SUMMARY
NR3A is the only NMDA receptor (NMDAR) subunit that down-regulates sharply prior to the onset of sensitive periods for plasticity, yet the functional importance of this transient expression remains largely unknown. To investigate the possibility that removal/replacement of juvenile NR3A-containing NMDARs is involved in experience-driven synapse maturation, we used a reversible transgenic system that allowed persistent NR3A expression in the postnatal forebrain. We found that removal of NR3A is required to develop strong NMDAR currents, full expression of long-term synaptic plasticity, a mature synaptic organization characterized by more synapses and larger postsynaptic densities, and the ability to form long-term memories. Deficits associated with prolonged NR3A were reversible, as late-onset suppression of transgene expression rescued both the synaptic and memory impairments. Our results suggest that NR3A behaves as a molecular brake to prevent the premature strengthening and stabilization of excitatory synapses, and that NR3A removal might thereby initiate critical stages of synapse maturation during early postnatal neural development.
INTRODUCTION
The development of functional neural circuits relies on the activity-dependent rearrangement of synaptic connectivity, likely via long-term plasticity mechanisms that stabilize some synapses while weakening or eliminating others. Most rearrangement activity occurs during sensitive periods of postnatal development, and typically diminishes as adult patterns of connectivity are established. The sequence of molecular events underlying the beginning and end of these sensitive periods is not well understood, but the replacement of “juvenile” with “mature” NMDAR subtypes (Carmignoto and Vicini, 1992; Crair and Malenka, 1995; Sheng et al., 1994) is thought to contribute by tuning the plasticity potential of individual synapses. This tuning helps determine which inputs will be strengthened and directs the overall balance between synapse elimination and stabilization (Perez-Otaño and Ehlers, 2004). Accumulating evidence suggests that failure to achieve the appropriate balance results in a number of CNS disorders ranging from schizophrenia to autism and mental retardation (Bagni and Greenough, 2005; Chao et al., 2007; Durand et al., 2007; Frankle et al., 2003). Thus, understanding the mechanisms linking NMDAR exchange to brain maturation is central for understanding not only neurodevelopment and information storage, but also how aberrations in NMDAR transmission lead to neural network dysfunctions.
NMDARs are heteromers containing NR1 subunits and combinations of four NR2 (A–D) and two NR3 (A–B) subunits. Most research to date has focused on the role of the developmental switch between predominantly NR2B- to NR2A-containing NMDARs because it occurs in many brain regions and can be driven by neural activity and experience (Barria and Malinow, 2002; Bellone and Nicoll, 2007; Philpot et al., 2001). In contrast, little is known about the importance of the removal/replacement of NR3A-containing NMDARs despite growing evidence that non-conventional NR3A subunits are well positioned to be potent regulators of NMDAR function. First, inclusion of NR3A results in the formation of heteromeric receptors (NR1/NR2/NR3A) with reduced Ca2+ permeability and low sensitivity to Mg2+ blockade (Perez-Otaño et al., 2001; Sasaki et al., 2002), thus modifying two major properties of classical NMDARs (NR1/NR2 heteromers). Second, NR3A is prominently expressed during a narrow temporal window of postnatal development that correlates with periods of intense synaptogenesis and pruning and later becomes down-regulated, just prior to the onset of critical period plasticity (Perez-Otaño et al., 2006; Wong et al., 2002). Third, genetic deletion of NR3A increases spine density, indicating that NR3A might participate in synapse formation or elimination (Das et al., 1998). Finally, removal of NR3A-containing NMDARs can be regulated by activity via selective recruitment of the endocytic adaptor PACSIN/syndapin 1 (Perez-Otaño et al., 2006), suggesting that regulated removal might occur at the level of individual synapses rather than non-selectively along entire dendritic branches or trees. Yet, if and how NR3A subunits modulate synaptic plasticity, synapse maturation, or learning is unknown, leaving an important gap in our understanding of NMDAR-mediated development. Assessing the role of NR3A may also be important for understanding human disease. For example, genetic variations in NR3A alter human brain function (Gallinat et al., 2007), and abnormal NR3A expression is associated with schizophrenia and bipolar disorder (Mueller and Meador-Woodruff, 2004).
To address the role of NR3A subunits in synapse development and plasticity, we generated transgenic mice in which NR3A expression could be prolonged beyond its natural time window in postnatal forebrain neurons. We found that prolonging NR3A expression results in synaptic NMDAR hypofunction, deficits in long-term potentiation (LTP), reduced postsynaptic maturation at Schaffer collateral-CA1 synapses of the hippocampus, and impairs behavioral flexibility and memory consolidation. Conversely, genetic deletion of NR3A yielded a premature concentration of NMDARs at postsynaptic sites, enhancing synaptic NMDAR currents and promoting an earlier developmental onset of LTP. Both the synaptic and cognitive deficits resulting from persistent NR3A expression could be reversed by suppressing transgene expression at later stages. These findings indicate that the presence of NR3A maintains synapses in an anatomically and functionally juvenile state that is refractory to the induction of LTP and structural plasticity, and incapable of supporting long-term memory storage.
RESULTS
Generation of GFPNR3A transgenic mice
We used the tetracycline-controlled transactivator (tTA) system to generate transgenic mice that express the NR3A coding region tagged with enhanced green fluorescent protein (GFPNR3A) under the control of the tetO promoter (for details and functionality of the tagged construct see Perez-Otaño et al., 2001, 2006). To achieve postnatal expression of the transgene, these animals were crossed to mice expressing tTA under the CaMKIIα promoter (Mayford et al., 1996) (Figure 1A). Resulting double transgenic mice were born with the expected Mendelian frequencies, were fertile and of normal weight, and grew to adulthood with no overt behavioral abnormalities. Direct or immunohistochemically-enhanced GFP visualization showed that GFPNR3A was expressed in forebrain areas including the hippocampus (mainly CA1 pyramidal neurons), regions of the neocortex, amygdala, striatum, and olfactory tubercle (Figures 1B and S1). At the subcellular level, GFPNR3A was localized in cell bodies and dendritic fields of CA1 and neocortical pyramidal neurons (Figure 1C). The regional pattern of GFPNR3A expression overlapped with neuronal populations where NR3A is endogenously expressed earlier in development (see comparative mapping analysis in Table S1). Temporally, GFPNR3A expression began around postnatal day 12 (P12) and steadily increased into adulthood, reaching plateau levels by P25, thus coinciding with and counteracting the developmental decline of endogenous NR3A expression in rodent forebrain (Figures S1 and S2, see Perez-Otaño et al., 2006; Wong et al., 2002). Administration of doxycycline for 5 days completely switched off transgene expression in both young (Figure 1D) and adult mice (data not shown).
Figure 1. Expression of GFPNR3A in transgenic mice.
(A) Constructs used for reversible production of GFPNR3A transgenic mice. The CaMKIIα promoter determines the temporal/spatial expression pattern of tTA. In the absence of doxycycline, the tTA protein drives expression of GFPNR3A linked to the tetO promoter (Gene ON). (B) Direct GFP visualization of GFPNR3A protein in the forebrain of double transgenic mice at postnatal day 25 (P25). Scale bar: 100 μm (25 μm in insets). Note high levels of expression in the striatum, specific cortical layers, and hippocampal CA1. (C) Dendritic targeting of GFPNR3A in pyramidal neurons of CA1 hippocampus (left, middle) and layer IV of the neocortex (right), shown by DAB-enhanced immunohistochemistry using anti-GFP antibody. Scale bars: 50 μm (left), 10 μm (middle, right). (D) Expression of transgenic GFPNR3A is regulated by doxycycline administration as shown by DAB-enhanced anti-GFP immunohistochemistry. Gene ON, P26 transgenic mice raised off doxycycline; Gene OFF, P26 transgenic mice shifted onto doxycycline at P21 (2 mg/ml in drinking water for 5 days) after allowing transgene expression at early stages. Abbreviations: St, striatum; SS1, primary somatosensory cortex; CA1, CA1 hippocampus; s rad, stratum radiatum; Cg, cingulate cortex; OT, olfactory tubercle; LS, lateral septum; Hi, hippocampus; Amy, amygdala. Scale bar: 500 μm.
Prolonged GFPNR3A expression did not affect gross brain cytoarchitecture or development. Histochemical examination did not reveal structural abnormalities in forebrain regions with high transgene expression such as the cortex, hippocampus, or striatum (Figure S1). No overt differences were found in dendritic morphologies of CA1 hippocampal neurons as shown by normal intensity and expression pattern of the dendritic marker MAP2 (Figure S1), or by Golgi impregnation (data not shown). We additionally stained for cytochrome oxidase to examine the patterned barrel structure of the primary somatosensory cortex, as the formation of these patterns has been reported to depend on NMDAR function (Iwasato et al., 2000), but no differences were detected (Figure S1).
Incorporation of GFPNR3A-containing NMDARs into synapses
Because the developmental down-regulation of NR3A coincides with major maturational changes at excitatory synapses, including changes in the kinetic properties and postsynaptic attachment of NMDARs, synaptic plasticity, and spine formation and growth, we examined the effects of prolonged NR3A expression in the CA1 region of the hippocampus at higher resolution (Groc et al., 2006; Harris and Teyler, 1984; Muller et al., 1989; Harris et al., 1992). Pyramidal neurons in CA1 express NR3A at peak levels between P6 – P15, but expression is markedly down-regulated by P25 and remains lower into adulthood as in other brain regions (Figure S2A, B).
A series of experiments demonstrated that GFPNR3A had incorporated into synaptic NMDARs by P25. First, immunohistochemical analysis showed that GFPNR3A expression was up-regulated in CA1 between P12–P25, coinciding with the decline in endogenous NR3A (Figure S2C). Western blots using an anti-NR3A antibody revealed a GFPNR3A band of the expected size in hippocampal membrane extracts of transgenic mice (~160 kDa, Figure S3A), which was absent in controls. GFPNR3A was enriched in synaptic fractions to a similar magnitude as endogenous NR3A (Figure S3A, Das et al., 1998; Perez-Otaño et al., 2006). Second, co-immunoprecipitation experiments showed that GFPNR3A interacts with NR1, NR2A and NR2B subunits, matching the behavior of native NR3A subunits and indicating that GFPNR3A assembles into heterotrimeric NMDAR complexes (Figure 2A, see Al Hallaq et al., 2002). The AMPAR subunit GluR2 was not detected in immunoprecipitates, demonstrating the specificity of subunit assembly. There were no discernible changes in the levels of NR1, NR2A or NR2B in total membranes of GFPNR3A transgenics relative to controls, or in light membrane fractions that contain intracellular organelles including endoplasmic reticulum and Golgi membranes (Figure S3C, D). Combined with the observation that synaptic enrichment of GFPNR3A was comparable to that of endogenous NR3A, these results indicate that intracellular trafficking of GFPNR3A subunits was essentially normal and that GFPNR3A expression did not cause non-specific intracellular sequestration of endogenous NMDAR subunits. Intriguingly, a selective decrease in the abundance of NR2B subunits was observed in synaptic fractions (Figure S3E).
Figure 2. GFPNR3A-containing NMDARs are functional and inserted into synapses.
(A) Transgenic GFPNR3A assembles with endogenous NMDAR subunits. Total membrane extracts from hippocampi of P25 double transgenic mice were solubilized, immunoprecipitated with anti-GFP antibody and immunoblotted with the indicated antibodies. Additionally, 5% of the lysate (Input) used for immunoprecipitation was loaded. (B–C) Effect of prolonged NR3A expression on synaptic NMDAR currents. Data are from pharmacologically-isolated NMDAR-mediated EPSCs recorded from CA1 pyramidal neurons and evoked by Schaffer collateral stimulation. (B) Input-output (I–O) curve demonstrating that the amplitude of synaptic NMDAR currents recorded at +40 mV is reduced in neurons from P21–P25 GFPNR3A transgenic mice (dt GFPNR3A). RMANOVA revealed a significant main effect of genotype (F(1, 17) = 10.56, p < 0.01). In this and all subsequent figures, error bars represent s.e.m. (C) Current-voltage (I–V) curve demonstrating reduced rectification of synaptic NMDAR currents measured in the presence of 4 mM Mg2+ in neurons from P21–P25 GFPNR3A transgenics. RMANOVA indicated a significant main effect of genotype (F(1, 25) = 4.22, p < 0.05). (D) Synaptic NMDAR currents recorded in CA1 neurons from NR3A knockout mice after Schaffer-collateral stimulation exhibit stronger rectification at early postnatal stages (P6–P8) but normalize afterwards. (E) Comparison of sensitivity to Mg2+ block indexes (1/C0) calculated for NMDARs in control, GFPNR3A transgenic and NR3A knockout mice at the ages indicated. Results are averages of 1/C0 values calculated from I/V curves fitted as described in Appendix S1 (n= 6–14 mice per group).
Third, at the subcellular level, dendritic GFPNR3A clusters colocalized with the postsynaptic marker Shank in the CA1 stratum radiatum (Figure S3B), confirming that GFPNR3A subunits were at synapses. GFPNR3A was also seen in peri- and extrasynaptic sites, as is the case for endogenous NR3A (Perez-Otaño et al., 2006).
Fourth, to assess if incorporation of GFPNR3A altered synaptic NMDAR function, we measured several features of NMDAR-mediated excitatory postsynaptic currents (EPSCs) in CA1 pyramidal neurons of brain slices from P21–25 transgenic and control mice; whole-cell patch-clamp recordings in transgenic mice were visually guided by GFP expression. As expected if a higher fraction of synaptic NMDARs contained the NR3A subunit (Sasaki et al., 2002; Tong et al., 2008), we found that GFPNR3A expression significantly reduced the overall amplitude of NMDAR EPSCs evoked by stimulating Schaffer collaterals and decreased the rectification in the current-voltage (I–V) relationship that is produced by Mg2+ blockade (Figure 2B, C). Together, these results indicate that transgenic GFPNR3A subunits assemble with native NR1 and NR2 subunits to form non-conventional, synaptically-activated NMDAR channels and corroborate previous findings that NR3A subunits incorporate into synapses (Perez-Otaño et al., 2006; Tong et al., 2008). Although longer single-channel open times have been observed for recombinant NR3A-containing NMDARs (Sasaki et al., 2002; Perez-Otaño et al., 2001), we did not detect differences in the duration of macroscopic NMDAR currents (Figure S4A). This observation is consistent with the lack of differences in the duration of macroscopic NMDAR currents in NR3A knockout mice (Sasaki et al., 2002), and with previous studies showing that decay kinetics are dominated by other factors such as glutamate unbinding rates (Pan et al., 1993).
GFPNR3A expression levels detected by biochemical techniques were low, and a precise quantification of the amount in CA1 pyramidal neurons relative to endogenous levels during development was precluded by contributions of a population of CA1 stratum oriens interneurons which retain high endogenous NR3A expression into adulthood (Figure S3A). To confirm functionally that sufficient levels of transgenic GFPNR3A subunits were being expressed to maintain synaptic NMDARs in their juvenile state, we took advantage of the decreased sensitivity to Mg2+ blockade of NR3A-containing receptors (Figure 2C, D). A single-parameter index of sensitivity to Mg2+ blockade (1/C0) was extracted from current-voltage relationships of NMDAR currents using a theory derived previously to describe current flux through NMDARs in the presence of Mg2+ (Jahr and Stevens, 1990; Appendix S1). The analysis showed that the sensitivity to Mg2+ block at synapses of P21–P25 GFPNR3A-expressing neurons was similar to the sensitivity at P6–P8 wild-type synapses (when endogenous NR3A expression is highest), and significantly less than at P21–P25 control synapses (when NR3A expression is low) or at P6–P8 NR3A knockout synapses (Figure 2E). These results provide functional evidence that transgenic GFPNR3A expression prolonged peak juvenile synaptic NR3A levels in the face of endogenous down-regulation, and that functional levels of synaptic NR3A in GFPNR3A transgenic mice were similar to levels expressed during early development in wild-type mice.
Altered long-term synaptic plasticity
We next examined whether prolonging NR3A expression altered long-term synaptic plasticity. In a blind fashion, we compared the magnitude of LTP measured in CA1 field potential recordings after high-frequency Schaffer collateral stimulation (HFS, three trains at 100 Hz, 1 sec). LTP was significantly reduced in slices from GFPNR3A transgenics when compared to control littermates (control, 167 ± 8%; GFPNR3A, 127 ± 8%, p = 0.0047, Figure 3A). To test whether the deficit could be overcome with a stronger LTP induction protocol, we measured the responses to three repeated applications of high-frequency stimulation trains, each separated by a 5-min interval. However, the LTP deficit was still observed (control, 208 ± 17 %; GFPNR3A, 157 ± 15%, p = 0.033, Figure 3B). Long-term depression (LTD) induced by low-frequency stimulation was unaffected (Figure 3C). Paired-pulse facilitation (PPF) ratios were also unchanged (Figure 3D), suggesting that release probability and presynaptic function are normal in GFPNR3A transgenics. These findings demonstrate that prolonging NR3A expression alters the ability of synapses to undergo potentiation, and that the effect is likely due to postsynaptic alterations. To determine if synaptic alterations were reversible, doxycycline was given to GFPNR3A transgenics starting at P26 (14 days, from P26–P40, scheme in Figure 3E). This administration protocol allowed GFPNR3A expression from its onset at P12 until P26, when defects in LTP are manifest, but suppressed GFPNR3A expression afterwards. Switching off transgene expression restored normal levels of LTP, which were indistinguishable from controls (Figure 3E).
Figure 3. Hippocampal LTP is attenuated in GFPNR3A transgenic mice.
(A) LTP evoked by HFS of Schaffer collaterals and measured with field excitatory postsynaptic potentials (fEPSPs) in the CA1 region of the hippocampus is attenuated in P25 GFPNR3A transgenic mice (p < 0.005, Student’s t-test). Inset: Representative traces from the average of 30 traces before and after HFS in slices from control and GFPNR3A transgenic mice. (B) A stronger LTP protocol (3 epochs of HFS, arrows) was unable to rescue the deficit in GFPNR3A transgenic mice (p < 0.05, Student’s t-test). Inset: Representative traces from the average of 30 traces before and after repeated HFS in slices from control and GFPNR3A transgenic mice. (C) No apparent defects in LTD induced by low-frequency stimulation (LFS). Inset: Representative traces from the average of 30 traces before and after LFS in slices from control and GFPNR3A transgenic mice. (D) PPF (interstimulus interval: 25 ms to 250 ms) data indicate that release probability is similar in neurons from P25 control and transgenic mice. Inset: Representative traces from control and GFPNR3A transgenic experiments taken from the 25 ms interval. (E) Administration of doxycycline for 14 days (from P26–P40, see scheme) normalizes LTP deficits in GFPNR3A transgenics.
Changes in the structure and function of excitatory synapses
The balance between LTP and LTD, or related NMDAR-dependent mechanisms, is thought to control the remodeling of neuronal circuitry during postnatal development by regulating synapse maturation and/or elimination. Thus, we examined if prolonged NR3A expression affected the number, structure, or composition of CA1 excitatory synapses.
Individual control and GFPNR3A-expressing CA1 pyramidal neurons were filled with the red fluorophore Alexa 568 in acute slices from P25 mice via the whole-cell patch-clamp recording pipette, and high-resolution confocal reconstructions of the filled neurons were obtained. Only a fraction of CA1 neurons expressed GFPNR3A, allowing the selection of control neurons from transgenic slices (Figure 4A, B). We found that spine density was significantly reduced in apical dendrites of GFPNR3A-expressing neurons (spines per 10 μm dendrite: control, 10.8 ± 0.9; GFPNR3A, 7.7 ± 0.6, p = 0.04, 29% decrease, Figure 4 C, D); effects were nominally more pronounced among the population of stable mushroom-type spines (control, 5.2 ± 0.6; GFPNR3A, 3.3 ± 0.2, p = 0.06, 36% decrease, Figure 4E). Reductions in spine number were confirmed in Golgi-stained CA1 pyramidal neurons from control and transgenic mice, where experiments and analysis were conducted blind to genotype. By P25, the average spine density was significantly lower in apical dendrites of CA1 neurons from GFPNR3A transgenic mice relative to controls (spines per 10 μm dendrite: control, 9.3 ± 0.3; GFPNR3A, 7.9 ± 0.3, p = 0.002, 15% decrease, Figure 4F, G). As expected, the magnitude of the defect was less than that detected in fluorescence fill experiments, because the Golgi silver impregnation does not allow distinction between GFPNR3A-expressing and non-expressing neurons.
Figure 4. Decreased spine density in CA1 hippocampal neurons of GFPNR3A transgenic mice.
(A) CA1 pyramidal neurons in acute hippocampal slices were filled with Alexa 568 (50 μM) via the patch pipette and transgenic neurons were identified by GFP-fluorescence. A maximum projection image of a filled control CA1 neuron is shown. Scale bar, 100 μm. (B) Only a fraction of CA1 pyramidal neurons express GFPNR3A. GFP-positive neurons (green) were counted on confocal hippocampal sections from double transgenic mice counterstained with TO-PRO-3 (blue) to label all nuclei. Scale bar, 50 μm. For quantitative analysis, brains from 10 double transgenic P25 mice were used and confocal CA1 images were acquired at various distances from the central line for neuronal counting (see schematic inset). Higher numbers of GFP-positive cells were found close to the subiculum. (C) Representative high magnification projections of Alexa 568-filled apical dendritic segments of P25 control and GFPNR3A-expressing CA1 neurons. Scale bar, 3 μm. (D) Mean spine densities in apical dendrites of P25 control and GFPNR3A-expressing CA1 neurons. Values from 3–10 secondary/tertiary dendrites were averaged to yield individual neuron parameters (* p < 0.05, ANOVA followed by Tukey’s t-test, n = 6–11 neurons per group). (E) Spines were grouped in different morphological categories and spine densities for the different categories were calculated as in D (see examples in C). (F) Representative apical dendritic segments of Golgi-impregnated CA1 pyramidal neurons from P25 control and GFPNR3A transgenic mice. Scale bar, 5 μm. (G) Mean spine densities in apical dendrites from CA1 pyramidal neurons. Values from 3–5 secondary/tertiary dendrites were averaged to calculate individual neuron parameters (* p < 0.005, ANOVA followed by Tukey’s t-test, n = 34–45 Golgi-stained neurons from 7–8 control and GFPNR3A transgenic mice).
At higher resolution, electron microscopy revealed a significant decrease in the number of asymmetric synapses in the CA1 stratum radiatum of P25 GFPNR3A transgenics, which was of similar magnitude to the changes in spine density detected by Golgi (synapses per field: control, 11.5 ± 0.1; GFPNR3A transgenic, 9.7 ± 0.1, p = 0.00001, 17% decrease, Figure 5A, B). In addition, synapses were smaller in transgenic mice, as shown by a shift in the overall synapse distribution toward synapses with shorter postsynaptic densities (PSDs) and a significantly reduced mean PSD length (Figure 5C). By P35, the number of synapses in transgenic mice was comparable to controls but the decrease in PSD length was maintained (Figure 5D, E). Taken together with the preferential decrease noted in the proportion of mature mushroom-type spines, these data indicate an inhibitory effect of NR3A on synapse/spine growth. To determine if decreases in PSD length were reversible, we treated mice with doxycycline (10 days, from P26–P35, scheme in Figure 5). Doxycycline administration completely reversed the effect of GFPNR3A on PSD length (Figure 5E), indicating that alterations in synapse size resulted from acutely altered levels of NR3A protein and did not reflect a permanent developmental deficit caused by increased NR3A expression during an earlier time window.
Figure 5. GFPNR3A transgenic mice have fewer and smaller synapses.
(A, B) Electron microscopy analysis shows reduced synapse density in the stratum radiatum of hippocampal CA1 from P25 GFPNR3A transgenic mice. s, spine. (C) Left, mean PSD length was significantly reduced in P25 GFPNR3A transgenics. Right, cumulative distribution plots reveal a shift towards synapses with shorter PSDs in P25 transgenic mice. (D, E) Synapse numbers (D) but not PSD length (E) returned to control levels by P35. Doxycycline administration for 10 days (from P26–35, see scheme) reversed the changes in PSD length. No differences in synapse number or size were observed between doxycycline-treated and untreated control mice (* p < 0.001, ANOVA followed by Tukey’s test, n = 6–8 mice per group, 10 fields of each animal were randomly acquired at the level of the central stratum radiatum and analyzed in a blind fashion).
Synapse size has been shown to correlate with AMPAR number and synaptic strength in some studies (Nusser et al., 1998; Takumi et al., 1999). Although we observed reductions in the size of evoked AMPAR-mediated EPSCs (Figure S4B) and in the frequency of spontaneous AMPAR miniature EPSCs (control, 0.50 ± 0.06 Hz; GFPNR3A, 0.34 ± 0.02 Hz, p < 0.02, 32% decrease, Figure 6A, B), we did not detect changes in quantal amplitude (control, 13.83 ± 0.70 pA; GFPNR3A, 13.43 ± 0.45 pA, Figure 6A, C). Further, the relative contribution of AMPAR-mediated EPSCs at synapses that are maintained in GFPNR3A transgenic mice was unchanged, as shown by lack of detectable changes in NMDAR/AMPAR ratios estimated from spontaneously occurring miniature EPSCs or synaptically evoked responses (Figure 6D). Together with the anatomical measurements, these results are consistent with NR3A expression altering the number of functional synapses without affecting the AMPAR content of remaining synapses.
Figure 6. Altered NR3A expression decreases functional synapse number.
(A–C) mEPSCs from P25 GFPNR3A transgenic mice have decreased frequency but unchanged amplitude. (A) Representative traces of mEPSC recordings made at −80 mV in the presence of 0.2 mM TTX and 0.05 mM picrotoxin in neurons from control and GFPNR3A transgenic mice. The middle panel is the average of all mEPSCs from a representative experiment in control and transgenic mice. (B) Averaged data and cumulative probability histograms of mEPSC frequencies demonstrating a lower probability of spontaneous events in neurons from GFPNR3A transgenic mice (* p < 0.02, Student’s t-test). Grey trace indicates control data, black trace represents data from GFPNR3A transgenic experiments. (C) Averaged data and cumulative probability histograms of mEPSC amplitudes. (D) Ratio of NMDAR to AMPAR current does not differ between P25 control and GFPNR3A transgenics. Ratios were calculated from spontaneous synaptic events recorded at +40 mV before and after application of APV to estimate each component (D1), or from synaptically evoked currents measuring the AMPAR component at −80 mV and the NMDAR component at +40 mV at a time (indicated by dotted line) when the AMPAR component had largely decayed (D2).
The above results support the hypothesis that the presence of juvenile NMDARs containing NR3A subunits limits functional and structural plasticity, preventing naïve synapses from undergoing maturation and stabilization. When NR3A levels are elevated during developmental stages of robust pruning, this leads to increased loss of immature, relatively inactive spines (Figure S5A, left). Conversely, removal of NR3A at active synapses would be expected to allow enhanced plasticity and thereby enable synapse maturation and stabilization as well as synapse and spine growth (Figure S5A, right). If this is true, one could predict that NR3A should be normally absent from large synapses. To test this, we analyzed the synaptic distribution of endogenous NR3A in wild-type mice using immunogold electron microscopy. NR3A was indeed preferentially localized to small synapses (Figure S5B). Remarkably, there was a sharp decrease in the number of NR3A immunogold particles with increasing synapse size, while NR1 particle numbers were roughly constant across synapse sizes as shown in previous studies (Figure S5C, Petralia et al., 1999; Takumi et al., 1999).
If NR3A acts as a “molecular brake” to prevent premature synapse strengthening, a second prediction would be that earlier postnatal down-regulation of NR3A should result in stronger LTP and accelerated maturation of synapses. In wild-type mice, LTP becomes easier to induce and of larger magnitude in hippocampal CA1 between P6–P8 and P15, coinciding with the onset of NR3A down-regulation and removal mechanisms (Harris and Teyler, 1984; Muller et al., 1989, Figure S2). Experiments in NR3A knockout mice showed that LTP was easier to induce in young (P6–P8) mice when NR3A was absent (Figure 7A), while LTD was unchanged (Figure S6). Further, a quantitative biochemical analysis showed that the postsynaptic abundance of NR1 subunits was higher in P6–P8 NR3A knockout mice. Total NR1 expression was unchanged, suggesting that synaptic concentration and stabilization of NMDARs, one of the initial events driving synapse maturation, also occurs earlier in the absence of NR3A (Figure 7B). This correlated with an earlier enhancement of synaptic NMDAR currents in NR3A knockout mice that is in agreement with previous results (Figure 7C, Sasaki et al., 2002).
Figure 7. Genetic deletion of NR3A accelerates the developmental onset of LTP and synaptic maturation.
(A) LTP evoked by HFS and measured in the CA1 region of the hippocampus is enhanced in P6-8 NR3A knockout mice when compared to control wild-type mice (p < 0.05, Student’s t-test), while the magnitude of LTP at P14–P18 and in adults is similar between genotypes. (B) Immunoblot analyses of NMDAR subunit abundance show that NR1 levels are significantly increased in forebrain synaptic fractions from P6-8 NR3A knockout mice (* p < 0.01 vs wild-type, Student’s t-test, values within bars represent sample sizes). Protein expression data are averages of densitometric values (OD) relative to protein loads. Representative immunoblots are shown. PNS, postnuclear supernatant, SPM, synaptic plasma membrane, PSDI, postsynaptic fraction. (C) Input-output (I–O) curve demonstrating that the amplitude of synaptic NMDAR currents recorded at +40 mV is enhanced in neurons from P6-8 NR3A knockout mice. RMANOVA revealed a significant main effect of genotype (F(1, 15) = 6.65, p < 0.05).
GFPNR3A transgenic mice learn but display altered long-term memory storage
To determine whether changes in synaptic plasticity and structure resulting from prolonged NR3A expression led to behavioral impairments, we tested adult GFPNR3A transgenics on learning tasks known to require NMDAR activation. Spatial memory was evaluated using the Morris water maze, in which mice are trained to find a hidden platform by using visual cues (“acquisition”). Both control and GFPNR3A transgenics learned to swim to the hidden platform (Figure 8A–C). GFPNR3A transgenics were initially more successful than controls, but by day 4 swim distances and times were similar (Figure 8B, C). During probe trials in which the platform was removed from the pool, both control and transgenic mice spent more time in the target quadrant searching for the platform (days 3 and 6 of training, Figure 8E), confirming that they had learned the platform location. However, probe trials conducted 21 days after training showed that long-term memory storage was significantly weaker in GFPNR3A transgenics (day 27, p < 0.03 vs control, Figures 8E and S7).
Figure 8. Disrupted long-term storage of spatial memory in GFPNR3A transgenic mice.
(A) Diagram showing training protocols used for assessment of spatial memory in the Morris water maze; remote memory tests are indicated by vertical arrows. (B, C) Adult GFPNR3A transgenic mice display normal acquisition of spatial reference memory, as shown by declines in swim distances and times to locate the hidden platform, but their performance is poorer than controls after platform reversal. Remote memory testing was conducted 21 days after the initial 6 day training period (day 27), and then mice were retrained for 3 days to restore performance. Transgenic mice showed longer swim distances during retraining, but by day 30 both control and transgenic mice returned to day 6 levels. After changing the platform location from the NE to the SW quadrant, swim distances and times were longer for both genotypes on day 31 and 32 relative to day 6 or 30. By day 33, controls returned to levels observed on day 30, but GFPNR3A transgenics needed more days of training to achieve these levels. (D) Control and transgenic mice showed no differences in learning to swim to a visible platform. (E) Probe trials for acquisition given at the end of test days 3, 6, and 27. Dashed lines indicate chance levels (25%). Note that both control and transgenic mice learned the hidden platform location, but remote memory was significantly impaired in GFPNR3A transgenics. (F) Probe trials for reversal testing given at the end of test days 33 and 36; remote memory was assessed 21 days later (day 57). Both control and transgenic mice learned the new platform location, as shown by a preference for the SW compared to NE quadrants on day 36 (* p < 0.05 control vs transgenics, Bonferroni corrected pairwise comparisons, n = 10 mice/genotype).
To test their flexibility in handling spatial information, GFPNR3A transgenics were trained to learn a new platform location (“reversal”) after a 3-day retraining interval. Reversal learning was significantly slower than in controls (Figure 8B, C, F). Further, probe trials conducted another 21 days later revealed that long-term memory of the new platform location was extinguished in the transgenics (day 57, p < 0.005 vs control, Figures 8F and S7). No differences were observed in the visible platform version of the task (Figure 8D) or in swim velocities and distances (Table S2), indicating normal sensory-motor function and coordination.
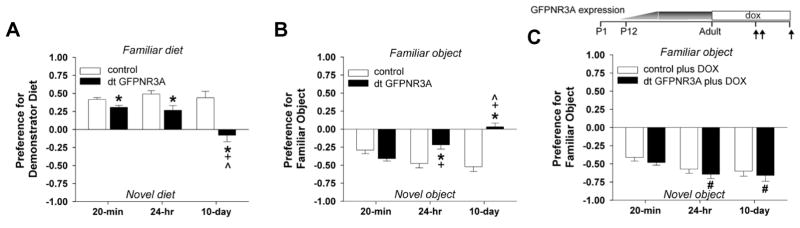
To evaluate the ability of GFPNR3A transgenics to form and store associative memories, we examined two types of non-spatial hippocampal-dependent tasks: social transmission of food preference and object recognition. Performance was measured at 20 min and after delays of 24 hrs and 10 days to assess both short-term and long-term memory. The social transmission of food preference task is based on the ability of rodents to develop a preference for foods that they smell on the breath of another individual. The task consisted of three stages: 1) a demonstrator mouse consumed a flavored diet; 2) tester mice were given 20 min to interact with the demonstrator; and 3) tester mice were given a choice to consume the familiar or a novel flavored diet. Although both genotypes preferred the familiar diet at 20 min and 24 hrs, GFPNR3A transgenics showed weaker preferences than controls (Figure 9A). By 10 days, the transgenics failed to display any preference whereas controls maintained a strong preference for the familiar diet (Figure 9A). There was a significant test day by genotype interaction (F(2,36) = 9.617, p < 0.001), reflecting poorer memory of GFPNR3A transgenics with longer retention delays. Differences could not be attributed to changes in feeding behavior because no genotype differences in latency to eat, bowl contacts and total amounts eaten were observed at 20 min or 24 hrs (Figure S8).
Figure 9. Deficits in long-term memory are reversible.
(A, B) Adult GFPNR3A transgenic mice exhibit anomalies in long-term memory in social transmission of food preference and object recognition tests. (A) Transgenic mice demonstrate reduced preferences for the familiar diet at 20 min and 24 hrs, and no preference for either the familiar or novel diet at 10 days relative to controls. Bonferroni post-hoc comparisons found significant differences between control and transgenic mice at 20 min (p < 0.036), 24 hrs (p < 0.006), and 10 days (p < 0.001). Control preference scores did not differ between test times, but transgenics showed a reduction in preference at 10 days compared to 20 min (p < 0.002) and 24 hrs (p < 0.001). (B) Transgenic mice display intact object recognition at 20 min but are impaired at 24 hrs and 10 days. Control preference scores did not differ between test times, whereas mutants showed a graded loss in preference over time. Hence, preferences for control and transgenic mice were similar at 20 min, but differed at 24 hrs (p < 0.016) and 10 days (p < 0.001). (C) Defects in long-term memory are reversible. Transgenic mice were raised off-doxycycline to allow GFPNR3A expression, and treated with doxycycline for 14 days before testing. Treatment was continued during the consolidation phase (see scheme, vertical arrows indicate test times). Note that adult transgenic mice display normal object recognition memory at all times following doxycycline treatment. Moreover, when compared to untreated transgenic mice, doxycycline-treated transgenics showed the same performance at 20 min, but enhanced memory for the novel object at 24 hrs and 10 days (n = 9–10 mice/genotype/treatment; *p < 0.05 from controls, +p < 0.05 from 20-min test, ^p < 0.05 from 24-hr test, #p < 0.05 from water-treated transgenics).
In the object recognition memory test, control mice displayed a strong preference for the novel object at all time points (Figure 9B). GFPNR3A transgenics also showed clear preference for the novel object at 20 min, but their ability to retain this preference had weakened at 24 hrs and was lost by 10 days (genotype by test day interaction, F(2,36) = 11.543, p < 0.001, Figure 9B). No genotype differences were noted for total object exploration time (20 min: control, 37.1 ± 3.5%, GFPNR3A, 39.4 ± 2.5%; 24 hrs: control, 42.6 ± 3.3%, GFPNR3A, 40.4 ± 2.5%; 10 days: control, 50.3 ± 2.5%, GFPNR3A, 48.9 ± 3.6%). Collectively, the behavioral data show that short-term memory processes appear intact in GFPNR3A transgenic mice, but their behavioral flexibility and ability to consolidate/retain the memories formed is severely impaired.
Defects in long-term memory are reversible
To evaluate if defects in long-term memory could be rescued, adult control and transgenic mice were treated with doxycycline (14 days) after allowing GFPNR3A expression at earlier stages, and examined in the novel object recognition task at 20 min, 24 hrs and 10 days. Both controls and doxycycline-treated GFPNR3A transgenic mice showed clear and statistically indistinguishable preferences for the novel object at all test times (Figure 9C). Comparison of the preference scores revealed that doxycycline-treated GFPNR3A transgenic mice had marked increases in preference for the novel object at both 24 hrs (p < 0.001) and 10 days (p < 0.001) relative to untreated transgenic mice. No differences were found between doxycycline-treated and untreated control animals. Similar conclusions based upon the reversibility seen for defects in LTP and synapse structure strongly indicate that memory deficits caused by prolonged expression of NR3A did not reflect permanent changes in neural circuitry. Moreover, they demonstrate that suppressing transgenic GFPNR3A expression is sufficient for the re-establishment of a functional neural network and recovery of learning and memory.
DISCUSSION
The aim of this study was to understand the functional importance of NR3A down-regulation in development by generating transgenic mice in which NR3A was persistently expressed in postnatal forebrain neurons. We found that prolonging NR3A expression prevents glutamatergic synapse maturation by limiting synapse potentiation and growth, and decreasing spine density, whereas knocking out endogenous NR3A conversely accelerates synaptic maturation events. Maturation deficits caused by altered NR3A expression were accompanied by major impairments in learning and memory processes. Importantly, however, both synaptic and cognitive deficits could be reversed at later stages, demonstrating that appropriate maturation of synapses can still be achieved if normal NR3A expression is restored. We propose that NR3A subunits place limits on the ability of synapses to potentiate and mature, and, due to their unique temporal pattern of expression, might act as a gatekeeper to ensure that the maturation of synapses is appropriately timed during postnatal brain development. Moreover, our results suggest that activity-dependent regulation of NR3A expression could serve as a switch to initiate selective synapse maturation by conferring the potential for localized, lasting changes in synaptic strength (Figure S5). These roles of NR3A are essential for the refinement and consolidation of neuronal networks.
NR3A: Regulator of synaptic maturation and pruning
Prolonged expression of NR3A subunits decreased the number and size of synaptic contacts, with persistent decreases in PSD length suggesting a dominant role of NR3A in limiting synaptic size. This notion is also supported by our observation that large synapses (PSD length > 250 nm) normally lack NR3A. Although the mechanism by which NR3A limits synapse size is currently unknown, one possibility is the reduced LTP at NR3A-expressing synapses. Stimuli that induce LTP are known to trigger spine growth and persistent PSD enlargement via activation of NMDARs (Engert and Bonhoeffer, 1999; Toni et al., 2001; Matsuzaki et al., 2004; Park et al., 2006), and lower Ca2+ influx through NR3-containing receptors likely decreases activity-dependent signaling and transcription, as well as cytoskeletal remodeling (West et al., 2002; Ackermann and Matus, 2003), two of the processes underlying the stabilizing effects of LTP on spine morphology. Consistent with this possibility, early removal of endogenous NR3A results in both robust LTP and enhanced stabilization and growth of excitatory synapses (this study and Das et al., 1998).
However, other genetic mutations of the NMDAR have been shown to modify LTP without altering synapse structure, suggesting dissociation between NMDAR-dependent plasticity and excitatory synapse maturation. For instance, CA1-specific NR1 knockout mice lack hippocampal LTP but have normal spine densities (Rampon et al., 2000; Tsien et al., 1996), and NR2B overexpression enhances LTP without changing spine density or size (Tang et al., 1999). Additionally, forebrain-specific NR1 deletion was reported to increase spine density in layer IV barrel neurons (Datwani et al., 2002, see also Adesnik et al., 2008), indicating that NMDAR activity limits, rather than promotes, synapse maturation. One key difference might be that selective prolonged expression or early removal of NR3A subunits alters the ability to induce LTP without modifying LTD, whereas previous NMDAR manipulations which eliminated NMDARs by targeting the NR1 locus also reduced or eliminated LTD, likely removing a critical mechanism for driving synapse pruning.
Alternatively, or concomitant with inhibiting LTP, NR3A may have a direct role in the destabilization and elimination of synapses because of its ability to mediate tonic, non-coincident calcium entry, one of the signals thought to contribute to synaptic pruning (Day et al., 2006), or through the recruitment via its intracellular domains (with limited homology to NR1 and NR2 subunits) of signaling complexes or actin regulators driving synapse disassembly. A number of intracellular binding partners of NR3A have already been identified. They include the catalytic subunit of protein phosphatase 2A, the endocytic adaptor and actin regulator PACSIN/syndapin 1, the scaffolding protein plectin, a cell cycle and apoptosis regulatory protein (CARP-1), and a regulator of G-protein signaling (GPS2/AMF1) (Chan and Sucher, 2001; Eriksson et al., 2007). Finally, the NR3A subunit can exert potent effects on receptor trafficking. For instance, NR3A can target NMDARs for endocytosis to facilitate synapse elimination (Perez-Otaño et al., 2006). In addition, NR3A-containing NMDARs are less firmly attached to PSDs than classical NMDARs (Perez-Otaño et al., 2006) and their diffusion properties may differ, keeping synapses in a labile, immature state (Figure S5). Impaired stabilization of NMDARs at postsynaptic sites as a result of persistent NR3A expression could contribute to the decrease in synaptic NMDAR currents and the displacement of NR2B from synapses observed in GFPNR3A transgenic mice, and would also explain why NMDARs concentrate earlier at NR3A knockout synapses.
Both the NMDAR and AMPAR component of synaptic currents were decreased in GFPNR3A transgenics, and NMDAR/AMPAR ratios indicated that the magnitude of these reductions was roughly proportional. Whereas one might initially have predicted that preventing developmental removal of NR3A might decrease this ratio—because NR1/NR2/NR3A channels with lower conductance would not be efficiently replaced by NR1/NR2 heteromers—, the lack of changes likely reflects decreases in AMPAR currents that could be the result of compensatory mechanisms, or of NR3-induced plasticity differences. For instance, prolonged NR3A expression might cause an increase in the fraction of NMDAR-only synapses; this is the expected outcome if maturation is partly achieved by adding AMPARs to NMDAR-only synapses (Durand et al. 1996). If so, any gains in the NMDAR component owing to an increase in NMDAR-only synapses could be offset by counteracting decreases due to the reduced conductance of NMDARs containing NR3. This scenario would be consistent with the trend towards a lower NMDA/AMPA ratio seen for spontaneous miniature events, the lack of a corresponding difference in the evoked ratio (Figure 6D), and the decrease in evoked AMPA responses, with no decrease in the amplitude of spontaneous AMPAR minis (Figure 6C). Proportional scaling could alternatively occur because AMPA and NMDA receptors tend to co-regulate in a homeostatic process (Watt et al., 2000). Further studies will be needed to distinguish between these two scenarios.
The transient nature of NR3A-induced changes in synapse density, but not in size, was unexpected. However, transient alterations in synapse/spine density have also been observed at similar stages of development in mouse models of fragile X and Rett syndromes (Nimchinsky et al., 2001; Chao et al., 2007). One possibility is that other cofactors that are permissive for spine pruning are also developmentally regulated. For example, sensory deprivation in visual cortex increases pruning mainly during the physiological critical period, reflecting a requirement for the proteolytic activity of tissue plasminogen activator (Mataga et al., 2004). Additionally or alternatively, the transient alteration in spine density could reflect a compensatory or homeostatic up-regulation of spine number triggered by prolonged NR3A expression; prior studies have shown that possibly relevant homeostatic mechanisms can be age-dependent and more pronounced in adult neurons (Kirov et al., 2004).
Cognitive development and structural plasticity
Cognitive development requires the remodeling and stabilization of synaptic connections in response to experience in order to support the acquisition and long-term storage of information. Pharmacological or genetic models based upon complete inhibition of NMDAR transmission disrupt both LTP and acquisition of spatial and associative memories (Morris, 1989; Rampon et al., 2000; Tsien et al., 1996). More selective manipulations such as genetic deletion or C-terminal truncation of NR2A subunits, which interfere with synaptic NMDAR stabilization as does prolonged GFPNR3A expression, reduce the magnitude of LTP to a lesser extent and impair flexible memory processing with, at most, subtle effects on acquisition (Sakimura et al., 1995; Steigerwald et al., 2000; Bannerman et al., 2008). This phenotype resembles behavioral abnormalities in GFPNR3A transgenics, which show normal to mildly impaired memory acquisition of spatial and associative tasks but are more severely impaired in tasks involving working memory (reversal learning).
Fewer studies support an involvement of NMDARs in long-term memory storage and their results have been controversial, in part because of difficulties in dissociating acquisition and consolidation. For instance, inducible genetic ablation of NR1 in CA1 pyramidal cells 1–7 days after training interferes with consolidation of spatial memories in the Morris water maze (Shimizu et al., 2000), but intra-ventricular AP5 infusion over the same time-scale does not (Morris, 1989). It has also been unclear whether NMDARs are required for the initial conversion of short- into long-term memories, a phase of memory consolidation which is completed in the hippocampus within a few hours of training, or for later phases of consolidation in the neocortex (Cui et al., 2004). Here we demonstrate that prolonged expression of NR3A-containing NMDARs dramatically impairs long-term memory in a variety of behavioral tasks assessing spatial or associative memory, and using single or repeated training events. Combined with the lack of short-term memory impairments, these results reveal a striking ability of NR3A subunits to disrupt memory consolidation. Further, the experiments using tasks which require brief training, such as object recognition or social transmission of food preference, showed that impairments in memory consolidation were already evident by 24 hours, suggesting that proper NMDAR transmission is required for the stabilization of initially fragile memory traces within the hippocampus.
However, because GFPNR3A expression was not restricted to the hippocampus, we cannot exclude contributions of inhibited synaptic stabilization in forebrain areas such as the prefrontal and anterior cingulate cortices, which express low levels of the transgene and participate in later phases of long-term memory storage (Frankland and Bontempi, 2005; Wiltgen et al., 2004). Such a contribution is also suggested by the observation that memories deteriorate over time, between 24 hours and 10 days, in GFPNR3A transgenics. In this context, our findings of altered PSD length and spine size/shape in GFPNR3A transgenics support a model whereby both early and late phases of memory consolidation are mediated by structural changes at postsynaptic sites (Lamprecht and LeDoux, 2004), and are consistent with studies linking long-term memory impairments with genetic manipulations of other molecules involved in structural plasticity such as PAK, a regulator of actin remodeling, or Arc/Arg 3.1 (Hayashi et al., 2004; Plath et al., 2006).
NR3A and diseases of development
Elevated NR3A levels have been reported in the brains of schizophrenic subjects, and our animal model shows that impaired NR3A expression results in an imbalance between synapse maturation and elimination, reproducing synaptic and cognitive problems typical of schizophrenia, such as defects in working memory, behavioral flexibility, and memory consolidation (Frantseva et al., 2007; Garey et al., 1998). In addition to schizophrenia, a number of postnatal developmental disorders are associated with immature spine formation, and we now provide evidence that an immature synaptic state can lead to profound cognitive deficits. Thus, transgenic mice with persistent NR3A expression might provide a “synaptic” model for schizophrenia and other cognitive disorders useful for exploring therapies based on NR3A regulation to overcome NMDAR dysfunction at the synapse. A key question surrounding therapeutic strategies to treat diseases of the synapse is whether abnormal synaptic phenotypes arising in development can be reversed in adulthood. Our study, along with other recent work (Guy et al., 2007; Ehninger et al., 2008), shows that restoring normal protein expression at either juvenile or adult ages results in robust phenotypic reversal. These findings demonstrate a remarkable capacity for adult plasticity at the level of the synapse and raise hopes that late pharmacological or genetic interventions can normalize behaviors in disorders of the synapse.
Supplementary Material
Acknowledgments
We thank Ana Fernández-Militino for advice on statistical analysis, Nobuki Nakanishi and Stuart Lipton for kindly providing the NR3A knockout mice, Jim Trimmer for the NR3A antibody, Jiechun Zhou, Aitor Zandueta, María José Lorente and Cristina Rodríguez-Viña for excellent technical help, Mike Ehlers, Tom Blanpied, Angel Barco, Jose Esteban, and Rafa Fernández-Chacón for critical readings of this manuscript. Work was supported by NARSAD, Marie Curie International Program, and UTE project CIMA (to I.P.O.), Spanish Ministry of Education and Science (SAF2006-10025, CSD2008-00005 to I.P.O., BFU2006-01896 to R.L.), Helen Lyng White, a NICHD training grant T32-HD40127 and NARSAD Fellowships (to A.C.R.), NIH and NARSAD (to B.D.P.), National Institute of Neurological Disorders and Stroke (to D.C.L.) and unrestricted funds (W.C.W.).
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann M, Matus A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat Neurosci. 2003;6:1194–1200. doi: 10.1038/nn1135. [DOI] [PubMed] [Google Scholar]
- Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA. NMDA receptors inhibit synapse unsilencing during brain development. Proc Natl Acad Sci USA. 2008;105:5597–5602. doi: 10.1073/pnas.0800946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hallaq RA, Jarabek BR, Fu Z, Vicini S, Wolfe BB, Yasuda RP. Association of NR3A with the N-methyl-D-aspartate receptor NR1 and NR2 subunits. Mol Pharmacol. 2002;62:1119–1127. doi: 10.1124/mol.62.5.1119. [DOI] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Niewoehner B, Lyon L, Romberg C, Schmitt WB, Taylor A, Sanderson DJ, Cottam J, Sprengel R, Seeburg PH, et al. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J Neurosci. 2008;28:3623–3630. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Cui Z, Wang H, Tan Y, Zaia KA, Zhang S, Tsien JZ. Inducible and reversible NR1 knockout reveals crucial role of the NMDA receptor in preserving remote memories in the brain. Neuron. 2004;41:781–793. doi: 10.1016/s0896-6273(04)00072-8. [DOI] [PubMed] [Google Scholar]
- Chan SF, Sucher NJ. An NMDA receptor signaling complex with protein phosphatase 2A. J Neurosci. 2001;21:7985–7992. doi: 10.1523/JNEUROSCI.21-20-07985.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- Datwani A, Iwasato T, Itohara S, Erzurumlu RS. NMDA receptor-dependent pattern transfer from afferents to postsynaptic cells and dendritic differentiation in the barrel cortex. Mol Cell Neurosci. 2002;21:477–492. doi: 10.1006/mcne.2002.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–5. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Li W, Fox K, Stryker MP, Silva AJ. Reversing neurodevelopmental disorders in adults. Neuron. 2008;60:950–960. doi: 10.1016/j.neuron.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Nilsson A, Samuelsson H, Samuelsson EB, Mo L, Akesson E, Benedikz E, Sundstrom E. On the role of NR3A in human NMDA receptors. Physiol Behav. 2007;92:54–59. doi: 10.1016/j.physbeh.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Lerma J, Laruelle M. The synaptic hypothesis of schizophrenia. Neuron. 2003;39:205–216. doi: 10.1016/s0896-6273(03)00423-9. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Fitzgerald PB, Chen R, Moller B, Daigle M, Daskalakis ZJ. Evidence for Impaired Long-Term Potentiation in Schizophrenia and Its Relationship to Motor Skill Leaning. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm151. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Gotz T, Kalus P, Bajbouj M, Sander T, Winterer G. Genetic variations of the NR3A subunit of the NMDA receptor modulate prefrontal cerebral activity in humans. J Cogn Neurosci. 2007;19:59–68. doi: 10.1162/jocn.2007.19.1.59. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Teyler TJ. Developmental onset of long-term potentiation in area CA1 of the rat hippocampus. J Physiol. 1984;346:27–48. doi: 10.1113/jphysiol.1984.sp015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi ML, Choi SY, Rao BS, Jung HY, Lee HK, Zhang D, Chattarji S, Kirkwood A, Tonegawa S. Altered cortical synaptic morphology and impaired memory consolidation in forebrain- specific dominant-negative PAK transgenic mice. Neuron. 2004;42:773–787. doi: 10.1016/j.neuron.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. A quantitative description of NMDA receptor-channel kinetic behavior. J Neurosci. 1990;10:1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov SA, Goddard CA, Harris KM. Age-dependence in the homeostatic upregulation of hippocampal dendritic spine number during blocked synaptic transmission. Neuropharmacology. 2004;47:640–648. doi: 10.1016/j.neuropharm.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Mataga N, Mizuguchi Y, Hensch TK. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44:1031–1041. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller HT, Meador-Woodruff JH. NR3A NMDA receptor subunit mRNA expression in schizophrenia, depression and bipolar disorder. Schizophr Res. 2004;71:361–370. doi: 10.1016/j.schres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Muller D, Oliver M, Lynch G. Developmental changes in synaptic properties in hippocampus of neonatal rats. Brain Res Dev Brain Res. 1989;49:105–114. doi: 10.1016/0165-3806(89)90063-1. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Tong G, Jahr CE. A false transmitter at excitatory synapses. Neuron. 1993;11:85–91. doi: 10.1016/0896-6273(93)90273-t. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otaño I, Ehlers MD. Learning from NMDA receptor trafficking: clues to the development and maturation of glutamatergic synapses. Neurosignals. 2004;13:175–189. doi: 10.1159/000077524. [DOI] [PubMed] [Google Scholar]
- Perez-Otaño I, Lujan R, Tavalin SJ, Plomann M, Modregger J, Liu XB, Jones EG, Heinemann SF, Lo DC, Ehlers MD. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci. 2006;9:611–621. doi: 10.1038/nn1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otaño I, Schulteis CT, Contractor A, Lipton SA, Trimmer JS, Sucher NJ, Heinemann SF. Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J Neurosci. 2001;21:1228–1237. doi: 10.1523/JNEUROSCI.21-04-01228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Sasaki YF, Rothe T, Premkumar LS, Das S, Cui J, Talantova MV, Wong HK, Gong X, Chan SF, Zhang D, et al. Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cortical neurons. J Neurophysiol. 2002;87:2052–2063. doi: 10.1152/jn.00531.2001. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tong G, Takahashi H, Tu S, Shin Y, Talantova M, Zago W, Xia P, Nie Z, Goetz T, Zhang D, et al. Modulation of NMDA receptor properties and synaptic transmission by the NR3A subunit in mouse hippocampal and cerebrocortical neurons. J Neurophysiol. 2008;99:122–132. doi: 10.1152/jn.01044.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Povilaitite P, Parisi L, Muller D. Remodeling of synaptic membranes after induction of long-term potentiation. J Neurosci. 2001;21:6245–6251. doi: 10.1523/JNEUROSCI.21-16-06245.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26:659–70. doi: 10.1016/s0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron. 2004;44:101–108. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Wong HK, Liu XB, Matos MF, Chan SF, Perez-Otaño I, Boysen M, Cui J, Nakanishi N, Trimmer JS, Jones EG, et al. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J Comp Neurol. 2002;450:303–317. doi: 10.1002/cne.10314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.