SUMMARY
Agonist binding to the A2 adenosine receptor (A2AR) and its regulation by guanine nucleotides was studied using the newly developed radioligand 125l-2-[4-(2-{2-[(4-ammnophenyl)methylcarbonylamino]ethylaminnocarbonyl}ethyl)phenyl]ethylamino-5′-N-ethylcarboxamidoadenosine (1251-PAPA-APEC) and its photoaffinity analog 125l-azido-PAPA-APEC. A single protein of Mr 45,000, displaying the appropriate A2AR pharmacology, is Iabeled in membranes from bovine striatum, PC12 cells, and frog erythrocytes. In DDT1 MF2 cells the labeled protein has a slightly lower molecular weight. Incorporation of 125l-azido-PAPA-APEC into membranes from rabbit striatum, however, reveals two specifically labeled peptides (Mr ~47,O00 and 38,000), both of which display A2AR pharmacology. Inhibition of protease activity leads to a decrease in the amount of the Mr 38,000 protein, with only the Mr 47,000 protein remaining. This suggests that the Mr 38,000 peptide is a proteolytic product of the Mr 47,000 A2AR protein. In membranes containing the intact undigested A2AR protein, guanine nucleotides induce a small to insignificant decrease in agonist binding, which is atypical of stimulatory Gs-coupled receptors. This minimal effect is observed in rabbit striatal membranes prepared in the presence of protease inhibitors, as well as in the other tissues studied. Binding to rabbit stnatal membranes that possess the partially digested receptor protein, however, reveals a 50% reduction in maximal specific agonist binding upon addition of guanine nucleotides. Inhibition of proteolysis in rabbit striatum, on the other hand, results in a diminished ability of guanine nucleotides to regulate agonist binding. Thus, the enhanced effectiveness of guanine nucleotides in rabbit striatal membranes is associated with the generation of the Mr 38,000 peptide fragment. Guanosine 5′-(β,γ-imido)triphosphate reduces photoaffinity labeling by 55% in the Mr 38,000 protein, whereas the labeling is decreased by only 28% in the Mr 47,000 receptor protein.
Our data, therefore, suggest that, unless proteolysis occurs, the A2AR in all tissues studied is tightly associated with the Gs protein and displays minimal guanine nucleotide modulation of agonist binding, which makes the A2AR an atypical stimulatory receptor.
ARs are known to mediate a wide range of physiological effects, including vasodilatation, suppression of cardiac rate and contractility, induction of sedation, and inhibition of platelet aggregability (1). Two subtypes of ARs have been defined, based on their pharmacological profiles, and termed A1AR and A2AR (2, 3). Over the past 5 years there have been dramatic advances in our understanding of the structure, function, and regulation of the A1AR (4–6). This receptor has been (a) studied by radioligand binding, where it was found to be tightly coupled to the Gi protein, both in membranes and following solubilization, and to be dramatically regulated under a variety of conditions, (b) photoaffinity labeled, with its glycoprotein nature being studied, and (c) purified to homogeneity (7–12). In contrast, little is known about the A2AR. Until 1989, there had been no selective high affinity radioligands to study the A2AR. [3H]NECA had been utilized as a radioligand, but its use was associated with many artifacts, in terms of binding to both A2AR and A1AR, as well as to multiple proteins that display characteristics of neither the A1AR nor the A2AR (13–15). Recently, we (16) and Jarvis et al. (17) have described the synthesis of two high affinity A2-selective agonist radioligands, 125I-PAPA-APEC and [3H]CGS 21680. We have used 125I-PAPA-APEC and its azide derivative to define the binding subunit structure of the A2AR and its glycoprotein characteristics in bovine striatal membranes, as well as to demonstrate that the binding subunit has the pharmacological properties expected of the A2AR (18, 19).
Early kinetic studies from Levitzki and co-workers (20, 21) and studies on the constituents of the AR signaling pathway in striatal membranes (22) suggested that the coupling of A2AR to its effector system, adenylate cyclase, was unusual, compared with that of other receptors that act through Gs to activate cyclase (20–23). These studies provided evidence that the A2AR was permanently coupled to its effector system and did not undergo an association/dissociation reaction as did the β-adrenergic receptor. We recently have found that guanine nucleotides have only a minimal effect on agonist binding in bovine striatal membranes (18). This is also atypical, compared with what has been described for other stimulatory receptors such as the glucagon and the D1 dopamine receptors (24, 25). Therefore, we undertook the present study to answer several specific questions. First, what is the A2AR subunit structure in different tissues and species? Second, is the A2AR sensitive to endogenous proteases and what functional consequences does this proteolysis produce? Third, is the bovine brain A2AR atypical, as it relates to the inability of guanine nucleotides to substantially decrease agonist binding?
Experimental Procedures
Materials
(R)-PIA, (S)-PIA, adenosine deaminase, and Gpp(NH)p were obtained from Boehringer-Mannheim. Soybean trypsin inhibitor, pepstatin A, leupeptin, PMSF, chloramine T, and HEPES-Na were from Sigma. NECA was generously donated by Dr. Ray Olsson (University of South Florida). SANPAH was purchased from Pierce. Na125I (carrierfree; 100 mCi/ml) was from Amersham Corp. Electrophoresis reagents were obtained from Bio-Rad Laboratories. All other chemicals were of the highest purity grade available.
Methods
Preparation of membranes from rabbit striatum
Rabbit brains were obtained from Pel-Freez or the Duke University Vivarium and were kept at –70°. After thawing, the corpus striatum was dissected and minced in ice-cold buffer (50 mm HEPES, 10 mm MgCl2, pH 7.4). The tissue was gently homogenized using a Teflon pestle, and membranes were centrifuged at 43,000 × g for 10 min. The pellet was washed once and finally resuspended to give a concentration of 400 mg of wet weight/ml. Aliquots were stored at –70° for use within 2 weeks. Rabbit striatal membranes prepared following the above protocol were designated as controls.
In order to limit endogenous proteolytic activity, the following buffer was used for preparation of rabbit striatal membranes: (in mm) HEPES, 50; EDTA, 5; PMSF, 0.1; (in μg/ml) soybean trypsin inhibitor, 100; leupeptin, 5; pepstatin A, 1; pH 7.9. Before storage, MgCl2 was added at a concentration of 6 mm, which was increased to 10 mm for the binding assay. The preparation of membranes from bovine striatum as well as DDT1 MF2 cells has been described and was carried out accordingly (18, 26). Membranes from frog erythrocytes were a generous gift from Dr. Marc G. Caron (Duke University Medical Center), and PC12 cell membranes were kindly provided by Dr. John W. Daly (National Institutes of Health). None of these membrane batches included protease inhibitors, except for the designated preparation from rabbit striatum.
Radioiodination of PAPA-APEC and synthesis of 125I-azido-PAPA-APEC
The parent compound PAPA-APEC, an A2AR-selective agonist, was synthesized as described (16). Iodination with Nat125I and synthesis of the azide derivative of PAPA-APEC have also been outlined in detail (18, 19). In brief, PAPA-APEC was iodinated by the chloramine T method and isolated by high pressure liquid chromatography, using a C18 μBondapak column. For isocratic elution, the mobile phase was composed of 60% methanol/40% ammonium formate (20 mm, pH 7.85). 125I-PAPA-APEC was either used as a radioligand or further processed to give the azide derivative. Briefly, the substance was dried down and redissolved in acetic acid (10 μl, 6 N). After reaction with sodium nitrite (20 μl, 20 mg/ml), sodium azide was added (10 μl, 5 mg/ml). In dimmed light, the incubation was run for 10 min and terminated by alkalinization (8 μl of ammonium hydroxide). Separation of 125I-azido-PAPA-APEC was performed by high pressure liquid chromatography, using an isocratic protocol (75% methanol/25% 20 mm ammonium formate, pH 7.85). The radioligand was assumed to have a specific activity of 2200 Ci/mmol on the day of radioiodination.
Photoaffinity labeling of A2AR
Photoaffinity labeling with the agonist photoaffinity/cross-linking probe 125I-azido-PAPA-APEC was performed as previously described (19). Frozen membranes were thawed, washed, and suspended in 50 mm HEPES, 10 mm MgCl2 (pH 6.8), in either the absence or the presence of protease inhibitors. After pretreatment with adenosine deaminase (1 unit/mg of protein/2 ml), incubations were run for 60 min at 37°, in a total volume of 1 ml consisting of 125I-azido-PAPA-APEC at ~1 nm, competitor or Gpp(NH)p at the indicated concentrations, and a total of 0.5–1 mg of membrane protein. The binding reaction was terminated by dilution of the mixture with ice-cold incubation buffer and centrifugation at 43,000 × g. The pellet was washed once, taken up in 1 ml, and exposed to UV light for 4 min at a distance of 1 cm from the light source (UVCG-25 mineral light). The entire procedure was performed in the dark. Upon photoincorporation, membranes were washed again, solubilized in 10% SDS-glycerol buffer, and subjected to electrophoresis on a 10% acrylamide gel and autoradiography.
Alternatively, photoaffinity labeling was performed with the radioligand 125I-PAPA-APEC, using SANPAH as the cross-linking agent (18).
Radioligand binding assay
125I-PAPA-APEC binding experiments were conducted as previously described (18). Membranes were prepared as for photoaffinity labeling assays. In saturation and competition binding experiments, incubations were for 60 min in a volume of 250 μl, containing 25–50 μg of membrane protein and radioligand, competitor, and/or Gpp(NH)p at the indicated concentrations. When the azide derivative was used as a radioligand, binding assays were carried out in foil-wrapped tubes. Separation of free and bound ligand was achieved by vacuum filtration over polyethylenirnine (0.3%)-treated glass fiber filters and rinsing with 3 × 4 ml of ice-cold buffer. The radioactivity was quantitated in a Packard Multi-Prias γ-counter at an efficiency of 75%.
Nonspecific binding assayed in the presence of 5 mm theophylline amounted to 50–60% of total binding in the KD concentration range. This binding component was not altered by the inclusion of guanine nucleotides. The variability of the data from triplicate determinations was less than 5% of the mean value.
Saturation and competition curves were analyzed using a nonlinear, least-squares, curve-fitting procedure, including a weighting routine and statistical analysis, as previously published.
In dissociation experiments, equilibrium binding was attained by preincubation of rabbit striaturn membranes (1 mg of protein) with 125I-PAPA-APEC (1–2 nm) for 45 min at 37°. Dissociation of radioligand binding was initiated by 50-fold dilution of the preincubation volume (1 ml), using buffer at 37° with or without 0.1 mm Gpp(NH)p. At the indicated time points, 3-ml aliquots were withdrawn, poured onto membrane filters, and rinsed with ice-cold incubation buffer. Evaluation of the experimental data and estimation of a koff value were achieved through fitting of the data to a monoexponential equation, based on nonlinear least-squares regression.
Protein determination was carried out according to the method of Bradford (27), with bovine serum albumin as a standard.
Results
Photoaffinity labeling of the A2AR was performed in membranes from rabbit and bovine striatum, rat PC12 and DDT1 MF-2 cells, and frog erythrocytes. The photoaffinity radioligand 125I-azido-PAPA-APEC labels distinct proteins, as shown by autoradiography after separation on SDS-PAGE (Fig. 1). Specific incorporation into the A2AR is completely suppressed by addition of 5 mm theophylline. As estimated from their migration profile, most of the A2AR species examined migrate with apparent Mr 44,000–47,000, whereas in DDT1 MF2 cells the A2AR has Mr 40,000. Specific labeling is confined to a single protein, except in the case of rabbit striatum membranes, wherein a doublet is observed at Mr 47,000 and 38,000 (Figs. 1 and 2).
Fig. 1.
Photoaffmnity labeling of A2ARs in membranes from rabbit and bovine stnatum, DDT, MF2 and PC12 cells, and frog erythrocytes (ERY). Membranes were incubated with the agonist photoaffinity probe 125I-azido-PAPA-APEC (1 nm) in the absence (C) or presence of 5 mm theophylline (T), as described in Experimental Procedures. After photoaffinity labeling, samples were solubilized and aliquots were subjected to SDS-PAGE and autoradiography. This autoradiograph is representative of two separate experiments.
Fig. 2.
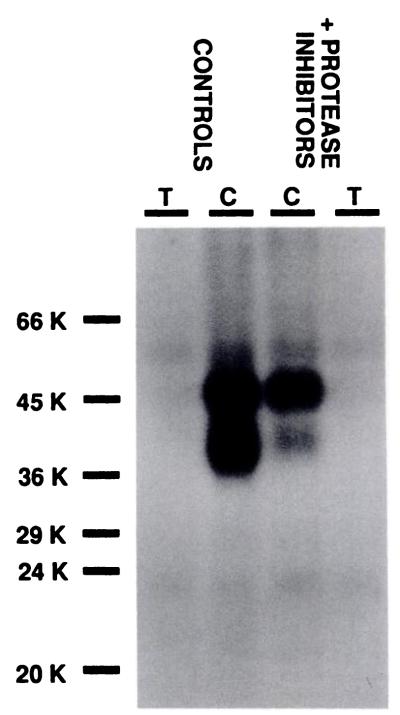
125I-Azido-PAPA-APEC labeling of A2ARs in control membranes from rabbit striatum and in membranes prepared with protease inhibitors (see Experimental Procedures). The inner lanes (C) show the result of photoaffinity labeling in the absence and the outer lanes (T) in the presence of 5 m theophylline. Equal amounts of protein from each sample (200 μg) were loaded onto the gel. This experiment was repeated several times.
This particular feature of the A2AR from rabbit striatum likely arises from partial enzymatic digestion of the receptor protein during cell disruption. Modification of the membrane preparation procedure to inhibit protease activity markedly reduces the amount of the Mr 38,000 protein, whereas labeling of the Mr 47,000 protein is similar or enhanced, compared with control (Fig. 2). The relative amount of radioactivity incorporated into each protein was quantified and gave identical results regardless of whether the azide derivative was used for direct photoaffinity labeling or, alternatively, 125I-PAPA-APEC was cross-linked into the receptor proteins using SANPAH as a cross-linking agent (data not shown).
Thus, inclusion of protease inhibitors and omission of Mg2+ induces a considerable shift in the ratio of upper to lower bands, from 1.6 (0.7–3.6, 95% confidence intervals) in control to 5.3 (2.9–9.4) under conditions in which proteolysis is inhibited.
Further evidence that the 125I-azido-PAPA-APEC-labeled Mr 38,000 protein originates from enzymatic cleavage of the Mr 47,000 A2AR comes from displacement experiments with AR agonists. The incorporation of the photoaffinity probe into both proteins is antagonized to a similar extent by NECA, (R)-PIA, and (S)-PIA, revealing a rank order of potency that is characteristic of the A2AR (Fig. 3). Competition binding curves with 125I-PAPA-APEC accordingly reflect the A2AR pharmacology. Ki values (nM) derived from experiments in control membranes are NECA, 18.0 ± 1.2; (R)-PIA, 343 ± 104; (S)-PIA, 7170 ± 2600; and theophylline, 7623 ± 5228 (means ± standard errors). Thus, the receptor fragment retains the binding affinity for agonists as well as antagonists, suggesting that the binding subunit remains functionally intact during enzymatic alteration of the receptor protein.
Fig. 3.
Inhibition of 125I-azido-PAPA-APEC photoaffinity labeling in control rabbit striatum membranes by AR agonists. Membranes were photoaffinity labeled with 125I-azido-PAPA-APEC alone (TOTAL) or in the presence of the indicated concentrations of the competing ligands. Autoradiography of the samples after separation on SDS-PAGE is shown, with the positions of molecular weight markers to the left. The results are representative of three similar experiments. THEO, theophylline.
A differentiating characteristic, however, is observed for the effect of guanine nucleotides on the incorporation of 125I-azido-PAPA-APEC into the doublet of A2AR proteins. Fig. 4 demonstrates the effect of the nonhydrolyzable guanine nucleotide Gpp(NH)p on photoaffinity labeling in membranes prepared in either the presence or the absence of protease inhibitors. In the smaller molecular weight peptide, labeling is clearly reduced by Gpp(NH)p (to 45 ± 12% of specific incorporation), whereas the effect appears small to insignificant in the larger protein (to 72 ± 10% of specific incorporation).
Fig. 4.
Effect of the guanine nucleotide analog Gpp(NH)p on the incorporation of the agonist photoaffinity probe 125I-azido-PAPA-APEC into the partially digested and the intact A2AR. Rabbit stnatum membranes were prepared as controls or in the presence of protease inhibitors (see Experimental Procedures). Membranes were incubated with 125I-azido-PAPA-APEC (1 nm) alone (TOTAL) or in the presence of Gpp(NH)p (0.1 mm) or theophylline (5 mm) (THEO). Autoradiography of the gel is shown, with the positions of molecular weight markers to the left. Three additional experiments gave similar results.
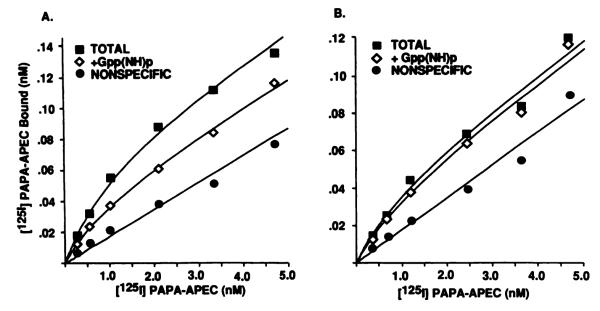
It has been well established that guanine nucleotides reduce high affinity agonist binding to G protein-coupled receptors (28). An increase in the agonist dissociation rate is considered to cause a shift to the low affinity binding state. In order to characterize the effect of guanine nucleotides on binding parameters, saturation experiments were performed with 125I-PAPA-APEC in rabbit striatal membranes. Fig. 5 displays saturable and monophasic binding isotherms in the absence as well as the presence of 0.1 mm Gpp(NH)p. As predicted from the results of photoaffinity labeling experiments, rabbit striatal membranes containing the intact receptor reveal almost superimposable binding curves. This is also observed in membranes that are demonstrated in Fig. 1 to harbor the undigested A2AR protein, i.e., from frog erythrocytes, DDT1 MF-2 cells, and PC12 cells (data not shown). In unprotected control membranes from rabbit striatum, however, 125I-PAPA-APEC binding is subject to G protein modulation. Upon addition of Gpp(NH)p, no change in receptor affinity becomes evident, whereas the receptor number decreases by 50% of maximum specific binding (Table 1). Because 125I-PAPA-APEC is a full agonist radioligand, it is not surprising that we do not detect a low affinity binding state in saturation experiments.
Fig. 5.
Effect of the guanine nucleotide analog Gpp(NH)p on reversible binding of the agonist radioligand 125I-PAPA-APEC to rabbit striatum membranes harboring either the intact or the partially digested A2AR protein. Control or protease-inhibited membrane preparations were used in the binding assay. Incubations were run in a volume of 250 μl containing 25–50 μg of membrane protein and 125I-PAPA-APEC, at the indicated concentrations, alone (TOTAL) or in the presence of Gpp(NH)p(0.1 mm)or theophylline(5 mm). Gpp(NH)p had no effect on the displacement of radioligand binding by theophylline. Experiments were performed in triplicate, and the data were fitted to an equation describing the interaction with a single binding site.
TABLE 1.
Parameter estimates for 125I PAPA-APEC binding to rabbit striatum values are mean ± standard error.
| Controls |
+Protease inhibitors |
|||
|---|---|---|---|---|
| KD | Receptor number | KD | Receptor number | |
| nm | fmol/mg | nm | fmol/mg | |
| No addition | 1.94 ± 0.54 | 248 ± 54 | 1.03 ± 0.08 | 197 ± 43 |
| +Gpp(NH)p | 1.43 ± 0.35 | 125 ± 23a | 1.57 ± 0.27 | 189 ± 40 |
| (0.1 mm) | (50 ± 11%b) | (96 ± 8%b) | ||
Significantly different from the value in the absence of Gpp(NH)p (p < 0.01).
Expressed as percentage of the total receptor number in the same experiment.
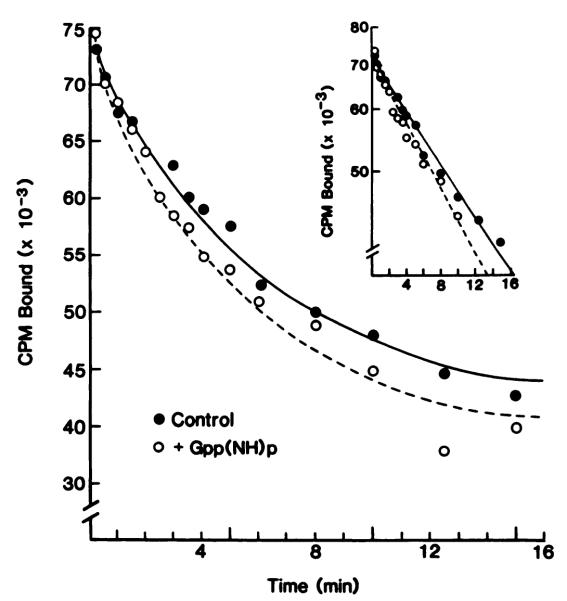
This finding is further confirmed in dissociation experiments. The resulting curves are shown in Fig. 6 and demonstrate a monophasic dissociation, according to an exponential decay that transforms to a straight line on a semilogarithmic plot. This indicates dissociation from a single affinity state of the receptor, giving a uniform koff of 0.16 ± 0.02 min–1 (six experiments). Gpp(NH)p creates a small, statistically nonsignificant, koff increase (0.20 ± 0.05 min–1), without altering the monophasic characteristics of the dissociation curve. In order to avoid interference from a time-dependent onset of guanine nucleotide effects, additional experiments were performed with Gpp(NH)p being present also during the preincubation period (data not shown). This modification would allow for dissociation from a supposedly low affinity state of the receptor but revealed koff values identical to those estimated in control experiments, providing evidence for the inability of the agonist radioligand 125I-PAPA-APEC to discern a low affinity state of the A2AR.
Fig. 6.
Dissociation of the agonist radioligand 125I-PAPA-APEC from the A2AR in control membranes from rabbit stnatum. Membranes prepared in the absence of protease inhibitors were preincubated with 1 nm 125I-PAPA-APEC, and dissociation was initiated by 50-fold dilution, without or with the addition of 0.1 mm Gpp(NH)p. Aliquots were withdrawn and filtered at the indicated time points. After fitting of the data, the lines were drawn according to an equation for monoexponential decay. Inset, data have been linearized for illustrative purposes. The results are representative of four experiments.
Discussion
In this paper we have provided information on the structure and function of A2ARs from different tissues and species. We have documented that (a) with a single exception the A2AR binding subunit resides on a Mr ~45,000 protein, (b) either the A2AR from the rabbit striatum is very sensitive to the effects of endogenous proteases or there are high levels of proteases in this species, and (c) this endogenous proteolysis has profound effects on the ability of guanine nucleotides to regulate agonist binding.
The A2AR of rabbit striatum is shown to be unstable in the presence of endogenous proteolytic enzymes (Fig. 2). Photoaffinity labeling with an A2AR-selective probe demonstrates two proteins are specifically labeled, both of which display the appropriate A2AR pharmacology. Inhibition of protease activity largely reduces the doublet to a single labeled protein of Mr 47,000. This protein, i.e., the undigested A2AR, reveals a molecular weight and pharmacological properties similar to those of the A2AR in bovine striatal membranes (18). Although the receptor protein from rabbit striatum is susceptible to endogenous proteolysis, A2ARs from other species and tissues, i.e., those in membranes from bovine striatum, frog erythrocytes, and PC 12 and DDT1 MF2 cells, have a structurally stable receptor protein. Thus, the rabbit striatum A2AR species appears to be unique among ARs, because neither A2ARs nor A1ARs have been reported to be susceptible to proteolysis (1, 18, 26).
It should be remembered that many transmembrane receptors, such as the α1- and the β-adrenergic receptors, have been found to be labile in the presence of tissue-specific proteases, a feature that has been described in several tissues (29, 30). Similarly, their degradation is inhibited by addition of protease inhibitors, particularly upon the removal of divalent metal cations.
Furthermore, our data suggest that alteration of the A2AR protein by proteolysis leads to a markedly enhanced modulation of agonist radioligand binding by guanine nucleotides. Treatment of membranes with protease inhibitors, on the other hand, results in an almost complete lack of effect of guanine nucleotides on agonist binding. Thus, the effectiveness of guanine nucleotides may be associated with the generation of the Mr 38,000 peptide fragment, as shown in Fig. 4. Prevention of proteolysis of the A2AR itself or of other membrane proteins appears to diminish the ability of guanine nucleotides to decrease agonist binding. This finding may be interpreted as tight coupling between receptor and G protein or, alternatively, as a uniform conformation of the receptor binding subunit regardless of G protein modulation. The latter concept could explain why the receptor does not display a low affinity state for the agonist PAPA-APEC unless the receptor structure is enzymatically altered.
Nevertheless, it might be argued that treatment of membranes with EDTA in the absence of Mg2+ to inactivate metal ion-dependent proteases might potentially perturb the A2AR-Gs-interaction and, thus, account for the loss of sensitivity to guanine nucleotides. Experiments with different conditions for the preparation of membranes from rabbit striatum (i.e., with and without Mg2+), however, led us to conclude that proteolysis and not alterations in the ion concentrations are responsible for the varying effects of guanine nucleotides (data not shown).
Provided that proteolysis is inhibited, the minimal effect of guanine nucleotides observed in rabbit striatal membranes is reminiscent of the results reported for 125I-PAPA-APEC binding in membranes from bovine striatum, wherein no proteolysis appears to occur and agonist binding is reduced by only ~10% in the presence of guanine nucleotides. This has also been found in DDT1 MF-2, PC12, and frog erythrocyte membranes and makes the A2AR distinctly unusual, compared with other receptors that activate adenylate cyclase. Although the common paradigm has been that guanine nucleotides totally ablate high affinity agonist binding (33), our data suggest that what initially appeared anomalous, i.e., a minimal effect of Gpp(NH)p on agonist binding in the bovine striatal A2AR, is actually typical for a number of A2ARs if endogenous proteolysis is inhibited.
Although our current understanding of the mode of coupling between the A2AR and the stimulatory G protein, Gs, is still rudimentary, there are data to suggest that there may be a very tight coupling between A2AR and Gs (20–23). For example, in turkey erythrocyte membranes it has been proposed that the A2AR is permanently coupled to the catalytic unit via the Gs protein, a model that is opposite to the transient nature of the β-adrenergic receptor-Gs interaction in the same membrane. Both models are, however, potentially in accord with the findings on the guanine nucleotide regulation of agonist binding. Whereas the β-adrenergic receptor-Gs complex apparently dissociates (at least functionally) in the presence of a GTP analog, which is associated with a complete loss of receptors in the agonist-specific high affinity state, there is virtually no effect of guanine nucleotides on agonist binding to the A2AR, as demonstrated in these studies. Taken together, these findings imply that A2AR and Gs do not functionally uncouple even in the presence of guanine nucleotides.
We and others have documented that the A1AR and Gi are tightly coupled, in that guanine nucleotides do not totally eliminate agonist-specific high affinity binding in membranes but do decrease binding by 40–60% (A1ARs are also not susceptible to endogenous proteolysis) (10, 34). Thus, ARs appear to be atypical in their responses to guanine nucleotides, with the A2AR being even more resistent to their effects than is the A1AR. In addition, it is clear that proteolysis of the membranes containing A2AR promotes guanine nucleotide sensitivity and, therefore, great care must be taken to avoid proteolysis, which can induce binding artifacts.
Acknowledgments
We would like to thank Linda Scherich for her assistance in the preparation of this manuscript.
C.N. is supported by a Postdoctoral Research Exchange Grant from the Max Kade Foundation, Inc. G.L.S. is supported in part by the National Heart, Lung and Blood Institute (Grant RO1-HL-35134) and Supplement and Grant-in-Aid (880662) from the American Heart Association and 3M Riker.
ABBREVIATIONS
- AR
adenosine receptor
- A1AR
A1 adenosine receptor
- A2AR
A2 adenosine receptor
- Gpp(NH)p
guanosine 5′-(β γ-imido)triphosphate
- NECA
5′-N-ethylcarboxamidoadenosine
- PAPA-APEC
2-[4-(2- 2-[(4-ammnophenyl)methylcarbonylamino]ethylammnocarbonyl]ethyyl)phenyljethylammno-5′-N-ethylcarboxamidoadenosmne
- (R)-PIA
(−)-N6-[(R)-1-methyl-2-phenylethyl]adenosmne
- (S)-PIA
(+)-N6-[(S)-1-methyl-2-phenylethyl]adenosine
- SANPAH
N-succmnimidyl 6-(4′-azido-2′-nitrophenylamino)hexanoate
- azido-PAPA-APEC
2-[4-[2-[2-[(4-azido-phenyl)methylcarbonylammnojethylaminocarbonyl]efthyl]phenyl]ethylammno-5′-N-ethylcarboxamido adenosine
- SOS
sodium dodecyl sulfate
- PAGE
polyacrylamide gel electrophoresis
- CGS 21680
2-(4-(2-carboxyethyl)phenylethylamino)-5′-N-ethylc&boxamidoadenosmne
- PMSF
phenylmethylsulfonyl fluoride
- HEPES
N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid
- G protein
guanine nucleotide-binding protein
References
- 1.Ramkumar V, Pierson G, Stiles GL. Adenosine receptors: clinical implications and biochemical mechanisms. Prog. Drug Res. 1988;32:195–247. doi: 10.1007/978-3-0348-9154-7_7. [DOI] [PubMed] [Google Scholar]
- 2.Van Calker D, Muller M, Hamprecht B. Adenosine inhibits the accumulation of cyclic AMP in cultured brain cells. Nature (Lond.) 1978;276:839–841. doi: 10.1038/276839a0. [DOI] [PubMed] [Google Scholar]
- 3.Londos C, Cooper DMF, Wolff J. Subclasses of external adenosine receptors. Proc. Nati. Acad. Sci. USA. 1980;77:2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stiles GL. Adenosine receptors: structure, function and regulation. Trends Phormacol Sci. 1986;87:486–490. [Google Scholar]
- 5.Stiles GL. Adenosine receptors and beyond: molecular mechanisms of physiological regulation. Clin. Res. 1990;38:10–18. [PubMed] [Google Scholar]
- 6.Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiol. Rev. 1990;70:761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- 7.Stiles GL. The A, adenosine receptor: solubilization and characterization of a guanine nucleotide-sensitive form of the receptor. J. Biol. Chem. 1985;260:6728–6732. [PubMed] [Google Scholar]
- 8.Stiles GL, Daly DT, Olsson RA. The A1 adenosine receptor: identification of the binding subunit by photoaffinity crosslinking. J. Biol. Chem. 1985;260:10806–10811. [PubMed] [Google Scholar]
- 9.Stiles GL. Photoaffinity crosslinked A1 adenosine receptor-binding subunits: homologous glycoprotein expression by different tissues. J. Biol. Chem. 1986;261:10839–10843. [PubMed] [Google Scholar]
- 10.Stiles GL. A1 adenosine receptor-G protein coupling in bovine brain membranes: effects of guanine nucleotides, salt and solubilization. J. Neurochem. 1988;51:1592–1598. doi: 10.1111/j.1471-4159.1988.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakata H. Purification of A1 adenosine receptor from rat brain membranes. J. Biol. Chem. 1989;264:16545–16551. [PubMed] [Google Scholar]
- 12.Olah ME, Jacobson KA, Stiles GL. Purification and characterization of bovine cerebral cortex A1-adenosine receptor. Arch. Biochem. Biophys. doi: 10.1016/0003-9861(90)90665-l. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeung SMH, Green RD. [3H]5′-N-Ethylcarboxamide adenosine binds to both Ra and Ri adenosine receptors in rat striatum. Naunyn-Schmiedeberg’s Arch. Pharrruzcol. 1984;325:218–225. doi: 10.1007/BF00495947. [DOI] [PubMed] [Google Scholar]
- 14.Lohse MJ, Elger B, Lindenborn-Fotinos J, Klotz K-N, Schwabe U. Separation of solubilized A2 adenosine receptors of human platelets from nonreceptor [3H]NECA binding sites by gel filtration. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1988;337:64–68. doi: 10.1007/BF00169478. [DOI] [PubMed] [Google Scholar]
- 15.Hutchison KA, Fox IH. Purification and characterization of the adenosine A2-like binding site from human placental membranes. J. Biol. Chem. 1989;264:19898–19903. [PubMed] [Google Scholar]
- 16.Jacobson KA, Pannell LK, Ji X-D, Jarvis MF, Williams M, Hutchison AJ, Barrington WW, Stiles GL. Agonist-derived molecular probes for A2 adenosine receptors. J. Mol. Recog. 1990;2:170–178. doi: 10.1002/jmr.300020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvis MF, Schutz R, Hutchison AJ, Do E, Sills MA, Williams M. [3H]-CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J. Pharmacol Exp. Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- 18.Barrington WW, Jacobson KA, Hutchison AJ, Williams M, Stiles GL. Identification of the A2 adenosine receptor binding subunit by photoaffinity crosslinking. Proc. Natl. Acad. Sci. USA. 1989;86:6572–6576. doi: 10.1073/pnas.86.17.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrington WW, Jacobson KA, Stiles GL. Glycoprotein nature of the A2-adenosine receptor binding subunit. Mol. Pharmacol. 38:177–183. [PMC free article] [PubMed] [Google Scholar]
- 20.Tolkovsky AM, Levitzki A. Coupling of a single adenylate cyclase to two (2) receptors: adenosine and catecholamine. Biochemistry. 1978;17:3811–3817. doi: 10.1021/bi00611a021. [DOI] [PubMed] [Google Scholar]
- 21.Rimon A, Hanski E, Braun S, Levitzki A. Mode of coupling between hormone receptors and adenylate cyclase elucidated by modulation of membrane fluidity. Nature (Lond.) 1978;276:394–396. doi: 10.1038/276394a0. [DOI] [PubMed] [Google Scholar]
- 22.Braun S, Levitzki A. Adenosine receptor permanently coupled to turkey erythrocyte adenylate cyclase. Biochemistry. 1979;18:2134–2138. doi: 10.1021/bi00577a045. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RA, Anand-Srivastava MB. Evidence for “tight coupling” of adenosine-receptors to adenylate cyclase from striatal membranes. Horm. Cell Regul. 1982;6:143–153. [Google Scholar]
- 24.Rojas FG, Birnbaumer L. Regulation of glucagon receptor binding. J. Biol. Chem. 1985;260:7829–7835. [PubMed] [Google Scholar]
- 25.Seeman P, Ulpian C, Gigoriadis D, Pri-Bar I, Bucham O. Conversion of dopamine D1 receptors from high to low affinity for dopamine. Biochem. Pharmacol. 1985;34:151–156. doi: 10.1016/0006-2952(85)90116-9. [DOI] [PubMed] [Google Scholar]
- 26.Ramkumar V, Barrington WW, Jacobson KA, Stiles GL. Demonstration of both A1 and A2 adenosine receptors in DDT1 MF-2 smooth muscle cells. Mol. Pharmacol. 1990;37:149–156. [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.De Lean P, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclasecoupled beta-adrenergic receptors. J. Biol. Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- 29.Leeb-Lundberg LM, Dickinson KE, Heald SE, Wikberg GES, Hagen PO, DeBernardis GT, Winn M, Arendson DL, Lefkowitz RJ, Caron MG. Photoaffinity labelling of mammalian α1-adrenergic receptors. J. Biol. Chem. 1984;259:2579–2588. [PubMed] [Google Scholar]
- 30.Benovic JL, Stiles GL, Lefkowitz RJ, Caron MG. Photoaffinity labelling of mammalian beta-adrenergic receptors: metal-dependent proteolysis explains apparent heterogeneity. Biochem. Biophys. Res. Commun. 1983;110:504–511. doi: 10.1016/0006-291x(83)91178-6. [DOI] [PubMed] [Google Scholar]
- 31.Bird SJ, Maguire ME. The agonist-specific effect of magnesium on binding by beta-adrenergic receptors in S49 lymphoma cells. J. Biol. Chem. 1978;253:8826–8834. [PubMed] [Google Scholar]
- 32.Williams LT, Mullikin D, Lefkowitz RJ. Magnesium dependence of agonist binding to adenylate cyclase-coupled hormone receptors. J. Biol. Chem. 1978;253:2984–2989. [PubMed] [Google Scholar]
- 33.Stiles GL, Caron MG, Lefkowitz RJ. β-Adrenergic receptors: biochemical mechanisms of physiological regulation. Physiol. Rev. 1984;64:661–743. doi: 10.1152/physrev.1984.64.2.661. [DOI] [PubMed] [Google Scholar]
- 34.Ströher M, Nanoff C, Schütz W. Differences in the GTP-regulation of membrane bound and solubilized A1 adenosine receptors. Naunyn-Schmiedeberg’s Arch. Phormacol. 1989;340:87–92. doi: 10.1007/BF00169212. [DOI] [PubMed] [Google Scholar]