Abstract
Background
Adult neurogenesis is coupled to angiogenesis in neurogenic niches in the dentate gyrus (DG) and increased by antidepressants in rodents. We hypothesized that, in major depressive disorder (MDD), antidepressants increase neural progenitor cells (NPCs) and capillaries in the human DG.
Methods
NPCs and capillaries, detected on hippocampal sections by immunohistochemistry for nestin, were quantified by stereology in matched MDDs (untreated, n=12), MDD treated with selective serotonin reuptake inhibitors (MDD*SSRI, n=6) or tricyclic antidepressants (MDD*TCA, n=6) and nonpsychiatric controls (n=12), all confirmed by psychological autopsy.
Results
MDD*SSRI had a larger capillary area and more NPCs versus MDDs (p=.034 and p=.008, respectively) and controls (p=.010 and p=.002, respectively) in the whole DG, more NPCs in the anterior (pes, p=.042) and central (mid-body, p=.004) DG, and greater capillary area in the pes (p=.002) and mid-body (p=.021). NPC number and capillary area correlated positively in the whole sample (R2=.454, p<.001) and in treated subjects (R2=.749, p=.001). We found no NPCs or antidepressant-related angiogenesis in CA1 and parahippocampal gyrus. DG volume correlated positively with NPC number (p=.004) and capillary area (p<.001), and differed between groups in whole hippocampus (p=.013) and mid-body (p=.036). Age negatively correlated with NPC number (p=.042), capillary area (p=.037) and bifurcations (p=.030). No sex effect was detected.
Conclusions
Antidepressants increase human hippocampal NPCs and angiogenesis selectively in the anterior and mid DG. These results raise the possibility of a causal relationship between angiogenesis and neurogenesis, as seen in other proliferating tissues, and support their possible role in the mechanism of action of antidepressants.
Keywords: neural progenitor cells, nestin, dentate gyrus, postmortem, stereology, immunohistochemistry
INTRODUCTION
Major depression (MDD) is among the ten leading causes of global disease burden (WHO). Its course is complicated by relapses, chronicity, poor and delayed treatment response. The cause of MDD and the mechanisms of antidepressant treatment efficacy are not well understood.
Adult neurogenesis is a mechanism of structural plasticity whereby new neurons are generated from the replication and maturation of neural progenitor cells (NPCs) in neurogenic niches in mammalian (20,31) and human (16) brain. Hippocampal neurogenesis plays a critical role in the response to antidepressants (48,50,70) and the behavioral adaptation to stress in rodents (53) and nonhuman primates (53,61). Impaired neurogenesis is hypothesized as contributing to MDD pathogenesis (34). Supporting this, hippocampal alterations are found in MDD: smaller volume in vivo reported by some (4,18,38,46,51,74,75,90), but not all (18,88,91) investigators; increased apoptosis in entorhinal cortex, subiculum, dentate gyrus (DG), CA1 and CA4 (44); fewer NPCs and mitotic cells in DG of older (45), but not younger (3,64) cases. Hence, hippocampal volume loss in MDD could result from less neurogenesis, poor survival or both. In MDD treated with selective serotonin reuptake inhibitors (SSRIs) we found more NPCs than in untreated MDDs and controls (3), but this was not replicated in older cases (45), suggesting cell proliferation and/or survival in response to antidepressants is age-dependent in MDD, as in rodents (9,54)
The replication, survival and differentiation of neural progenitor cells (NPCs) into mature neurons occurs in neurogenic niches that are source of vascularization (14,59) and trophic factors (43). Neurogenesis and angiogenesis are regulated by the same growth factors including vascular endothelial growth factor (VEGF) (29), fibroblast growth factor-2 (19), brain derived neurotrophic factor (BDNF) (35) and neuropeptide Y (25,99). The angiogenic niche is also a source of NPCs which, in Macaca fuscata, originate in part from vascular pericytes and adventitial cells (96). The exercise-induced increase of DG cerebral blood volume (CBV) in vivo correlates with cognitive function in humans (60). It is not known whether angiogenesis correlates with neurogenesis in human DG, but it is known that angiogenesis is necessary for tumor proliferation (8) and angiogenesis arising from the subventricular zone has a role in post-stroke brain remodeling (36,87). Vascular disease contributes to geriatric depression, cognitive impairment (73) and poor antidepressant response (73), supporting that vascularization plays a crucial role in depressive symptomatology and antidepressant response.
Nestin (NEural STem cell proteIN) is a class VI intermediate filament protein expressed during development, until around postnatal day 11 in rat cortex (30) and gradually replaced by intermediate filament proteins specific for mature cells, such as glial fibrillary acidic protein (GFAP) in glial cells and other types of neurofilaments in neurons (see (37) for review). Similarly, in adult rodent brain proliferating cells initially express nestin and subsequently develop the morphology and antigenic properties of neurons or astrocytes (65). In transgenic adult mice expressing green fluorescent protein under the control of regulatory regions of the nestin gene, nestin-positive cells eventually express polysialated neuronal cell adhesion molecule (PSA-NCAM) (95), doublecortin and the transcription factor NeuroD (82). In culture, nestin-immunoreactive cell spheres differentiate into neurons and glia (27). Moreover, nestin-positive type II NPCs, but not GFAP-positive type I NPCs, increase their proliferation in response to antidepressants (15). Therefore, nestin seems an ideal marker to examine neurogenesis within the adult brain. However, nestin re-expression in glial cells can be induced by cerebral ischemia (13), traumatic brain injury (68), de-afferentation of DG cells (5), or neurotoxicity (97), although in these cases reactive astrocytes express both nestin and GFAP (97). In human subgranular zone (SGZ), we showed (3) that nestin-immunoreactive cells do not have the morphology of astrocytes and most do not express GFAP; the few nestin-immunoreactive cells expressing GFAP had the morphology of quiescent, type I, NPCs with apical processes extending toward the molecular layer of the DG, crossing the granule cell layer (GCL); GFAP also labeled astrocytes in all subregions of the hippocampus. While CD31 (also known as PECAM, platelet/endothelial cell adhesion molecule) labels endothelial cells (7) and collagen IV labels vascular basement membranes, nestin is a marker of neovascularization: it is expressed only in newly formed vessels after pituitary infarcts and not in adenohypophyses and pituitary adenomas without infarction or infarcts without vascular response (3,52,69), is expressed after transient heart ischemia with myocardial regeneration and neovascularization (3,52,69). To confirm the vascular localization of nestin, we triple labeled hippocampal sections with nestin/CD31/DAPI and nestin/collagen-IV/DAPI. To detect proliferating NPCs, nestin-immunoreactive cells were double labeled with Ki-67 (31), which co-localizes with BrdU (64). We quantified nestin-immunoreactive capillaries and NPCs in DG, and, for comparison, in CA1 and parahippocampal gyrus and examined the hypothesis that DG NPC number will correlate with the amount of capillaries in the DG, but not in the control regions, and that NPCs and vascularization will be more in antidepressant-treated MDDs compared with untreated MDDs.
METHODS AND MATERIALS
Tissue was obtained from the Macedonian/New York State Psychiatric Institute brain collection. Research was conducted with IRB approval. At autopsy, 2 cm-thick coronal blocks of both hemispheres were frozen in dichlorodifluoromethane (−30°C) and stored at −80°C, selected brain areas were formalin-fixed for neuropathological examination, brain pH determined (23) and toxicology performed on cerebellar samples, as described elsewhere (3,77). See Supplement on toxicological screening. Psychological autopsy (33) was performed with informed consent on all cases, including controls.
We studied 12 triplets of subjects matched for age, sex and postmortem interval: 12 untreated MDDs, 12 MDDs treated with selective serotonin reuptake inhibitors (MDD*SSRI, n=6) or tricyclic antidepressants (MDD*TCA, n=6) and 12 non-psychiatric controls (Table 1). Matching was performed because there is a relationship between age and neurogenesis (3,63,77), estrogens affect NPCs and their differentiation (85) and PMI can affect protein antigenicity (42).
Table 1. Demographic and clinical characteristics of subjects.
Inclusion criteria for MDD were history of and a depressive episode within four months prior to death; unmedicated MDD had negative toxicology and no psychotropic drug prescription in the last three months of life; antidepressant-treated MDD had toxicology positive for SSRIs or TCAs and same drug prescription for at least three prior to death, we do not have data on how long subjects were taking the same antidepressant prior to the three months before death; normal controls died by accident or sudden natural causes, had no psychotropic drugs prescription and negative toxicology and psychiatric history. Exclusion criteria for all groups were: alcohol or drug dependence or abuse, positive toxicology for alcohol or drugs of abuse, mental retardation, AIDS, positive brain neuropathology, undetermined death, resuscitation with prolonged (>10 min) hypoxia. Among the 12 untreated MDD, eight had no lifetime antidepressants prescription, one had fluoxetine prescription three years before death (lasting four months); one had perphenazine prescription 18 years before death (lasting two months); one had paroxetine and amytriptyline prescription one year before death (lasting one month); one had amytriptyline and haloperidol prescription 14 years before death (lasting four years). We have no information about past treatment compliance.
| Group | Age (y) | PMI (h) | Brain pH | Sex (F:M) | Suicide (Yes) | Cause of death | Axis I | Brain Toxicology |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | ||||||||
| C (N= 12) | 41.8 ± 14.6 | 15.3 ± 4.8 | 6.43 ± 0.33 | 7: 5 | 0 | 5 CV 3 MVA 3 Homicide 1 Peritonitis |
12 None | 12 Clear |
| MDD (N= 12) | 43.6 ± 13.3 | 16.0 ± 5.9 | 6.48 ± 0.26 | 6: 6 | 9 | 9 Suicide 3 RF |
12 MDD | 12 Clear |
| MDD*SSRI (N= 6) | 38.8 ± 13.8 | 15.8± 7.5 | 6.56 ± 0.44 | 3: 3 | 5 | 5 Suicide 1 AO |
6 MDD | 1 Fluox 1 Sert 1 Sert/Fluox 1 Parox/Sert/Fluox |
| MDD*TCA (N= 6) | 46.2 ± 17.1 | 12.1 ± 5.2 | 6.48 ± 0.19 | 4: 2 | 3 | 3 Suicide 1 CV 1 AO |
6 MDD | 3 Nor 2 Clomi/BDZ 1 Nor/Venla/BDZ |
C: non-psychiatric controls; MDD: major depressive disorder; MDD*SSRI: MDD treated with selective serotonin reuptake inhibitors; MDD*TCA: MDD treated with tricyclic antidepressants; CV: cardiovascular; MVA: motor vehicle accident; RF: respiratory failure; AO: accidental overdose; Fluox: fluoxetine; Sert: sertraline; Parox: paroxetine; Nor: nortriptyline; Clomi= clomipramine; BDZ: benzodiazepines; Venla: venlafaxine.
The entire hippocampal formation was dissected from right hemisphere consecutive frozen coronal blocks, fixed in 4% paraformaldehyde phosphate buffer saline at 4°C, cryoprotected in 30% sucrose, sectioned at 50μm on a freezing microtome (Microm HM440E) and stored in 40-well boxes at −20°C in cryoprotectant (30% ethylene glycol in 0.1M phosphate buffer). Sections at 1-mm interval stained with cresyl violet at the time of sectioning (Figure S1 in the Supplement) were the reference to align nestin-stained sections along the anterior-posterior axis of the hippocampus.
Immunohistochemistry and immunohistofluorescence
Immunohistochemistry for nestin was performed as previously described (3). See immunohistofluorescence method in the Supplement.
Stereology and confocal microscopy
On sections at 2-mm interval throughout the hippocampal pes, mid-body and posterior body (Figure S1 in the Supplement) processed for nestin brightfield immunohistochemistry (Figure 1), we estimated NPC number, capillary area, volume, length and bifurcations in DG (the region of interest), CA1 and parahippocampal gyrus (the control regions), using the optical disector with fractionator method (StereoInvestigator, MBF Biosciences Inc., Williston, VT), as previously described (3). See Supplement for detailed stereology method. We quantified the percentage of NPCs in contact with blood vessels on one section per case, selecting the section of each case with the highest number of nestin-immuoreactive cells. Fluorescent double/triple-labeled capillaries and cells were analyzed with 60x oil-immersion objective on a confocal microscope (FV1000, Olympus, Center Valley, PA, Figures 2 and 3).
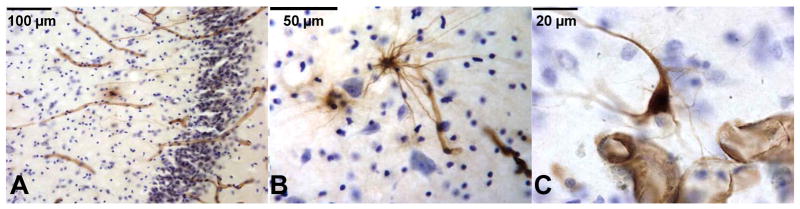
Figure 1. Immunohistochemistry for nestin in the human hippocampus.
A: Nestin-immunoreactive cells and capillaries (in brown) were found in the subgranular zone (SGZ), usually clustered at the junction of the upper and lower blade of the DG. The dentate gyrus (DG) granule cells are stained for Nissl with Cresyl Violet. Blood vessels (in brown) show their characteristic morphology. B: Nestin-immunoreactive cell next to a capillary. Nestin-immunoreactive cells exhibit their characteristic multipolar appearance. C: Nestin- immunoreactive vessels sectioned perpendicularly to their longitudinal axis. Nestin-immunoreactive cell shows immunostained perikarya and multiple processes, including some touching the blood vessels.
Figure 2. Confocal images of nestin/Ki-67/DAPI immunofluorescence in the human dentate gyrus.
A: Nestin-immunoreactive (green) neural progenitor cell (NPC) in the subgranular zone (SGZ), its dendrites are also labeled with nestin. The NPC nucleus labels for Ki-67 and DAPI (purple) showing it is in mitosis (red arrow). Another mitotic nucleus in the SGZ (white arrow) labels for Ki-67 and DAPI (purple) but not nestin, suggesting it is a mitotic cell that is not an amplifying NPC; its phenotype is unknown. Nestin also stains capillaries (green) and one shows a cell nucleus stained with DAPI (blue) inside of it. Ki-67 also labels endothelial cells undergoing regular cell division (yellow arrow). All cell nuclei stain with DAPI (blue), as seen in the granule cell layer (GCL). Co-labeling was confirmed by acquiring z-stacks at 1-μm intervals and creating orthogonal views. Lateral views of the same cell are visible in the inserts demonstrating the co-localization of nestin and Ki-67. B: Two cell nuclei stained for Ki-67 (red). C: Nestin-immunoreactive cell (green), and capillary portions. NPC dendrites are also stained for nestin and touch the capillary. D: Cell nuclei stained with DAPI in the GCL and in a vessel (white arrow)
Figure 3. Nestin/PECAM/DAPI and nestin/collagen-IV/DAPI immunofluorescence triple labeling of the human dentate gyrus.
A: Nestin (green) and PECAM (or CD31, red) labeling of capillaries and endothelial cells in the subgranular zone (SGZ). Endothelial cells labeled by PECAM (red) are seen inside a capillary stained with nestin (green). Nuclei stain with DAPI (blue) and are visible in the granule cell layer (GCL) and inside the capillary (possibly the nucleus of an endothelial cell). Co-labeling was confirmed by acquiring z-stacks at 1-μm intervals and creating orthogonal views. B: Endothelial cells labeled by CD31 (red); C: Nestin labels a capillary; D: Nuclei stained with DAPI (blue) in the GCL and capillary (arrow); E: Collagen IV co-localizes in the vasculature with nestin (yellow), further confirming the vascular localization of nestin. Examples of vessels where nestin is expressed on portions of the vessel (red, arrows) that do not express collagen IV (green), confirming that nestin, but not collagen IV is expressed in remodeling capillaries. Nuclei are stained with DAPI (blue). Co-labeling was confirmed by acquiring z-stacks at 1-μm intervals and creating orthogonal views. F: Vessels stained with nestin (red); C: Vessels stained with collagen IV (green); D: Nuclei stained with DAPI (blue); some show an elongated shape, and are probably nuclei of endothelial cells as they are inside a vessel (arrows in H, G, F).
Statistical Analysis
Data analysis was performed using SPSS (version 18.0.3 for Mac) and R (CRAN, http://cran.r-project.org) software. Regression analysis was used to test the correlation between NPC number, age, PMI, capillary area, volume, length and bifurcations in DG, CA1 and parahippocampal gyrus; between-groups comparisons were performed using univariate (ANOVA) and multivariate analysis of variance (MANOVA) with Tukey post-hoc analysis for pair-wise comparisons, age as covariate, and p<.05 for significance level, correcting for multiple comparisons. Data are expressed as mean ± SEM. Since cases were 12 matched triplets, we also used a mixed generalized linear regression model (62), with NPC number, capillary area and group as dependent variables, age and sex as covariates.
RESULTS
The hippocampal formation was approximately 40 mm long and the DG 28 mm long (Figure S1A in the Supplement). Nestin-immunoreactive NPCs (Figure 1A) in contact with capillaries in the SGZ (Figure 1B, 1C) were 85% average in the whole sample, ranging from 50 to 100% across subjects.
Fluorescence triple-labeling immunohistochemistry for nestin/Ki-67/DAPI, analyzed by confocal microscopy, showed actively cycling NPCs and Ki-67 labeling on endothelial cells undergoing regular cell division, (Figure 2). Nestin/CD31/DAPI and nestin/collagen-IV/DAPI demonstrated the vascular localization of nestin and the latter confirmed a differential expression of nestin and collagen IV (Figure 3).
Effect of antidepressant treatment on NPCs, capillary area and DG volume
Antidepressant-treated MDD showed abundant vasculature and nestin-immunoreactive cells with prominent processes compared to untreated MDDs (Figure 4). In the whole DG, NPC number was higher in MDD*SSRI compared with untreated MDDs (p=.008), controls (p=.002) and MDD*TCA (p=.014); DG capillary area (mm2) was higher in MDD*SSRI compared with untreated MDDs (p=.034) and controls (p=.010), but not MDD*TCA; DG volume (mm3) was highest in MDD*SSRI, without significant between-group differences on post-hoc tests and no difference between untreated MDDs and controls (Table 2).
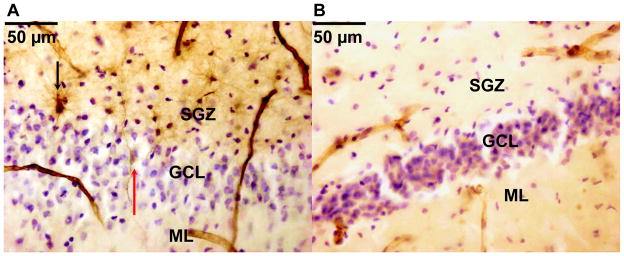
Figure 4. Nestin-immunoreactive cells and vessels in the dentate gyrus from an untreated subject with major depression (MDD) and an MDD treated with antidepressants.
The fluoxetine-treated MDD (in A) shows more prominent nestin-immunoreactive cells, processes, and capillaries (in brown) compared with the untreated MDD (in B). Granule cells and glia are stained for Nissl with Cresyl violet. The subgranular zone (SGZ), granule cell layer (GCL) and molecular layer (ML) are indicated. A: 28-year-old female with MDD treated with fluoxetine (positive toxicology and antidepressant prescription in the three months prior to death). Nestin-immunoreactive cells are visible in the SGZ (black arrow) showing a multipolar morphology, and in the GCL (red arrow) showing a bipolar morphology. Nestin-immunoreactive vessels are present in the neurogenic niche. B: 33-year-old female with MDD who was not on medication (clear toxicology and no antidepressant prescription in the three months prior to death). Vessels are much less prominent and nestin-immunoreactive cells are not visible in this part of the DG.
Table 2.
Effect of antidepressant treatment on neural progenitor cell (NPC) number, capillary area and dentate gyrus (DG) volume in the whole DG and its sub-regions.
| NPC number | ||||
|---|---|---|---|---|
| Whole DG | DG pes | DG mid body | DG posterior body | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| Controls | 594 ± 165 | 518 ± 179 | 120 ± 41 | 42 ± 31 |
| MDD | 455 ± 83 | 198 ± 208 | 127 ± 48 | 224 ± 79 |
| MDD*SSRI | 9435 ± 5351* | 4564 ± 2735** | 2812 ± 1584*** | 3275 ± 1880 |
| MDD*TCA | 3453 ± 2168 | 965 ± 1113 | 1007 ± 1111 | 3149 ± 3213 |
| F | 3.628 | 3.239 | 5.817 | 2.925 |
| ANOVA p | .007 | .042 | .004 | .060 |
| Capillary area (mm2) | ||||
| Whole DG | DG pes | DG mid body | DG posterior body | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| Controls | 2.2 ± .21 | 2.2 ± .20 | 0.22 ± 0.03 | 0.29 ± 0.07 |
| MDD | 2.8 ± .22 | 1.9 ± 1.6 | 0.26 ± .004 | 0.52 ± 0.16 |
| MDD*SSRI | 3.7 ± .49# | 3.1 ± .37## | 0.43 ± 0.05### | 0.38 ± 0.07 |
| MDD*TCA | 1.9 ± .25 | 1.5 ± .37 | 0.26 ± 0.02 | 0.25 ± 0.05 |
| F | 7.598 | 6.648 | 3.842 | 0.944 |
| ANOVA p | .013 | .002 | .021 | .446 |
| DG Volume (mm3) | ||||
| Whole DG‡ | DG pes | DG mid body‡ | DG posterior body | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| Controls | 109.6 ± 18.2 | 58.7 ± 10.5 | 41.9 ± 8.2 | 20.8 ± 5.5 |
| MDD | 110.1 ± 11.7 | 47.2 ± 7.7 | 40.9 ± 8.4 | 32.1 ± 7.4 |
| MDD*SSRI | 185.1 ± 16.2 | 65.3 ± 7.8 | 85.8 ± 11.2 | 43.4 ± 11.4 |
| MDD*TCA | 123.1 ± 28.1 | 42.6 ± 15.2 | 31.4 ± 13.2 | 51.4 ± 12.4 |
| F | 5.034 | 0.941 | 4.107 | 2.567 |
| ANOVA p | .013 | .436 | .019 | .093 |
MDD: subjects with major depression;
MDD*SSRI: MDD treated with selective serotonin reuptake inhibitors (SSRI);
MDD*TCA: MDD treated with selective serotonin reuptake inhibitors (TCA);
df: 3, 35;
MDD*SSRI > Controls, MDD, MDD*TCA, p < .050;
MDD*SSRI > MDD, p < .050;
MDD*SSRI > Controls, MDD, p < .050;
MDD*SSRI > Controls, MDD, p < .050;
MDD*SSRI > MDD, MDD*TCA, p < .050;
MDD*SSRI > Controls, MDD*TCA, p < .050;
No significant differences on post-hoc tests between groups.
Regional changes in NPCs, capillary area and DG volume associated with antidepressant treatment
MDD*SSRI had more NPCs in the pes compared with untreated MDDs (p=.041), and in the mid-body compared with controls (p=.004) and untreated MDDs (p=.004, Figure S2 in the Supplement); with no between-group differences in the posterior body (Table 2).
Capillary area was larger in MDD*SSRI compared with MDD (p=.007) and MDD*TCA (p=.003) in the pes; larger in MDD*SSRI than controls (p=.047) and MDD*TCA (p=.021), and at a trend level compared with untreated MDDs (p=.055) in the mid-body (Figure S2 in the Supplement), with no between-group differences in the posterior body (Table 2).
DG volume did not differ between groups in the pes, differed between groups in the mid-body with a trend for larger volume in MDD*SSRI than in untreated MDDs (p=.051) and MDD*TCA (p=.052, Figure S2 in the Supplement); with no between-group differences in the posterior body (Table 2, Figure S2 in the Supplement).
Correlations between NPCs, capillary area and DG volume
In the whole DG, in treated MDDs, NPC number correlated with capillary area (R2=.55, F=26.928, df=1,9, p=.006, b1=2.0, Figure S3A in the Supplement) and DG volume (R2=.521, F=11.283, df=1,9, p=.008, b1=124.8, Figure S3B in the Supplement); in the whole sample (Table S1 in the Supplement), NPC number and capillary area were positively correlated, DG volume correlated with NPC number and capillary area (Figure S3C in the Supplement). The positive correlation between NPC number and total capillary area was also found using the mixed generalized linear regression model (t=3.774, p=.0018) after log transformation of the data and removal of outliers.
In DG pes and mid-body, but not in posterior body, NPCs and capillary area were correlated; mid-body DG volume correlated with NPC number in DG pes and mid-body (Table S1 in the Supplement).
Other capillary measures: effect of antidepressants, correlation with capillary area and NPC number
In the whole DG, capillary area correlated with capillary volume and length, which correlated with NPC number (Table S1 in the Supplement). We used capillary area as our primary outcome measure but DG total capillary length also differed between groups (F=6.705, df=3, 35, p=.004), being greater in MDD*SSRI compared with untreated MDDs (p=.009), MDD*TCA (p=.038) and controls (p=.009).
Effect of age and sex on DG NPC number and capillaries
In the whole sample, age negatively correlated with capillary bifurcations (R2=.132, F=5.1573, df=1,34, p=.030, b1=−.001), but had no effect on whole DG capillary area (R2=.114, p=.115) or NPC number (R2=.088, p=.078), pes capillary area (R2=.151, p=.067) or NPC number (R2=.102, p=.137), mid-body capillary area (R2=.053, p=.290) or NPC number (R2=.092, p=.160). Including age as covariate and sex as fixed factor in a MANOVA confirmed NPC number and capillary area between-group differences, showed no effect of sex, and a trend to a negative effect of age on NPC number (F=3.936, df=1,35, p=.058). The mixed generalized linear regression model, which accounts for the matching of cases into triplets, confirmed a negative effect of age on NPC number (t=−2.224, p=.042).
Effect of nicotine exposure on DG NPC number and capillaries
There was one smoker in MDD*TCA and no smokers in MDD*SSRI, therefore we could not test tobacco exposure effects in antidepressant-treated MDDs. In untreated MDDs, smokers (n=4) and not smokers (n=8) did not differ for NPC number or capillary measures. In controls, smokers (n=7) had fewer NPCs than non-smokers (n=5) in the whole DG (non-smokers: 1014±281; smokers: 293±108; F=7.283; df:1,10; p=.022) and in DG pes (non-smokers: 912±293; smokers: 202±89; F=6.548; df:1,10; p=.038), with no differences in capillary measures.
NPCs and capillaries in control regions
NPCs were not observed in CA1 or parahippocampal gyrus. We detected no effect of treatment or MDD on angiogenesis in either of these comparison brain regions. Moreover, we did not find correlations between NPC number in DG and capillary measures in either CA1 or parahippocampal gyrus.
DISCUSSION
This study confirms and extends our previous finding that MDD*SSRI have more DG NPCs and larger DG volume than untreated MDDs or MDD*TCA (3), showing more NPCs selectively in DG pes and mid-body. This expanded sample includes the cases reported earlier (3) and is made up of 12 matched triplets, each comprised of a non-psychiatric control, an untreated MDD and an MDD on either SSRls or TCAs. In addition, we find more angiogenesis in association with SSRI treatment in the DG of adult humans with MDD. MDD*SSRl have larger capillary area in DG pes and mid-body and larger DG mid-body volume than untreated MDDs and MDD*TCA. There are no between-group differences in NPCs, capillaries or DG volume in the posterior DG. The degree of angiogenesis correlates with the NPC number in the whole DG, DG pes and mid-body. Greater NPC number and capillary area correlate with larger DG volume. Number of bifurcations per capillary and NPCs are fewer with increasing age, but there is no effect of age on capillary area. Sex did not show an effect on NPC number or capillary area.
The nestin-immunoreactive cells counted in the present study found in the SGZ of the human DG, did not show vertical processes crossing the GCL, ending in elaborate arbors in the ML, a characteristic of quiescent (type 1) NPCs, but showed instead the morphology of amplifying (type 2) NPCs (15), as previously shown (3). Amplifying, but not quiescent, NPCs increase their symmetric divisions in response to fluoxetine administration in rodents and are positive for nestin, while quiescent progenitors are positive for GFAP but not nestin (15). Therefore, based on morphology and antigenicity, the nestin-immunoreactive cells we counted appear to be amplifying NPCs.
NPC number and capillary area correlated positively, consistent with the hypothesis that common factors regulate angiogenesis and neurogenesis in human brain. We found that, on average, 85% of NPCs were in contact with capillaries in SGZ. The establishment of a vascular niche may promote neurogenesis (59) by increasing BDNF expression from astrocytes and endothelial cells (41,79), by VEGF increasing cell replication or vessel permeability (59), which maximize access to fibroblast growth factor-2 (92) and insulin-like growth factor-I (1). After chronic stress, VEGF and its receptor Flk-1 decrease in the DG, paralleling NPCs reduction, suggesting that lower VEGF decreases cell proliferation (24). Conversely, intracerebroventricular administration of VEGF in rat increases BrdU labeling of SGZ cells, and VEGFR2/Flk-1 receptors are found on cells expressing the immature neuronal marker doublecortin (28). Exercise increases hippocampal neurogenesis (89) and blood volume in animals and humans (60). The exercise-induced neurogenesis increase is inhibited in rodents by blocking VEGF alone (17). In our human sample, DG capillary area correlates with capillary volume and length, which correlate with NPC number; capillary length is greater in MDD*SSRI compared with untreated MDDs, MDD*TCA and controls, indicating that larger DG capillary area in MDD*SSRI is not due to vasodilatation, but to capillary proliferation.
More NPCs and capillaries are found in the DG of adult humans with MDD exposed to SSRI until the time of death and during the last three months of life. Fluoxetine increases DG proliferation in rodents (48,70), restores the hippocampal regulation of the hypothalamic-pituitary-adrenal (HPA) axis and reverses the behavioral effects of unpredictable chronic stress with a neurogenesis-dependent mechanism (84). VEGF expression (39) and Flk-1 signaling (21) are required for antidepressant behavioral effects and cell proliferation (93). Since we find more NPCs and vascularization in MDD*SSRI but not in MDD*TCA, it is inferred that the proliferative effect is related to serotonin, and that norepinephrine effects of TCAs may block their serotonergic effect on angiogenesis. NPC number in MDD*TCA (Table 2) is suggestive of an effect of TCAs on NPCs, but with no significant between-group differences, probably because of the large standard deviation, but DG volume and capillary area are similar to those of controls and untreated MDDs. Serotonin affects cell proliferation, regulates cell growth in human neoplasms (12,26,80,81) and exerts complex actions on blood vessels, dependent on different serotonin receptors (56). Serotonin depletion and inhibition of 5HT2B receptors suppress tumor angiogenesis; cancer cells show attenuated growth in serotonin transporter knockout mice (2); restoration of serotonin synthesis in tryptophan hydroxylase knockout mice, restores colon cancer allograft growth via regulation of angiogenesis (57). Serotonin increases vascular permeability, through enhancement of TR3/Nur77, a nuclear transcription factor with vascular permeabilizing and angiogenic activity up-regulated by VEGF (98). The observation that angiogenesis is necessary for cancer growth is in agreement with the role of angiogenesis in supporting cell growth in other conditions, including neurogenesis, as it also happens in the subventricular zone in post-stroke conditions (36).
Inhibiting DG neurogenesis blocks some behavioral effects of antidepressants (70), suggesting that it may be necessary for antidepressant action. DG irradiation abolished fluoxetine behavioral effects after repeated separation in primates (53,61). Hippocampal activity and neurogenesis may mediate antidepressant effects (11). Nevertheless, neurogenesis-dependent and independent antidepressant responses exist in rodents (10). Moreover, several stages are involved in the progression from stem cells to mature neurons (proliferation, differentiation, maturation, survival) and the efficiency of a drug on adult neurogenesis cannot be assessed analyzing one stage alone. We cannot assume that more NPCs will result in more neurons.
Greater DG volume, NPCs number and capillary area in MDD*SSRI are found selectively in the mid and anterior hippocampus, which in primates (86), and rodents, (ventral hippocampus) (66) connects with the frontal lobe-amygdala circuit and regulates neuroendocrine responses to psychological stress (55,66). This functional differentiation of the anterior hippocampus suggests that neurogenesis in the DG pes may be involved in emotional responses (67). Hence, the regional-specific changes we observe with SSRI treatment may have an effect on mood. The length of the human hippocampal formation and DG measured in this study is in agreement with previous reports (47), indicating we had an anatomically complete representation of them.
DG volume was not smaller in MDDs than in controls. Most (4,18,38,46,74,74,75,90), but not all (18,88,91) magnetic resonance (MRI) studies find smaller hippocampal volume in MDD. Most MRI studies did not report DG volume, but hippocampal formation volume, which includes Ammon’s horn: DG and CA1–CA4 pyramidal subfields, subiculum and entorhinal cortex. Antidepressant effects on DG volume are less studied. In patients with MDD, hippocampal volume was negatively correlated with days spent in a major depressive episode while untreated, but not with time spent depressed while taking antidepressants (72). Therefore, antidepressants may have a neuroprotective effect during depression. We found larger DG volume in MDD*SSRI, which is proportional to NPC number and regional vascularization, without a change in capillary density or volume fraction. In agreement with our findings, MDDs on antidepressants have larger MRI hippocampal body volume than healthy controls and unmedicated patients (49). MDD also show increased bilateral hippocampal volume after electroconvulsive shock, suggesting the hippocampus may play a central role in depression treatment (58). We acknowledge that contributors to larger DG volume in antidepressant-treated MDDs may be larger neuropil, more glial cells, or less apoptosis, not yet studied in human postmortem brain.
The relationship between MDD, neurogenesis and angiogenesis is not known. Deficient adult hippocampal neurogenesis is hypothesized as contributing to MDD pathogenesis (34), but we are not finding an effect of MDD on NPC number or vascularization, as we previously reported (3). Most untreated MDDs of our sample had no lifetime antidepressant exposure, four had some years before death; it is not likely that such past treatment explains no difference in NPCs between untreated MDDs and controls. Neurogenesis level is very low in untreated adults and differences between controls and MDDs are much harder to detect than increased levels as in antidepressant-treated cases. On the other hand, it is possible that later stages of NPC maturation or cell survival are compromised in MDD. According to this hypothesis, NPCs would not be fewer in MDD vs. controls, but neuroblasts or mature granule cells would be. Moreover, a deficit of neuropil could contribute to smaller DG in MDD. We are currently testing these hypotheses. Conversely, stress vulnerability associated with MDD, might be related to poor neurogenesis and represent an endophenotype of MDD. In fact, stress-sensitive rats show low proliferation in baseline conditions, while stress paradigms do not change neurogenesis or BDNF expression in other strains (22). In neurogenesis-deficient mice (by transgenic or radiation methods), compared with intact mice, glucocorticoid levels are slower to recover after stress and less suppressed by dexamethasone, and depressive-like behaviors are evident in novelty suppressed feeding, forced swim test, and sucrose preference task, a measure of anhedonia (78). Adult neurogenesis is critical for the hippocampal negative control of the HPA axis and it is required for effective behavioral responses to stress (78). The effect of stress conditions on human DG neurogenesis in treated and untreated MDDs should be studied.
We observe a negative effect of age on NPC number and capillary bifurcations in the DG. Accordingly, increased DG proliferation was not found in older MDDs (45), while we reported more NPCs and mitotic cells in antidepressant-treated MDD of younger age (3). In the rat, dog and marmoset (40,63,77) neurogenesis declines with age and fluoxetine effect on neurogenesis in mice is age-dependent (9,54). Reduced angiogenesis might affect NPC replication or survival in older age, contributing to the declining antidepressant response reported in older MDD (32). In fact, fitness, which increases neurogenesis via VEGF action (17) and cerebral blood volume in humans (60), protects against hippocampus volume loss occurring with age (71).
A limitation of any postmortem study is the cross-sectional analysis, therefore causes and consequence cannot be discerned. The relationship between neurogenesis and clinical improvement of MDD cannot be studied postmortem and, at the moment, there are no feasible methods to assess human adult neurogenesis in vivo. We do not have a measure for severity of MDD in the psychological autopsy and therefore cannot correlate the clinical impact of treatment with neurogenesis or angiogenesis levels. Suicides numbered one in MDD*SSRI, three in MDD*TCA and three in untreated MDD. We did not observe an effect of suicide on NPCs or capillaries in any group, although NPC number was more than double in non-suicide MDD*TCA than in suicide MDD*TCA. Group variability was too high to reach statistical significance. Suicide effect on hippocampal neurogenesis should be tested in larger samples. Factors possibly affecting group variability were nicotine and benzodiazepine use. In the control group, nicotine-exposed subjects had fewer NPCs in the whole DG and pes, according to the literature in lower mammals (6,71). Nicotine effect could not be tested in antidepressant-treated MDDs, which had only one smoker; untreated MDDs had double the number of non-smokers than controls, possibly blunting control-MDD differences in NPCs. Benzodiazepines negatively affect proliferation, differentiation and survival (76,94). One untreated-MDD, two MDD*SSRI and three MDD*TCA received benzodiazepines, possibly affecting group results. Cases were too few to analyze benzodiazepine effect, which should be addressed in future studies.
CONCLUSIONS
We show the first evidence of an association between angiogenesis and NPC proliferation in adult human DG, as predicted from animal studies. Angiogenesis and neurogenesis are higher in SSRI-treated MDDs than in untreated MDD or in absence of psychiatric disease and treatment. Angiogenesis and neurogenesis co-regulation in human could open new ways to enhance cell proliferation and survival, which may be relevant for treating mood disorders and neurodegenerative diseases. Control of angiogenic activity may be key to obtain phenotype consolidation and full integration into host neural circuitry of stereotactically transplanted stem cells, which can differentiate into functional excitatory neurons under optogenetic stimulation (83).
Supplementary Material
Acknowledgments
We thank Mihran J. Bakalian for the data management and graphics preparation, Kelly M. Burke for the stereology work, Laika R. Simeon for the laboratory work and graphics preparation. Supported by NYSTEM contract # N08G-184 FAU # 0804180400, AFSP, NARSAD, MH83862, MH94888.
Footnotes
FINANCIAL DISCOSURES/CONFLICTS OF INTEREST
Dr. René Hen receives compensation as a consultant for Roche and Lundbeck. Dr. J. John Mann received grants from GlaxoSmithKline and Novartis. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, et al. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24:23–40. doi: 10.1016/s1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 2.Asada M, Ebihara S, Yamanda S, Niu K, Okazaki T, Sora I, et al. Depletion of serotonin and selective inhibition of 2B receptor suppressed tumor angiogenesis by inhibiting endothelial nitric oxide synthase and extracellular signal-regulated kinase 1/2 phosphorylation. Neoplasia. 2009;11:408–417. doi: 10.1593/neo.81630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John MJ, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Brook GA, Perez-Bouza A, Noth J, Nacimiento W. Astrocytes re-express nestin in deafferented target territories of the adult rat hippocampus. Neuroreport. 1999;10:1007–1011. doi: 10.1097/00001756-199904060-00021. [DOI] [PubMed] [Google Scholar]
- 6.Bruijnzeel AW, Bauzo RM, Munikoti V, Rodrick GB, Yamada H, Fornal CA, et al. Tobacco smoke diminishes neurogenesis and promotes gliogenesis in the dentate gyrus of adolescent rats. Brain Res. 2011;1413:32–42. doi: 10.1016/j.brainres.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 7.Christensen K, Aaberg-Jessen C, Andersen C, Goplen D, Bjerkvig R, Kristensen BW. Immunohistochemical expression of stem cell, endothelial cell, and chemosensitivity markers in primary glioma spheroids cultured in serum-containing and serum-free medium. Neurosurgery. 2010;66:933–947. doi: 10.1227/01.NEU.0000368393.45935.46. [DOI] [PubMed] [Google Scholar]
- 8.Cook KM, Figg WD. Angiogenesis inhibitors: Current strategies and future prospects. CA Cancer J Clin. 2010;60:222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couillard-Despres S, Wuertinger C, Kandasamy M, Caioni M, Stadler K, Aigner R, et al. Ageing abolishes the effects of fluoxetine on neurogenesis. 2009. [DOI] [PubMed] [Google Scholar]
- 10.David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 12.Dizeyi N, Bjartell A, Hedlund P, Tasken KA, Gadaleanu V, Abrahamsson PA. Expression of serotonin receptors 2B and 4 in human prostate cancer tissue and effects of their antagonists on prostate cancer cell lines. Eur Urol. 2005;47:895–900. doi: 10.1016/j.eururo.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Duggal N, Schmidt-Kastner R, Hakim AM. Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain Res. 1997;768:1–9. doi: 10.1016/s0006-8993(97)00588-x. [DOI] [PubMed] [Google Scholar]
- 14.Duijvestijn AM, van Goor H, Klatter F, Majoor GD, van Bussel E, van Breda V. Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest. 1992;66:459–466. [PubMed] [Google Scholar]
- 15.Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 17.Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 18.Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh A, Greenberg ME. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 20.Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Sci. 1982;218:474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- 21.Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology. 2009;34:2459–2468. doi: 10.1038/npp.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanson ND, Owens MJ, Boss-Williams KA, Weiss JM, Nemeroff CB. Several stressors fail to reduce adult hippocampal neurogenesis. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison PJ, Heath PR, Eastwood SL, Burnet PWJ, McDonald B, Pearson RCA. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: Selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995;200:151–154. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- 24.Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- 25.Howell OW, Doyle K, Goodman JH, Scharfman HE, Herzog H, Pringle A, et al. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. J Neurochem. 2005;93:560–570. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- 26.Ishizuka J, Beauchamp RD, Townsend CM, Jr, Greeley GH, Jr, Thompson JC. Receptor-mediated autocrine growth-stimulatory effect of 5-hydroxytryptamine on cultured human pancreatic carcinoid cells. J Cell Physiol. 1992;150:1–7. doi: 10.1002/jcp.1041500102. [DOI] [PubMed] [Google Scholar]
- 27.Itoh T, Satou T, Nishida S, Hashimoto S, Ito H. Cultured rat astrocytes give rise to neural stem cells. Neurochem Res. 2006;31:1381–1387. doi: 10.1007/s11064-006-9186-8. [DOI] [PubMed] [Google Scholar]
- 28.Jin K, Mao XO, Sun Y, Xie L, Greenberg DA. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest. 2002;110:311–319. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalman M, Ajtai BM. A comparison of intermediate filament markers for presumptive astroglia in the developing rat neocortex: immunostaining against nestin reveals more detail, than GFAP or vimentin. Int J Dev Neurosci. 2001;19:101–108. doi: 10.1016/s0736-5748(00)00058-7. [DOI] [PubMed] [Google Scholar]
- 31.Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 32.Keller MB, Lavori PW, Klerman GL, Andreasen NC, Endicott J, Coryell W, et al. Low levels and lack of predictors of somatotherapy and psychotherapy received by depressed patients. Arch Gen Psychiatry. 1986;43:458–466. doi: 10.1001/archpsyc.1986.01800050064007. [DOI] [PubMed] [Google Scholar]
- 33.Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;94:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- 34.Kempermann G, Kronenberg G. Depressed new neurons--adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Li Q, Hempstead BL, Madri JA. Paracrine and autocrine functions of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain-derived endothelial cells. J Biol Chem. 2004;279:33538–33546. doi: 10.1074/jbc.M404115200. [DOI] [PubMed] [Google Scholar]
- 36.Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, et al. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- 37.Krupkova O, Jr, Loja T, Zambo I, Veselska R. Nestin expression in human tumors and tumor cell lines. Neoplasma. 2010;57:291–298. doi: 10.4149/neo_2010_04_291. [DOI] [PubMed] [Google Scholar]
- 38.Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med. 2004;34:1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- 39.Lee JS, Jang DJ, Lee N, Ko HG, Kim H, Kim YS, et al. Induction of neuronal vascular endothelial growth factor expression by cAMP in the dentate gyrus of the hippocampus is required for antidepressant-like behaviors. J Neurosci. 2009;29:8493–8505. doi: 10.1523/JNEUROSCI.1321-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Gould TD, Yuan P, Manji HK, Chen G. Post-mortem interval effects on the phosphorylation of signaling proteins. Neuropsychopharmacology. 2003;28:1017–1025. doi: 10.1038/sj.npp.1300112. [DOI] [PubMed] [Google Scholar]
- 43.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 44.Lucassen PJ, Muller MB, Holsboer F, Bauer J, Holtrop A, Wouda J, et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucassen PJ, Stumpel MW, Wang Q, Aronica E. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology. 2010;58:940–949. doi: 10.1016/j.neuropharm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 46.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. 3. New York, New York: Academic Press; 2008. [Google Scholar]
- 48.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malykhin NV, Carter R, Seres P, Coupland NJ. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci. 2010;35:337–343. doi: 10.1503/jpn.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manev H, Uz T, Smalheiser NR, Manev R. Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur J Pharmacol. 2001;411:67–70. doi: 10.1016/s0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- 51.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 52.Mokry J, Ehrmann J, Karbanova J, Cizkova D, Soukup T, Suchanek J, et al. Expression of intermediate filament nestin in blood vessels of neural and non-neural tissues. Acta Medica (Hradec Kralove) 2008;51:173–179. doi: 10.14712/18059694.2017.20. [DOI] [PubMed] [Google Scholar]
- 53.Morley-Fletcher S, Mairesse J, Soumier A, Banasr M, Fagioli F, Gabriel C, et al. Chronic agomelatine treatment corrects behavioral, cellular, and biochemical abnormalities induced by prenatal stress in rats. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2280-x. [DOI] [PubMed] [Google Scholar]
- 54.Navailles S, Hof PR, Schmauss C. Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. J Comp Neurol. 2008;509:372–381. doi: 10.1002/cne.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nettles KW, Pesold C, Goldman MB. Influence of the ventral hippocampal formation on plasma vasopressin, hypothalamic-pituitary-adrenal axis, and behavioral responses to novel acoustic stress. Brain Res. 2000;858:181–190. doi: 10.1016/s0006-8993(99)02281-7. [DOI] [PubMed] [Google Scholar]
- 56.Nishikawa T, Tsuno NH, Shuno Y, Sasaki K, Hongo K, Okaji Y, et al. Antiangiogenic effect of a selective 5-HT4 receptor agonist. J Surg Res. 2010;159:696–704. doi: 10.1016/j.jss.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Nocito A, Dahm F, Jochum W, Jang JH, Georgiev P, Bader M, et al. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 2008;68:5152–5158. doi: 10.1158/0008-5472.CAN-08-0202. [DOI] [PubMed] [Google Scholar]
- 58.Nordanskog P, Dahlstrand U, Larsson MR, Larsson EM, Knutsson L, Johanson A. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J ECT. 2010;26:62–67. doi: 10.1097/YCT.0b013e3181a95da8. [DOI] [PubMed] [Google Scholar]
- 59.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 60.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, et al. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6:e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pinheiro JC, Bates DM. Mixed effects models in S and S-PLUS. New York: Springer Verlag; 2000. [Google Scholar]
- 63.Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5:545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- 64.Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 65.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Sci. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- 67.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 68.Sahin KS, Mahmood A, Li Y, Yavuz E, Chopp M. Expression of nestin after traumatic brain injury in rat brain. Brain Res. 1999;840:153–157. doi: 10.1016/s0006-8993(99)01757-6. [DOI] [PubMed] [Google Scholar]
- 69.Salehi F, Kovacs K, Cusimano MD, Horvath E, Bell CD, Rotondo F, et al. Immunohistochemical expression of nestin in adenohypophysial vessels during development of pituitary infarction. J Neurosurg. 2008;108:118–123. doi: 10.3171/JNS/2008/108/01/0118. [DOI] [PubMed] [Google Scholar]
- 70.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of Hippocampal neurogenesis for the behavioral effects of antidepressants. Sci. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 71.Scerri C, Stewart CA, Breen KC, Balfour DJ. The effects of chronic nicotine on spatial learning and bromodeoxyuridine incorporation into the dentate gyrus of the rat. Psychopharmacology (Berl) 2006;184:540–546. doi: 10.1007/s00213-005-0086-4. [DOI] [PubMed] [Google Scholar]
- 72.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 73.Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sinner B, Friedrich O, Zausig Y, Bein T, Graf BM. Toxic effects of midazolam on differentiating neurons in vitro as a consequence of suppressed neuronal Ca2+-oscillations. Toxicology. 2011;290:96–101. doi: 10.1016/j.tox.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 77.Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88:249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 80.Sonier B, Arseneault M, Lavigne C, Ouellette RJ, Vaillancourt C. The 5-HT2A serotoninergic receptor is expressed in the MCF-7 human breast cancer cell line and reveals a mitogenic effect of serotonin. Biochem Biophys Res Commun. 2006;343:1053–1059. doi: 10.1016/j.bbrc.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 81.Sonier B, Lavigne C, Arseneault M, Ouellette R, Vaillancourt C. Expression of the 5-HT2A serotoninergic receptor in human placenta and choriocarcinoma cells: mitogenic implications of serotonin. Placenta. 2005;26:484–490. doi: 10.1016/j.placenta.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 82.Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, Kempermann G. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–814. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- 83.Stroh A, Tsai HC, Wang LP, Zhang F, Kressel J, Aravanis A, et al. Tracking stem cell differentiation in the setting of automated optogenetic stimulation. Stem Cells. 2011;29:78–88. doi: 10.1002/stem.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 87.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 88.Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, et al. Hippocampal volume in primary unipolar major depression: A magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- 89.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 90.Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 92.Wagner JP, Black IB, Cicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu CS, Wang SC, Chang IS, Lin KM. The association between dementia and long-term use of benzodiazepine in the elderly: nested case-control study using claims data. Am J Geriatr Psychiatry. 2009;17:614–620. doi: 10.1097/JGP.0b013e3181a65210. [DOI] [PubMed] [Google Scholar]
- 95.Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- 96.Yamashima T, Tonchev AB, Vachkov IH, Popivanova BK, Seki T, Sawamoto K, et al. Vascular adventitia generates neuronal progenitors in the monkey hippocampus after ischemia. Hippocampus. 2004;14:861–875. doi: 10.1002/hipo.20001. [DOI] [PubMed] [Google Scholar]
- 97.Yoo YM, Lee U, Kim YJ. Apoptosis and nestin expression in the cortex and cultured astrocytes following 6-OHDA administration. Neurosci Lett. 2005;382:88–92. doi: 10.1016/j.neulet.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 98.Zhao D, Qin L, Bourbon PM, James L, Dvorak HF, Zeng H. Orphan nuclear transcription factor TR3/Nur77 regulates microvessel permeability by targeting endothelial nitric oxide synthase and destabilizing endothelial junctions. Proc Natl Acad Sci U S A. 2011;108:12066–12071. doi: 10.1073/pnas.1018438108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, et al. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–195. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.