Table I.
Effect of Functionalization on the Activity of 1,3-Dialkyl-8-(p-hydroxyphenyl)xanthines at A1- and A2-Adenosine Receptors
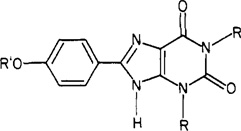 | ||||
|---|---|---|---|---|
|
Ki,a nM |
||||
| compd | R′ | A1 receptor | A2 receptors | ratio A1/A2 |
| R = CH3 | ||||
| 2a | H | 50 ± 20 | 130 ± 30 | 2.6 |
| 8a | HO2CCH2 | 500 ± 200 | 430 ± 80 | 0.86 |
| 9a | 26 ± 5 | 20 ± 7 | 0.77 | |
| R = n-C3H7 | ||||
| 2b | H | 2.9 ± 0.8 | 50 ± 10b | 17 |
| 8b | HO2CCH2 | 58 ± 3 | 34 ± 13 | 0.59 |
| 9b | 36 ± 23 | 750 ± 370 | 21 | |
| 9c | 4.1 ± 1.5 | 62 ± 37 | 15 | |
| 10 | CH3CH2OCOCH2 | 42 ± 3 | 30 ± 12 | 0.71 |
| 11 |  |
96 ± 25 | > 1000 (30% inhibn) | > 10 |
| 12 |  |
9.0 ± 0.7 | 40 ± 14 | 4.4 |
| 13a | H2NCOCH2 | 6.0 ± 1.0 | 47 ± 2 | 7.8 |
| 13b | (CH3)2 NCOCH2 | 32 ± 7 | 68 ± 39 | 2.1 |
| 13c | H2N(CH2)2NHCOCH2 | 1.2 ± 0.5 | 49 ± 17 | 41 |
| 13d | H2N( CH2)8NHCOCH2 | 5 ± 2 | 470 ± 170 | 94 |
| 13e | H2NNHCOCH2 | 59 ± 0.7 | 32 ± 4 | 0.54 |
| 14a | CH3CONH(CH2)2NHCOCH2 | 24 ± 3.5 | 62 ± 3 | 2.6 |
| 14b | 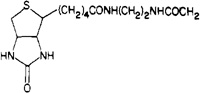 |
54 ± 2 | 180 ± 80 | 3.3 |
| 16 | 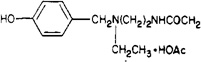 |
2.2 ± 0.7 | 320 ± 70 | 145 |
IC50 values for A1 receptors were obtained from antagonism of binding of 1 nM [3H]cyclohexyladenosine to rat cerebral cortical membranes. IC50 values for A1 receptors were obtained from antagonism of 3H-labeled cyclic AMP accumulation elicited by 15 µM 2-chloroadenosine in [3H]adenine-labeled guinea pig cerebral cortical slices. Ki values were calculated from the equation Ki = IC50/(1 + concentration of adenosine analogue/Ka for adenosine analogue). Values are means ± SEM for two to four experiments. Each experiment contained triplicate determinations.
Values from ref 4.
