Abstract
Tumors are highly complex tissues composed of neoplastic cells and different kinds of stromal cells. Tumor stromal cells, especially fibroblasts, play important roles during the multistep development of tumors. In this review, the two-faced characteristics of tumor stromal fibroblasts are discussed in the light of our current knowledge. For one thing, fibroblasts act as an “inflammation regulator” by secretion of cytokines and regulation of tumor immunity; for another, they act as a “damage healer” for cure of wounds by remodeling extracellular matrix or taking a part in the “foreign body reaction”. Since the properties of fibroblasts are complicated, both aspects of fibroblasts for tumor development should be considered carefully in clinical studies to target cancer-associated fibroblasts.
Keywords: Tumor stroma, Fibroblasts, Inflammation, Tissue damage
Introduction
The solid tumors are heterogeneous with a reactive stroma accompanying cancer cells. Similar to the stromal cells in normal epithelial tissue, the tumor stromal cells include a variety of cell types, including immune cells such as macrophages, granulocytes, and T cells, as well as endothelial cells and fibroblasts [1–3].
Fibroblasts are a type of cells that synthesizes the extracellular matrix (ECM), functions as an important regulator in inflammation, and also plays critical roles in wound healing [4–8]. As tumors are recognized as “wounds that do not heal” [9], it is possible that fibroblasts are activated and play an important role also during tumor development. The notion that fibroblasts within the tumor stroma acquire an activated phenotype, similar to fibroblasts in wound healing, has been studied since the 1970s [10–12].
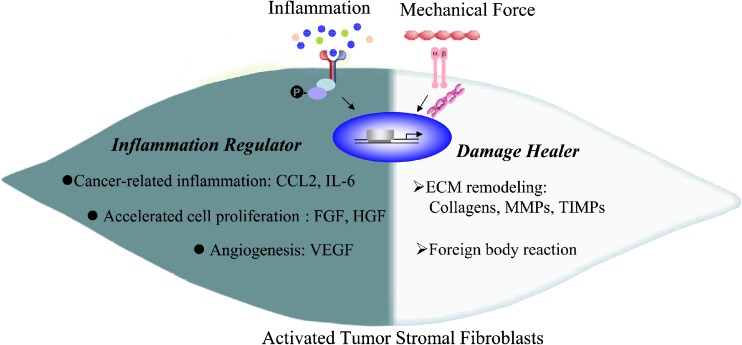
In this review, the behaviors of cancer-associated fibroblasts (CAFs) are artificially divided into two parts: as an “inflammation regulator” by secretion of cytokines, which promote cancer-associated inflammation, facilitate premalignant cell proliferation and contribute to angiogenesis [1, 2, 13]; as a “damage healer” for cure of wounds, which contain the capability of remodeling ECM, producing collagen or collagenase [14–16] (Fig. 1).
Fig. 1.
Multifaceted tumor stromal fibroblasts. In tumor microenvironment, fibroblasts are activated by the inflammatory cytokines and mechanical forces. The two-faced characteristics of activated fibroblasts in tumor are shown. For one side, activated fibroblasts act as an “inflammation regulator” by secretion of cytokines, e.g. CCL-2 and IL-6 to promote cancer-related inflammation, FGF and HGF to accelerate premalignant cell proliferation and VEGF to promote angiogenesis. For another, activated fibroblasts act as a “damage healer” who is responsible for ECM remodeling and foreign body reaction
Activation of Fibroblasts in Tumor Stroma
Activated fibroblasts express α-smooth-muscle actin and have more outstretched appearance, leading to the term ‘myofibroblasts’, are found in healing wounds, and are also associated with cancers [16]. Activated fibroblasts get the abilities of proliferation, migration, remodeling ECM and secreting cytokines. But how do they perceive the injury and get activated? One mechanism is that the injury causes inflammation [17], and various of inflammatory cytokines produced during this process will stimulate fibroblasts to a reactive status. Another is the mechanical stimulus, which is driven by the damage of tissue integrity, namely broken of intercellular adhesions, cell contractility, and forces generated within the microenvironment [18]. When the tissue integrity is impaired by injury, fibroblasts will sense the change of the mechanical force and be activated via integrins, which are specialized focal adhesion sites and physically link the ECM to the cytoskeleton [19–21] (Fig. 1).
Inflammation and Fibroblast Activation
Chronic inflammation is a hallmark of cancer [22], a lot of factors in cancer-associated inflammation are reported to induce the activation of fibroblasts. Among them transforming growth factor beta (TGF-β) and platelet-derived growth factor (PDGF) are the most well investigated ones to activate fibroblasts. TGF-β has been reported to be capable of inducing fibroblasts to differentiate into activated smooth-muscle reactive fibroblasts, termed myofibroblasts; while PDGF is in favor of the proliferation of myofibroblasts [2, 23, 24]. Erez et al. have found that Interleukin (IL)-1 treatment on fibroblasts activated the nuclear factor (NF)-κB signaling pathway and induced the expression of pro-inflammatory genes [25]. Similarly, hepatic stellate cells (HSCs), the main kind of fibroblasts in liver, were found sensitive to LPS derived from the intestinal tract, which resulted in the increased activation and survival rate of HSCs, and contributed to liver fibrosis and hepatocellular carcinoma [26, 27]. Surprisingly, cytokine signaling through JAK kinases is involved in generating actomyosin contractility in CAFs [28]. Except for cytokines, mitochondrial oxidative stress can also facilitate cancer-associated fibroblasts to produce lactate and promote the tumor metabolism [29, 30].
Mechanical Forces and Fibroblast Activation
It was previously reported that primary cultured fibroblasts on petri dishes were activated “automatically” [31], and this phenomenon implies mechanical stimuli may acting as a driving force for myofibroblast differentiation. Recent studies have shown that mechanical force induces latent TGF-β activation and myofibroblast differentiation [20]. Fibroblasts were also reported to secrete higher levels of tumor necrosis factor (TNF)-α in response to compression [19]. Integrin–mediated activation of fibroblasts triggered by mechanical stress is also viewed as the major mechanism by several groups [20, 32]. It has been clearly reported that stiffened ECM, accompanied by collagen cross linking, facilitate epithelium invasion by promoting its focal adhesions and enhanced PI3 kinase (PI3K) activity [33]. However, it is still elusive about the exact mechanism for mechanically induced fibroblast activation in tumor microenvironment.
Stromal Fibroblasts in Tumor Initiation and Promotion
Many studies have demonstrated a direct involvement of activated fibroblasts in tumor initiation and promotion. By employing FSP-Cre; TGF-βRIIflox/flox mice, Bhowmick et al. have found that fibroblasts participated in tumor initiation by modulates the oncogenic potential of adjacent epithelia [34]. The loss of TGF-β responsiveness in fibroblasts resulted in intraepithelial neoplasia in prostate and invasive squamous cell carcinoma of the forestomach in these mice, both associated with an increased abundance of stromal cells. The results show that TGF-β signaling in fibroblasts modulates the growth and oncogenic potential of adjacent epithelia in selected tissues. Other groups also found that genetic inactivation of Pten in stromal fibroblasts accelerated the initiation of mammary epithelial tumors [35, 36]. The characters of fibroblasts during tumor initiation and promotion are quite complicated and we will make some classifications in the following context, hoping this will be helpful to our understanding of the nature of this elusive stromal cell.
As an Inflammation Regulator
Fibroblasts are proved to promote tumor initiation, which relies on facilitating tumor-associated chronic inflammation. Our previous work has demonstrated that fibroblasts promoted skin carcinogenesis via maintaining chemokine (C-C motif) ligand (CCL)-2-mediated macrophage infiltration and chronic inflammation. The inflammatory reaction was impaired after the selective depletion of proliferating fibroblasts in the skin of FSP-TK transgenic mice. This led to reduced local CCL-2 concentration and impaired macrophage infiltration. As a result of down-regulated inflammation, the incidence of skin tumor was reduced [37]. In a spontaneous tumor model of K14-HPV16 mice, the inflammatory signature was already activated in CAFs isolated from the initial hyperplastic stage in multistep skin tumorigenesis. CAFs from this pathway promoted macrophage recruitment, neovascularization, and tumor growth in a NF-κB signaling-dependent way [38]. It was also reported that overexpression of TGF-β and/or hepatocyte growth factor in mouse fibroblasts induced the initiation of breast cancer within the normal human epithelium [39].
CAFs helped tumor cells to escape from immune-mediated rejection by recruiting myeloid-derived suppressor cells (MDSCs), T regulatory cells (Tregs) and promoting Th2 polarization of the tumor microenvironment [40]. Haniffa et al. showed that IFN-γ–stimulated fibroblasts can also suppress allogeneic T cell proliferation by upregulated indoleamine 2,3-dioxygenase and accelerated tryptophan metabolism in fibroblasts. Kraman et al. found that ablation of fibroblasts in the established tumor aroused adaptive tumor immunity involving IFN-γ and TNF-α [41]. These indicate that fibroblasts may have the immunosuppressive properties [42].
As a Damage Healer
Lots of tumor inducing agents, such as microorganisms or many kinds of carcinogens as well as growing tumors will produce tissue damage. Back to 80 years ago, G. Friedrich [43] and R. Rössle [44] found in a series of patients (frequently smokers) that lung carcinomas grew at sites of scars. They suggested that scars predisposed to cancer be termed Narbenkrebs (scar cancer). Scars within the carcinomas had an immature phenotype (increased collagen type 3 content) indicative for an early stage of fibrotic process, whereas scars at some distance from the neoplasm revealed a mature, late stage of the fibrotic process (decreased type 3 and increased type 1 and 4 collagen) [45].
Polycyclic aromatic hydrocarbons (PAH) are a group of environmental pollutants some of which (e.g. Benzo(a)pyrene) have been shown to cause human cancers [46]. Methylcholanthrene (MCA), another PAH molecule, has been widely used in mice to study chemical induced carcinogenesis [47–49]. Injection of MCA/oil induced a series of local reactions against the carcinogen emulsion [50], including the infiltration of inflammatory cells, the recruitment and proliferation of fibroblasts and finally the encapsulation of MCA by ECM which is called “foreign body reaction” [51]. The “foreign body reaction” is characterized by encapsulation of foreign materials. It is phylogenetically one of the oldest defense mechanisms predating adaptive immunity, a major protective mechanism in invertebrates and usually observed as a pathological reaction in humans [14]. Further investigation shows that treatment with collagenase led to destruction of the MCA encapsulation and then a rapid tumor development in the long term “tumor free” mice. Fibroblasts protected epithelial cells from DNA damage, epithelial malignancy occurred in the absence of local activating fibroblasts (unpublished data). Besides chemical carcinogen, it appears that fibroblasts-derived fibrotic capsule is also able to enclose neoplasm. In clinical cases, mainly in hepatocellular carcinomas and mammary carcinomas, the presence of a capsule around neoplastic cells is recognized as an indication of good prognosis [52–54]. Encapsulated tumors have low growth rate, or none at all. Once the capsule is disrupted, growth of tumor resumes [55, 56].
Our results indicate that inflammation and scarring, both suspected to contribute to malignancy, prevent cancer in certain situations [14]. Whether scar cancer results from inefficient encapsulation of carcinogen is not yet known, however, benzo(a)pyrene was detected in substantial amounts in lung tissues of smokers [45, 57] and former smokers retain a substantial risk of developing lung cancer [58].
Stromal Fibroblasts in Tumor Progression
Co-injection of CAFs with tumor cells has already well demonstrated the tumor-promoting potential of fibroblasts nearly 20 years ago [59], and the refined mechanism of fibroblasts influence on tumor growth, angiogenesis, and metastasis has recently been investigated more intensively [60–62]. As reviewing the CAF-associated proteins which were reported to influence the tumor development in the past 10 years, we found most of them could be divided into two parts: immune-derived, e.g. chemokine (C-X-C motif) ligand (CXCL)-14 [63], CXCL-12 [62], IL-1 [64], and IL-6 [65, 66], and conventional activated fibroblast-derived, e.g. hyaluronidases [67, 68] and matrix metalloproteinase (MMPs). So, in this section of tumor progression, we will also discuss the fibroblasts in two parts which mentioned above.
As an Inflammation Regulator
CAFs promote tumor progression through producing a cancer cell-favorable inflammatory microenvironment. Fibroblast-derived cytokines such as IL-1 and CXCL-14 also have been shown to play vital roles as immune modulators [63, 64]. CAF-derived CXCL-12 was shown to be responsible for recruiting endothelial progenitor cells to breast tumors, which stimulated tumor blood vessel growth [62]. Vascular endothelial growth factor (VEGF) has been reported as an important tumor angiogenesis factor in many studies, which can be secreted by tumor cells, macrophages, mast cells and fibroblasts [69]. Studies by Fukumura et al. have shown that VEGF promoter activity is high in stromal fibroblasts in the transplant and spontaneous mammary tumor models [70], indicating that fibroblasts may be the major producer of VEGF and therefore be crucial for tumor angiogenesis in specific tumor models. Consistent with that, our study also shows that stromal fibroblasts express VEGF at both the RNA and protein levels. Further studies showed that fibroblasts promoted tumor growth when coinjected with tumor cells. However, the presence of IFN-γ, well known to promote immunologically induced rejection of transplanted tumor cells [71], significantly impaired the ability of fibroblasts to promote tumor growth due to a reduced angiogenesis. The mechanism relied mainly on the IFN-γ-mediated down-regulation of VEGF production by fibroblasts [13]. Importantly, PDGF-C from fibroblast may rescue angiogenesis in some anti-VEGF resistant tumors [72]. Several factors secreted by fibroblasts, such as epidermal growth factor, hepatocyte growth factor, insulin-like growth factor and IL-6, can stimulate cancer cell proliferation, maintain their survival and enhance their invasive properties [24, 65].
As a Damage Healer
Fibroblasts decorate the tumor microenvironment through direct cell-to-cell contacts and secretion of MMPs, tissue inhibitors of metalloproteinase (TIMPs), and ECM components, such as collagen and hyaluronan [73]. It was reported that tumor cells can get signals from the former enzymes and their targets, be stimulated to get epithelial–mesenchymal transition, migrate along tracks made of ECM collagen fibers, and then cross tissue boundaries and escape the primary tumor site, and finally achieve the metastasis with the help of fibroblasts to creating a niche [28, 74–78]. Actually, CAF derived MMP-1, 2, 3, 9, 11, 13, 14, 19, ADAMT-5 and TIMP-1, 2, 3 are reported involving in angiogenesis and the proliferation, adhesion, migration, differentiation and apoptosis of tumor cells or stromal cells [75].
The biomechanical remodeling of the microenvironment by fibroblasts is also important for the progression of tumor. Goetz et al. found Caveolin-1 positive fibroblasts are enriched in stroma of human breast, kidney and colon carcinoma and melanoma metastasis. Due to decreased CAF contractility, Caveolin-1-deficient mice got disorganized stromal tissue architecture [18]. It was also reported that cytokine signaling through the receptor subunit GP130-IL6ST generated contractile force in stromal fibroblasts to remodel the ECM and promote tumor invasion [28]. Similar to “foreign body reaction”, fibroblasts repressed the early stages of tumor progression by facilitating the formation of gap junctions between activated fibroblasts and thereby exerting contact inhibition on cancer cells [79, 80].
Further Discussion
Though the role of tumor stromal fibroblasts has been divided into two parts in this review artificially, to some extent, usually both sides of the dual characters of fibroblasts could influence each other and contribute to the final outcome of tumor in one given stroma. As discussed above, the “foreign body reaction” towards MCA is dependent on IFN-γ, a pleotropic inflammatory cytokine [14]. For another example, ECM degradation will promote release of growth factors bound to the ECM [81].
It should be noticed that the specific roles of fibroblasts are microenvironment-dependent. It has been reported that normal human fibroblasts at different locations in the body should be considered as distinct differentiated cell types [82], which indicates the existence of different subsets of CAFs in different tumors. But whether different subsets of fibroblasts exist in one specific tumor type is still largely unknown. In conclusion, the role of fibroblasts during tumor development is doubtless but intricate. And many studies have shown their therapeutic opportunities by targeting CAF-derived pro-tumorigenic factors or interfering with the recruitment of CAFs [3, 72, 83, 84]. However, the ideal therapeutic methods should take account of the two-faced characteristics of fibroblasts in tumor stroma and remove cancer-promoting properties of fibroblasts but retain cancer-resistant ones.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81030049 and 31071261), Ministry of Science and Technology of China (2012CB917103). And we thank Jingjing Deng and Chen Ni for critical reading and helpful discussion.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 2.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 3.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/S1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 6.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 7.Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, Salmon M, Buckley CD. A stromal address code defined by fibroblasts. Trends Immunol. 2005;26:150–156. doi: 10.1016/j.it.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 9.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 10.Ryan GB, Cliff WJ, Gabbiani G, Irle C, Statkov PR, Majno G. Myofibroblasts in an avascular fibrous tissue. Lab Invest. 1973;29:197–206. [PubMed] [Google Scholar]
- 11.Durning P, Schor SL, Sellwood RA. Fibroblasts from patients with breast cancer show abnormal migratory behaviour in vitro. Lancet. 1984;2:890–892. doi: 10.1016/S0140-6736(84)90653-6. [DOI] [PubMed] [Google Scholar]
- 12.Tsukada T, McNutt MA, Ross R, Gown AM. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol. 1987;127:389–402. [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Yang W, Qin C, Zhang L, Deng J, Liu S, Qin Z. Responsiveness of stromal fibroblasts to IFN-gamma blocks tumor growth via angiostasis. J Immunol. 2009;183:6413–6421. doi: 10.4049/jimmunol.0901073. [DOI] [PubMed] [Google Scholar]
- 14.Qin Z, Kim HJ, Hemme J, Blankenstein T. Inhibition of methylcholanthrene-induced carcinogenesis by an interferon gamma receptor-dependent foreign body reaction. J Exp Med. 2002;195:1479–1490. doi: 10.1084/jem.20011887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worthley DL, Giraud AS, Wang TC. Stromal fibroblasts in digestive cancer. Cancer Microenviron. 2010;3:117–125. doi: 10.1007/s12307-009-0033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 17.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Goetz JG, Minguet S, Navarro-Lerida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibanez T, Pellinen T, Echarri A, Cerezo A, Klein-Szanto AJ, Garcia R, Keely PJ, Sanchez-Mateos P, Cukierman E, Pozo MA. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kook SH, Jang YS, Lee JC. Human periodontal ligament fibroblasts stimulate osteoclastogenesis in response to compression force through TNF-alpha-mediated activation of CD4+ T cells. J Cell Biochem. 2011;112:2891–2901. doi: 10.1002/jcb.23205. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Hagood JS, Lu B, Merryman WD, Murphy-Ullrich JE. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J Biol Chem. 2010;285:22382–22393. doi: 10.1074/jbc.M110.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci. 2006;119:508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007;35:661–664. doi: 10.1042/BST0350661. [DOI] [PubMed] [Google Scholar]
- 24.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-Dependent manner. Cancer Cell. 2009;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 26.Seki E, Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 27.Luedde T, Schwabe RF. NF-kappaB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanz-Moreno V, Gaggioli C, Yeo M, Albrengues J, Wallberg F, Viros A, Hooper S, Mitter R, Feral CC, Cook M, Larkin J, Marais R, Meneguzzi G, Sahai E, Marshall CJ. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell. 2011;20:229–245. doi: 10.1016/j.ccr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Taddei ML, Giannoni E, Raugei G, Scacco S, Sardanelli AM, Papa S, Chiarugi P. Mitochondrial oxidative stress due to complex I dysfunction promotes fibroblast activation and melanoma cell invasiveness. J Signal Transduct. 2011;2012:684592. doi: 10.1155/2012/684592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balliet RM, Capparelli C, Guido C, Pestell TG, Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Chiavarina B, Pestell RG, Howell A, Sotgia F, Lisanti MP. Mitochondrial oxidative stress in cancer-associated fibroblasts drives lactate production, promoting breast cancer tumor growth: understanding the aging and cancer connection. Cell Cycle. 2011;10:4065–4073. doi: 10.4161/cc.10.23.18254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong WI, Park O, Radaeva S, Gao B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44:1441–1451. doi: 10.1002/hep.21419. [DOI] [PubMed] [Google Scholar]
- 32.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 35.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, Naidu S, Wei G, Sharma SM, Stephens JA, Fernandez SA, Gurcan MN, Weinstein MB, Barsky SH, Yee L, Rosol TJ, Stromberg PC, Robinson ML, Pepin F, Hallett M, Park M, Ostrowski MC, Leone G. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bronisz A, Godlewski J, Wallace JA, Merchant AS, Nowicki MO, Mathsyaraja H, Srinivasan R, Trimboli AJ, Martin CK, Li F, Yu L, Fernandez SA, Pecot T, Rosol TJ, Cory S, Hallett M, Park M, Piper MG, Marsh CB, Yee LD, Jimenez RE, Nuovo G, Lawler SE, Chiocca EA, Leone G, Ostrowski MC. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol. 2011;14:159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Chen L, Xiao M, Wang C, Qin Z. FSP1+ fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am J Pathol. 2011;178:382–390. doi: 10.1016/j.ajpath.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-Dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 39.Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4 T1 murine breast cancer model. PLoS One. 2009;4:e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 42.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 43.Friedrich G. Periphere Lungenkrebse auf dem Boden pleuranaher Narben. Virchows Arch Pathol Anat. 1939;304:230–247. doi: 10.1007/BF02595199. [DOI] [Google Scholar]
- 44.Rössle R. Die Narbenkrebse der Lungen. Schweiz Med Wochenschr. 1943;39:1200–1203. [Google Scholar]
- 45.Madri JA, Carter D. Scar cancers of the lung: origin and significance. Hum Pathol. 1984;15:625–631. doi: 10.1016/S0046-8177(84)80286-5. [DOI] [PubMed] [Google Scholar]
- 46.Baird WM, Hooven LA, Mahadevan B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ Mol Mutagen. 2005;45:106–114. doi: 10.1002/em.20095. [DOI] [PubMed] [Google Scholar]
- 47.Noguchi Y, Jungbluth A, Richards EC, Old LJ. Effect of interleukin 12 on tumor induction by 3-methylcholanthrene. Proc Natl Acad Sci U S A. 1996;93:11798–11801. doi: 10.1073/pnas.93.21.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 49.Qin Z, Blankenstein T. A cancer immunosurveillance controversy. Nat Immunol. 2004;5:3–4. doi: 10.1038/ni0104-3. [DOI] [PubMed] [Google Scholar]
- 50.Hu WJ, Eaton JW, Ugarova TP, Tang L. Molecular basis of biomaterial-mediated foreign body reactions. Blood. 2001;98:1231–1238. doi: 10.1182/blood.V98.4.1231. [DOI] [PubMed] [Google Scholar]
- 51.Zmener O. Tissue response to a new methacrylate-based root canal sealer: preliminary observations in the subcutaneous connective tissue of rats. J Endod. 2004;30:348–351. doi: 10.1097/00004770-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Ooi LP, Crawford DH, Gotley DC, Clouston AD, Strong RW, Gobe GC, Halliday JW, Bridle KR, Ramm GA. Evidence that “myofibroblast-like” cells are the cellular source of capsular collagen in hepatocellular carcinoma. J Hepatol. 1997;26:798–807. doi: 10.1016/S0168-8278(97)80245-0. [DOI] [PubMed] [Google Scholar]
- 53.Bridle KR, Crawford DH, Powell LW, Ramm GA. Role of myofibroblasts in tumour encapsulation of hepatocellular carcinoma in haemochromatosis. Liver. 2001;21:96–104. doi: 10.1034/j.1600-0676.2001.021002096.x. [DOI] [PubMed] [Google Scholar]
- 54.Ng IO, Lai EC, Ng MM, Fan ST. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer. 1992;70:45–49. doi: 10.1002/1097-0142(19920701)70:1<45::AID-CNCR2820700108>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 55.Vaage J. Fibrosis in immune control of mammary-tumor growth. Int J Cancer. 1992;51:325–328. doi: 10.1002/ijc.2910510225. [DOI] [PubMed] [Google Scholar]
- 56.Barsky SH, Gopalakrishna R. Increased invasion and spontaneous metastasis of BL6 melanoma with inhibition of the desmoplastic response in C57 BL/6 mice. Cancer Res. 1987;47:1663–1667. [PubMed] [Google Scholar]
- 57.Seto H, Ohkubo T, Kanoh T, Koike M, Nakamura K, Kawahara Y. Determination of polycyclic aromatic hydrocarbons in the lung. Arch Environ Contam Toxicol. 1993;24:498–503. doi: 10.1007/BF01146169. [DOI] [PubMed] [Google Scholar]
- 58.Enstrom JE, Heath CW., Jr Smoking cessation and mortality trends among 118,000 Californians, 1960–1997. Epidemiology. 1999;10:500–512. doi: 10.1097/00001648-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Dimanche-Boitrel MT, Vakaet L, Jr, Pujuguet P, Chauffert B, Martin MS, Hammann A, Roy F, Mareel M, Martin F. In vivo and in vitro invasiveness of a rat colon-cancer cell line maintaining E-cadherin expression: an enhancing role of tumor-associated myofibroblasts. Int J Cancer. 1994;56:512–521. doi: 10.1002/ijc.2910560410. [DOI] [PubMed] [Google Scholar]
- 60.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 62.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 63.Augsten M, Hagglof C, Olsson E, Stolz C, Tsagozis P, Levchenko T, Frederick MJ, Borg A, Micke P, Egevad L, Ostman A. CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth. Proc Natl Acad Sci U S A. 2009;106:3414–3419. doi: 10.1073/pnas.0813144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 65.Studebaker AW, Storci G, Werbeck JL, Sansone P, Sasser AK, Tavolari S, Huang T, Chan MW, Marini FC, Rosol TJ, Bonafe M, Hall BM. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 2008;68:9087–9095. doi: 10.1158/0008-5472.CAN-08-0400. [DOI] [PubMed] [Google Scholar]
- 66.Hugo HJ, Lebret S, Tomaskovic-Crook E, Ahmed N, Blick T, Newgreen DF, Thompson EW, Ackland ML (2012) Contribution of fibroblast and mast cell (Afferent) and tumor (Efferent) IL-6 effects within the tumor microenvironment. Cancer Microenviron 5:83–93 [DOI] [PMC free article] [PubMed]
- 67.Tuhkanen H, Anttila M, Kosma VM, Yla-Herttuala S, Heinonen S, Kuronen A, Juhola M, Tammi R, Tammi M, Mannermaa A. Genetic alterations in the peritumoral stromal cells of malignant and borderline epithelial ovarian tumors as indicated by allelic imbalance on chromosome 3p. Int J Cancer. 2004;109:247–252. doi: 10.1002/ijc.11733. [DOI] [PubMed] [Google Scholar]
- 68.Stern R. Hyaluronidases in cancer biology. Semin Cancer Biol. 2008;18:275–280. doi: 10.1016/j.semcancer.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 69.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 70.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/S0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 71.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 72.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 74.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 75.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 77.Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, Jain RK. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 79.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol. 2007;39:666–671. doi: 10.1016/j.biocel.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 82.Chang HY, Chi JT, Dudoit S, Bondre C, Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 84.Ostermann E, Garin-Chesa P, Heider KH, Kalat M, Lamche H, Puri C, Kerjaschki D, Rettig WJ, Adolf GR. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin Cancer Res. 2008;14:4584–4592. doi: 10.1158/1078-0432.CCR-07-5211. [DOI] [PubMed] [Google Scholar]