Abstract
Human mucosal-associated invariant T (MAIT) cells express the semi-invariant T cell receptor Vα7.2 and are restricted by the MHC-Ib molecule MR1. While MAIT cells share similarities with other innate T cells the extent to which MAIT cells are innate and their capacity to adapt is unknown. We evaluated the function of Vα7.2+ T cells from the thymus, cord blood, and peripheral blood. While antigen-inexperienced MAIT cells displayed a naive phenotype these had intrinsic effector capacity in response to Mycobacterium tuberculosis infected cells. Vα7.2+ effector thymocytes contained sjTREC suggesting limited replication and thymic origin. In evaluating the capacity of Mtb-reactive MAIT cells to adapt, we found that those from peripheral blood demonstrated a memory phenotype and had undergone substantial expansion suggesting they responded to antigenic stimulation. MAIT cells, an evolutionarily conserved T cell subset that detects a variety of intracellular infections, share features of innate and adaptive immunity.
Introduction
The immune recognition of intracellular infection plays a critical role in the host response to both viral and bacterial infection. While innate mechanisms, such as those exemplified by Toll-like receptor (TLR) and NOD-like receptor family members, have evolved to directly detect the presence of microbial products, these mechanisms are often insufficient in the ultimate containment of intracellular infection. In contrast, cytolytic CD8+ T cells, by virtue of sampling the intracellular environment via MHC-Ia molecules, can play a definitive role in the recognition and ultimate containment of these infections.
MHC-Ia-restricted CD8+ T cells use a broad array of T cell receptors (TCR) to discriminate among MHC molecules bound to a vast array of peptide ligands. Adaptively acquired CD8+ T cells have the advantages of specificity and longevity. However, the transition from low-frequency naïve T cells to high-frequency effector cells can take several days, allowing intracellular pathogens, many of which employ evasion strategies, to establish life-long residency and escape detection and elimination by host immune cells. In this regard, work performed on the recognition of the intracellular pathogen Mycobacterium tuberculosis (Mtb) is illustrative. Adaptively acquired CD4+ and CD8+ T cells are critical for the containment of Mtb, and others and we have found that CD8+ T cells reflect the presence and abundance of this pathogen (1–4). However, the acquisition of adaptive T cell immunity in TB is delayed relative to that seen in viral infection, and hence provides a window of opportunity for mycobacterial persistence (1, 5, 6).
In contrast, innate T cells operate through the coordinated use of a limited family of TCRs in conjunction with restriction by MHC-Ib molecules that have limited polymorphism. While MHC-Ia restricted T cells acquire effector function in the periphery following antigenic stimulation, innate T cell effector function is acquired in the thymus in the absence of exogenous antigenic exposure. This results in the ability of innate T cells to respond rapidly to intracellular infection, thereby controlling the pathogen as well as facilitating the acquisition of effective immunity. In the mouse, T cells restricted by the MHC-Ib molecule H2-M3 (7, 8) are present in the thymus. Similarly CD1d-restricted invariant natural killer T cells (iNKT) cells (9) are present in the mouse and human thymus (10, 11). Additionally, we have previously found that the human thymus contains Mtb-reactive CD8+ T cells of unknown restriction specificity (12).
MR1-restricted MAIT cells share a number of characteristics that are associated with innate T cells. Human MAIT cells express a semi-invariant TCR (in humans Vα7.2) (13, 14)}, are restricted by the monomorphic MHC-Ib molecule MR1, and are selected in the thymus on a hematopoietic cell type that remains to be defined (14, 15). MR1 is the most evolutionarily conserved MHC-like molecule among mammals (16). MR1 is predicted to be structurally similar to classical MHC-Ia molecules that have antigen presentation function (17).
Although the identity of the MR1 ligand(s) is unknown, it is clear that MAIT cells are capable of detecting host cells infected with a variety of microbes including Gram+ and Gram− bacteria, fungi, and mycobacteria in an MR1-restricted fashion (18, 19). Thus the combination of the semi-invariant TCR, the monomorphic MR1 molecule and ligand must in concert allow for the detection of this wide array of microbes. As such, we believe the use of Mycobacterium tuberculosis, as a model antigen to stimulate MAIT cells, is generally applicable to the study of MAIT cells.
Nonetheless, whether or not human antigen-inexperienced MR1-restricted T cells have innate functional capacity is unknown. Furthermore, increasing evidence suggests that, like other T cell subsets, MAIT cells can adapt following thymic egress. Following microbial colonization, mouse MAIT cells expand (14, 19), suggesting activation and possibly differentiation as a consequence of peripheral stimulation. In humans, MAIT cells are decreased in the blood of patients with active TB or other bacterial pneumonias (18, 19). Whether or not these changes are associated with long-term adaptation is not known.
To address the hypothesis that human MAIT cells are innate we assessed the capacity of antigen-inexperienced MAIT cells to perform as direct ex vivo effectors. Here, we have used the model pathogen Mtb to compare and contrast the function and phenotype of human, antigen-inexperienced, thymic-resident MAIT cells with those in cord blood and those in the peripheral circulation. We found that Mtb-reactive Vα7.2+ CD8+ MAIT cells were capable of short-term cytokine production in the thymus and cord blood of all donors tested. Moreover, we found that functional MAIT thymocytes contained high levels of sjTREC indicating these cells had undergone minimal replication. Because MR1 is necessary for the intrathymic selection of MAIT cells, we sought to determine the cellular source of this ligand. Robust expression of MR1 was present exclusively on a subset of CD3+ thymocytes suggesting that these T cells could serve to select MAIT cells in the human thymus. In evaluating the adaptive capacity of MAIT cells we found that in contrast to the naïve phenotype of effector MAIT cells from thymus and cord blood, peripheral blood Mtb-reactive MAIT cells expanded and expressed a memory T cell phenotype suggesting that MAIT cells are capable of maturation in response to peripheral antigenic stimulation. From these data, we conclude that human MAIT cells can be considered both innately and adaptively acquired.
Results
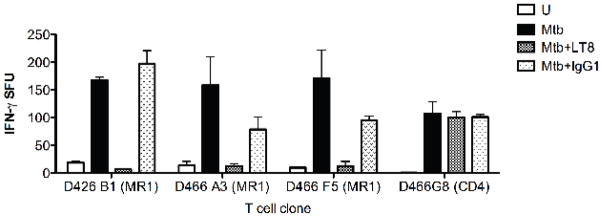
We previously demonstrated that MR1-restricted Mtb-reactive MAIT cells from peripheral blood are present in the CD8+ but not the CD4−CD8− (DN) T cell subset (18). Consequently, we sought to evaluate the requirement for CD8 co-receptor engagement for MAIT cell recognition of Mtb-infected APCs. Figure 1 shows that CD8 blockade abrogated the ability of three independently derived CD8+ Mtb-reactive MR1-restricted clones (18) to detect infected cells. Similar results were obtained using Mtb-infected epithelial cells as APCs (not shown). In contrast, CD8 blockade had no effect on an Mtb-specific MHC-II-restricted clone (Figure 1). These data demonstrate that CD8 is required for the activation of these MAIT cells.
Figure 1. CD8 co-receptor blockade prevents the detection of Mtb-infected DC by human MR1-restricted Mtb-reactive CD8+ T cell clones.
D466 DC were left uninfected (U) or infected with Mtb (MOI 30) overnight and incubated (10,000/well) with Mtb-reactive MR1-restricted CD8+ T cell clones (D426B1, D466A3, D466F5) (10,000/well) or the Mtb-specific CD4+ T cell clone (D466G8) (5000/well) in the presence of CD8 blocking antibody LT8 or control msIgG1 antibody (2.5ug/ml each). IFN-γ production was detected the following day by ELISPOT. Each bar and error bars represent the mean and SEM respectively of IFN-γ spot forming units (SFU) from duplicate wells. Similar results were obtained in 3 independent experiments.
To establish the relationship between prior antigenic exposure and the prevalence and phenotype and MR1-restricted MAIT cells, we performed flow cytometric analysis of MAIT cells derived from human thymus (n = 6), cord blood (n = 4), and peripheral blood (n = 6). We first determined the total frequency of CD8+ Vα7.2+ cells (figure 2B). CD8+ Vα7.2+ cells were detectable from all tissue sources and from all donors (figure 2A and 2B). The frequencies of CD8+ T cells that expressed Vα7.2+ in the thymus (n = 6; mean: 1.8; SEM: 0.6) were lower compared to those in cord blood (n = 4; mean 4.2; SEM: 1; P = 0.05) and peripheral blood (n = 6; mean: 4.3; SEM: 1; P = 0.04).
Figure 2. Human MAIT cells from the thymus and cord blood have innate ex vivo effector capacity.
CD4-depeletd T cells from the thymus, cord blood, and peripheral blood (500,000/well) were incubated with stimulator A549 cells left uninfected or infected with Mtb (1.5e6/2 cm2 well) and tested for the intracellular production of TNF-α as described in material and methods. Briefly, after overnight incubation, a protein transport inhibitor was added for the final 6 hrs of the assay after which cells were surface stained for expression of the Vα7.2 TCR and CD8, then fixed and permeabilized before staining for intracellular TNF-α. As TNF-α+ Vα7.2+ is not present in the CD8-negative subset, all frequencies were determined from CD8 positive gated events only.
A) Dot plot analyses from the intracellular cytokine staining assay demonstrating representative responses to uninfected A549 (left column) or Mtb-infected A549 (right column) from an individual thymus, cord blood and adult donor. Cells are gated on CD8+ events. The x-axis represents TNF-α; the y-axis represents Vα7.2. Numbers represent the frequency of CD8+ events within each gate.
B) Frequencies of Vα7.2+ CD8+ cells from the thymus (n = 6; mean: 1.8; SEM: 0.6), cord blood (n = 4; mean 4.2; SEM: 1.0) and peripheral blood (n = 6; mean: 4.3; SEM: 1.0). Results for each donor were derived using the frequencies of cells derived from the red gate (right panel). Significant differences were detected between thymus and cord blood (P = 0.05) and thymus and peripheral blood (P = 0.04).
C) Frequencies of Vα7.2+ CD8+ cells that produced TNF-α in response to Mtb-infected A549 cells. Thymus (n = 6; mean: 3.02; SEM: 0.58), cord blood (n = 4; mean 0.92; SEM: 0.52) and peripheral blood (n = 6; mean: 6.20; SEM: 2.80). Results for each donor were derived using the percentage of cells in the black gate as a proportion of those in the red gate (right panel). Significant differences were observed between thymus and cord blood (P =0.04).
D) Frequencies of Vα7.2+ cells among Mtb-reactive TNFα+ CD8+ cells. Thymus (n = 6; mean: 6.07; SEM: 1.14), cord blood (n = 4; mean 15.68; SEM: 5.89) and peripheral blood (n = 6; mean: 26.60; SEM: 5.12). Results for each donor were derived using the percentage of cells in the black gate as a proportion of those in the red gate (right panel). Significant differences were observed between thymus and peripheral blood (P =0.002).
The unpaired two-tailed, t-test was used to assess significant differences between groups. A single asterisk represents a P value < 0.05 and two asterisks < 0.01.
However, antibody detection of Vα7.2+ T cells alone does not allow for the identification of functional MR1-restricted MAIT cells. The anti-Vα7.2 antibody that was previously published (clone 3C10) (15) as well as the recently developed anti Vα7.2 antibody (clone OF-5A12) (Supp Figures S1 & S2) label all T cells that express the Vα7.2 TCR including those that are MHC-Ia restricted. Therefore to establish the frequency and phenotype of functional MR1-restricted Mtb-reactive Vα7.2+ cells ex-vivo, we employ an assay that uses Mtb-infected A549 epithelial cells as stimulators (18).
We evaluated the frequency of Mtb-reactive MAIT cells from the thymus, cord blood, and peripheral blood using CD4-depleted T cells that contain both CD8+ and DN T cells. CD4-depleted T cells from all donors were incubated with Mtb-infected A549 cells. Effector MAIT cells were enumerated using intracellular staining for TNF-α in conjunction with cell surface staining for the Vα7.2 TCR that defines MAIT cells using OF-5A12 (Supp Figures S1 & S2). Representative flow cytometric plots from the thymus, cord blood, and peripheral blood are depicted in figure 2A demonstrating that uninfected A549 cells do not elicit non-specific responses. Consistent with our prior report (18), we found pathogen-reactive MAIT cells from thymus and cord blood, in addition to peripheral blood, were present in the CD8+ population and were MR1-restricted (Supp Figure S3 and data not shown). On average, Mtb-reactive TNF-α+ MAIT cells comprised 3.0 % of Vα7.2+ CD8+ T cells from the thymus, 0.92% from cord blood, and 6.2% from peripheral blood (Figure 2C). Mtb-reactive MAIT cells from antigen-inexperienced sources were detected from all donors at frequencies substantially higher than those expected from naïve conventional CD8+ T cells (20).
While our data clearly established the presence of Mtb-reactive MAIT cells in the thymus, it was possible these cells were derived from a peripheral antigen-experienced population (21). To confirm that MAIT cells from the thymus and cord blood were naïve, we quantified the content of signal joint TCR gene excision circles (sjTREC) in Vα7.2+ T cells. sjTRECs are circular DNA products resulting from intrathymic V(D)J T-cell receptor gene recombination (22). As sjTREC do not replicate during mitosis they are diluted during cell division. Consequently, sjTREC levels are highest in thymocytes that have recently rearranged their T cell receptor, lower in peripheral naïve T cells and undetectable in previously activated T cells that have by definition undergone extensive division. As such, the prevalence of sjTREC can be used to assess the replicative history of a T cell population.
Using quantitative PCR, we estimated sjTREC content from live, FACS-sorted CD8 single positive (SP) Vα7.2+ or Vα7.2− from the thymus, cord, and peripheral blood (23). As expected, sjTREC levels were undetectable in Vα7.2+ and Vα7.2− CD8+ T cells isolated from adult peripheral blood that contain predominantly antigen-experienced T cells (figure 3A). We detected similar levels of sjTREC from both Vα7.2+ and Vα7.2−, cord-blood derived CD8+ cells (figure 3A). Notably, Vα7.2+ and Vα7.2− CD8 SP thymocytes had levels of sjTREC similar to total CD8 SP thymocytes indicating these cells had not undergone extensive cell division (figure 3A). Nonetheless, sjTREC levels from Vα7.2+ CD8 SP thymocytes were significantly (P = 0.02) reduced compared to those from the Vα7.2− CD8 SP thymocyte subset (figures 3A & 3B).
Figure 3. Human Vα7.2+ thymocytes and functional MAIT thymocytes retain sjTREC.
A) sjTREC content per 104 cells from the following FACS-sorted subsets; total CD8+ thymocytes (n = 2); paired CD8+Vα7.2+ and Vα7.2− populations from thymus (n = 7), cord blood (n = 3) and PBMC (n = 3). B) Analysis of thymocyte data in A) demonstrating that CD8+ Vα7.2+ thymocytes have significantly less sjTREC than their paired CD8+ Vα7.2− thymocytes (P= 0.02). C Representative dot plot of Vα7.2 and LAMP expression on CD8+ CD4−, PI−, γδTCR− thymocytes stimulated without (left) or with (right) PMA/ionomycin for 2 hours. D) sjTREC analysis from FACS-sorted subsets. Significant differences (P = 0.02) were observed between the LAMP+ and LAMP− CD8+ CD4−, PI−, γδTCR− thymocytes that were Vα7.2+ but not Vα7.2−.
The paired two-tailed, t-test was used to assess significant differences between groups. A single asterisk represents a P value < 0.05.
One possible explanation for the reduced sjTREC content in the Vα7.2+ CD8 SP thymocytes was that these represented a heterogeneous population of cells containing both naïve and antigen-experienced T cells. Antigen-experienced T cells from the periphery can recirculate back to the thymus (21). Such previously activated T cells could perform as short-term effectors but would have undetectable sjTREC. In contrast, we presumed that naïve Vα7.2+CD8 SP thymocytes with innate effector capacity would retain quantifiable sjTREC.
To evaluate the sjTREC content from live functional Vα7.2+ CD8 SP effectors from the thymus we performed a short-term (two-hour) stimulation with PMA and ionomycin. We previously showed that Mtb-reactive thymocytes express granzyme, a component of the granule exocytosis pathway (12). In order to be able to FACS-isolate live cells with the capacity to degranulate we utilized the CD107a/LAMP assay (24) after confirming concordance between CD107a/LAMP expression and TNF-α in Mtb-reactive thymocytes (not shown). Figure 3C shows a representative staining of CD107a/LAMP by Vα7.2+ and Vα7.2− CD8 SP thymocytes in response to PMA/ionomycin stimulation (right panel). We FACS-sorted four populations of live CD8 SP thymocytes (n = 4) based on Vα7.2 and LAMP expression and sjTREC were assessed. We found sjTREC were detectable from all subsets regardless of their ability to degranulate (figure 3D) indicating that functional MAIT cells had not undergone extensive proliferation and were unlikely to be derived from peripheral blood (figure 3D). Equivalent sjTREC content was present in LAMP+ and LAMP-Vα7.2− CD8 SP subsets (p = 0.61). However, Vα7.2+ LAMP+ cells had a 32 % reduction in sjTREC compared to their LAMP− counterparts (P = 0.02; n = 4). In combination, these data suggest that as a population, functional MAIT thymocytes are not derived from peripheral blood but undergo additional intrathymic cell division.
We next addressed phenotypic changes that MAIT cells undergo once they exit the thymus and encounter peripheral antigen(s). A complex relationship exists between microbes and MAIT cells. MAIT cells are undetectable in the gut lamina propria of germ-free mice, but can be induced to expand after microbial colonization (14, 19). Furthermore, MAIT cells predominate in tissue sites including the lung (18), the liver (25) and gut lamina propria (14). Together, these findings suggest a close interrelationship between MAIT cells and microbes located in the mucosa. Therefore to determine if MAIT cells adapt to their peripheral environment we compared the maturation state of Mtb-reactive MAIT cells in the thymus, cord blood, and peripheral blood. Cell surface expression of CD45RA, CCR7, and CD62L expression were used to define naïve, effector, and memory states of MAIT cells.
The CD45RA isoform on T cells has been used to broadly delineate naïve T cells, while CD45RO expression in conjunction with the absence of CD45RA is associated with antigen-experience. In the human thymus however, the majority of CD8 SP thymocytes express the CD45RO isoform and only 20% express CD45RA (26, 27). The upregulation of the CD45RA isoform on CD8 SP thymocytes is thought to coincide with their final maturation step allowing thymic egress (26–28). In evaluating thymocytes, we found identical expression of CD45RA on Mtb-reactive MAIT cells, Va7.2+ CD8+ T cells, and total CD8 SP thymocytes (figure 4 left column) suggesting the functional capacity of MAIT cells in the thymus is achieved prior to final commitment for thymus egress.
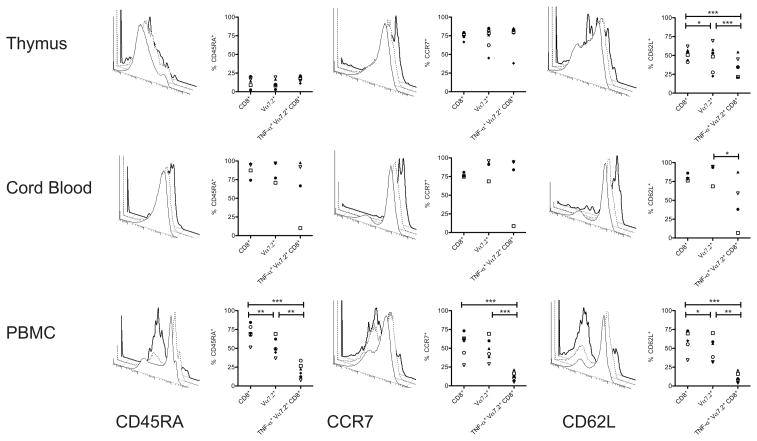
Figure 4. Antigen-inexperienced MAIT cells are inherently innate effectors with a naïve phenotype while peripheral blood MAIT cells display a phenotype associated with antigen exposure.
The intracellular cytokine-staining assay was performed as described in figure 1. In addition, cells were surface stained with antibodies for CD45RA, CD62L, and CCR7. Frequencies and representative histograms represent expression of CD45RA (left column), CCR7 (middle column) or CD62L (right column) on total CD8+ cells (solid line), Vα7.2+ CD8+ cells (dashed line), and TNFα+ Vα7.2+ CD8+ cells (bold line) from thymus (n = 6), (top row), cord blood (n = 4), (middle row) and peripheral blood (n = 6) (bottom row). Within each graph frequencies from each donor are represented by the same symbol. The paired two-tailed, t-test was used to assess significant differences between groups. A single asterisk represents a P value < 0.05; two asterisks < than 0.01; and three asterisks < 0.001.
Cord blood primarily contains naïve T cells that are defined by the expression of the CD45RA isoform. We found no significant differences in the frequencies of CD45RA+ cells between total CD8 SP, CD8+ Vα7.2+, and Mtb-reactive Vα7.2+ CD8+ cord blood T cells further suggesting that functional MAIT cells have a naïve phenotype. In peripheral blood total CD8+ T cells and Vα7.2+ CD8+ cells contained both CD45RA+ and CD45RA− cells. In contrast, Mtb-reactive MAIT cells were uniformly CD45RA negative, a phenotype associated with antigenic stimulation (figure 4 left column). Furthermore, Vα7.2+ CD8+ cells in the periphery were proportionally expanded within the total non-classical Mtb-reactive CD8+ T cells (Figure 2D). These data suggest that once in the periphery, MAIT cells have the capacity to divide and differentiate following exposure to antigenic and/or environmental stimuli.
To further define their cell-surface phenotype, we evaluated expression of CCR7 and CD62L on MAIT cells. Conventionally, naïve T cells co-express the chemokine receptor CCR7 and the selectin CD62L while effector T cells lack surface expression both molecules. As shown in figure 4 (middle panel), the majority of Mtb-reactive MAIT cells in the thymus and cord blood expressed CCR7 while those in peripheral blood completely lacked expression of CCR7. This dichotomy once again reflected the naïve phenotype of Mtb-reactive MAITs from the thymus and cord blood in contrast to the antigen-experienced phenotype in the peripheral blood Mtb-reactive MAIT cells.
However, in contrast to CCR7, we found significant decreases in the frequencies of Mtb-reactive MAIT cells from thymus and CB that expressed the CD62L marker typically present on naïve T cells (figure 4 right panel). On thymocytes, expression of CD62L is characteristically associated with their imminent egress from the thymus (28, 29). However, CD8+ mature thymocytes lacking CD62L may preferentially home to non- lymphoid tissues (30). In peripheral blood, CD62L expression on Mtb-reactive MAIT cells was absent consistent with an antigen-experienced phenotype. Thus, the overall phenotype of Mtb-reactive MAIT cells from the thymus and cord blood is consistent with a naïve phenotype, but one that may preferentially allow these cells to home to tissue sites.
As with conventional T cells, iNKT cells and MAIT cells are selected in the thymus in an MHC-dependent fashion on CD1d and MR1 respectively. However, unlike conventional T cells that are selected on thymic epithelial cells (28), MHC-Ib restricted T cells including H2-M3 restricted T cells, (8, 31) iNKT cells (11), and MAIT cells (15) are selected on hematopoietic cells. For thymic selection of iNKT cells, CD4+CD8+ double positive (DP) cortical thymocytes that express CD1d are required (11). Similarly, MAIT cell selection is thought to occur on a hematopoietic cell subset (15) that remains to be identified. Given the requirement for MHC in thymic selection we reasoned that candidate hematopoietic cells that positively select MAIT cells would express MR1.
Although MR1 transcription and translation are thought to be ubiquitous in all cell types (16, 32) endogenous MR1 cell surface expression has historically been difficult to detect. Modest levels of endogenous MR1 have been detected on mouse cells after the addition of an anti-MR1 antibody that stabilizes cell surface MR1 (33). In our studies, we detected surface MR1 on epithelial cell line A549 after infection with Mtb (18).
To determine whether or not endogenous MR1 is expressed in the human thymus, we performed flow cytometric analysis of human thymus single cell suspensions (n = 4). CD45 expression was used to delineate hematopoietic from non-hematopoietic cells. Figure 5 shows a representative analysis demonstrating that MR1 cell surface was only detectable in the CD45+ subset. We did not detect MR1 on CD45− cells from any of the donors (n = 5). We also found that MR1 expression was highly restricted to cells expressing the T cell marker CD3 (Figure 5B). Further analysis of MR1-expressing cells indicated these were primarily CD4+CD8+ DP thymocytes. However, MR1+ cells could be found in all T cell subsets except the CD4−CD8− DN subset (Figure 5C). These results suggest that, as with CD1d-restricted iNKT cells (34), an MR1-expressing T cell could serve as the selecting cell for MAIT cells in the human thymus.
Figure 5. Endogenous MR1 is expressed at high levels on a subset of CD45+ CD3+ thymocytes.
Representative analysis of single cell suspensions of thymocytes (n = 4) stained with antibodies for pan-CD45, CD3, CD4, CD8 and MR1 (clone 26.5) and analyzed by flow cytometry. A) Dot plots of CD45 expression (y-axis) and msIgG2a isotype control or MR1 (x-axis) from live cells. B) Histogram of CD3 expression on MR1+ cells. C) Dot plot of CD8 (x-axis) and CD4 expression (y-axis) from total live thymocytes (grey) or MR1-gated cells (red). The mean frequency of MR1+ cells from total live thymocytes (n =4) was 0.37% (SEM: 0.02). Similar results were seen from all 4 thymocyte donors.
Discussion
We demonstrate that semi-invariant human MR1-restricted Vα7.2+ MAIT cells are innately pathogen reactive.. First, we demonstrate that a population of Vα7.2+ T cells from the human thymus and cord blood, have the capacity to respond to bacterial infection in the absence of prior antigenic exposure. As such these cells display functional immediacy, a key feature of innate immunity. We note that the thymuses used in these studies were derived from neonates within days of birth. Thus it is unlikely exogenous microbial exposures could account for our results. Furthermore, we found that functional Vα7.2+ cells in the thymus contain sjTREC at levels similar to naïve conventional thymocytes supporting the hypothesis that these Vα7.2+ T cells are not simply derived from peripheral blood effectors. Nonetheless, in comparison to CD8 SP Vα7.2− T cells, those cells expressing Vα7.2 had undergone modest but increased intrathymic cell division. This observation suggests that MAIT cells, like iNKT cells (11), undergo some cell division in the thymus prior to release into the periphery. This intrathymic cell division is likely to contribute to readily detectable frequencies of MAIT cells, which in combination with their intrinsic effector function, suggests MAIT cells can contribute to early control of intracellular microbial infection. In mice, MR1-dependent control of Klebsiella pneumoniae was demonstrated within the first 4 days of infection (35).
A distinguishing feature of innate T cells is the manner in which they are selected in the thymus. Specifically, innate T cells are selected in the thymus on hematopoietic cells in contrast to conventional T cells that are selected on thymic epithelial cells. T cell selection by hematopoietic cells has been shown for MHC-Ib restricted T cells notably H2-M3 (8) and CD1d-restricted T cells (11). Like human MAIT cells, both of these cell types can be found as thymic effectors. Furthermore selection by hematopoietic cells resulted in H2-M3 restricted T cells with an activated phenotype with potent effector function, in contrast to those selected on thymic epithelial cells (31). Thus, a correlation exists between hematopoietic cell selection and innate T cell programming. It is known that MAIT cells are selected in the thymus in an MR1-dependent manner on a hematopoietic cell (14, 15). Here we show that MR1 is uniquely expressed on subset of CD3+ CD45+ hematopoietic T cells. Moreover, MR1 is expressed at relatively high levels on these cells as compared to other cell subsets both as we have observed (data not shown) and as reported by others (33, 36). In addition, these data are consistent with those recently published by Chua et al. in which mouse DP thymocytes were capable of MR1-dependent direct stimulation of MAIT cell hybridomas (33). We suggest that MR1-restricted innate Vα7.2+ T cells are positively selected in the thymus via endogenous MR1 on this CD3+ MR1+ thymocyte subset.
Innate T cells are often enriched in specific tissue sites. For example iNKT cells are preferentially distributed to the liver in a manner dependent on the transcription factor PLZF (37, 38) (39). We have observed the enrichment of MR1-restricted Mtb-reactive Vα7.2+ T cells in the lung (18). The relative absence of CD62L of the surface of Mtb-reactive thymic-resident MAIT suggests that MAIT cells are inherently programmed to traffic to tissue sites (30).
The relative expansion of Vα7.2+ T cells as a proportion of the total Mtb-reactive non-classical CD8+ T cell response suggests that microbial-reactive MAIT cells have the capacity to adapt to the peripheral environmental signals. In the thymus, MAIT cells accounted for 6% of the Mtb-reactive non-classical CD8+ subset. This proportion increased in cord blood to 16% and ultimately reached 27% in the periphery. These findings are similar to those previously published in ruminants and mice. Neonatal ruminants have detectable frequencies of MAIT cells in the thymus but low frequencies of MAIT cells in the periphery. After birth MAIT cells accumulate to high frequencies in the mucosa and the spleen but remain stable in the thymus (40). In the mouse, MAIT cells are dependent on microbial colonization for their peripheral expansion (14, 19). Thus our data support the hypothesis that microbial flora serve as stimulators for MAIT cells either by directly providing antigen or indirectly by altering the local environment. Here we demonstrate that following thymic egress MAIT cells can adapt, and speculate that this is a response to peripheral microenvironmental signals. This contrasts with iNKT cells whose abundance in the peripheral blood of humans does not change from birth to adulthood (41). Thus MAIT cell adaptation in the periphery may differ from other innate non-classical T cell responses.
In further support of the relationship of MAIT cells with environmental signals, we found that thymic as well as cord blood Mtb-reactive MAIT cells have a naïve phenotype. These findings are similar to those from Martin et al who previously showed that a proportion of Vα7.2+ CD161+ thymocytes and all cord blood cells expressed CD45RA (15). In sharp contrast, peripheral blood Mtb-reactive MAIT cells lost cell surface expression of CD45RA and CCR7 and were uniformly of a memory phenotype. Furthermore, the lack of detectable sjTREC from peripheral Vα7.2+ T cells confirms these cells had undergone multiple rounds of cellular division. The combination of peripheral cell expansion and cell surface phenotypic changes is consistent with MAIT cell adaptation in response to an encounter with peripheral antigen(s).
This study demonstrates that CD8 co-receptor engagement is required for the activation of MR1-restricted CD8+ Mtb-reactive Vα7.2+ T cell clones. Furthermore, our ex vivo analysis of innate effector function demonstrates that virtually all the Mtb-reactive MR1-restricted Vα7.2+ T cells from the thymus and cord blood express CD8. Controversy exists regarding the capacity of MR1 to interact with CD8. Riegert et al. (16) have argued that the mouse and human MR1 Ig-like α3 domains lack all the amino acid residues thought necessary for MHC/CD8 interactions. In contrast, Walter et al (42) have found that the deduced amino acid sequence of the rat MR1 homologue contains the prototypic CD8 binding sites. Thus, pathogen-reactive MAIT cells appear to depend on CD8 for their activation but this may not be the case with all MAIT cells.
As discussed above, we have consistently observed that Mtb-reactive MR1-restricted Vα7.2+ T cells from human peripheral blood express CD8 ((18) and data not shown). By contrast, human MAIT cells have been defined on the basis on the co-expression of Vα7.2+ and CD161, and have been found in the DN and CD8 single positive populations (13–15, 18, 25). Furthermore, MAIT cells were originally defined by the expression of a canonical TCR comprised of the semi-invariant Vα7.2/Jα33+ TCRα chain (13, 14). However, 25% of Mtb-reactive MR1-restricted T cell clones we sequenced express the Vα7.2 TCRα chain in combination with Jα20 ((18) and data not shown). These observations suggest that pathogen-reactive MR1-restricted MAIT cells may be functionally distinct from Va7.2/CD161 positive, DN MAIT cells that produce IL-17 (25). At present, a role for DN MAIT cells in MR1-dependent microbial recognition remains to be defined.
Pathogen-reactive MAIT cells that share features of innate and adaptive immunity are poised to play an important role in early containment of microbial infection. Representative of innate T cells, pathogen-reactive MAIT cells possess intrinsic effector functions that would allow them to act early in the response to infections. Also similar to other innate cells that localize to specific tissue sites, pathogen-reactive MAIT cells are found in the lung where microbial entry frequently occurs. Pathogen-reactive MAIT cells can detect microbial intracellular infection of non-professional antigen presenting cells such as epithelial cells (18), further supporting a role for MAIT cells in peripheral tissues.. Nonetheless, in addition to these innate characteristics, MAIT cells may contribute to early pathogen control in manner akin to memory T cells. Immunological memory is represented by increased frequencies of antigen-specific cells that display enhanced functionality after primary antigenic exposure. The mature phenotype of pathogen-reactive MAIT cells in the periphery suggests these cells have been altered by antigenic stimulation and the increases in pathogen-reactive MAIT cells in the periphery may allow for improved microbial control. Thus human pathogen-reactive MR1-restricted MAIT cells share qualities of both innate and adaptive immunity that suggests they have functional diversity in their ability to control infection.
METHODS
Human subjects
All tissue and blood were obtained under protocols approved by the Institutional Review Board at Oregon Health & Science University. Human thymuses were obtained from children of less than 4 months of age undergoing cardiac surgery at Doernbecher Children’s Hospital in Portland, Oregon. The TB disease incidence in Oregon in 2009 was 2.3/100,000 only one of which was a pediatric case (Annual Tuberculosis Report, Oregon 2009). As such, neonatal thymus donors were considered TB unexposed. Single cell suspensions were generated as previously described (12). Umbilical cord blood was obtained from the placenta of uncomplicated term pregnancies after delivery. Within 24 hours of collection into CPT tubes (BD), the blood was processed and CBMC were obtained after centrifugation. PBMC were obtained by aphaeresis from healthy adult donors with informed consent. Adult subjects lacked any detectable responses to Mycobacterium tuberculosis immunodominant antigens CFP-10 and ESAT-6 and were considered TB unexposed.
Mycobacteria
The H37Rv strain of Mycobacterium tuberculosis was used for all live Mtb infections (ATCC), prepared as previously described (43), and infected at multiplicity of infection (moi) of 30 unless stated otherwise (44).
Cells
A549 cells (ATCC CCL-185) were used as stimulators for direct ex vivo determination of Mtb-reactive T cells. Monocyte-derived dendritic cells were prepared as previously described (18).
Generation of anti-Vα7.2 antibody OF-5A12
Animal care and immunization procedures were performed in accordance with a protocol approved by the Oregon Health & Science University Institutional Animal Care and Use Committee. A modified subtractive immunization strategy (45, 46) was used for generation of the OF-5A12 hybridoma. Briefly, mice were injected with (6e6 CD8+ T cells clones lacking the Vα7.2 TCR. At 24 and 48 hours after the initial immunization, mice were treated with cyclophosphamide to ablate the response to the T cells lacking Vα7.2 TCR. Three and 6 weeks following the initial immunization, mice were injected with 2e6 CD8+ MAIT cell clones expressing the Vα7.2 TCR, from the same donor. The MAIT cell clone was confirmed to express Vα7.2 by positive 3C10 staining (18). Furthermore this clone was confirmed to be MR1 restricted by antibody blockade (not shown). The fusion of splenocytes with SP2/0 was performed at 5 days following the final boost using standard cell-cell fusion techniques. Confirmation of OF-5A12 antibody specificity was performed by flow cytometry on an extensive panel of Vα7.2 positive and negative-expressing cells that were also stained with a previously characterized monoclonal antibody specific for the Vα7.2 TCR (Supp Fig. S1). The specificity of OF-5A12 for the Vα7.2 TCR was confirmed by blocking the ability of 3C10 to bind MAIT cells after OF-5A12 pre-incubation (Supp Fig. S2).
Isolation of CD8+ Vα7.2+ T cells
CD3+ CD4− T cells from PBMC and CB were enriched by negative selection using magnetic bead separation according to the manufacturer’s instructions (Miltenyi) except that for thymocytes we doubled the amount of CD4 beads due to the high frequency of CD4+ cells in the thymus. FACS was used to isolate Vα7.2+ CD8+(SP) and CD8−CD4− (DN) cells that lacked CD4 (Biolegend) and the γδTCR (Thermo).
Intracellular Cytokine Staining Assay
CD8+ and DN T cells from PBMC and CB were selected using negative selection of T cells then depleted of CD4+ cells by positive selection using magnetic bead separation according to the manufacturer’s instructions (Miltenyi). CD4-depleted T cells were added to Mtb-infected or uninfected A549 cells at ratio of 3:1 and incubated for 16 hrs. GolgiStop (BD Pharmingen) was added for the final 6 hrs of the assay. Cells were first stained with LIVE/DEAD® Fixable Dead Cell Stain Kit (Invitrogen) before being surface stained for expression of the Vα7.2 TCR using anti Vα7.2 antibody (clone OF-5A12 -described below and Supp figures S1 and S2) CD8α, CD45RA, CCR7, CD62L and subsequently fixed and permeabilized with Cytofix/CytoPerm (BD Pharmingen) then stained in the presence of Perm/Wash (BD Pharmingen), with fluorochrome-conjugated antibodies to TNF-α (Beckman Coulter). Acquisition was performed with an LSRII flow cytometer with FACS Diva software (BD). All analyses were performed using FlowJo software (TreeStar).
Generation and isolation of functional MAIT cells
CD4-depleted thymocytes were added to uninfected A549 cells at ratio of 3:1 and incubated for 2 hrs in the presence of phorbol myristate acetate (20ng/ml; Sigma) and ionomycin (1uM; Sigma), 0.5ng/ml IL-2 and anti-CD107α (LAMP)-PE (10ul/ml; BD Biosciences). Cells were then stained with fluorochrome-conjugated antibodies to CD8α (Biolegend), Vα7.2 (OF-5A12) CD4 (Biolegend), γδTCR (Thermo) and propridium iodide (PI) (BD Biosciences). γδTCR−, CD4−, PI−, CD8+ cells were sorted into 4 subsets (a minimum of 50,000 cells each) based on Vα7.2 and LAMP expression using a FACS Vantage and sjTREC analysis performed as described below.
sjTREC content
sjTREC content was quantified as described by Haines et al (23) using plasmids generously provided to us by David B. Lewis. Briefly, total cellular DNA was isolated using a QIAamp DNAMini kit (QIAGEN) and used in 20.0 ul real-time PCR reactions in 96 well plates analyzed in a Step One Plus Real Time PCR instrument (Applied Biosystems). Reactions included oligonucleotide primers and a TAMRA-FAM labeled internal probe for the δ Rec- ΨJ α signal joint (47). A TAMRA-FAM labeled internal probe for the C α region of the TCR- α gene locus was used for detection of C α copy number. Plasmids were used in standard curves ranging from 1.0 × 101 to 1.0 × 105 copies per reaction (23, 47).
Supplementary Material
Nine MR1-restricted and nine HLA-Ia restricted CD8+ T clones were stained for cell surface expression of the Vα7.2 TCR. The previously characterized monoclonal antibody 3C10 (15) conjugated to biotin was used at 2.5ug/ml followed by secondary Streptavidin PECy5.5 (1:1000) (Biolegend). The OF-5A12 antibody was used at (0.5ug/ml per 1e6 cells) followed by secondary anti-msIgG1 (BD Pharmingen) used at (1:2000 dilution). Restriction of MR1 clones was confirmed by antibody blockade using the anti-MR1 antibody 26.5 as previously performed (18). HLA-Ia –restricted CD8+ T cells clones were previously characterized (48).
Four MR1-restricted and one HLA-Ia restricted T cell clones were stained with either OF-5A12 (left column) or 3C10 (middle column) anti-Vα7.2 specific antibodies as in S1. To assess the specificity of OF-5A12 (right column) the clones were pre-incubated with OF-5A12 (0.5ug/ml per 1e6 cells) for 15 minutes 4C, washed, then incubated with the biotinylated 3C10 antibody (2.5ug/ml 15 min 4C). Cells were washed and biotin detected using by flow cytometry using Steptavidin-PECy5.5. Filled histograms represent the Steptavidin-PECy5.5 fluorescence intensity after pre-incubation with OF-5A12. The solid line represents staining with 3C10 alone.
Thymocytes were depleted of CD4+ T cells, incubated overnight with A549 cells that were either uninfected (1st column), Mtb-infected (moi 30; 2nd column) or Mtb-infected in the presence of anti-MR1 (clone 26.5; 3rd column) or IgG2a control antibody (4th column; both antibodies used at 5ug/ml). Golgi Stop was added for the final 8 hrs of the assay. Cells were surface stained for expression of the Vα7.2 TCR then fixed and permeabilized before staining for TNF-α, CD8 and CD4 expression. Controls performed to test for specific TNF-α staining showed no background responses (data not shown). Plots are gated on CD8+ CD4− thymocytes. The number on the upper left of each plot represents the percentage of CD8+ T cells that expressed the Vα7.2 TCR. The number in the box on the right represents the percentage of CD8+ Vα7.2+ thymocytes cells that produced TNF-α in response to target cells. Similar results were seen with 3 individual thymus donors.
Acknowledgments
We thank Dr. David B. Lewis for kindly providing us with plasmids and protocols to perform sjTREC analyses, Dr. Ted H. Hansen for his generous gift of the anti-MR1 antibody, members of the Operating Room Team at Doernbecher’s Children’s Hospital for their collaboration in collecting thymus for our studies, Dr. Sally Segel & the members of the Labor and Delivery Ward at OHSU for their collaboration in collecting cord blood for our studies, and Erin Merrifield for her expert assistance with human subjects protocols. Funding was provided in part by the Department of Veterans Affairs and with resources and the use of facilities at the Portland VA Medical Center, National Institutes of Health (NIH) grants AI078965 and AI48090, and NIH contract HHSN266200400081C.
Footnotes
Disclosure.
The authors have no conflicts of interest to declare.
Contributor Information
Tarek Eid, Email: teid@standford.edu.
Sue Smyk-Pearson, Email: smykpearson@ohsu.edu.
Yvonne Eberling, Email: eberling@ohsu.edu.
Gwendolyn M. Swarbrick, Email: swarbric@ohsu.edu.
Stephen M. Langley, Email: langleys@ohsu.edu.
Philip R. Streeter, Email: streetep@ohsu.edu.
Deborah A. Lewinsohn, Email: lewinsde@ohsu.edu.
References
- 1.Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138(1):293–8. Epub 1987/01/01. [PubMed] [Google Scholar]
- 2.Kamath A, Woodworth JS, Behar SM. Antigen-specific CD8+ T cells and the development of central memory during Mycobacterium tuberculosis infection. J Immunol. 2006;177(9):6361–9. doi: 10.4049/jimmunol.177.9.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billeskov R, Vingsbo-Lundberg C, Andersen P, Dietrich J. Induction of CD8 T cells against a novel epitope in TB10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J Immunol. 2007;179(6):3973–81. doi: 10.4049/jimmunol.179.6.3973. Epub 2007/09/06. [DOI] [PubMed] [Google Scholar]
- 4.Lewinsohn DM, Tydeman IS, Frieder M, Grotzke JE, Lines RA, Ahmed S, et al. High resolution radiographic and fine immunologic definition of TB disease progression in the rhesus macaque. Microbes Infect. 2006;8(11):2587–98. doi: 10.1016/j.micinf.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, Fountain JJ, et al. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc Natl Acad Sci U S A. 2008;105(31):10961–6. doi: 10.1073/pnas.0801496105. Epub 2008/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205(1):105–15. doi: 10.1084/jem.20071367. Epub 2007/12/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu NM, Wang B, Kerksiek KM, Kurlander R, Pamer EG, Wang CR. The selection of M3-restricted T cells is dependent on M3 expression and presentation of N-formylated peptides in the thymus. J Exp Med. 1999;190(12):1869–78. doi: 10.1084/jem.190.12.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urdahl KB, Sun JC, Bevan MJ. Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat Immunol. 2002;3(8):772–9. doi: 10.1038/ni814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2(10):971–8. doi: 10.1038/ni710. Epub 2001/09/11. [DOI] [PubMed] [Google Scholar]
- 10.Berzins SP, Cochrane AD, Pellicci DG, Smyth MJ, Godfrey DI. Limited correlation between human thymus and blood NKT cell content revealed by an ontogeny study of paired tissue samples. Eur J Immunol. 2005;35(5):1399–407. doi: 10.1002/eji.200425958. Epub 2005/04/09. [DOI] [PubMed] [Google Scholar]
- 11.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 12.Gold MC, Ehlinger HD, Cook MS, Smyk-Pearson SK, Wille PT, Ungerleider RM, et al. Human innate Mycobacterium tuberculosis-reactive alphabetaTCR+ thymocytes. PLoS Pathog. 2008;4(2):e39. doi: 10.1371/journal.ppat.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4–8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178(1):1–16. doi: 10.1084/jem.178.1.1. Epub 1993/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–9. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 15.Martin E, Treiner E, Duban L, Guerri L, Laude H, et al. Stepwise Development of MAIT Cells in Mouse and Human. PLoS Biology. 2009;7(3):e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riegert P, Wanner V, Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J Immunol. 1998;161(8):4066–77. [PubMed] [Google Scholar]
- 17.Huang S, Gilfillan S, Cella M, Miley MJ, Lantz O, Lybarger L, et al. Evidence for MR1 antigen presentation to mucosal-associated invariant T cells. J Biol Chem. 2005;280(22):21183–93. doi: 10.1074/jbc.M501087200. [DOI] [PubMed] [Google Scholar]
- 18.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, et al. Human Mucosal Associated Invariant T Cells Detect Bacterially Infected Cells. PLoS Biol. 2010;8(6):e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–8. doi: 10.1038/ni.1890. Epub 2010/06/29. [DOI] [PubMed] [Google Scholar]
- 20.Alanio C, Lemaitre F, Law HK, Hasan M, Albert ML. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood. 2010;115(18):3718–25. doi: 10.1182/blood-2009-10-251124. Epub 2010/03/05. [DOI] [PubMed] [Google Scholar]
- 21.Hale JS, Fink PJ. Back to the thymus: peripheral T cells come home. Immunol Cell Biol. 2009;87(1):58–64. doi: 10.1038/icb.2008.87. Epub 2008/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 23.Haines CJ, Giffon TD, Lu LS, Lu X, Tessier-Lavigne M, Ross DT, et al. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med. 2009;206(2):275–85. doi: 10.1084/jem.20080996. Epub 2009/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281(1–2):65–78. doi: 10.1016/s0022-1759(03)00265-5. Epub 2003/10/29. [DOI] [PubMed] [Google Scholar]
- 25.Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–9. doi: 10.1182/blood-2010-08-303339. Epub 2010/11/19. [DOI] [PubMed] [Google Scholar]
- 26.Fujii Y, Okumura M, Inada K, Nakahara K, Matsuda H. CD45 isoform expression during T cell development in the thymus. Eur J Immunol. 1992;22(7):1843–50. doi: 10.1002/eji.1830220725. Epub 1992/07/01. [DOI] [PubMed] [Google Scholar]
- 27.Fukuhara K, Okumura M, Shiono H, Inoue M, Kadota Y, Miyoshi S, et al. A study on CD45 isoform expression during T-cell development and selection events in the human thymus. Hum Immunol. 2002;63(5):394–404. doi: 10.1016/s0198-8859(02)00379-8. [DOI] [PubMed] [Google Scholar]
- 28.Spits H. Development of alphabeta T cells in the human thymus. Nat Rev Immunol. 2002;2(10):760–72. doi: 10.1038/nri913. [DOI] [PubMed] [Google Scholar]
- 29.Campbell JJ, Pan J, Butcher EC. Cutting edge: developmental switches in chemokine responses during T cell maturation. J Immunol. 1999;163(5):2353–7. Epub 1999/08/24. [PubMed] [Google Scholar]
- 30.Holder JE, Kimpton WG, Washington EA, Cahill RN. L-selectin expression on thymic emigrants defines two distinct tissue-migration pathways. Immunology. 1999;98(3):422–6. doi: 10.1046/j.1365-2567.1999.00882.x. Epub 1999/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho H, Bediako Y, Xu H, Choi HJ, Wang CR. Positive selecting cell type determines the phenotype of MHC class Ib-restricted CD8+ T cells. Proc Natl Acad Sci U S A. 2011;108(32):13241–6. doi: 10.1073/pnas.1105118108. Epub 2011/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto K, Hirai M, Kurosawa Y. A gene outside the human MHC related to classical HLA class I genes. Science. 1995;269(5224):693–5. doi: 10.1126/science.7624800. [DOI] [PubMed] [Google Scholar]
- 33.Chua WJ, Kim S, Myers N, Huang S, Yu L, Fremont DH, et al. Endogenous MHC-Related Protein 1 Is Transiently Expressed on the Plasma Membrane in a Conformation That Activates Mucosal-Associated Invariant T Cells. J Immunol. 2011;186(8):4744–50. doi: 10.4049/jimmunol.1003254. Epub 2011/03/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J Immunol. 2000;164(5):2412–8. doi: 10.4049/jimmunol.164.5.2412. Epub 2000/02/29. [DOI] [PubMed] [Google Scholar]
- 35.Georgel P, Radosavljevic M, Macquin C, Bahram S. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol. 2011;48(5):769–75. doi: 10.1016/j.molimm.2010.12.002. Epub 2010/12/31. [DOI] [PubMed] [Google Scholar]
- 36.Gozalbo-Lopez B, Gomez del Moral M, Campos-Martin Y, Setien F, Martin P, Bellas C, et al. The MHC-related protein 1 (MR1) is expressed by a subpopulation of CD38+, IgA+ cells in the human intestinal mucosa. Histol Histopathol. 2009;24(11):1439–49. doi: 10.14670/HH-24.1439. Epub 2009/09/18. [DOI] [PubMed] [Google Scholar]
- 37.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9(9):1055–64. doi: 10.1038/ni.1641. Epub 2008/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29(3):391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med. 2011 doi: 10.1084/jem.20102630. Epub 2011/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldfinch N, Reinink P, Connelley T, Koets A, Morrison I, Van Rhijn I. Conservation of mucosal associated invariant T (MAIT) cells and the MR1 restriction element in ruminants, and abundance of MAIT cells in spleen. Vet Res. 2010;41(5):62. doi: 10.1051/vetres/2010034. Epub 2010/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bienemann K, Iouannidou K, Schoenberg K, Krux F, Reuther S, Feyen O, et al. iNKT cell frequency in peripheral blood of Caucasian children and adolescent: The absolute iNKT cell count is stable from birth to adulthood. Scand J Immunol. 2011 doi: 10.1111/j.1365-3083.2011.02591.x. Epub 2011/06/16. [DOI] [PubMed] [Google Scholar]
- 42.Walter L, Gunther E. Isolation and molecular characterization of the rat MR1 homologue, a non-MHC-linked class I-related gene. Immunogenetics. 1998;47(6):477–82. doi: 10.1007/s002510050385. [DOI] [PubMed] [Google Scholar]
- 43.Lewinsohn DM, Zhu L, Madison VJ, Dillon DC, Fling SP, Reed SG, et al. Classically restricted human CD8+ T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigenic specificity. J Immunol. 2001;166(1):439–46. doi: 10.4049/jimmunol.166.1.439. [DOI] [PubMed] [Google Scholar]
- 44.Lewinsohn DA, Heinzel AS, Gardner JM, Zhu L, Alderson MR, Lewinsohn DM. Mycobacterium tuberculosis-specific CD8+ T cells preferentially recognize heavily infected cells. Am J Respir Crit Care Med. 2003;168(11):1346–52. doi: 10.1164/rccm.200306-837OC. [DOI] [PubMed] [Google Scholar]
- 45.Sleister HM, Rao AG. Strategies to generate antibodies capable of distinguishing between proteins with >90% amino acid identity. J Immunol Methods. 2001;252(1–2):121–9. doi: 10.1016/s0022-1759(01)00346-5. Epub 2001/05/04. [DOI] [PubMed] [Google Scholar]
- 46.Williams CV, Stechmann CL, McLoon SC. Subtractive immunization techniques for the production of monoclonal antibodies to rare antigens. Biotechniques. 1992;12(6):842–7. Epub 1992/06/01. [PubMed] [Google Scholar]
- 47.Hazenberg MD, Verschuren MC, Hamann D, Miedema F, van Dongen JJ. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J Mol Med. 2001;79(11):631–40. doi: 10.1007/s001090100271. [DOI] [PubMed] [Google Scholar]
- 48.Lewinsohn DA, Winata E, Swarbrick GM, Tanner KE, Cook MS, Null MD, et al. Immunodominant tuberculosis CD8 antigens preferentially restricted by HLA-B. PLoS Pathog. 2007;3(9):1240–9. doi: 10.1371/journal.ppat.0030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nine MR1-restricted and nine HLA-Ia restricted CD8+ T clones were stained for cell surface expression of the Vα7.2 TCR. The previously characterized monoclonal antibody 3C10 (15) conjugated to biotin was used at 2.5ug/ml followed by secondary Streptavidin PECy5.5 (1:1000) (Biolegend). The OF-5A12 antibody was used at (0.5ug/ml per 1e6 cells) followed by secondary anti-msIgG1 (BD Pharmingen) used at (1:2000 dilution). Restriction of MR1 clones was confirmed by antibody blockade using the anti-MR1 antibody 26.5 as previously performed (18). HLA-Ia –restricted CD8+ T cells clones were previously characterized (48).
Four MR1-restricted and one HLA-Ia restricted T cell clones were stained with either OF-5A12 (left column) or 3C10 (middle column) anti-Vα7.2 specific antibodies as in S1. To assess the specificity of OF-5A12 (right column) the clones were pre-incubated with OF-5A12 (0.5ug/ml per 1e6 cells) for 15 minutes 4C, washed, then incubated with the biotinylated 3C10 antibody (2.5ug/ml 15 min 4C). Cells were washed and biotin detected using by flow cytometry using Steptavidin-PECy5.5. Filled histograms represent the Steptavidin-PECy5.5 fluorescence intensity after pre-incubation with OF-5A12. The solid line represents staining with 3C10 alone.
Thymocytes were depleted of CD4+ T cells, incubated overnight with A549 cells that were either uninfected (1st column), Mtb-infected (moi 30; 2nd column) or Mtb-infected in the presence of anti-MR1 (clone 26.5; 3rd column) or IgG2a control antibody (4th column; both antibodies used at 5ug/ml). Golgi Stop was added for the final 8 hrs of the assay. Cells were surface stained for expression of the Vα7.2 TCR then fixed and permeabilized before staining for TNF-α, CD8 and CD4 expression. Controls performed to test for specific TNF-α staining showed no background responses (data not shown). Plots are gated on CD8+ CD4− thymocytes. The number on the upper left of each plot represents the percentage of CD8+ T cells that expressed the Vα7.2 TCR. The number in the box on the right represents the percentage of CD8+ Vα7.2+ thymocytes cells that produced TNF-α in response to target cells. Similar results were seen with 3 individual thymus donors.