Abstract
Increasing evidence suggests that enteric glial cells (EGCs) are critical for enteric neuron survival and functions. In particular, EGCs exert direct neuroprotective effects mediated in part by the release of glutathione. However, other glial factors such as those identified as regulating the intestinal epithelial barrier and in particular the omega6 fatty acid derivative 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) could also be involved in EGC-mediated neuroprotection. Therefore, our study aimed to assess the putative role of EGC-derived 15d-PGJ2 in their neuroprotective effects. We first showed that pretreatment of primary cultures of enteric nervous system (ENS) or human neuroblastoma cells (SH-SY5Y) with 15d-PGJ2 dose dependently prevented hydrogen peroxide neurotoxicity. Furthermore, neuroprotective effects of EGCs were significantly inhibited following genetic invalidation in EGCs of the key enzyme involved in 15d-PGJ2 synthesis, i.e. L-PGDS. We next showed that 15d-PGJ2 effects were mediated by an Nrf2 dependent pathway but were not blocked by PPARγ inhibitor (GW9662) in SH-SY5Y cells and enteric neurons. Finally, 15d-PGJ2 induced a significant increase in glutamate cysteine ligase expression and intracellular glutathione in SH cells and enteric neurons. In conclusion, we identified 15d-PGJ2 as a novel glial-derived molecule with neuroprotective effects in the ENS. This study further supports the concept that omega-6 derivatives such as 15d-PGJ2 might be used in preventive and/or therapeutic strategies for the treatment of enteric neuropathies.
Key points
Enteric glial cells play a major role in the regulation of enteric neuronal functions.
15-Deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) is an omega-6 fatty acid derivative that was recently identified as a novel glial-derived mediator involved in the control of the proliferation and differentiation of intestinal epithelial cells.
In this study, we show that 15d-PGJ2 has neuroprotective properties during oxidative stress in the enteric nervous system.
We furthermore show that 15d-PGJ2 neuroprotective effects are dependent on the activation of the Nrf2 pathway and are associated with an increase of glutathione synthesis in neurons.
This study supports the concept that omega-6 derivatives might be used in preventive and/or therapeutic strategies for the treatment of enteric neuropathies.
Introduction
The enteric nervous system (ENS) is an integrative neuronal network located within the gastrointestinal tract involved in the regulation of gastrointestinal functions such as motility and secretion (Schemann & Neunlist, 2004; Furness, 2006). The ENS is composed of neurons and enteric glial cells (EGCs). In general, EGCs largely outnumber neurons (Hoff et al. 2008) and are likely to represent the ENS counterpart of central nervous system (CNS) astrocytes as they resemble astrocytes both morphologically and immunohistochemically (Jessen & Mirsky, 1980; Gabella, 1981; Ferri et al. 1982).
Emerging evidence suggests a central role for EGCs in the control of enteric neuronal functions. EGCs control neuronal phenotype (Aube et al. 2006), neuronal survival (Abdo et al. 2010) and are suggested to be involved in neurogenesis (Laranjeira et al. 2011). Transgenic models in which EGCs are altered display changes in enteric neurochemical phenotype (Aube et al. 2006) or enteric neuronal loss (Bush et al. 1998). More recently, a neuroprotective effect of EGCs has been demonstrated (Abdo et al. 2010). Using co-culture experiments of EGCs with primary cultures of rat ENS or human neuroblastoma cells, we have shown that EGCs prevent hydrogen peroxide or dopamine neurotoxicity. These neuroprotective effects are partially but not completely mediated by glutathione (GSH), suggesting that other glial factors are involved.
We have recently identified EGCs as a source of 15d-PGJ2, a lipid mediator that exerts anti-proliferative and pro-differentiation effects on intestinal epithelial cells (Bach-Ngohou et al. 2010). 15d-PGJ2 is a derivative of omega-6 polyunsaturated fatty acids and is produced from the non-enzymatic dehydration of PGD2, which is synthesized from prostaglandin H2 by prostaglandin D synthase (PGDS) (Straus & Glass, 2001). Identified initially as an endogenous ligand for peroxisome proliferator-activated receptor-γ (PPARγ), 15d-PGJ2 can also activate other signalling pathways, among which is the Nrf2 (nuclear factor erythroid-derived 2) pathway. Once activated, the Nrf2 transcription factor increases the expression of anti-oxidant genes such as NAD (P)H:quinone oxidoreductase 1 (NQO1), haem oxygenase 1 (HO1) or glutamate cysteine ligase (GCL) (Jaiswal, 2004; Kansanen et al. 2009). Recently, 15d-PGJ2 has been shown to exert protective effects during ischaemia and NMDA-induced excitotoxicity (Lin et al. 2006; Ou et al. 2006; Zhao et al. 2006). Whether 15d-PGJ2 is involved in EGC-mediated protection of enteric neurons has not been studied yet. Therefore, we aimed to characterize the putative neuroprotective role of EGC-derived 15d-PGJ2 in enteric neurons and to identify the signalling pathways involved.
Methods
Cell culture models
Enteric glial cells (EGCs)
Non-transformed EGCs (NT) were obtained from Dr A. Rühl and prepared as previously described (Ruhl et al. 2001). EGCs (REG or rat′s enteric glia) were obtained from adult rat myenteric plexus in our laboratory. Briefly, longitudinal muscle/myenteric plexus layers from adult rat small intestine were treated for enzymatic and mechanical dissociation in GentleMACS tubes C (MiltenyiBiotec, Paris, France). Individual ganglia were selected and cultured in 0.5% gelatin-coated plates. After 2 weeks of culture, up to 95% of cells expressed typical markers of EGCs such as Sox10, GFAP and S100β. EGCs were cultured in DMEM medium (Invitrogen, Cergy-Pontoise, France) supplemented with 10% (v/v) heat inactivated fetal bovine serum (FBS, BioWest, Nuaillé, France), 1% (v/v) glutamine (Invitrogen), 50 μg ml−1 streptomycin (Invitrogen) and 50 U ml−1 penicillin (Invitrogen).
PGD2 synthase deficient enteric glial cell lines
EGCs were transfected with pLKO.1-puro shPTGDS vector plasmids coding for the small hairpin (sh) RNA against prostaglandin D2 synthase (Sigma, Saint Quentin Fallavier, France). Cell transfection was performed using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Medium was renewed after 24 h and cells were treated with puromycin (10 μg ml−1, Invitrogen) until the stable expression of transgene. The efficient shRNA sequence was (shPTGDS): CCGGCAGTGT GAGACCAAGATCATCTCGAGATGATCTTGGTCTCACCTGGTTTTT. An inefficient shRNA construction was used as a negative control (shMOCK): CCGGGCCCCAACTTTCAACAAGACAACTCGAGTTGTCTTGTTGAAAGTTGGGCTTTTT.
Neuroblastoma cell line
Neuroblastoma SH-SY5Y cells were purchased from ATCC and maintained in SH-SY5Y medium: DMEM/F12 (Invitrogen) supplemented with 15% (v/v) FBS, 1% (v/v) glutamine, 1% (v/v) non-essential amino acids (Invitrogen), 50 U ml−1 penicillin and 50 μg ml−1 streptomycin.
Co-culture experiments
EGCs, shPTGDS EGCs or shMOCK EGCs were seeded onto Transwell porous filters (Corning, Avon, France) at a density of 0.16 × 105 cells cm−2. After 24 h, EGC filters were placed in the presence of SH-SY5Y (0.16 × 105 cells cm−2) seeded into the bottom of 12-well plates and cultured for 96 h. EGCs and culture medium were removed prior to incubation of SH-SY5Y cells for 24 h with fresh SH-SY5Y medium containing 200 μm H2O2.
Primary cultures of ENS
Primary cultures of ENS were prepared from intestines of embryonic day (E)15 rat embryos and used after 13 days of culture as previously described (Chevalier et al. 2008; Abdo et al. 2010). Pregnant Sprague–Dawley rats were purchased (CERJ, Le Genest St Isle, France) and manipulated in compliance with the French institutional guidelines. These procedures were approved by the local institutional animal research committee (Agreement E. 44011; INSERM, Nantes, France). Efforts were made to minimize animal suffering and the number of animals used. Pregnant rats were killed by an overdose of isoflurane followed by neck dislocation. The embryos (E15; 35–45 per isolation from 3 pregnant rats) were removed and killed by decapitation. The intestines of the embryos were then removed and finely diced in Hank's buffered salt solution (HBSS, Sigma). Tissue fragments were collected in 5 ml of medium (DMEM–F12 1:1 medium) and digested at 37°C for 15 min in 0.1% trypsin (Sigma). The trypsin reaction was stopped by adding 10 ml of medium containing 10% (v/v) FBS and then treated by 0.01% DNase I (Sigma) for 10 min at 37°C. After triturating with a 10 ml pipette, cells were centrifuged at 110 g for 10 min. Cells were counted and then seeded at a density of 2.4 × 105 cells cm−2 on 12- or 24-well plates previously coated for 6 h with a solution of 0.5% (v/v) gelatin (Sigma) in sterile phosphate buffered saline (PBS). After 24 h, the medium was replaced with the same medium without FBS but containing 1% N-2 supplement (Life Technologies, Cergy Pontoise, France). Cells were maintained in culture for 13 days. Half of the medium was replaced every other day.
Primary cultures of ENS were treated with varying concentrations of 15d-PGJ2 (1, 2 and 3 μm) for 24, 48 and 72 h. Thereafter, cultures were incubated with fresh medium for 24 h with or without 200 μm H2O2.
Assessment of cell viability
Flow cytometry analysis of 7amino-actinomycin D permeable cells
Both floating and attached SH-SY5Y cells were collected. Attached SH-SY5Y cells were dissociated following treatment with trypsin-EDTA. Cell suspension was then centrifuged for 5 min at 550 g and resuspended in 500 μl PBS. Membrane permeability was evaluated using 7amino-actinomycin D (50 ng μl−1, 7-AAD) as a fluorescent marker for DNA. SH-SY5Y cell suspensions (200 μl) were incubated with 5 μl 7-AAD for 10 min before flow cytometry using BD FACSArray (Le Pont de Claix, France). Results were expressed as percentage of total cells.
Detection of neuron specific enolase (NSE)
NSE released in the culture medium was quantified by immunoradiometric assay (Prolifigen NSE IRMA, Diasorin; Stillwater, USA) according to the manufacturer's protocol. Results are expressed in nanograms per millilitre.
Immunohistochemical analysis
SH-SY5Y cells and primary cultures of ENS were fixed in PBS containing 4% paraformaldehyde at room temperature for 1 h. Cells were then washed in PBS and permeabilized for 30 min in PBS/NaN3 containing 0.5% (v/v) Triton X-100 (Sigma) and 4% (v/v) horse serum before incubation overnight at 4°C with mouse anti-β-tubulin III (1:1000, Sigma), mouse anti Human Neuronal Protein HuC/ HuD (HuC/D) (1:200, Molecular Probes, Invitrogen), rabbit anti-Nrf2 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or goat anti-glutamate cysteine ligase catalytic subunit GCLc (1:200, Santa Cruz Biotechnology). Cells were then washed in PBS and incubated for 30 min with the following secondary antibodies coupled to fluorophores (donkey anti-rabbit, anti-mouse or anti-goat IgG conjugated to carboxymethylindocyanine (1:500; Jackson Laboratories, purchased from Immunotech, Marseille, France), anti-mouse IgG conjugated to FluoProbes 488 (1:200; Interchim, Montluçon, France)) or with DAPI (1 mg l−1 in PBS; Merck, Fontenay-sous-Bois, France) for 10 min.
Images were acquired with a digital camera (model DP71, Olympus, France) coupled to a fluorescence microscope (Olympus IX 50) and analysed with Cell B software (Soft Imaging System, Olympus).
In primary cultures of ENS, we have quantified Nrf2 and GCLc in situ expression using ImageJ software based analysis (http://rsb.info.nih.gov/ij/). The mean fluorescence intensity of Nrf2 staining was measured in the region of interest (ROI) of each individual enteric neuron (identified as Hu-positive cell). This value was determined from two independent experiments for control ENS cultures (19–49 enteric neurons per image, 9 images, 305 enteric neurons analysed) and for 2 h 15d-PGJ2-treated ENS cultures (21–39 enteric neurons per image, 10 images, 292 enteric neurons analysed). Mean fluorescence intensity of GCLc staining was measured in the ROI of individual enteric neurons in control ENS cultures (24–37 enteric neurons per image, 3 images, 97 enteric neurons analysed) and in ENS cultures treated for 24 h with 15d-PGJ2 (24–37 enteric neurons per image, 3 images, 97 enteric neurons analysed) and for 24 h 15d-PGJ2-treated ENS cultures (17–27 enteric neurons per image, 3 images, 69 enteric neurons analysed). Mean fluorescence intensity was corrected by measuring the background for each image. The mean fluorescence intensity was determined as the sum of the grey values of all the pixels in the ROI divided by the number of pixels of the ROI using ImageJ. The area of the ROI of enteric neurons was similar between all conditions analysed (control and 15d-PGJ2-treated ENS cultures; data not shown).
Western blot
EGCs transfected or not transfected with shRNA PTGDS plasmids were harvested with RIPA (Millipore, Molsheim, France) containing a cocktail of protease inhibitors (Complete, Roche, Meylan, France). Following a short centrifugation, insoluble material was removed and discarded. Equal amounts of lysate (soluble fraction) were separated using the Invitrogen NuPage Novex Bis Tris MiniGels before electrophoretic transfer with the iBlot Dry Blotting System, also from Invitrogen. Membranes were blocked for 1 h at room temperature in Tris-buffered saline (TBS) (100 mm NaCl, 10 mm Tris, pH 7.5) with 5% non-fat dry milk. Membranes were incubated overnight at 4°C with the primary antibodies. Rabbit anti-lipocalin-type-PGDS (1:1000, Cayman, Interchim, Montluçon, France), rabbit anti-Nrf2 (1:1000, Santa Cruz Biotechnology) and mouse anti-β-actin antibodies (1:5000; Sigma) were used in this study. Bound antibodies were detected with horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies (Amersham, Les Ulis, France; 1:5000) and visualized by enhanced chemiluminescent detection (ECL plus, Amersham). The relevant immunoreactive bands were quantified with laser-scanning densitometry and analysed with NIH ImageJ software. The values of Change to L-PGDS and Nrf2 immunoreactivity were normalized to the amount of β-actin immunoreactivity in the same sample.
RT–quantitative PCR
Total RNA extraction from cells was performed with RNA nucleospin kit (Macherey-Nagel, Hoerdt, France) according to the manufacturer's instructions. For reverse transcription, 2 μg of purified total RNA was denaturated and subsequently processed for reverse transcription using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer's recommendations. PCR amplifications were performed using the Absolute Blue SYBR green fluorescein kit (ABgene, Courtaboeuf, France) according to the manufacturer's protocol and run on MyiQ thermocycler (Biorad, Marnes-la-Coquette, France).
The following primers were used. S6 (NM_001010): forward primer, 5′-CCAAGCTTATTCAGCGTCTTGTTACTCC-3′; reverse primer, 5′-CCCTCGAGTCCTTCATTCTCTTGGC-3′. GCLc (NM_001498): forward primer, 5′-AGGTGACATTCCAAGCCTGC-3′; reverse primer, 5′-CCCCAGCGACAATCAATGTC-3′. NQO1 (NM_000903): forward primer, 5′-CATGAACGAGGCTGCTGCAGC-3′; reverse primer, 5′-CCAGCCTTTC AGAATGGCAGG-3′. Nrf2 (NM_006164): forward primer, 5′-GTTTCTTCGGCTACGTTTCAG-3′; reverse primer, 5′-ACAGGGGCTACCTGAGCAAC-3′.
Relative quantification of gene expression was determined by using the standard curve method and endogenous control ribosomal protein S6 mRNA. The ratios GCLc/S6, NQO1/S6 or Nrf2/S6 were compared to control experimental conditions.
Intracellular glutathione assessment
In SH-SY5Y cells, total glutathione (reduced and oxidized forms) was measured in a microtitre plate assay using a glutathione assay kit (Sigma). The content of glutathione was normalized to the protein content quantified by the Bio-Rad protein assay.
In primary cultures of ENS, glutathione was identified in situ using monochlorobimane (MCB, Sigma) (Chatterjee et al. 1999). Following treatment, MCB (60 μmol l−1) was directly added to the medium and incubated for 20 min at 37°C before fixation with 4% PFA and standard immunostaining procedures.
Reagents
15d-PGJ2, hydrogen peroxide, 7-amino actinomycin D (7-AAD), rosiglitazone, GW9662 and luteolin were all purchased from Sigma.
Statistics
Data are expressed as means ± SEM. Results were analysed by t test or one-way ANOVA followed by Tukey's post hoc test or two-way ANOVA followed by Bonferroni's post hoc test. Prism 4.0 (GraphPad Software Inc., La Jolla, USA) was used for statistical analysis.
Results
EGC-derived 15d-PGJ2 has neuroprotective effects
We first studied whether 15d-PGJ2 exerts neuroprotective effects in the ENS. Pre-incubation of primary cultures of ENS with 15d-PGJ2 significantly and dose-dependently reduced the NSE release induced by H2O2 (Fig. 1A). Furthermore, 15d-PGJ2 prevented H2O2-induced morphological alterations of the enteric neuronal network (Fig. 1B). In order to assess a direct neuroprotective effect of 15d-PGJ2, SH-SY5Y cells were pre-treated with 15d-PGJ2 before challenging them with H2O2. Under these conditions, 15d-PGJ2 dose-dependently reduced the proportion of 7-AAD positive SH-SY5Y cells induced by H2O2 as compared to controls (SH-SY5Y cells not pre-treated with 15d-PGJ2) (Fig. 1C).
Figure 1. EGC-derived 15d-PGJ2 (exerts neuroprotection against oxidative stress-induced cell death.

A, primary cultures of ENS were treated or not treated with H2O2 (200 μm; 24 h; filled and open bars, respectively) with or without (control) previous treatments with 15d-PGJ2 (1, 2 or 3 μm; 72 h). NSE released in the medium was measured by immunoradiometric assay. Values are the mean ± SEM of from 6 to 11 independent experiments (#P < 0.05 as compared to cultures without H2O2; *P < 0.05 as compared to primary cultures treated with H2O2 but without 15d-PGJ2 pretreatment; two-way ANOVA followed by Bonferroni's post hoc test). B, primary cultures of ENS were treated or not treated with H2O2 (200 μm, 24 h), with or without previous treatment with 15d-PGJ2 (3 μm; 72 h). Immunostaining was performed with anti-β-tubulin III antibody. Images are representative of 4 independent experiments. Scale bar: 100 μm. C, SH-SY5Y cells were treated or not treated with H2O2 (200 μm; 24 h; filled and open bars, respectively), with or without (control) previous treatments with different concentrations of 15d-PGJ2 (0.1, 1 or 5 μm; 24 h). Neuronal cell death was analysed by measuring the cell permeability to 7-amino-actinomycin D (% 7-AAD+ cells) by flow cytometry. Values are the mean ± SEM from four independent experiments (#P < 0.05 as compared to conditions without H2O2; *P < 0.05 as compared to conditions treated with H2O2 but without 15d-PGJ2 pretreatment; two-way ANOVA followed by Bonferroni's post hoc test). D, EGCs were transfected with shRNA PTGDS (EGC shPTGDS) or with the shRNA PTGDS inefficient construction (EGC shMOCK). Inset: different non-transformed EGCs (NT; ROG) express L-PGDS. Immunoblot analysis using antibodies against change to L-PGDS or βactin were carried out from EGC extracts. Quantitative analysis was performed by measuring band densities with ImageJ. Values are the mean ± SEM from four independent experiments (*P < 0.05 as compared to EGCs or EGC shMOCK; one-way ANOVA followed by Tukey's post hoc test). E, SH-SY5Y cells were treated or not treated with H2O2 (200 μm; 24 h; filled and open bars, respectively) after 96 h co-culture with EGCs, EGC shPTGDS or EGC shMOCK. Neuronal cell death was analysed by measuring the cell permeability to 7-amino-actinomycin D (% 7-AAD+ cells) by flow cytometry. Values are the mean ± SEM from five independent experiments (#P < 0.05 as compared to control without H2O2, *P < 0.05 as compared to H2O2-treated SH-SY5Y cells alone; †P < 0.05 as compared to SH-SY5Y cells co-cultured with EGCs or EGC shMOCK; one-way ANOVA followed by Tukey's post hoc test).
As previously described in transformed EGCs (Bach-Ngohou et al. 2010), non-transformed EGCs (NT or REG) also express L-PGDS, the key enzyme in the production of 15d-PGJ2 (Fig. 1D). Expression of L-PGDS was silenced in EGCs using shRNA. L-PGDS protein level was significantly decreased in EGC shPTGDS shRNA but and was not modified in EGC shMOCK (Fig. 1D). We tested the effects of L-PGDS invalidation of EGCs in co-cultures with SH-SY5Y cells. The protective effects of EGCs upon SH-SY5Y cells were significantly reduced when cells were co-cultured in the presence of EGCs transfected with PTGDS shRNA but not with EGCs transfected with mock shRNA (Fig. 1E).
15d-PGJ2 neuroprotective effects are independent of the PPARγ pathway
We next aimed at identifying signalling pathways in SH-SY5Y cells involved in the 15d-PGJ2 neuroprotective effects. We first focused on the PPARγ pathway, as 15d-PGJ2 is a natural ligand of PPARγ receptors (Scher & Pillinger, 2005). We showed that treatment of SH-SY5Y cells with the PPARγ agonist rosiglitazone did not modify the proportion of 7-AAD positive SH-SY5Y cells induced by H2O2 as compared to control (Fig. 2A). Consistently, treatment of SH-SY5Y with a PPARγ inhibitor (GW9662) did not modify the neuroprotective effects of 15d-PGJ2 (Fig. 2B).
Figure 2. 15d-PGJ2 neuroprotective effects are independent of the PPARγ pathway.

A, SH-SY5Y cells were treated or not treated with H2O2 for (200 μm; 24 h; filled and open bars, respectively) after previous treatment with rosiglitazone (5 μm; 24 h). Neuronal cell death was analysed by measuring the cell permeability to 7-amino-actinomycin D (% 7-AAD+ cells) by flow cytometry. Values are the mean ± SEM from six independent experiments (#P < 0.05 as compared to untreated SH-SY5Y cells (control, open bar); one-way ANOVA followed by Tukey's post hoc test). B, SH-SY5Y cells were treated or not treated with H2O2 (200 μm; 24 h; filled and open bars, respectively) after treatment with 15d-PGJ2 (5 μm; 24 h), with or without 24 h pre-treatment with GW9662 (10 μm). Neuronal cell death was analysed by measuring the cell permeability to 7AAD (% 7AAD+ cells) by flow cytometry. Values are the mean ± SEM from four independent experiments (#P < 0.05 as compared to untreated conditions without H2O2 (open bar); *P < 0.05 as compared to untreated conditions with H2O2 (filled bar); one-way ANOVA followed by Tukey's post hoc test).
15d-PGJ2 neuroprotective effects are associated with the activation of the Nrf2 pathway
Among other signalling pathways putatively involved in EGC neuroprotective effects and known to be modulated by 15d-PGJ2 is the Nrf2 pathway (Kansanen et al. 2009).
We first demonstrated that Nrf2 protein expression is significantly increased in SH-SY5Y cells co-cultured with EGCs but not with SH-SY5Y cells as compared to control (SH-SY5Y cells cultured alone) (Fig. 3A). Moreover, treatment of SH-SY5Y cells with 15d-PGJ2 induced a significant increase in Nrf2 protein expression (Fig. 3B and C). We next determined whether 15d-PGJ2 effects were mediated by Nrf2 dependent pathways using luteolin, an inhibitor of Nrf2 (Tang et al. 2011). We first showed that luteolin significantly reduced Nrf2 expression in SH-SY5Y cells both in control conditions and also after treatment with 15d-PGJ2 (Supplemental Fig. S1A, available online only). We next showed that neuroprotective effects of 15dPGJ2 were abolished in the presence of luteolin (Supplemental Fig. S1C).
Figure 3. 15d-PGJ2 neuroprotective effects are associated with activation of the Nrf2 pathway.

A, SH-SY5Y cells were cultured alone or co-cultured with EGCs or SH-SY5Y cells for 96 h. Immunoblot analysis using antibodies against Nrf2 or βactin was performed from SH-SY5Y extracts and was quantified by measuring band densities with ImageJ. Values are the mean ± SEM from seven independent experiments (*P < 0.05 as compared to control; one-way ANOVA followed by Tukey's post hoc test). B, SH-SY5Y cells were treated or not treated (control) with 15d-PGJ2 (5 μm; 5 h). Protein extracts were analysed for quantification with immunoblots using anti-Nrf2 and anti-βactin antibodies. Quantitative analysis was performed by measuring band densities with ImageJ. Values are the mean ± SEM of seven independent experiments (*P < 0.05 as compared to control; t test). C, SH-SY5Y cells were treated or not treated (control) with 15d-PGJ2 (5 μm; 5 h). Immunocytochemistry with anti-Nrf2 antibodies and DAPI labelling were performed and images are representative of three independent experiments. Scale bar: 100 μm. D, SH-SY5Y cells were treated or not (control) with 15d-PGJ2 (5 μm) for different time periods (1, 2, 4, 6 and 24 h). Quantification of GCLc mRNA was performed by RT–quantitative PCR as described in Methods. Values are the mean ± SEM of four independent experiments (*P < 0.05 as compared to control; one-way ANOVA followed by Tukey's post hoc test). E, SH-SY5Y cells were treated with varying concentrations of 15d-PGJ2 (1, 2.5 or 5 μm) for 24 h. Quantification of total glutathione was measured using enzymatic glutathione assay. Values are the mean ± SEM of four independent experiments (*P < 0.05 as compared to control; one-way ANOVA followed by Tukey's post hoc test).
We next determined whether 15d-PGJ2 also modulates the expression of anti-oxidant genes regulated by Nrf2 such as GCLc (catalytic subunit of GCL), the key enzyme in glutathione biosynthesis. We showed that 15d-PGJ2 time-dependently increased the expression of GCLc mRNA in SH-SY5Y cells (Fig. 3D). We also demonstrated that 15d-PGJ2 dose-dependently increased intracellular glutathione levels in SH-SY5Y cells (Fig. 3E). Interestingly, luteolin prevented the 15d-PGJ2 increase in GCLc mRNA expression in SH-SY5Y cells (Supplemental Fig. S1B). Moreover, 15d-PGJ2 increased NQO1 mRNA expression in a concentration- and time-dependent fashion (Supplemental Fig. S2).
15d-PGJ2 neuroprotective effects in primary cultures of ENS are associated with Nrf2 pathway and glutathione synthesis
In the final part of this study, we determined whether 15d-PGJ2 activates a similar pathway in the ENS. Treatment of ENS primary culture with 15d-PGJ2 induced a significant increase in both Nrf2 (Fig. 4A and B) and GCLc (Fig. 4C and D) expressions in enteric neurons (identified by their immunoreactivity for Hu). Using MCB to measure in situ intracellular glutathione, we showed that treatment with 15d-PGJ2 induced an increase in MCB intensity in enteric neurons as compared to control (Fig. 4E).
Figure 4. 15d-PGJ2 neuroprotective effects on primary cultures of ENS are associated with Nrf2 pathway and glutathione synthesis.
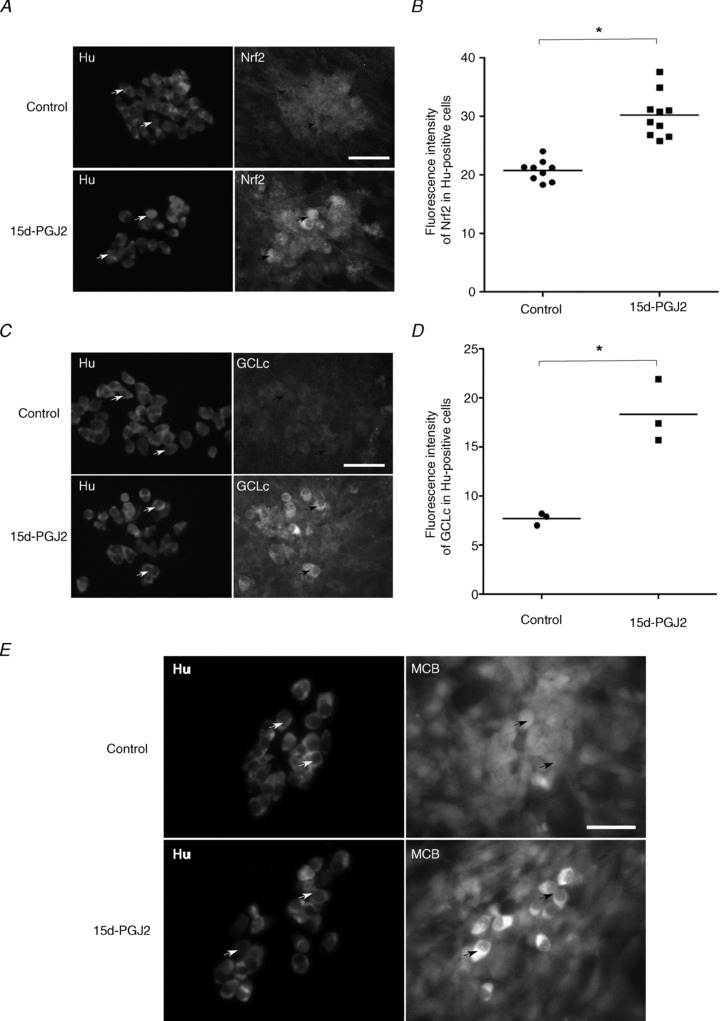
A, primary cultures of ENS were treated or not (control) with 15d-PGJ2 (3 μm, 2 h) and analysed by immunostaining with anti-Nrf2 or anti-Hu antibodies. Arrows represent examples of co-expression of Hu and Nrf2 proteins. Scale bar: 100 μm. B, quantitative analysis of Nrf2 protein expression in enteric neurons. Mean fluorescence intensity of Nrf2 corresponding to each enteric neuron (Hu-positive cell) was determined from ImageJ software analysis of control ENS cultures (9 images, 19–49 enteric neurons per image, two independent experiments, 305 enteric neurons analysed) and after 2 h of 15d-PGJ2-treatment (10 images, 21–39 enteric neurons per image, two independent experiments, 292 enteric neurons analysed). Mean fluorescence intensity was corrected by measuring background for each image. Each symbol represents the mean value of fluorescence intensity in enteric neurons per analysed image (*P < 0.001 as compared to control; t test). C, primary cultures of ENS were treated or not treated (control) with 15d-PGJ2 (3 μm; 24 h) and analysed by immunostaining with anti-GCLc or anti-Hu antibodies. Arrows represent examples of co-expression of Hu and GCLc proteins. Scale bar: 100 μm. D, quantitative analysis of GCLc protein expression in enteric neurons. Mean fluorescence intensity of GCLc corresponding to each enteric neuron (Hu-positive cell) was determined from ImageJ software analysis of control ENS cultures (3 images, 24–37 enteric neurons per image, 97 enteric neurons analysed) and after 2 h of 15d-PGJ2-treatment (3 images, 17–27 enteric neurons per image, 69 enteric neurons analysed). Mean fluorescence intensity was corrected by measuring background for each image. Each symbol represents the mean value per analysed image of fluorescence intensity in enteric neurons (*P < 0.01 as compared to control; t test). E, primary cultures of ENS were treated or not treated (control) with 15d-PGJ2 (3 μm; 24 h). Following treatment, monochlorobimane (MCB, 60 μm) was directly added to the medium and incubated for 20 min at 37°C before fixation and standard immunostaining procedures with anti-Hu antibodies. Treatment of primary cultures of ENS with 3 μm 15d-PGJ2 for 24 h induced an increase of total glutathione conjugated to MCB in enteric neurons (Hu-positive cells) as compared to untreated cultures (Control). Arrows represent examples of co-expression of Hu and conjugated MCB. Scale bar: 100 μm.
Discussion
In this study, we identified EGC-derived 15d-PGJ2 as a novel glial mediator with major neuroprotective effects upon enteric neurons. We further showed that 15d-PGJ2 effects were associated with the activation of the Nrf2 pathway and an increase in neuronal intracellular production of glutathione. Therefore, the use of omega6 derivatives such as 15d-PGJ2 might be a novel preventive and therapeutic strategy for the treatment of enteric neuropathies.
A major finding of our study is the demonstration of the neuroprotective effects of 15d-PGJ2 in the ENS. Indeed, 15d-PGJ2 prevented H2O2-induced neuronal cell death in primary cultures of ENS in a dose-dependent manner. However, whether 15d-PGJ2 neuroprotective effects also occur in vivo in enteric neurons needs to be confirmed in future studies. Nevertheless, 15d-PGJ2 can limit in vivo acute brain damage induced by experimental ischaemia in rats (Lin et al. 2006; Ou et al. 2006) or spinal cord injury in mice (Kerr et al. 2008). The direct neuroprotective effects of 15d-PGJ2 during oxidative stress demonstrated in our study were previously observed in undifferentiated neurons or neuroblastoma cells (Aoun et al. 2003; Lim et al. 2004; Lin et al. 2006; Kim et al. 2008). Interestingly, 15d-PGJ2 can also protect rat cortical neurons in vitro and in vivo from NMDA-induced excitotoxicity (Zhao et al. 2006). Similarly, 15d-PGJ2 can limit acute brain damage induced by experimental ischaemia in rat, probably due to its neuroprotective effects (Lin et al. 2006; Ou et al. 2006). The protective effects of 15d-PGJ2 observed in neurons also extend to other cell types such as endothelial cells (Levonen et al. 2001; Hosoya et al. 2005) or epithelial cells (Garg & Chang, 2004; Poncin et al. 2008) during various stress conditions.
Another finding was that co-culture with EGCs and 15d-PGJ2 increased the expression of transcription factor Nrf2 in enteric neurons and in SH-SY5Y cells. The increase of Nrf2 protein expression could be due to in Nrf2 protein stability. Indeed, 15d-PGJ2 can dissociate Keap1 (cytosolic Nrf2 inhibitor) and Nrf2 proteins preventing Nrf2 ubiquitinylation and subsequent degradation by the proteasome (Nguyen et al. 2004; Kansanen et al. 2009). Our study further suggested that 15d-PGJ2 neuroprotective effects were mediated by Nrf2 pathways as luteolin prevented these effects. Indeed, luteolin was previously described to inhibit Nrf2 by increasing Nrf2 mRNA degradation in different cell types (Tang et al. 2011). In our study with SH-SY5Y cells, we showed that luteolin also reduced Nrf2 mRNA. However, one cannot exclude that other pathways also involved in 15d-PGJ2 neuroprotection might also be altered by luteolin. In contrast, our study suggests that 15d-PGJ2 neuroprotection does not involve a PPARγ dependent pathway, as its effect is not blocked by the specific antagonist GW9662 nor reproduced by the PPARγ agonist rosiglitazone. This is in agreement with other studies suggesting that the protective effects of 15d-PGJ2 are independent of the PPARγ pathway (Aoun et al. 2003; Lim et al. 2004) but in these studies, pathways responsible for neuroprotection remained unidentified.
Our study also showed that the increase in Nrf2 expression in enteric neurons and SH-SY5Y cells was associated with an increase in one of its target genes GCL. This is consistent with known binding of Nrf2 to antioxidant response elements (AREs) and the ARE-mediated regulation of anti-oxidant enzyme gene expression such as NQO1, HO1 or GCL (Jaiswal, 2004; Kansanen et al. 2009). Furthermore, 15d-PGJ2-enhanced GCL gene expression was associated with an increase in intracellular concentration of glutathione in enteric neurons and SH-SY5Y cells. Similarly, activation of Nrf2 leads to an increase of glutathione in neurons of mouse brain (Escartin et al. 2011), in astrocytes (Shih et al. 2003), PC12 cells (Lim et al. 2004; Chen et al. 2006) or even endothelial cells (Levonen et al. 2001).
This increase of glutathione induced by 15d-PGJ2 could be responsible for enhanced neuroprotection against oxidative stress. However, other factors released by EGCs such as glial cell-derived neurotrophic factor (GDNF) (Steinkamp et al. 2003; von Boyen et al. 2006) could also increase glutathione synthesis in enteric neurons. Indeed, GDNF protects PD cybrids (cytoplasmic hybrid cells from patients with idiopathic Parkinson's Disease) from H2O2-induced neuronal death by enhancing intracellular GSH (Onyango et al. 2005). In addition, GDNF treatment can also enhance GSH levels in rat striatum (Chao & Lee, 1999).
This study also further highlights the central role of GSH in EGC-mediated neuroprotective effects. Indeed, we previously showed that EGCs can directly synthesize and release glutathione. The constitutive release of GSH by EGCs might be responsible for neuroprotective effects in the extracellular microenvironment of neurons. Concomitantly, in this study, we showed that EGCs, via the secretion of factors such as 15d-PGJ2, can also enhance the neuronal concentration of glutathione, further improving neuroprotection by acting in part in the intracellular environment of neurons.
In conclusion, this study reinforces the central role of EGCs in ENS neuroprotection and in the maintenance of gut homeostasis (Neunlist et al. 2007; Savidge et al. 2007; Abdo et al. 2010; Flamant et al. 2011; Van Landeghem et al. 2011). In particular, via production of the omega-6 derivative 15d-PGJ2, EGCs can act on both enteric neurons (by the Nrf2 pathway) and intestinal epithelial cells (via the PPARγ pathway; Bach-Ngohou et al. 2010). Interestingly, recent studies have shown that omega3 fatty acid derivatives can also modulate the ENS phenotype (de Quelen et al. 2011), further identifying lipid molecules as novel modulators of ENS and gut functions.
Acknowledgments
H.A. was supported by a grant from INSERM Pays de la Loire and SanTDige Foundation. M.M.M. is supported by a grant from Nantes-Métropole. B.L. is funded by the Centre National de la Recherche Scientifique (CNRS).
Glossary
Abbreviations
- 7-AAD
7-amino actinomycin D
- CNS
central nervous system
- 15d-PGJ2
15-deoxy-Δ12,14-prostaglandin J2
- EGCs
enteric glial cells
- ENS
enteric nervous system
- FBS
fetal bovine serum
- GCL
glutamate cysteine ligase
- GCLc
GCL catalytic subunit
- GSH
glutathione
- HO1
haem oxygenase 1
- L-PGDS
lipocalin-prostaglandin D synthase
- MCB
monochlorobimane
- NQO1
NAD(P)H:quinone oxidoreductase
- NSE
neuronal specific enolase
- PPARγ
peroxisome proliferator-activated receptor γ
Author contributions
All the data appearing in this manuscript were collected in the INSERM 913 research unit at the University of Nantes. H.A. designed and performed experiments, collected and analysed the data, and contributed to writing the manuscript. M.M.M. performed experiments, collected data, and reviewed/edited the manuscript. P.D. and K.B.-N. contributed to the discussion and reviewed/edited the manuscript. M.N. contributed to writing the manuscript and reviewing/editing it. B.L. designed the study, supervised the project and contributed to writing the manuscript. All authors approved the final version of the manuscript.
Supplementary material
Figure S1
Figure S2
References
- Abdo H, Derkinderen P, Gomes P, Chevalier J, Aubert P, Masson D, Galmiche JP, Vanden Berghe P, Neunlist M, Lardeux B. Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J. 2010;24:1082–1094. doi: 10.1096/fj.09-139519. [DOI] [PubMed] [Google Scholar]
- Aoun P, Watson DG, Simpkins JW. Neuroprotective effects of PPARγ agonists against oxidative insults in HT-22 cells. Eur J Pharmacol. 2003;472:65–71. doi: 10.1016/s0014-2999(03)01867-3. [DOI] [PubMed] [Google Scholar]
- Aube AC, Cabarrocas J, Bauer J, Philippe D, Aubert P, Doulay F, Liblau R, Galmiche JP, Neunlist M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut. 2006;55:630–637. doi: 10.1136/gut.2005.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach-Ngohou K, Mahé MM, Aubert P, Abdo H, Boni S, Bourreille A, Denis MG, Lardeux B, Neunlist M, Masson D. Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-Δ12,14-prostaglandin J2. J Physiol. 2010;588:2533–2544. doi: 10.1113/jphysiol.2010.188409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Chao CC, Lee EHY. Neuroprotective mechanism of glial cell line derived neurotrophic factor on dopamine neurons: role of antioxidation. Neuropharmacology. 1999;38:913–916. doi: 10.1016/s0028-3908(99)00030-1. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Noack H, Possel H, Keilhoff G, Wolf G. Glutathione levels in primary glial cultures: monochlorobimane provides evidence of cell type-specific distribution. Glia. 1999;27:152–161. [PubMed] [Google Scholar]
- Chen ZH, Yoshida Y, Saito Y, Sekine A, Noguchi N, Niki E. Induction of adaptive response and enhancement of PC12 cell tolerance by 7-hydroxycholesterol and 15-deoxy-Δ12,14-prostaglandin J2 through up-regulation of cellular glutathione via different mechanisms. J Biol Chem. 2006;281:14440–14445. doi: 10.1074/jbc.M600260200. [DOI] [PubMed] [Google Scholar]
- Chevalier J, Derkinderen P, Gomes P, Thinard R, Naveilhan P, Vanden BergheP, Neunlist M. Activity-dependent regulation of tyrosine hydroxylase expression in the enteric nervous system. J Physiol. 2008;586:1963–1975. doi: 10.1113/jphysiol.2007.149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quelen F, Chevalier J, Rolli-Derkinderen M, Mourot J, Neunlist M, Boudry G. n3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J Physiol. 2011;589:4341–4352. doi: 10.1113/jphysiol.2011.214056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Won SJ, Malgorn C, Auregan G, Berman AE, Chen P-C, Déglon N, Johnson JA, Suh SW, Swanson RA. Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. J Neurosci. 2011;31:7392–7401. doi: 10.1523/JNEUROSCI.6577-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri GL, Probert L, Cocchia D, Michetti F, Marangos PJ, Polak JM. Evidence for the presence of S100 protein in the glial component of the human enteric nervous system. Nature. 1982;297:409–410. doi: 10.1038/297409a0. [DOI] [PubMed] [Google Scholar]
- Flamant M, Aubert P, Rolli-Derkinderen M, Bourreille A, Neunlist MR, Mahé MM, Meurette G, Marteyn B, Savidge T, Galmiche JP, Sansonetti PJ, Neunlist M. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut. 2011;60:473–484. doi: 10.1136/gut.2010.229237. [DOI] [PubMed] [Google Scholar]
- Furness HB. The Enteric Nervous System. Blackwell Publishing; 2006. [Google Scholar]
- Gabella G. Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells. Neuroscience. 1981;6:425–436. doi: 10.1016/0306-4522(81)90135-4. [DOI] [PubMed] [Google Scholar]
- Garg TK, Chang JY. 15-deoxy-delta 12, 14-Prostaglandin J2 prevents reactive oxygen species generation and mitochondrial membrane depolarization induced by oxidative stress. BMC Pharmacol. 2004;4:6. doi: 10.1186/1471-2210-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff S, Zeller F, von Weyhern CW, Wegner M, Schemann M, Michel K, Ruhl A. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J Comp Neurol. 2008;509:356–371. doi: 10.1002/cne.21769. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Warabi E, Noguchi N, Itoh K, Yamamoto M. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem. 2005;280:27244–27250. doi: 10.1074/jbc.M502551200. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980;286:736–737. doi: 10.1038/286736a0. [DOI] [PubMed] [Google Scholar]
- Kansanen E, Kivelä AM, Levonen AL. Regulation of Nrf2-dependent gene expression by 15-deoxy-Δ12,14-prostaglandin J2. Free Radic Biol Med. 2009;47:1310–1317. doi: 10.1016/j.freeradbiomed.2009.06.030. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Girolami EI, Ghasemlou N, Jeong SY, David S. The protective effects of 15-deoxy-Δ-12,14-prostaglandin J2 in spinal cord injury. Glia. 2008;56:436–448. doi: 10.1002/glia.20630. [DOI] [PubMed] [Google Scholar]
- Kim JW, Li MH, Jang JH, Na HK, Song NY, Lee C, Johnson JA, Surh YJ. 15-Deoxy-Δ12,14-prostaglandin J2 rescues PC12 cells from H2O2-induced apoptosis through Nrf2-mediated upregulation of heme oxygenase-1: Potential roles of Akt and ERK1/2. Biochem Pharmacol. 2008;76:1577–1589. doi: 10.1016/j.bcp.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P, Pachnis V. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011;121:3412–3424. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levonen AL, Dickinson DA, Moellering DR, Mulcahy RT, Forman HJ, Darley-Usmar VM. Biphasic effects of 15-deoxy-Δ12,14-prostaglandin J2 on glutathione induction and apoptosis in human endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:1846–1851. doi: 10.1161/hq1101.098488. [DOI] [PubMed] [Google Scholar]
- Lim SY, Jang JH, Na HK, Lu SC, Rahman I, Surh YJ. 15-Deoxy-Δ12,14-prostaglandin J2 protects against nitrosative PC12 cell death through up-regulation of intracellular glutathione synthesis. J Biol Chem. 2004;279:46263–46270. doi: 10.1074/jbc.M406555200. [DOI] [PubMed] [Google Scholar]
- Lin TN, Cheung WM, Wu JS, Chen JJ, Lin H, Liou JY, Shyue SK, Wu KK. 15d-prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:481–487. doi: 10.1161/01.ATV.0000201933.53964.5b. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, Naveilhan P, Ruhl A, Lardeux B, Savidge T, Paris F, Galmiche JP. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-β1-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2007;292:G231–G241. doi: 10.1152/ajpgi.00276.2005. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004;37:433–441. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Onyango IG, Tuttle JB, Bennett JP., Jr Brain-derived growth factor and glial cell line-derived growth factor use distinct intracellular signaling pathways to protect PD cybrids from H2O2-induced neuronal death. Neurobiol Dis. 2005;20:141–154. doi: 10.1016/j.nbd.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Ou Z, Zhao X, Labiche LA, Strong R, Grotta JC, Herrmann O, Aronowski J. Neuronal expression of peroxisome proliferator-activated receptor-gamma (PPARγ) and 15d-prostaglandin J2-mediated protection of brain after experimental cerebral ischemia in rat. Brain Res. 2006;1096:196–203. doi: 10.1016/j.brainres.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Poncin S, Gerard AC, Boucquey M, Senou M, Calderon PB, Knoops B, Lengele B, Many MC, Colin IM. Oxidative stress in the thyroid gland: from harmlessness to hazard depending on the iodine content. Endocrinology. 2008;149:424–433. doi: 10.1210/en.2007-0951. [DOI] [PubMed] [Google Scholar]
- Ruhl A, Franzke S, Collins SM, Stremmel W. Interleukin6 expression and regulation in rat enteric glial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1163–G1171. doi: 10.1152/ajpgi.2001.280.6.G1163. [DOI] [PubMed] [Google Scholar]
- Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl. 1):55–59. doi: 10.1111/j.1743-3150.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Scher JU, Pillinger MH. 15d-PGJ2: the anti-inflammatory prostaglandin? Clin Immunol. 2005;114:100–109. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkamp M, Geerling I, Seufferlein T, von Boyen G, Egger B, Grossmann J, Ludwig L, Adler G, Reinshagen M. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology. 2003;124:1748–1757. doi: 10.1016/s0016-5085(03)00404-9. [DOI] [PubMed] [Google Scholar]
- Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med Res Rev. 2001;21:185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- Tang X, Wang H, Fan L, Wu X, Xin A, Ren H, Wang XJ. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic Biol Med. 2011;50:1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Van Landeghem L, Chevalier J, Mahé MM, Wedel T, Urvil P, Derkinderen P, Savidge T, Neunlist M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol. 2011;300:G976–G987. doi: 10.1152/ajpgi.00427.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boyen GBT, Steinkamp M, Geerling I, Reinshagen M, Schäfer KH, Adler G, Kirsch J. Proinflammatory cytokines induce neurotrophic factor expression in enteric glia: a key to the regulation of epithelial apoptosis in Crohn's disease. Inflamm Bowel Dis. 2006;12:346–354. doi: 10.1097/01.MIB.0000219350.72483.44. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ou Z, Grotta JC, Waxham N, Aronowski J. Peroxisome-proliferator-activated receptor-gamma (PPARγ) activation protects neurons from NMDA excitotoxicity. Brain Res. 2006;1073–1074:460–469. doi: 10.1016/j.brainres.2005.12.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


