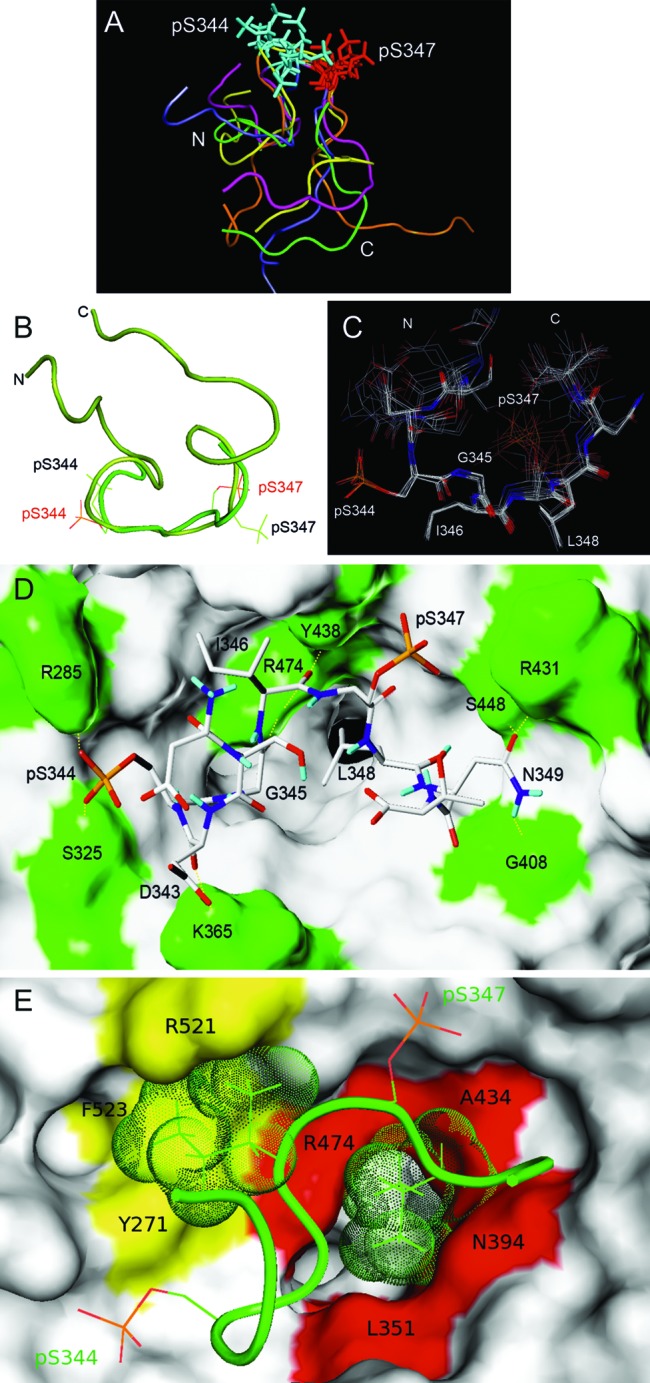
TRNOE-derived structures of the 2P-hNrf2 peptide bound to β-TrCP and analysis of the docking interactions. (A) Of 50 TRNOE-derived structures, the five lowest-energy conformations are shown. Superimpositions are shown on the DpSGIpS motif. (B) Nrf2 docked structure (light green) superimposed on the TRNOE bound structure (dark green) on the DpSGIpS motif. Phosphoserine residues are labeled in red in the docked structure and black in the TRNOE structure. (C) The seven lowest-score configurations that are representative of the bound structures of Nrf2. The superimposition was made on the DpSGIpS motif. (D and E) Structure of the complex of β-TrCP with the best docking conformation of Nrf2 deduced from the NMR data. β-TrCP is shown in surface representation. (D) β-TrCP residues that participate in hydrophilic intermolecular interactions including Arg285, Ser325, Lys365, Gly408, Arg431, Tyr438, Ser448, and Arg474 (green). H bonds are shown with dashed yellow lines. (E) Amino acids of β-TrCP and Nrf2 that participate in hydrophobic intermolecular interactions. The hydrophobic cluster located at the entrance of the central channel of β-TrCP (red), interacts with the side chain of Leu348 (green dots). A second hydrophobic cluster (yellow) interacts with the side chain of Ile346 (green dots).
