Summary
Recent structural characterizations of classical and nonclassical major histocompatibility complex class II (MHCII) proteins have provided a view into the dynamic nature of the MHCII peptide binding groove and the role that structural changes play in peptide loading processes. Although there have been numerous reports of crystal structures for MHCII-peptide complexes, a detailed analysis comparing all the structures has not been reported, and subtle conformational variations present in these structures may not have been fully appreciated. We compared the 91 MHCII crystal structures reported in the PDB to date, including a HLA-DR mutant particularly susceptible to DM-mediated peptide exchange, and reviewed experimental and computational studies of the effect of peptide binding on MHCII structure. These studies provide evidence for conformational lability in and around the α subunit 310 helix at residues α48-51, a region known to be critical for HLA-DM-mediated peptide exchange. A biophysical study of MHC-peptide hydrogen bond strengths and a recent structure of the nonclassical MHCII protein HLA-DO reveal changes in the same region. Conformational variability was observed also in the vicinity of a kink in the β subunit helical region near residue β66 and in the orientation and loop conformation in the β2 Ig domain. Here, we provide an overview of the regions within classical and nonclassical MHCII proteins that display conformational changes and the potential role that these changes may have in the peptide loading/exchange process.
Keywords: major histocompatibility complex, human lymphocyte antigen, antigen presentation, peptide binding, protein structure, conformational change
Introduction
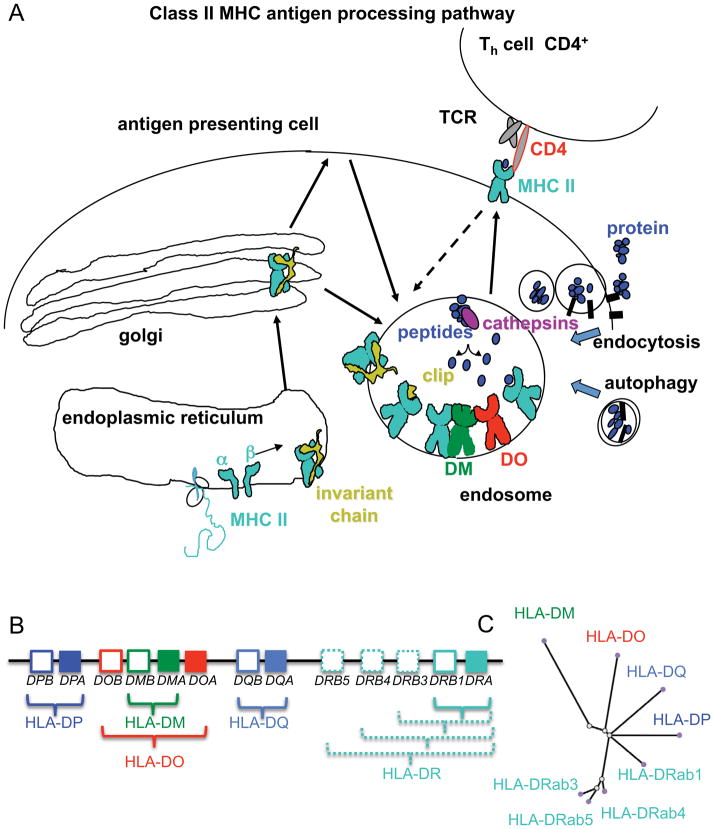
Major histocompatibility complex class II (MHCII) proteins are ~50 kDa heterodimeric transmembrane glycoproteins that comprise a critical part of the adaptive immune response to foreign pathogens. MHCII proteins are expressed by so-called ‘professional antigen-presenting cells’ such as dendritic cells, macrophages, and B cells, where they bind peptides derived from self and foreign proteins and display them at the cell surface for interaction with CD4+ T cells (Fig. 1). Newly synthesized MHCII α and β subunits are translocated to the lumen of the endoplasmic reticulum where they associate with a trimeric chaperone protein known as the class II MHC-associated invariant chain (1)(Unlike the MHCII α and β subunit, the invariant chain is not polymorphic). The resultant nonameric complex (2) is sorted to the endosomal compartment via AP-1 (3). Except for the regions involved in trimer formation and MHCII association, the invariant chain is unstructured and very sensitive to proteolysis (4) and quickly succumbs to degradation by endosomal-resident proteases known as cathepsins (5, 6). The majority of the invariant chain is removed, leaving a small peptide fragment know as CLIP (class II invariant chain peptide) bound in the MHCII peptide binding groove (4, 7).
Fig. 1. Class II MHC antigen processing pathway.
(A) Newly synthesized MHCII proteins associate in the endoplasmic reticulum with invariant chain chaperone that guides transport to endosomal compartments. Endosomal proteases cleave the invariant chain to leave a small peptide (clip) in the MHCII binding site. DM acts as a catalytic peptide exchange factor to release clip and promote binding of endosomal peptides derived from self and foreign proteins brought into the compartment by endocytosis or autophagy. Peptide-loaded MHCII proteins traffic to the cell surface for interaction with CD4+ T cells. MHCII can also be loaded with peptide at the cell surface or after recycling. (B) Arrangement of classical (HLA-DP, HLA-DQ, HLA-DR) and nonclassical (HLA-DM, HLA-DO) human MHCII α and β subunit genes in the major histocompatibility complex (MHC) locus on chromosome 6. In most haplotypes, DRB1 and one of the DRB3, DRB4, or DRB5 genes are expressed. (C) Phylogenetic tree showing sequence similarity relationships between MHC genes. Calculated for concatenated α and β sequences. Values in parenthesis show pairwise amino acid identity with HLA-DR. Classical MHCII are highly polymorphic. Similarities were calculated for 0101 alleles of all genes except for DQB*0501, using IMGT (71), BLAST (72), and TreeDyn (73).
Peptides destined for endosomal loading onto MHCII proteins can derive from self or foreign proteins brought into the endosomal pathway by fluid-phase endocytosis, receptor-medicated endocytosis, or autophagy (Fig. 1A). The relative efficiency of these pathways depends on the type and maturation state of the antigen-presenting cell. Regardless of the source of peptide, CLIP peptide must be removed from MHCII to allow antigenic peptide binding. This process is facilitated by the nonclassical MHCII protein HLA-DM (H2-M in mice). Nonclassical in this context means non-peptide binding and non-polymorphic, in comparison to the classical human MHCII proteins HLA-DR, HLA-DP, HLA-DQ and classical murine MHCII proteins I-E and I-A, which are highly polymorphic and bind peptides. In vitro, DM serves as a catalyst to promote both peptide binding and release (8, 9) and so can be considered as an enzyme (10, 11). In vivo, DM is required for efficient peptide loading, and in its absence, many cell-surface MHCII still carry CLIP (12). Despite numerous studies (discussed below), the mechanism by which DM mediates peptide exchange still has not been resolved. After loading, MHCII–peptide complexes traffic to the cell surface, where they can interact with T-cell receptors on CD4+ T cells. Recycling MHCII proteins can be reloaded by a similar process (13)
In some types of antigen-presenting cells, the regulation of peptide exchange by DM is modulated by another nonclassical MHCII protein HLA-DO (DO). The role played by DO in antigen presentation currently is poorly understood, although the unique pattern of expression in thymocytes, trophoblasts, certain immature dendritic cells, and B cells until germinal center entry, has led to suggestions of a role in induction or maintenance of T-cell tolerance (14). The observation of decreased autoimmunity upon DO overexpression in a mouse diabetes model is consistent with this idea (15). Most in vitro and in vivo studies of DO action have revealed an inhibitory function, with DO inhibiting DM-mediated catalysis of peptide binding or peptide release (16–19). However, some exceptions suggesting a more complicated role for DO have appeared (20, 21). Recent work from our laboratory has shown that DO inhibits DM-mediated peptide exchange through a competitive inhibition mechanism, with DO acting as substrate analog (data not shown).
The class II region of the major histocompatibility locus (MHC) comprises genes for both classical and nonclassical MHC proteins (Fig. 1B,C). With our recent determination of a crystal structure for DO (in complex with DM) (data not shown), structural information now is available for an example of each of the MHC locus proteins. Crystal structures of the nonclassical MHCII proteins, DM and DO, have revealed these accessory proteins to be structural homologues of classical MHCII proteins, with the largest deviations in structural similarities along the MHCII peptide binding groove. There have been 91 classical MHCII - peptide crystal structures solved to date (Table 1), and although there is remarkable similarity among them all, there are subtle differences in key regions of the MHCII known to be important for peptide binding and DM-mediated peptide exchange.
Table 1.
| PDB ID | Protein | Peptide | Res. |
|---|---|---|---|
| 1K8I | H2-M | 3.1 | |
| 1HDM | HLA-DM | 2.5 | |
| 2BC4 | HLA-DM | 2.27 | |
| 3LQZ | HLA-DP2 | DRa | 3.25 |
| 3PL6 | HLA-DQ1 (A1*0102/B1*0502) | MBP | 2.55 |
| 1S9V | HLA-DQ2 (A1*0501/B1*0201) | deam. gliadin | 2.2 |
| 4D8P | HLA-DQ2.3 (A1*0301/B1*0201) | Gliadin | 3.05 |
| 1UVQ | HLA-DQ6 (A1*0102/B1*0602) | hypocretin | 1.8 |
| 1JK8 | HLA-DQ8 (A1*0301/B1*0302) | human insulin | 2.4 |
| 2NNA | HLA-DQ8 (A1*0301/B1*0302) | deam. glutein | 2.1 |
| 3QXA | HLA-DR1 | CLIP | 2.71 |
| 3QXD | HLA-DR1 | CLIP | 2.3 |
| 3S4S | HLA-DR1 | HA | 2.4 |
| 3S51 | HLA-DR1 | HA | 2.1 |
| 1AQD | HLA-DR1 (B1*0101) | endogenous | 2.45 |
| 1DLH | HLA-DR1 (B1*0101) | HA | 2.8 |
| 1FYT | HLA-DR1 (B1*0101) | HA | 2.6 |
| 1HXY | HLA-DR1 (B1*0101) | HA | 2.6 |
| 1JWM | HLA-DR1 (B1*0101) | HA | 2.7 |
| 1JWS | HLA-DR1 (B1*0101) | HA | 2.6 |
| 1JWU | HLA-DR1 (B1*0101) | HA | 2.3 |
| 1KGO | HLA-DR1 (B1*0101) | EBV | 2.65 |
| 1KLG | HLA-DR1 (B1*0101) | TPI | 2.4 |
| 1KLU | HLA-DR1 (B1*0101) | TPI | 1.93 |
| 1LO5 | HLA-DR1 (B1*0101) | HA | 3.2 |
| 1PYW | HLA-DR1 (B1*0101) | HA variant | 2.1 |
| 1R5I | HLA-DR1 (B1*0101) | MAM | 2.6 |
| 1SEB | HLA-DR1 (B1*0101) | endogenous | 2.7 |
| 1SJE | HLA-DR1 (B1*0101) | HIV 16mer | 2.45 |
| 1SJH | HLA-DR1 (B1*0101) | HIV 13mer | 2.25 |
| 1T5W | HLA-DR1 (B1*0101) | synthetic | 2.4 |
| 1TX5 | HLA-DR1 (B1*0101) | synthetic | 2.5 |
| 2FSE | HLA-DR1 (B1*0101) | collagen II | 3.1 |
| 2G9H | HLA-DR1 (B1*0101) | HA | 2 |
| 2IAM | HLA-DR1 (B1*0101) | TPI | 2.8 |
| 2IAN | HLA-DR1 (B1*0101) | TPI | 2.8 |
| 2ICW | HLA-DR1 (B1*0101) | HA | 2.41 |
| 2IPK | HLA-DR1 (B1*0101) | HA variant | 2.3 |
| 2OJE | HLA-DR1 (B1*0101) | HA | 3 |
| 2WBJ | HLA-DR1 (B1*0101) | ENGA | 3 |
| 3L6F | HLA-DR1 (B1*0101) | MART-1 | 2.1 |
| 3PDO | HLA-DR1 (B1*0101) | CLIP | 1.95 |
| 3PGC | HLA-DR1 (B1*0101) | CLIP (Flipped) | 2.66 |
| 3PGD | HLA-DR1 (B1*0101) | CLIP | 2.72 |
| 1FV1 | HLA-DR2a (B5*0101) | MBP | 1.9 |
| 1H15 | HLA-DR2a (B5*0101) | EBV | 3.1 |
| 1ZGL | HLA-DR2a (B5*0101) | MBP | 2.8 |
| 1HQR | HLA-DR2a (B5*0101) | MBP | 3.2 |
| 1BX2 | HLA-DR2b (B1*1501) | MBP | 2.6 |
| 1YMM | HLA-DR2b (B1*1501) | MBP | 3.5 |
| 1A6A | HLA-DR3 (B1*0301) | CLIP | 2.75 |
| 3T0E | HLA-DR4 (B1*0401) | MBP | 4 |
| 1D5M | HLA-DR4 (B1*0401) | pep mimetic | 2 |
| 1D5X | HLA-DR4 (B1*0401) | pep mimetic | 2.45 |
| 1D5Z | HLA-DR4 (B1*0401) | pep mimetic | 2 |
| 1D6E | HLA-DR4 (B1*0401) | pep mimetic | 2.45 |
| 1J8H | HLA-DR4 (B1*0401) | HA | 2.4 |
| 2SEB | HLA-DR4 (B1*0401) | Collagen II | 2.5 |
| 2XN9 | HLA-DR4 (B1*0401) | HA | 2.3 |
| 3O6F | HLA-DR4 (B1*0401) | MBP | 2.8 |
| 2Q6W | HLA-DR52a (B3*0101) | platelet integ. | 2.25 |
| 3C5J | HLA-DR52c (B3*0301) | UNP | 1.8 |
| 1LNU | I-Ab | 3K (Eα variant) | 2.5 |
| 1MUJ | I-Ab | CLIP | 2.15 |
| 3C5Z | I-Ab | 3K | 2.55 |
| 3C60 | I-Ab | 3K | 3.05 |
| 3C6L | I-Ab | 3K | 3.4 |
| 1IAO | I-Ad | Ova | 1.6 |
| 2IAD | I-Ad | HA | 2.4 |
| 3RDT | I-Ab | 3K | 2.7 |
| 1ES0 | I-Ag7 | GAD65 | 2.6 |
| 1F3J | I-Ag7 | HEL | 3.1 |
| 3CUP | I-Ag7 | GAD | 3.09 |
| 3MBE | I-Ag7 | HEL | 2.89 |
| 1D9K | I-Ak | Conalbumin | 3.2 |
| 1IAK | I-Ak | HEL | 1.9 |
| 1JL4 | I-Ak | Ovotransferrin | 4.3 |
| 1K2D | I-Au | MBP | 2.2 |
| 1U3H | I-Au | MBP | 2.42 |
| 2P24 | I-Au | MBP | 2.15 |
| 2PXY | I-Au | MBP | 2.23 |
| 2Z31 | I-Au | MBP | 2.7 |
| 1FNE | I-EK | HB | 1.9 |
| 1FNG | I-EK | HB | 1.9 |
| 3QIU | I-EK | MCC | 2.7 |
| 3QIW | I-EK | MCC | 3.3 |
| 1I3R | IE-k | Hemoglobin b2 | 2.4 |
| 1IEA | IE-k | HB | 2.3 |
| 1IEB | IE-k | HSP70 | 2.7 |
| 1KT2 | IE-k | MCC | 2.8 |
| 1KTD | IE-k | PCC | 2.4 |
| 1R5V | IE-k | artificial pep. | 2.5 |
| 1R5W | IE-k | artificial pep. | 2.9 |
| 3QIB | IE-k | MCC | 2.7 |
MHC structural overview
The first crystal structure of an MHCII protein, determined almost two decades ago, was for HLA-DR1 [DRA*01:01, B1*01:01; see Marsh et al. (22) or http://hla.alleles.org for a description of the HLA allele nomenclature], bound to a mixture of B-cell-derived naturally processed peptides (23). The structure revealed an architecture composed of a membrane distal peptide-binding groove formed by two α helices flanking an 8-strandedβ pleated sheet floor, with both α and β subunits contributing approximately equal halves (Fig. 2A). The α1 and β1 domains together comprise the peptide binding domain (Fig. 2B). The membrane proximal regions consist of two immunoglobulin (Ig)-like domains, α2 and β2, one from each subunit. Membrane-spanning and short cytosolic domains were not included in that structure and have not been characterized since for any MHCII protein. Although this structure was generated from an MHCII protein in complex with a mixture of peptides in the binding groove, the extended polyproline type II conformation of the peptide backbone was evident (24). This conformation can be thought of as a twisted β strand with an approximate three-residue repeat, which directs some of the bound peptide side chains toward pockets in the MHCII peptide binding site, with the intervening residues exposed for interaction with TCR (Fig. 2C). The pockets conventionally are numbered relative to the largest HLA-DR1 pocket termed P1, which accommodates a hydrophobic residue near the peptide N-terminus. Pockets were also observed to accommodate the P4, P6, and P9 residues (25). The same basic peptide conformation has been observed in all subsequent structures, including one in which the peptide has been reported to bind in the opposite orientation (26). The MHCII peptide-binding groove was shown to be open-ended, thereby allowing peptides of variable length to bind. Subsequently, it has been shown that MHCII proteins can bind peptides with as little as two residues (27) as well as full length unfolded proteins (28), although studies of the spectrum of naturally processed peptides shown that most fall into the range of 13–25 residues, with an average length of ~15 (29).
Fig. 2. MHCII structure.
(A) Crystal structure for HLA-DR1 (PDB ID 1DLH) with HA peptide shown in stick representation, with cartoon representation for the α chain shown in sand, with the 3–10 helical region in brown, and the for β chain shown in cyan. (B) Top view of the peptide binding groove shown in surface representation with HA peptide shown. The N-terminus of the peptide is at the left and the α1 helix is at the top. (C) 90° rotation of the surface representation shown in panel (B) cutaway to show the P1, P4, P6 and P9 side chain specificity pockets.
Since the first crystal structure was solved, there have been an additional 91 MHCII–peptide complexes deposited in the PDB (Table 1). In addition to many peptide complexes of HLA-DR1, structures for other mouse and human MHCII proteins have allowed for a comparative structural approach. Subsequent studies of MHCII structures have confirmed the role of four main side chain specificity pockets, P1, P4, P6, and P9, with additional contributions from P3, P7, and P10 (30, 31). Due to the polymorphic nature of MHCII proteins, there are differences in the architecture, charge, and shape of the binding pockets, with consequent differences in peptide binding specificity. In many cases, the contributions of the pockets to the overall peptide-binding affinity can be considered independent, leading the use of simple position-specific scoring matrices (i.e. motifs) in predicting relative peptide binding affinities for many MHCII alleles (32). However, the predictive ability of these algorithms is somewhat limited as compared to similar approaches applied to MHCI proteins (22, 33), and cooperative effects have been shown to modulate binding affinity for at least some peptides (34).
The canonical conformation of peptides bound to MHCII proteins appears to be maintained by a hydrogen-bonding network that extends from the main chain of the peptide to both main chain and conserved side chain residues of the MHCII (24, 25) (Fig. 3). The hydrogen bond network is highly conserved despite differences in peptide sequences and MHCII proteins and therefore confers a stereotyped mode of binding across the spectrum of peptide-MHCII interactions. The disruption of the hydrogen bond network has been posited as a key determinant for DM-mediated peptide exchange. Because DM must work on highly polymorphic proteins, disruption of the hydrogen bonds that are invariant would provide a unifying mechanism that could extend across the repertoire of peptide-MHCII complexes (8). However, numerous studies have suggested a more complicated mechanism of action for DM (discussed below).
Fig. 3. Hydrogen bonding network.
(A) Top view of the peptide binding groove shown in cartoon representation with HA peptide backbone shown in stick representation, colored as in Fig. 2. MHCII conserved residues that contribute to the peptide-MHC hydrogen bonding network are shown in stick representation. Dashed lines indicate conserved hydrogen bonds. (B) Chemical diagram of the peptide-MHC hydrogen bonding. MHCII residues are noted and the positions of the peptide side chains are indicated in red.
Structural alignments of all MHCII show heterogeneity in the α subunit 310 helix and the β subunit helical kink
We have examined the 91 MHCII-peptide complex structures deposited in the PDB in order to determine regions of conformational heterogeneity. Although overall the structures are highly similar, alignments of the peptide binding domains of these MHCII proteins revealed structural heterogeneity in three regions: the α subunit 3–10 helical region α45-54 (Fig. 4A,B), the β2 Ig-like domain (Fig. 4C), and the pronounced kink in the β subunit helical region β62-71 (Fig. 5A).
Fig. 4. Conformational variability in MHCII structures.
MHCII structures in the PDB (Table 1) were aligned to the peptide binding domain of 1DLH (residues α1-81 and β1-90). (A) End view of the aligned structures looking down the bound peptide from N- to C-termini, with variable region around the α subunit 3–10 helix near the peptide N-terminus boxed. (B) Closeup of 3–10 helical region. Outliers from an otherwise tightly grouped cluster of structures are shown in red (HLA-DQ, 3PL6), blue (HLA-DQ, 1UVQ), green (HLA-DQ, 1SV9), orange (HLA-DQ, 4DP8), magenta (HLA-DQ, 2NNA), cyan (HLA-DR1 F54C, 3QXD, chains d–f), and purple (I-Ag7, 1F3J). (C) MHCII were aligned to the β2 Ig domain 1DLH (residues 91–180). After domain alignment structural heterogeneity is restricted to the lower loops.
Fig. 5. Conformational variability in the β chain helical region.
(A,B) MHCII structures were aligned as in Fig. 4. (A) Top view of the aligned structures with peptide N-terminus at left and variable region around the β subunit helical kink at residues 62–70 boxed. (B) Outliers tilting distal to the peptide binding groove indicated by red arrow: red (HLA-DR5b, H15), cyan (HLA-DR5b, 1ZGL). Outliers tilting inward towards the peptide binding groove indicated by open arrow: blue (I-Ag7,3CUP), slate (I-Au, 1U3H), chocolate (I-Au, 2P24), purple (I-Ag7, 1F3J), salmon (I-Ag7, 1ES0), yellow (I-Au, 2PXY), magenta (I-Ag7, 3MBE), orange (I-Ag7, 2Z31), and wheat (I-Ak, 1IAK). (C) Ribbon diagram of two molecules in the asymmetric unit of 1H15 showing reorientation in the β subunit helical kink region (arrow) induced by a crystal contact (green) at residue Asp β66. (D) Top view of the two molecules shown in panel C.
The largest degree of structural heterogeneity is found in the β2 Ig-like domain, whose orientation relative to the peptide binding domain varies by ~10 degrees (Fig. 4A, curved arrow). The motion is best described as a pivot about a point near the attachment to the peptide-binding domain and thus represents a rigid body motion rather than a conformational change within the Ig domain. After alignment of the Ig domains (Fig. 4C), less variation is observed, but there is still substantial conformational heterogeneity in the lower loops connecting the beta strands. The largest variation is seen for the A–B loop between the first two strands of the Ig domain, residues 105–112 (Fig. 4A,C). Residues in this region also tend to exhibit higher atomic displacement factors (B-factors) as compared to the rest of structure. Whether or not this sort of flexibility will be present for the full-length membrane-embedded protein is not known.
Outside the end of the β2-domain, the greatest structural heterogeneity observed within the set of MHCII crystal structures is in the α subunit 310 helical region (Fig. 4A,B). In this region, residues α45-51 form a short 310 helix, at the N-terminal end of the peptide binding groove and roughly perpendicular to it, which connects the β sheet platform with the long α helix that defines the binding groove (Figs 2A and 3A). The 310 helical conformation is similar to a conventional alpha helical conformation but wound more tightly, with 3 rather than 3.4 residues per turn and NH-CO hydrogen bonds between i and i+3 residues forming 10-atom rings. The 310 helix is thought to be less stable than the alpha helix and extended 310 helices are relatively rare in protein structures (35). In this region, the majority of the MHCII structures group together with Cα deviation of about 2Å. There are however large deviations for the HLA-DQ proteins and one of the mouse I-Ag7 structures (Fig. 4B, arrow). The greatest variance in distance is seen for the HLA-DQ protein structure 3PL6 at residue α50, which deviates from the HLA-DR1 structure 1DLH by 6.7 Å at this position. Interestingly, another HLA-DQ protein structure 1UVQ deviates greatly in this region as well, with multiple residues deviating from 1DLH by 4 Å. Even though both of these HLA-DQ alleles share the same α chain, HLA-DQA*01:02, they show as much variation between each other as they do when compared to 1DLH. Unlike the other HLA-DQ alleles, this α chain contains 2 glycines at the α52 and α53 positions that may confer structural lability in this region allowing multiple conformers to be adopted.
Aside from the HLA-DQ alleles carrying the Gly-Gly sequence at α52-α53, the other MHCII protein structures exhibit smaller but still substantial deviations in the 310 helix α45-51 region. These deviations are not due to insertions or deletions. Although there is sequence variation in this region, only one protein, HLA-DQ (1SV9), has a deletion (at theα52 residue). Interestingly, insertion of a residue to repair the deletion restores DM susceptibility to this otherwise resistant allele (36). In addition to 1SV9, the largest variations in this region are observed for the HLA-DQ proteins 1UVQ, 4DP8, 2NNA, 3PL6, the mouse IAg-7 protein 1F3J, and the HLA-DR1αF54C mutant 3QXD. The variation is best described by a partial unwinding of the 310 helical structure, which is more pronounced in the C-terminal region of the helix. The largest outliers are 3PL6 and 1F3J, with α48-51 NH-CO i+3 hydrogen bonds lengths greater than 4.5 Å, and a conformation intermediate between 310 and conventional alpha helix (Fig. 4B, grey arrow). Both of these structures do however conform to a 310 helix for one turn between residues α45-48. The other HLA-DQ structures that vary from the normal distribution in this region have 310 helices that extend fully from α45-51, although they all have an increased NH-CO i+3 hydrogen bond lengths of ~3.8A, as compared to 1DLH and the other structures for which this distance is ~3.3 Å.
Aligned with the 310 helix is another similarly oriented short helical region from the β subunit that correspondingly connects the β1 domain α helix to the lower β2 Ig domain (Fig. 4A,B). This region however is found in a conventional α helical conformation and is much less conformationally heterogeneous in MHCII structures determined to date. Some structural variation is observed in this region for the several I-Ad (1IAO) and I-Ab (1MUJ, 3C60, 3RDT, 1LNU, 3C5Z, 3C6L) structures, which all have proline at position β86.
The third region that exhibits significant structural variation in MHCII protein structure lies within the β1 helix at the kinked region from residues β62-70 (Fig. 5). This region has extensive polymorphoric variation and is also a site that has been implicated as undergoing peptide-induced conformational changes (see below). We aligned the peptide binding domains (α1-85 and β1-90) of all the structures to 1DLH in order to examine regions of conformational variability (Fig. 5A,B). The majority of structures cluster together with Cα deviations throughout the peptide binding site of less than 2Å. The major exception to this (in addition to the 310 helix) is in the region around Asp β66, which shows a 3.7Å range even between structures that cluster tightly throughout the rest of the peptide binding domain (Fig. 5B). There are two groups of outliers in this region. One consists of several I-A proteins that have a two-residue deletion in the β63-67 region, which causes the kinked region to tilt inward toward the peptide binding groove by approximately 4Å (Fig. 5B, open arrow). The other group is consists of the two HLA-DRB5*01:01 structures 1H15 and 1ZGL. This group is displaced in the opposite direction at Asp β66, moving up an away from the peptide by ~3Å. In both cases in the crystal lattice there are intermolecular contacts at Asp β66, one by a crystal contact (1H15) and one by a TCR contact (1ZGL). The latter is described in detail by Mariuzza’s group (37), who compare the MHCII structure with and without TCR and find that the peptide binding groove is opened by 1.5Å. They note the extensive contact at the β kinked region and the change induced there by the TCR Vβ contact. The conformational changes described by Li et al. (37) in the TCR-bound HLA-DRB5*01:01 are very similar to those induced in the other HLA-DRB5*0101 structure (1H15) at the crystal contact. We aligned the two molecules in the asymmetric unit of 1H15 and found that the crystal contact induces a 3Å displacement of the beta Aspβ66 away from the peptide binding domain (Fig. 5C,D). There are extensive crystal contacts at other regions in this structure that do not result in conformational changes, which suggests this region in particular is labile. This alternate conformation exists despite the fact that this structure was constrained with an r.m.s. of 0.5Å for all C α atoms (38).
Dynamic nature of the HLA-DR1 αF54C mutant MHCII-peptide complex
The structural variation in published MHCII-peptide structures could be due to structural differences induced by peptide or MHC sequence changes. However, in some cases the structural variation is observed even for multiple copies of the same protein within the crystallographic asymmetric unit, and so is likely to represent actual structural lability rather than peptide-dependent changes or allelic variation. For example, the β2 Ig domain hinge motions were noted even in the first MHCII structures, which contained a dimer in the asymmetric unit (23, 25).
We recently reported the 2.3Å crystal structure of HLA-DR1 (αF54C) bound to CLIP, along with WT HLA-DR1 bound to CLIP that crystallized in the same unit cell (39). The αF54C point mutation in the peptide binding site makes the protein exquisitely susceptibility to DM-mediated peptide release, more than 100-fold compared to WT HLA-DR1, and also increases the affinity for DM. The crystal structure revealed that the mutation facilitated a conformational change in one molecule of the asymmetric unit. The conformational change involved reorientation of the α-subunit 310 helix, along with concerted rotamer changes in residues Leu α45, Phe α48, Phe α51, and Phe β89 (Fig. 5B). Similar rotamer changes for residues Leu α45, Phe α48, and Phe α51 are seen for all HLA-DQ structures that have the HLA-DQA*0301α chain, 2NNA, 1JK8, and 4D8P. The conformational change in the αF54C mutant appeared to be induced by a crystal contact at Phe αF51, which contacted the β2 domain of another molecule in the crystal. The ability to adopt the altered conformation was induced by the mutation, as the WT HLA-DR1-CLIP crystal had similar crystal contacts, but no change was induced in the WT structure. The Phe α51 side chain is known from mutagenesis experiments in the WT protein to be an important DM contact site (40), and we speculated that the increased susceptibility to DM and increased DM affinity induced by the αF54C mutation were due to increased tendency to adopt the alternate conformation (39).
Evidence for increased lability in solution for αF54C and not just in crystals was obtained by hydrogen-deuterium exchange mass spectrometry (39). In this experiment, borrowed from the arsenal of tools used to investigate protein folding, peptide bond amide NH groups exchange with D2O at different rates depending on their exposure to solvent and their participation in hydrogen-bonding interactions (Fig. 7A). We incubated pre-formed MHCII-peptide complexes in D2O for various times, before transfer to cold acidic buffer to stop further exchange and strip the peptide from MHC for analysis by multi-dimensional mass spectrometry. The location of individual deuterated amide NH groups was determined by electron transfer dissociation (ETD) fragmentation (39). We found that amide bonds flanking the P1 pocket exchanged very slowly, implicating in particular the strong H-bonds involving the MHCII Asn β82 side chain and Serα53 main chain (Fig. 7B). A much earlier study evaluating the effects of mutation of H-bonding residues on MHC-peptide dissociation revealed essentially the same phenomenon as a striking difference in the roles of Asn α 69 and Asnβ82 (41). For the HLA-DR1 αF54C mutation, faster exchange relative to WT HLA-DR1 was noted at the very stable P2 amide NH hydrogen bond involving Asn β82 (Fig. 6B). Since the αF54C crystal structure retained the intact canonical hydrogen bond network, we interpreted this difference in H-D exchange rates as evidence for dynamic flexibility in the αF54C peptide binding groove, in particular around the P1 site.
Fig. 7. Hydrogen-deuterium exchange mass spectrometry provides information on stability of MHCII-peptide hydrogen bonds.
(A) Mass spectra of HA peptide in H2O (left), HA peptide in D2O (center), and HA peptide bound to MHCII after incubation in D2O for 30 seconds. With increasing time in D2O additional exchange is observed, depending on amide hydrogen bonding and exposure. Cartoons above spectra are intended to indicate general effect of MHC-peptide hydrogen-bonding on hydrogen-deuterium exchange. (B) Summary of hydrogen-deuterium electron transfer dissociation data for amide NH groups of HA peptide bound to HLA-DR1, from Painter et al. (39). MHC-peptide hydrogen bond network shown by dashed lines. NH groups were characterized as having slow (red), medium (orange), or fast (green) exchange kinetics based on degree of exchange of NH at several time points: slow, less than 30% at all time points; medium, less than 80% exchanged at 128 minutes; fast, more than 80% exchanged at 2 minutes and/or unprotected. Star indicates the NH group that experienced less protection for HA bound to the αF54C mutant of HLA-DR1, where it was assigned a medium exchange rate value.
Fig. 6. HLA-DR1 αF54C mutant structure showing conformational change between two molecules in the asymmetric unit.
Two molecules in asymmetric unit are shown in green and salmon. (A) Top view with residues that undergo conformational changes shown in stick representation. (B) 90° rotation of the top panel showing the altered pitch of the 3–10 alpha helix and rotomer changes
Conformational changes in MHCII induced by peptide binding and release
No crystal or NMR structure has been reported for any MHCII protein in the absence of peptide, but biophysical and biochemical studies have shown that the peptide-free or ‘empty’ form of MHCII has significant conformational changes relative to the canonical peptide-bound form. For HLA-DR1 at least, peptide-free species can be obtained by refolding purified α and β subunits expressed separately in E. coli (42). Peptide binding stabilizes the protein against SDS-induced chain dissociation and thermal denaturation (42), but even in the absence of the peptide HLA-DR1 is thermally stable (Tm ~70°C) and folded correctly as shown by CD spectroscopy and conformation-dependent antibody binding (42, 43). Peptide binding induces changes in hydrodynamic behavior consistent with ~10–15% reduction in hydrodynamic radius, as observed by gel filtration, dynamic light scattering, and analytical ultracentrifugation (42), together with alterations in the CD spectrum consistent with a small increase in helical content. Together these were interpreted in terms of local conformational changes and/or small domain reorientations (43). Several monoclonal antibodies specifically recognize the empty protein (44, 45). Epitopes for these antibodies have been mapped to two regions, one including residues β53-67 near the β subunit helical kink, and the other at one including residues β182-190 at the end of the β2 Ig domain (44). Because of the way these antibodies were raised, only β subunit specificities were obtained. Evidence for peptide-induced conformational changes in the alpha subunit was obtained by a differential chemical modification approach, which identified Lys α67 and Arg α50, in addition to Lys β98 and Arg β189, as sites of peptide-induced changes in reactivity or accessibility (46). Overall, these studies provide clear evidence for peptide-driven conformational changes in HLA-DR1. Although these studies provide only relatively low resolution information, they appear to implicate the same regions for which conformational variation was observed in the crystal structure, viz. the α subunit 310 helix, the β1 domain helical kink, and β2 Ig domain.
In the absence of experimental high-resolution structural information on the peptide-free MHCII conformation, several groups have used molecular dynamics and other computational approaches to gain insight into peptide-dependent conformational changes (47–50). The simulations all showed changes throughout the peptide-binding groove upon removal of peptide (47, 49, 50). A normal mode analysis of the peptide-free protein revealed similar motions (48). In one study, we removed the peptide from a HLA-DR1-peptide crystal structure and ran molecular dynamics simulations to explore conformations accessible to the peptide-free form (49). This simulation revealed conformational changes near the α subunit 310 helix and β subunit helical kink. Theα51-59 change was a refolding of the extended strand region from the side of the binding groove into the position usually occupied by peptide (Fig. 8A). Strikingly, these alterations in structure maintained the general shape and electrostatic environment of the entire peptide binding groove, in particular, with the αF54 side chain engaging the P1 pocket in a similar manner as the original peptide P1 side chain (Fig. 8B). In addition, the canonical P-1 to P4 hydrogen bonding network was recapitulated between the conserved residues of the β chain and the reoriented α51-59 region. Experimental support for this model was generated using monoclonal antibodies and a bacterial superantigen that bound epitopes on HLA-DR1 predicted to have altered accessibility due to the conformational changes induced upon peptide removal. This study also revealed a reorientation of the α subunit 310 helix and a rigid body motion around the β1 domain helical kink (Fig. 8A). Other molecular dynamics simulations of peptide-free MHCII are in agreement with a conformational change in the β kinked region as well as with more lability in the α subunit 310 region, although these studies did not find the same degree of reorientation of the α51-59 strand region (47, 48, 50).
Fig. 8. Comparison of crystal structure of MHCII – peptide complex and molecular dynamics model of peptide-free MHCII.
(A) Peptide-binding domain of HLA-DR1 with HA peptide (1DLH, cyan) and peptide-free model from molecular dynamics simulation (49). Side chain specificity pockets are labeled P1-P9. In the peptide-free model the α chain has partially unwound and the extended strand region has moved into the peptide-binding groove. Residues α51 and α59 that define the extent of the conformational rearrangement are indicated). (B) Surface representation cutaway view of peptide binding site (same orientation as Fig. 1C) with the P1 pocket engaged by the HA peptide tyrosine in the MHCII-peptide complex or by the extended strand region αF54 side chain in the peptide-free model.
Detailed kinetics studies from several groups have showed that peptide-free MHCII proteins can adopt (at least) two forms that differ in their ability to bind peptide (43, 51, 52), providing evidence for some degree of structural lability even in the absence of peptide. These experiments show a time-dependent decrease in MHCII peptide-binding capacity after removal of a bound peptide and have been interpreted as showing that a peptide-receptive form is generated immediately upon peptide release, which slowly and reversibly converts to a peptide-averse state over a few minutes (52). A role for DM in converting between these species has been suggested, but this is controversial (53, 54). Although there is ample evidence for distinct kinetic species, structural correlates of the peptide-receptive and peptide-averse forms have not been defined. An attractive model for the peptide-averse form is the molecular dynamics-derived structure described above with the α51-59 region folded into the peptide binding site. In that scenario, engagement of the P1 pocket by the αF54 side chain and involvement of the conserved MHC hydrogen bonding residues would occlude the N-terminal end of the groove and interfere with peptide binding. However, this idea remains to be tested experimentally.
Nonclassical MHCII: HLA-DM and HLA-DO
Crystal structures for the nonclassical MHCII protein HLA-DM and its murine counterpart H-2M were first reported in 1998 (55, 56), with a higher resolution DM structure in 2006 (57). The protein fold overall is similar to classical MHCII, but with a distinctly collapsed upper domain (Fig. 9). DM is not known to bind peptides or other ligands, and no binding cleft is apparent in the upper domain [a small ‘vestigial’ pocket in the vicinity of MHCII P4 region is apparent, but is believe not to have functional role (55)]. In this aspect, DM is like some nonclassical MHCI proteins, which do not appear to bind peptide or other ligands and which also have closed binding sites (58). However, unlike these nonclassical MHCI proteins, DM does not interact at the cell surface with binding partners on other cells but instead acts to facilitate MHCII-peptide exchange by interacting with classical MHCII peptide complexes in the same membrane (59). The pattern of mutations known to disrupt DM-MHCII interaction (40, 60, 61) and studies of tethered complexes (62, 63) support a side-by-side orientation of DM in its interaction with MHCII. How this interaction leads to facilitated peptide binding and release is still a mystery, despite extensive efforts in many laboratories, and several mechanisms have been proposed. A mechanism in which HLA-DM mediates peptide catalysis by disruption of one or more hydrogen bonds between the peptide and the MHCII has been the focus of a number of studies (8, 64, 65). However, recent mutagenesis studies suggest that none of the individual hydrogen bonds that extend from the peptide to the MHCII side chains are responsible for the increased rate of peptide catalysis by HLA-DM (66, 67). Other work suggesting a compare-and-exchange mechanism in which DM mediates exchange of free peptide with MHC-bound peptide through a two-peptide intermediate state has been put forth (68). A number of studies have focused on the ability of HLA-DM to bind to alternate conformers of MHCII. One study suggests HLA-DM recognizes a ‘floppy’ P1 pocket (69). In addition, HLA-DM has been proposed to recognize MHCII that has a vacant P1 pocket (60). The HLA-DR1 αF54C mass spectrometry work from our lab suggests that HLA-DM preferentially binds MHCII with conformational flexibility in the 310 helical region, leading to binding of HLA-DM to conformers of MHCII regardless of the peptide occupancy (39). A recent report showing that peptide-loaded MHCII isomers can be substrates for HLA-DM could be interpreted such that conformational heterogeneity of the MHCII peptide complex, rather than the occupancy of the P1 pocket, is a crucial determinant for HLA-DM peptide catalysis (70).
Fig. 9. Structural similarities between classical MHCII (HLA-DR1) and nonclassical MHCII (HLA-DO and HLA-DM).
α chains shown in brown, beta chains in cyan, and peptide (for HLA-DR1) shown in red. (A) Top view of peptide binding site. (B) Same view as top panel with surface representation. (C) 90° rotation of the top panel.
Recently we determined the crystal structure of the other nonclassical MHCII protein, DO, in complex with DM (data not shown). The overall fold is very much like a classical MHCII protein and distinctly different from that of DM (Fig. 9). The upper domain is not collapsed as for DM and still retains the contours of an apparent binding cleft, although density was not observed in the binding site and DO has not been reported to bind peptides or other ligands. Interestingly, large structural differences relative to classical MHCII were mostly restricted to the α subunit 310 region and β2 Ig-like domain, i.e. the same regions where MHCII proteins have conformational variability. The conformational alterations at the N-terminal side of the peptide-binding site would be expected to interfere with peptide binding, at least by analogy with MHCII, and might help explain DO’s tight binding to DM.
Conclusions
Analysis of available structural information on MHCII proteins reveals three areas of conformational lability: the region around the α subunit 310 helix, the β1 domain helical kink, and the β2 Ig domain lower loops. Several sources of information, including conformational variation observed for crystal structures of different MHCII – peptide complexes, differences between identical molecules in different crystallographic asymmetric units, biochemical studies of peptide-induced conformational changes, and computational modeling of empty and peptide-loaded MHCII proteins, all point to structural lability in the same regions. At least for the α subunit 310 helix region, the changes appear to be consistent with a concerted motion between stable conformers, rather than a continuum of related conformations. This region, along with the distal end of the β2 domain Ig region, has been implicated in DM catalysis (40, 60, 62). How DM works still is not clear, but most studies point to important role for peptide-MHC interaction at N-terminus of the binding site in DM function. The DR1 αF54C mutant, which exhibits greatly increased susceptibility to DM action, increased structural lability of the α subunit 310 helix, and weakened MHC-peptide hydrogen bonding around the P1 pocket, provides additional support for the importance of this region. The recent structure of HLA-DO bound to HLA-DM provides another example of conformational variation within the overall MHCII fold, with alterations in the α subunit 310 helix and adjacent regions that appear to be responsible for tight binding to DM. Although the DO structure shows larger conformational alterations than observed within the current set of classical MHCII – peptide crystal structures, the changes are in the same regions, and molecular dynamics studies suggest that similar and even larger changes in the same regions are accessible to MHCII proteins in the absence of peptide. Given the strong homology between DO and classical class II MHC proteins, is it possible that the conformational alterations in MHCII proteins described represent snapshots along a trajectory for DM-MHCII interaction and subsequent peptide exchange.
Acknowledgments
Supported by NIH AI38996 and AI48833.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 2.Roche PA, Marks MS, Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991;354:392–394. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- 3.Santambrogio L, Potolicchio I, Fessler SP, Wong SH, Raposo G, Strominger JL. Involvement of caspase-cleaved and intact adaptor protein 1 complex in endosomal remodeling in maturing dendritic cells. Nat Immunol. 2005;6:1020–1028. doi: 10.1038/ni1250. [DOI] [PubMed] [Google Scholar]
- 4.Jasanoff A, Park SJ, Wiley DC. Direct observation of disordered regions in the major histocompatibility complex class II-associated invariant chain. Proc Natl Acad Sci USA. 1995;92:9900–9904. doi: 10.1073/pnas.92.21.9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avva RR, Cresswell P. In vivo and in vitro formation and dissociation of HLA-DR complexes with invariant chain-derived peptides. Immunity. 1994;1:763–774. doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh CS, deRoos P, Honey K, Beers C, Rudensky AY. A role for cathepsin L and cathepsin S in peptide generation for MHC class II presentation. J Immunol. 2002;168:2618–2625. doi: 10.4049/jimmunol.168.6.2618. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh P, Amaya M, Mellins E, Wiley DC. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457–462. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- 8.Weber DA, Evavold BD, Jensen PE. Enhanced dissociation of HLA-DR-bound peptides in the presence of HLA-DM. Science. 1996;274:618–620. doi: 10.1126/science.274.5287.618. [DOI] [PubMed] [Google Scholar]
- 9.Sherman MA, Weber DA, Jensen PE. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 10.Sloan VS, et al. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 11.Vogt AB, Kropshofer H, Moldenhauer G, Hammerling GJ. Kinetic analysis of peptide loading onto HLA-DR molecules mediated by HLA-DM. Proc Natl Acad Sci USA. 1996;93:9724–9729. doi: 10.1073/pnas.93.18.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 13.Pathak SS, Lich JD, Blum JS. Cutting edge: editing of recycling class II: peptide complexes by HLA-DM. J Immunol. 2001;167:632–635. doi: 10.4049/jimmunol.167.2.632. [DOI] [PubMed] [Google Scholar]
- 14.Jensen PE. Antigen processing: HLA-DO--a hitchhiking inhibitor of HLA-DM. Curr Biol. 1998;8:R128–131. doi: 10.1016/s0960-9822(98)70988-1. [DOI] [PubMed] [Google Scholar]
- 15.Yi W, Seth NP, Martillotti T, Wucherpfennig KW, Sant’Angelo DB, Denzin LK. Targeted regulation of self-peptide presentation prevents type I diabetes in mice without disrupting general immunocompetence. J Clin Invest. 2010;120:1324–1336. doi: 10.1172/JCI40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfonso C, Williams GS, Han JO, Westberg JA, Winqvist O, Karlsson L. Analysis of H2-O influence on antigen presentation by B cells. J Immunol. 2003;171:2331–2337. doi: 10.4049/jimmunol.171.5.2331. [DOI] [PubMed] [Google Scholar]
- 17.Denzin LK, Sant’Angelo DB, Hammond C, Surman MJ, Cresswell P. Negative regulation by HLA-DO of MHC class II-restricted antigen processing. Science. 1997;278:106–109. doi: 10.1126/science.278.5335.106. [DOI] [PubMed] [Google Scholar]
- 18.Liljedahl M, et al. Altered antigen presentation in mice lacking H2-O. Immunity. 1998;8:233–243. doi: 10.1016/s1074-7613(00)80475-6. [DOI] [PubMed] [Google Scholar]
- 19.van Ham SM, et al. HLA-DO is a negative modulator of HLA-DM-mediated MHC class II peptide loading. Curr Biol. 1997;7:950–957. doi: 10.1016/s0960-9822(06)00414-3. [DOI] [PubMed] [Google Scholar]
- 20.Kropshofer H, Hammerling GJ, Vogt AB. The impact of the nonclassical MHC proteins HLA-DM and HLA-DO on loading of MHC class II molecules. Immunol Rev. 1999;172:267–278. doi: 10.1111/j.1600-065x.1999.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 21.Perraudeau M, et al. Altered major histocompatibility complex class II peptide loading in H2-O-deficient mice. Eur J Immunol. 2000;30:2871–2880. doi: 10.1002/1521-4141(200010)30:10<2871::AID-IMMU2871>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 22.Marsh SG, et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010;75:291–455. doi: 10.1111/j.1399-0039.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown JH, et al. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993;364:33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- 24.Jardetzky TS, et al. Crystallographic analysis of endogenous peptides associated with HLA-DR1 suggests a common, polyproline II-like conformation for bound peptides. Proc Natl Acad Sci USA. 1996;93:734–738. doi: 10.1073/pnas.93.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern LJ, et al. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994;368:215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- 26.Gunther S, et al. Bidirectional binding of invariant chain peptides to an MHC class II molecule. Proc Natl Acad Sci USA. 2010;107:22219–22224. doi: 10.1073/pnas.1014708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato AK, et al. Determinants of the peptide-induced conformational change in the human class II major histocompatibility complex protein HLA-DR1. J Biol Chem. 2000;275:2165–2173. doi: 10.1074/jbc.275.3.2165. [DOI] [PubMed] [Google Scholar]
- 28.Runnels HA, Weber DA, Moore JC, Westerman LE, Jensen PE. Intact proteins can bind to class II histocompatibility molecules with high affinity. Mol Immunol. 1997;34:471–480. doi: 10.1016/s0161-5890(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 29.Chicz RM, et al. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992;358:764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- 30.Zavala-Ruiz Z, Strug I, Anderson MW, Gorski J, Stern LJ. A polymorphic pocket at the P10 position contributes to peptide binding specificity in class II MHC proteins. Chem Biol. 2004;11:1395–1402. doi: 10.1016/j.chembiol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Zavala-Ruiz Z, et al. Exploration of the P6/P7 region of the peptide-binding site of the human class II major histocompatability complex protein HLA-DR1. J Biol Chem. 2003;278:44904–44912. doi: 10.1074/jbc.M307652200. [DOI] [PubMed] [Google Scholar]
- 32.Sturniolo T, et al. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat Biotechnol. 1999;17:555–561. doi: 10.1038/9858. [DOI] [PubMed] [Google Scholar]
- 33.Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol. 2008;4:e1000048. doi: 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrante A, Gorski J. Cooperativity of hydrophobic anchor interactions: evidence for epitope selection by MHC class II as a folding process. J Immunol. 2007;178:7181–7189. doi: 10.4049/jimmunol.178.11.7181. [DOI] [PubMed] [Google Scholar]
- 35.Vieira-Pires RS, Morais-Cabral JH. 3(10) helices in channels and other membrane proteins. J Gen Physiol. 2010;136:585–592. doi: 10.1085/jgp.201010508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou T, et al. An insertion mutant in DQA1*0501 restores susceptibility to HLA-DM: implications for disease associations. J Immunol. 2011;187:2442–2452. doi: 10.4049/jimmunol.1100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Huang Y, Lue J, Quandt JA, Martin R, Mariuzza RA. Structure of a human autoimmune TCR bound to a myelin basic protein self-peptide and a multiple sclerosis-associated MHC class II molecule. EMBOJ. 2005;24:2968–2979. doi: 10.1038/sj.emboj.7600771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang HL, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002;3:940–943. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 39.Painter CA, Negroni MP, Kellersberger KA, Zavala-Ruiz Z, Evans JE, Stern LJ. Conformational lability in the class II MHC 310 helix and adjacent extended strand dictate HLA-DM susceptibility and peptide exchange. Proc Natl Acad Sci USA. 2011;108:19329–19334. doi: 10.1073/pnas.1108074108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doebele RC, Busch R, Scott HM, Pashine A, Mellins ED. Determination of the HLA-DM interaction site on HLA-DR molecules. Immunity. 2000;13:517–527. doi: 10.1016/s1074-7613(00)00051-0. [DOI] [PubMed] [Google Scholar]
- 41.McFarland BJ, Katz JF, Beeson C, Sant AJ. Energetic asymmetry among hydrogen bonds in MHC class II*peptide complexes. Proc Natl Acad Sci USA. 2001;98:9231–9236. doi: 10.1073/pnas.151131498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frayser M, Sato AK, Xu L, Stern LJ. Empty and peptide-loaded class II major histocompatibility complex proteins produced by expression in Escherichia coli and folding in vitro. Protein Expr Purif. 1999;15:105–114. doi: 10.1006/prep.1998.0987. [DOI] [PubMed] [Google Scholar]
- 43.Zarutskie JA, et al. A conformational change in the human major histocompatibility complex protein HLA-DR1 induced by peptide binding. Biochemistry. 1999;38:5878–5887. doi: 10.1021/bi983048m. [DOI] [PubMed] [Google Scholar]
- 44.Carven GJ, et al. Monoclonal antibodies specific for the empty conformation of HLA-DR1 reveal aspects of the conformational change associated with peptide binding. J Biol Chem. 2004;279:16561–16570. doi: 10.1074/jbc.M314315200. [DOI] [PubMed] [Google Scholar]
- 45.Santambrogio L, Sato AK, Carven GJ, Belyanskaya SL, Strominger JL, Stern LJ. Extracellular antigen processing and presentation by immature dendritic cells. Proc Natl Acad Sci USA. 1999;96:15056–15061. doi: 10.1073/pnas.96.26.15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carven GJ, Stern LJ. Probing the ligand-induced conformational change in HLA-DR1 by selective chemical modification and mass spectrometric mapping. Biochemistry. 2005;44:13625–13637. doi: 10.1021/bi050972p. [DOI] [PubMed] [Google Scholar]
- 47.Gupta S, et al. Anchor side chains of short peptide fragments trigger ligand-exchange of class II MHC molecules. PLoS One. 2008;3:e1814. doi: 10.1371/journal.pone.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nojima H, Takeda-Shitaka M, Kurihara Y, Kamiya K, Umeyama H. Dynamic flexibility of a peptide-binding groove of human HLA-DR1 class II MHC molecules: normal mode analysis of the antigen peptide-class II MHC complex. Chem Pharm Bull. 2003;51:923–928. doi: 10.1248/cpb.51.923. [DOI] [PubMed] [Google Scholar]
- 49.Painter CA, Cruz A, Lopez GE, Stern LJ, Zavala-Ruiz Z. Model for the peptide-free conformation of class II MHC proteins. PLoS One. 2008;3:e2403. doi: 10.1371/journal.pone.0002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yaneva R, Springer S, Zacharias M. Flexibility of the MHC class II peptide binding cleft in the bound, partially filled, and empty states: a molecular dynamics simulation study. Biopolymers. 2009;91:14–27. doi: 10.1002/bip.21078. [DOI] [PubMed] [Google Scholar]
- 51.Sadegh-Nasseri S, Stern LJ, Wiley DC, Germain RN. MHC class II function preserved by low-affinity peptide interactions preceding stable binding. Nature. 1994;370:647–650. doi: 10.1038/370647a0. [DOI] [PubMed] [Google Scholar]
- 52.Rabinowitz JD, et al. Formation of a highly peptide-receptive state of class II MHC. Immunity. 1998;9:699–709. doi: 10.1016/s1074-7613(00)80667-6. [DOI] [PubMed] [Google Scholar]
- 53.Zarutskie JA, Busch R, Zavala-Ruiz Z, Rushe M, Mellins ED, Stern LJ. The kinetic basis of peptide exchange catalysis by HLA-DM. Proc Natl Acad Sci USA. 2001;98:12450–12455. doi: 10.1073/pnas.211439398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grotenbreg GM, et al. Empty class II MHC created by peptide photolysis establishes role of DM in peptide association. J Biol Chem. 2007;282:21425–21436. doi: 10.1074/jbc.M702844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mosyak L, Zaller DM, Wiley DC. The structure of HLA-DM, the peptide exchange catalyst that loads antigen onto class II MHC molecules during antigen presentation. Immunity. 1998;9:377–383. doi: 10.1016/s1074-7613(00)80620-2. [DOI] [PubMed] [Google Scholar]
- 56.Fremont DH, Crawford F, Marrack P, Hendrickson WA, Kappler J. Crystal structure of mouse H2-M. Immunity. 1998;9:385–393. doi: 10.1016/s1074-7613(00)80621-4. [DOI] [PubMed] [Google Scholar]
- 57.Nicholson MJ, et al. Small molecules that enhance the catalytic efficiency of HLA-DM. J Immunol. 2006;176:4208–4220. doi: 10.4049/jimmunol.176.7.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson IA, Bjorkman PJ. Unusual MHC-like molecules: CD1, Fc receptor, the hemochromatosis gene product, and viral homologs. Curr Opin Immunol. 1998;10:67–73. doi: 10.1016/s0952-7915(98)80034-4. [DOI] [PubMed] [Google Scholar]
- 59.Zwart W, et al. Spatial separation of HLA-DM/HLA-DR interactions within MIIC and phagosome-induced immune escape. Immunity. 2005;22:221–233. doi: 10.1016/j.immuni.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Anders AK, et al. HLA-DM captures partially empty HLA-DR molecules for catalyzed removal of peptide. Nat Immunol. 2011;12:54–61. doi: 10.1038/ni.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pashine A, et al. Interaction of HLA-DR with an acidic face of HLA-DM disrupts sequence-dependent interactions with peptides. Immunity. 2003;19:183–192. doi: 10.1016/s1074-7613(03)00200-0. [DOI] [PubMed] [Google Scholar]
- 62.Stratikos E, Mosyak L, Zaller DM, Wiley DC. Identification of the lateral interaction surfaces of human histocompatibility leukocyte antigen (HLA)-DM with HLA-DR1 by formation of tethered complexes that present enhanced HLA-DM catalysis. J Exp Med. 2002;196:173–183. doi: 10.1084/jem.20020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Busch R, Pashine A, Garcia KC, Mellins ED. Stabilization of soluble, low-affinity HLA-DM/HLA-DR1 complexes by leucine zippers. J Immunol Methods. 2002;263:111–121. doi: 10.1016/s0022-1759(02)00034-0. [DOI] [PubMed] [Google Scholar]
- 64.Narayan K, et al. HLA-DM targets the hydrogen bond between the histidine at position beta81 and peptide to dissociate HLA-DR-peptide complexes. Nat Immunol. 2007;8:92–100. doi: 10.1038/ni1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stratikos E, Wiley DC, Stern LJ. Enhanced catalytic action of HLA-DM on the exchange of peptides lacking backbone hydrogen bonds between their N-terminal region and the MHC class II alpha-chain. J Immunol. 2004;172:1109–1117. doi: 10.4049/jimmunol.172.2.1109. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Z, Callaway KA, Weber DA, Jensen PE. Cutting edge: HLA-DM functions through a mechanism that does not require specific conserved hydrogen bonds in class II MHC-peptide complexes. J Immunol. 2009;183:4187–4191. doi: 10.4049/jimmunol.0901663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrante A, Gorski J. Cutting edge: HLA-DM-mediated peptide exchange functions normally on MHC class II-peptide complexes that have been weakened by elimination of a conserved hydrogen bond. J Immunol. 2009;184:1153–1158. doi: 10.4049/jimmunol.0902878. [DOI] [PubMed] [Google Scholar]
- 68.Ferrante A, Anderson MW, Klug CS, Gorski J. HLA-DM mediates epitope selection by a “compare-exchange” mechanism when a potential peptide pool is available. PLoS One. 2008;3:e3722. doi: 10.1371/journal.pone.0003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chou CL, Sadegh-Nasseri S. HLA-DM recognizes the flexible conformation of major histocompatibility complex class II. J Exp Med. 2000;192:1697–1706. doi: 10.1084/jem.192.12.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferrante A, Gorski J. A Peptide/MHCII conformer generated in the presence of exchange peptide is substrate for HLA-DM editing. Sci Rep. 2012;2:386. doi: 10.1038/srep00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robinson J, et al. The IMGT/HLA database. Nucleic Acids Res. 2009;37:D1013–1017. doi: 10.1093/nar/gkn662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Altschul SF, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chevenet F, Brun C, Banuls AL, Jacq B, Christen R. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics. 2006;7:439. doi: 10.1186/1471-2105-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]