Summary
The lateral habenula (LHb) has recently been identified as a key regulator of the reward system by driving inhibition onto dopaminergic neurons. However, the nature and potential modulation of the major input to the LHb originating from the basal ganglia are poorly understood. Although the output of the basal ganglia is thought to be primarily inhibitory, here we show that transmission from the basal ganglia to the LHb is excitatory, glutamatergic and suppressed by serotonin. Behaviorally, activation of this pathway is aversive, consistent with its role as an ‘anti-reward’ signal. Our demonstration of an excitatory projection from the basal ganglia to the LHb explains how LHb-projecting basal ganglia neurons can have similar encoding properties as LHb neurons themselves. Our results also provide a link between ‘anti-reward’ excitatory synapses and serotonin, a neuromodulator implicated in depression.
Introduction
Recent studies in monkeys have shown that neurons in the LHb become active when an animal fails to receive an expected reward or if the animal receives a signal indicating a negative outcome (Matsumoto and Hikosaka, 2007), i.e. these neurons encode ‘anti-reward’ conditions and compute reward prediction errors – the difference between the amount of reward expected and the amount of reward received, a computation that is thought to drive reinforcement learning (Sutton and Barto, 1998). LHb neurons have also been shown to inhibit dopaminergic neurons in the ventral tegmental area (VTA) (Ji and Shepard, 2007), which encode ‘reward’ conditions (Schultz, 1997; but see Matsumoto and Hikosaka, 2009). These findings are consistent with the view that an ‘anti-reward’ (LHb) nucleus inhibits a reward (VTA) center and drives negative reward signals in dopamine neurons. However, the nature of the inputs that drive aversive responses in the LHb, as well as their possible modulation by other neurotransmitters, is poorly understood.
The globus pallidus internus (GPi), an output region of the basal ganglia, and its non-primate homologue, the entopeduncular (EP) nucleus, are major sources of input to the primate (Kim et al., 1976; Parent et al., 2001) and rodent (Herkenham and Nauta, 1977) LHb, respectively, as well as the thalamus (Filion and Harnois, 1978; Harnois and Filion, 1982; Parent et al., 2001). The GPi and EP are thought to be primarily inhibitory brain regions since neurons in both structures express GABAergic molecular markers (Oertel et al., 1984; Stephenson et al., 2005) and lesions of the EP greatly reduce these markers in the LHb and thalamus (Penney and Young, 1981; Vincent et al., 1982). Recently, it was found that most LHb-projecting pallidal neurons have reward-modulated activity that begins before that of LHb neurons themselves, consistent with upstream control of LHb neurons (Hong and Hikosaka, 2008). Surprisingly, LHb-projecting pallidal neurons display ‘anti-reward’ characteristics, similar to LHb neurons (Hong and Hikosaka, 2008). This finding suggests either that inhibitory projections out of the basal ganglia disynaptically disinhibit LHb neurons or that a previously unidentified excitatory projection exists from the basal ganglia to the LHb.
Here we test the hypothesis that an excitatory projection exists from the EP to the LHb that signals aversive events. We use a combination of optogenetics and immunohistochemistry to show that the projection from the EP to the LHb is predominantly excitatory, glutamatergic and aversive. We also show that the excitatory projection from the EP to the LHb is suppressed by low concentrations of serotonin, providing a link between aversive signaling in the LHb and a neuromodulator involved in mood disorders.
Results
Excitatory, glutamatergic projections from the EP to the LHb
To test the hypothesis that the projection from the EP to the LHb is excitatory, we injected AAV driving expression of the light-inducible cation channel, channelrhodopsin-2 (Boyden et al., 2005), tagged with YFP (ChR2-YFP), into the EP in vivo. Two weeks after injection, we prepared coronal brain slices, which displayed localized fluorescence in neuronal cell bodies in the EP (Fig. 1a and Supplementary Fig. 1) as well as fluorescent fibers in the projection region, the lateral aspect of the LHb (Fig. 1b and Supplementary Fig. 1). To test for excitation of LHb neurons by EP inputs, we obtained whole-cell current clamp recordings from neurons in the lateral aspect of the LHb and stimulated the EP inputs to the LHb with brief (0.5–5 ms) pulses of 470 nm light through an LED-coupled optic fiber placed over the LHb. Consistent with the EP providing excitatory input to the LHb, light stimulation produced depolarizing synaptic responses at resting potentials (Fig. 1c,d; 13/13 cells depolarized). To maximize detection of any hyperpolarizing synaptic response, we injected depolarizing current and raised the membrane potential close to 0 mV. Even in these conditions the slope of the postsynaptic response remained positive for 12 out of 13 cells (Fig. 1c,d), indicating dominant depolarizing synaptic input. Bath application of NBQX largely blocked the excitatory response indicating its mediation by AMPA-type glutamate receptors (Fig. 1e), although we could detect GABA-mediated currents when cells were clamped at positive holding potentials (Supplementary Fig. 1). Voltage-clamp recordings in the presence of picrotoxin (100 µM) showed that the excitatory EP input consisted of inwardly rectifying AMPA responses and minimal NMDA responses (Fig. 1f,g).
Figure 1.
Stimulation of EP axons in the LHb excites LHb neurons via activation of glutamate receptors. (a) Example of injection of AAV-ChR2-YFP (green) into EP (insets). (b) Example of EP projections expressing ChR2-YFP in LHb (insets). Projections are from the same injection shown in (a) (see Supplementary Fig. 1 for higher magnification pictures). (c) Left, light-evoked depolarization at resting potential. Right, Light-evoked depolarization of same cell at depolarized potential. Light pulse (2 ms) delivered at t=0. Bold line indicates regions used to compute the postsynaptic potential (PSP) slope. (d) Light-evoked PSP slopes at resting potential (circles) and depolarized potentials (triangles) for 13 cells. (e) Light-evoked responses reduced by NBQX (3 µM). Light pulses indicated by marks above traces, here and in Fig. 4. (f) Example of light-evoked excitatory currents at Vclamp = -50 mV blocked by NBQX (see Supplementary Fig. 1 for example of light-evoked GABA-A response). (g) I-V curves for NMDA and AMPA responses fitted with second-order polynomials and normalized to the AMPA response at −60 mV (n = 9 cells).
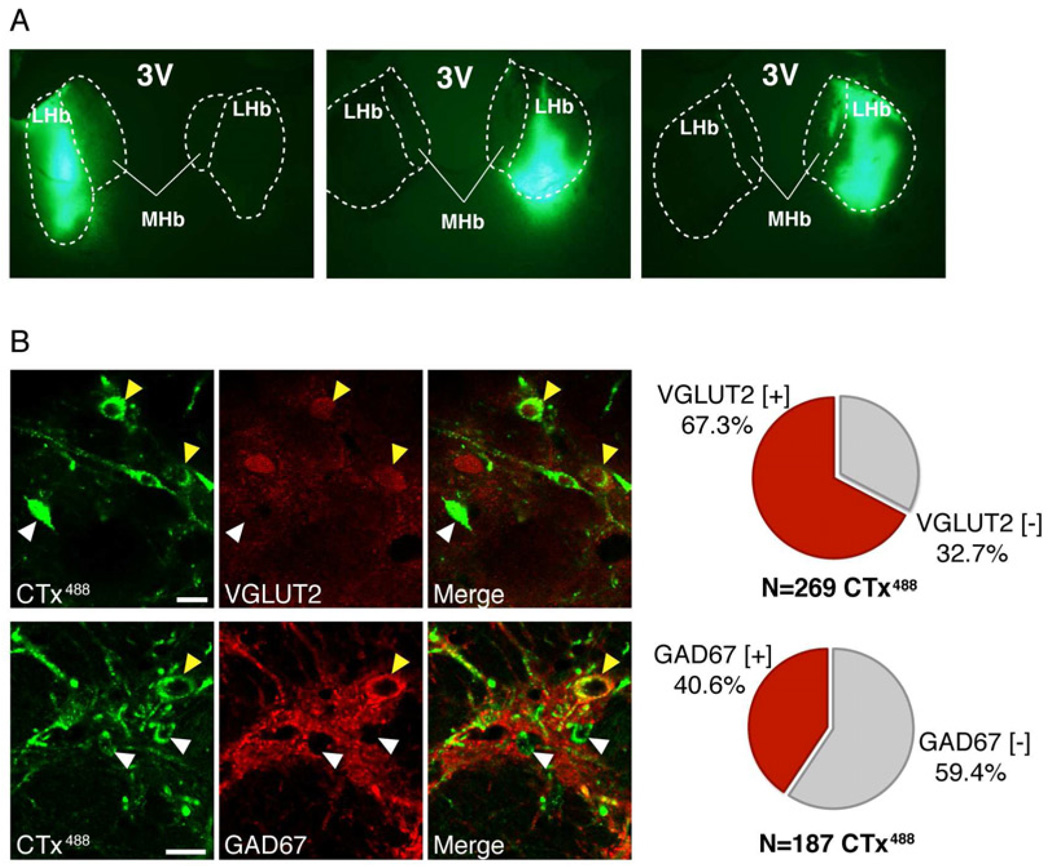
To confirm the electrophysiological results, we injected in vivo the retrograde tracer cholera toxin subunit B conjugated with Alexa 488 (CTx488) into the LHb (Fig. 2a), followed by immunohistochemistry of the EP. Consistent with the electrophysiological results, we found about 2/3 of retrogradely labeled cell bodies in the EP expressed the vesicular glutamate transporter, VGLUT2, a marker of glutamatergic neurons, and a minority expressed the GABAergic marker, GAD67 (Fig. 2b). These results indicate substantial excitatory, glutamatergic projections from the basal ganglia to the LHb, projections which likely contribute to the ‘anti-reward’ responses of LHb neurons (Hong and Hikosaka, 2008; Matsumoto and Hikosaka, 2007).
Figure 2.
The majority of LHb-projecting EP neurons are glutamatergic. (a) Cholera toxin (CTx488; green) injection sites. LHb, lateral habenula; MHb, medial habenula; 3V, third ventricle. (b) Confocal images of EP neurons retrogradely labeled by LHb injections of CTx488 (green) that co-stained for the glutamatergic marker, VGLUT2, or the GABAergic marker, GAD67 (red; yellow arrowheads) or not (white arrowheads). Bars = 20 µm.
Selective, optical stimulation of EP inputs to the LHb is aversive
The majority of neurons in the basal ganglia that project to the primate LHb are excited by aversive stimuli, similar to LHb neurons themselves (Hong and Hikosaka, 2008). This suggests that output neurons of the basal ganglia that project to the LHb are driving LHb neurons’ responses to aversive stimuli, and predicts that stimulation of fibers from the EP to the LHb is aversive. To allow selective activation of the EP-LHb pathway in vivo, we injected AAV that drives expression of ChR2-YFP into the rat EP and implanted chronic dual fiberoptic cannulae that provided optical access to the LHb bilaterally (Supplementary Fig. 2). Three weeks later, we optically stimulated ChR2-YFP-expressing axons in the LHb (that originated from cell bodies in the EP) via a fiberoptic cable connected to the implanted cannulae and coupled to a blue laser. To determine whether stimulation of the EP-LHb pathway is aversive or rewarding, we tested rats for directed place preference using a two-compartment (A and B) shuttle box (see Experimental Procedures and Fig. 3a). During a baseline period of 10 minutes the animals spent equal time in A and B compartments. Subsequently, during the next 30 minutes light pulses (20 Hz) were delivered to the LHb when the animal was in compartment A. Animals developed a clear avoidance of compartment A during this period (Fig. 3b). This aversive effect was reversible, since optogenetic activation of the EP-LHb pathway while animals were in compartment B reversed the avoidance (Fig. 3c-e); delivery of light alone had no effect (Fig. 3f,g). These results indicate that the EP-LHb pathway provides aversive signals to the animal consistent with EP driving excitatory, ‘anti-reward’ signals of the LHb.
Figure 3.
Stimulation of EP axons in the LHb is aversive. (a) Schematic of the directed place preference paradigm (see Experimental Procedures for details). Rats were allowed to move between both sides of the test cage during the entire 40 minute session. No stimulation was delivered during the 10 minute baseline period. After this baseline period, optical stimulation of EP axons in the LHb was delivered when the rat was in Context A (see Supplementary Fig. 2 for surgical design and location of optic fiber cannulae). (b) Light has no effect in mCherry control group but causes avoidance of Context A in rats expressing ChR2-YFP (ChR2-YFP, n = 7; mCherry, n = 5). (c) Schematic of the reversal of directed place preference (see Experimental Procedures for details). Throughout the entire 1 hour session, the rat was allowed to move freely between both sides of the test cage. No stimulation was delivered during the first 10 minute baseline period. After this baseline period, optical stimulation of EP axons in the LHb was delivered every time the rat was in context A. This optical stimulation paired with context A lasted 20 minutes. After this period, optical stimulation was reversed and now paired with context B for the next 20 mintues and then paired back again with context A for the last 10 minutes. (d) Individual avoidance scores (gray lines) over time and mean (dark line) for ChR2- YFP-expressing rats. (e) Mean avoidance scores for ChR2-YFP-expressing rats for each period (n = 6 rats). (f) Individual avoidance scores for mCherry-expressing rats as in (d). (g) Mean avoidance scores for mCherry-expressing rats for each period (n = 5 rats). n.s., not significant, * P < .05, and ** P < .001.
Neuromodulation of excitatory input from the EP to the LHb
The LHb has been implicated in the pathophysiology of depression (Hikosaka, 2010; Li et al., 2011), potentially by reducing the output of brainstem aminergic neurons (Ferraro et al., 1996; Hikosaka, 2010; Ji and Shepard, 2007). However, the neuromodulation of transmission that drives LHb neurons is poorly understood. Because of its well established role in depression, we tested if serotonin could modulate the synaptic and/or intrinsic excitability of LHb neurons. We focused on neurons in the lateral aspect of the LHb which received input from the ChR2-YFP labeled EP. To examine the synaptic target of serotonin we recorded responses to paired light pulses (separated by 100 ms) in voltage-clamp. Synaptic currents were reduced by bath application of low concentrations of serotonin (Fig. 4a; 27 ± 6% depression after first light pulse; p = .001, n = 10 cells), but not dopamine (Fig. 4a; 7 ± 5%; p = .26, n = 7 cells), and the ratio of the second to first response increased after serotonin application (Fig. 4b,c; 22 ± 9% increase; p = 0.02), consistent with a reduction in the probability of neurotransmitter release. In contrast to serotonin’s effect on synapses, we observed no change in the response to depolarizing current injection (Fig. 4d,e; all p > 0.1, n = 13 cells) and no change in resting potential (Vm before serotonin, −56 ± 2 mV; after serotonin, −56 ± 2 mV; p > 0.6; n = 13 cells). These results indicate that serotonin provides presynaptic inhibition to excitatory input from the EP to the LHb.
Figure 4.
Serotonin depresses excitatory input to LHb from EP. (a) Serotonin (1 µM; n = 10 cells), but not dopamine (1–10 µM; n = 7), depresses light-evoked excitatory currents. Bar indicates time of drug application. (b) Serotonin increases the paired pulse ratio (i.e., amplitude of second response/amplitude of first response) of light-evoked excitatory currents (n = 10 cells). (c) Example of serotonin-mediated depression of light-evoked excitatory currents. Scale, 100 ms, 200 pA. (d) No effect of serotonin (1 µM) on spiking during current injection (as indicated; n = 13 cells). (e) Example of current-evoked spiking before and after serotonin. Scale, 50 ms, 40 mV. * P = .02.
Discussion
Here we investigate the physiological and behavioral function of basal ganglia outputs to the LHb by in vivo labeling of the EP nucleus with ChR2-YFP. We find that this pathway is primarily excitatory and glutamatergic and provides an aversive stimulus, consistent with upstream control of LHb ‘anti-reward’ responses. Our results explain how a basal ganglia output, traditionally thought to be inhibitory (Oertel et al., 1984) can display similar encoding properties as its target nucleus, the LHb (Hong and Hikosaka, 2008).
We also examined the impact of serotonin on neurons in the LHb, a nucleus that provides inhibitory influence over brainstem aminergic nuclei (Ferraro et al., 1996; Hikosaka, 2010; Ji and Shepard, 2007), including dopaminergic neurons (Ji and Shepard, 2007). We show that the excitatory EP input to the LHb is suppressed by serotonin, suggesting that serotonin inhibits upstream synapses responsible for decreasing dopamine output. Our findings provide a link between a neuromodulator relevant to mood disorders and an ‘anti-reward’ circuit.
Our discovery of a direct, glutamatergic projection from the EP to the LHb is consistent with a recent study showing expression of VGLUT2 mRNA in the EP (Barroso-Chinea et al., 2008). This study found high VGLUT2 mRNA expression in the rostral EP which preferentially targets the LHb (Araki et al., 1984), but also VGLUT2 mRNA in neurons that project to the thalamus. We extend this finding by demonstrating the presence of a strong, excitatory, glutamatergic projection from the EP to the LHb, as well as VGLUT2 expression in the majority of LHb-projecting EP neurons. We also show that stimulation of the excitatory projection from the EP to the LHb is aversive, suggesting that glutamatergic inputs from the EP to the LHb drive LHb neuronal responses to aversive events. Consistent with previous results (Oertel et al., 1984; Vincent et al., 1982), we also found electrophysiological and immunohistochemical evidence of a GABAergic projection from the EP to the LHb. Our immunohistochemistry suggested that a minority of LHb-projecting EP neurons are GABAergic, consistent with a previous pharmacohistochemical study (Araki et al., 1984), although we cannot rule out the possibility that our GAD67 antibody did not label all GABAergic EP neurons. The function of this GABAergic projection is unclear. It may control the gain of LHb neuronal activity, or given that LHb neurons are inhibited by unexpected, rewarding stimuli (Hong and Hikosaka, 2008; Matsumoto and Hikosaka, 2007), these inputs may signal the presence of reward. Future studies that selectively manipulate GABAergic inputs to the LHb are needed to address this issue.
To our knowledge, this is the first study to isolate and characterize the synapses of a specific projection thought to participate in the computation of reward prediction errors – the difference between the amount of reward expected and the amount of reward received, a computation thought to drive reinforcement learning (Sutton and Barto, 1998). Given that both LHb-projecting basal ganglia neurons and LHb neurons are excited by the presence of unexpected, aversive events (Hong and Hikosaka, 2008; Matsumoto and Hikosaka, 2007) and our finding that projections from the basal ganglia to the LHb are excitatory and aversive, it is likely that excitatory projections from the basal ganglia to the LHb signal negative reward prediction errors. Interestingly, the activity of these excitatory projections was suppressed by low concentrations of serotonin. This suggests that synapses that transmit reward prediction errors are subject to neuromodulation and opens new avenues for the study of the interaction between mood and learning.
Experimental Procedures
Animals
Male Sprague-Dawley rats, age 28–32 days at time of surgery (42–46 days when sacrificed for slice experiments), were housed 1–4/cage and kept on a 12/12 hour light-dark cycle (lights on/off at 6 am/6 pm). All procedures involving animals were approved by the Institute Animal Care and Use Committees of the University of California, San Diego.
Virus preparation, injection and cannula implant
cDNA encoding flexed version of ChR2(H134R)-eYFP was kindly provided by Dr. Karl Deisseroth (Stanford). To make nonflexed version, ChR2(H134R)-eYFP sequence was PCR amplified flanked by KpnI and EcoRI sites and subcloned into pAAV-EF1α-flexed-ChR2(H134R)-eYFP-WPRE vector using same restriction sites. Sequencing confirmed gene sequence integrity. AAV-EF1α-ChR2(H134R)-eYFP-WPRE (serotype 1; 9 × 1011 GC ml−1) (named AAV-ChR2-YFP in the text) and AAV-CMV-mCherry were made by Salk Vector Core (La Jolla, CA).
Rats were anesthetized with isoflurane for stereotaxic bilateral injection of AAV-ChR2-YFP into the EP (A-P: −2.1 mm from bregma; M-L: 2.55–2.70 mm; D-V: −6.3 – −6.9 mm from dura). 0.1 – 0.5 µL of virus were injected into each hemisphere over 8–20 minutes using a picospritzer. Control rats for behavior experiments were injected with an AAV encoding mCherry. The injection pipette was not removed until 10 minutes after the end of the infusion to allow diffusion of the virus. Subjects for the behavioral experiment were injected with virus as described above and dual fiberoptic cannulae (Doric Lenses, Canada) were implanted in order to have the tip of the fiberoptic cannulae (200 µm, 0.22 NA) above the left and the right LHb (A-P: −3.6 mm from bregma; M-L: +/−0.75 mm; D-V: −4.0 mm from dura) (see Supplementary Fig. 2) and secured to the skull with screws and dental cement. Rats were injected subcutaneously with 5 mg/kg carprofen (NSAID) after surgery.
Freely moving directed place preference (DPP)
Rats (n = 7 in ChR2-YFP group, n = 5 in mCherry control group) used for DPP underwent surgery at 4–7 weeks old and behavior experiments were conducted at least three weeks after surgery. DPP was carried out in a shuttle box (50 cm W × 25 cm D × 30 cm H; Coulbourne Instrument) equipped with a door separating the two halves and photocell detectors. Walls were modified in order to present different patterns to provide contextual differences. Photocell detectors allowed automatic monitoring of rat location in the cage for the duration of testing. Optical activation of ChR2-YFP-expressing axons was performed using an optical fiber coupled to a 473 nm solid-state laser diode (OEM laser system, MI) with 20 mW of output from the 200 µm fiber.
Directed place preference was designed in order to monitor preference/aversion induced by optical stimulation of the LHb. Throughout the full duration of the test, rats were free to explore both sides of the cage. The first 10 min allowed us to measure preference for either context without manipulation. No preference was found during this first 10 min. After this 10 min baseline period, optical stimulation (continuous 20Hz, 5 msec pulse duration) was delivered while the animal was in one context (defined as “context A”). For the next 30 min, optical stimulation of the LHb occurred whenever the rat was located in context A. Optical stimulation was stopped when the animal was in the other side of the cage (context B). Avoidance scores were measured by taking time spent in context B minus time spent in context A divided by total time (120 sec). Student’s T-test compared avoidance score from period 10–40 min to baseline (0–10 min period). In a different set of DPP testing, pairing of the optical stimulation with context A (20 min) was switched to context B for an another 20 min and then paired again with context A for the last 10 min of the 1 hour session (see schematic in Fig. 3). One ChR2-YFP-expressing rat lost its cannula before the DPP reversal test, so only 6 ChR2-YFP-expressing rats were tested for reversal of DPP. Student’s T-test compared avoidance score from periods 10–30, 30–50, and 50–60 to baseline period (0–10 min).
Electrophysiology
Two weeks after surgery, rats were anesthetized with isoflurane before decapitation and brain removal. Brains were chilled in ice-cold dissection buffer (110.0 mM choline chloride, 25.0 mM NaHCO3, 1.25 mM NaH2PO4, 2.5 mM KCl, 0.5 mM CaCl2, 7.0 mM MgCl2, 25.0 mM glucose, 11.6 mM ascorbic acid, 3.1 mM pyruvic acid; gassed with 95%O2/5%CO2) and cut in 400 micron thick coronal slices through the EP and LHb. Slices were transferred to 35 °C ACSF (118 mM NaCl, 2.5 mM KCl, 26.2 mM NaHCO3, 1 mM NaH2PO4, 20 mM Glucose, 4 mM MgCl2, 4 mM CaCl2; 22°–25°C; pH 7.4; gassed with 95%O2/5%CO2) for 30 minutes. After an additional 30 minutes of recovery at room temperature, slices were transferred to the recording chamber and constantly perfused with 27 °C ACSF.
Recordings were made from cells in the lateral half of the LHb, where ChR2-YFP expression was highest, using an Axopatch 1D amplifier with a 5kHz sampling frequency and a filter set at a −3dB cutoff frequency of 5kHz. For current-clamp recordings the intracellular solution consisted of (in mM): 130 K-Gluconate, 5 KCl, 10 Hepes, 2.5 MgCl2, 4 Na2ATP, 0.4 Na3GTP, 10 Na-phosphocreatine, 0.6 EGTA (pH 7.2). For voltage-clamp recordings the intracellular solution consisted of (in mM): 7.5 QX314, 115 cesium methanesulfonate, 20 CsCl, 10 HEPES, 2.5 MgCl2, 4 Na2-ATP, 0.4 Na-GTP, 10 Na-phosphocreatine, and 0.6 EGTA (pH 7.2). 470 nm light pulses were delivered to the LHb via an optic fiber attached to an LED. To examine the effect of serotonin (1 µM in all experiments) on excitability, we also injected current (−100, 0, 100, 200, 300 pA) for 300 ms – one current injection every 10 seconds in current-clamp. To examine the effect of serotonin on light-evoked currents, cells were clamped at −50 mV and 0.5–5 ms light pulses were given in pairs, 100 ms between each pulse, every 10 seconds. Picrotoxin (100 µM) was included in the extracellular recording solution during voltage clamp but not current clamp recordings. For I-V recordings, NBQX (3 µM) and APV (100 µM) were added during the recordings taken at −60, 0, and 40 mV (minimum 10 sweeps taken at each potential before and after each drug) to determine the contribution of AMPA and NMDA receptors to excitatory currents. After recordings were completed, slices were fixed in 4% formaldehyde overnight and mounted on slides for localization of the injection site and labeled projection to the LHb.
Data analysis
To measure the effects of EP fiber stimulation on membrane potential for each cell, the slope of the postsynaptic potential (PSP) was measured (average of 3 sweeps) at resting potential. The same start and peak times were used to compute the PSP slope during depolarization (average of 3 sweeps).
The time intervals used to determine the effect of serotonin on excitability were 0–150 s before serotonin and 7.5–10 min after serotonin.
Excitatory current amplitude was measured as the peak amplitude in a 2 ms window between 3–20 ms after the light pulse.
Statistical analysis
All values given in the text and figures indicate mean ± SEM. Student’s t test was used with p < 0.05 deemed significant.
Tracer injection, perfusion and tissue processing
To label LHb-projecting EP neurons, 0.5 µl of Cholera toxin subunit B conjugate to the AlexaFluor488 (CTx488) (2 mg/ml in phosphate-buffered saline, PBS pH 7.4) was unilaterally injected in the LHb (AP: −3.6 mm, ML: 0.7 mm, DV: −4.8 mm) over 5–7 minutes. Rats were allowed to survive for 40 hours, were perfused and brains processed for immunohistochemistry.
For perfusion, rats were deeply anesthetized using a mix of ketamine/dexdomitor (75 and 5 mg/Kg respectively IP) and transcardially perfused with saline followed by a solution of phosphate buffer 0.1 M (PB, pH 7.4) containing 4 % paraformaldehyde. Brains were postfixed overnight in the same solution, rinsed with PB and cryoprotected by immersion in PB/30% sucrose solution for three days. Frozen brains were sectioned at 50 µm with a sliding microtome in the coronal plane.
For each brain, 3 slices encompassing the entopeduncular nucleus were chosen for immunohistochemistry. Free floating slices were first blocked in TN (Tris 0.1M, 1% NaCl, pH 7.4) buffer containing 10% normal goat serum and 0.2% triton X-100 for 3 hours. After blocking, slices were incubated with the following antibodies diluted in TN/3% NGS/0.2% Triton X-100 solution; anti-VGLUT2 (Millipore) or anti-GAD67 (Millipore) for 48 hours at room temperature. After 3 washes in TN buffer, slices were incubated with secondary antibody Alexa Fluor647 goat anti-mouse (Invitrogen) in TN/3% NGS/0.2% Triton X-100 for 4 hours at RT. Slices were washed and mounted using Vectashield mounting medium (Vector Laboratories). Images were taken with a FV1000 confocal microscope (Olympus), adjusted for brightness using Fluoview software and assembled in Adobe Illustrator.
Highlights.
-
-
Input to the lateral habenula from the basal ganglia is excitatory.
-
-
Excitatory input to the lateral habenula from the basal ganglia is glutamatergic.
-
-
Input to the lateral habenula from the basal ganglia is aversive.
-
-
Excitatory input to the lateral habenula from the basal ganglia is suppressed by serotonin.
Shabel et al. characterize a major input to the lateral habenula, a brain region involved in reward, and show that this input is aversive and suppressed by serotonin, providing a link between aversive signaling and a neuromodulator involved in depression.
Supplementary Material
Acknowledgments
We thank Dr. Karl Deisseroth for providing ChR2 cDNA and Dr. Chihye Chung for expert technical assistance. Support provided by NIH (SJS, RM) and a postdoctoral award from the Instituts de Recherche en Santé du Canada (C.D.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
S.J.S., C.D.P. A.T., and R.T.M. performed and analyzed experiments; S.J.S. and C.D.P made the figures; S.J.S., C.D.P and R.M. designed the study; S.J.S., C.D.P. and R.M. wrote the manuscript.
References
- Araki M, McGeer PL, McGeer EG. Retrograde HRP tracing combined with a pharmacohistochemical method for GABA transaminase for the identification of presumptive GABAergic projections to the habenula. Brain Res. 1984;304:271–277. doi: 10.1016/0006-8993(84)90330-5. [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Rico AJ, Perez-Manso M, Roda E, Lopez IP, Luis-Ravelo D, Lanciego JL. Glutamatergic pallidothalamic projections and their implications in the pathophysiology of Parkinson's disease. Neurobiol Dis. 2008;31:422–432. doi: 10.1016/j.nbd.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Ferraro G, Montalbano ME, Sardo P, La Grutta V. Lateral habenular influence on dorsal raphe neurons. Brain Res Bull. 1996;41:47–52. doi: 10.1016/0361-9230(96)00170-0. [DOI] [PubMed] [Google Scholar]
- Filion M, Harnois C. A comparison of projections of entopeduncular neurons to the thalamus, the midbrain and the habenula in the cat. J Comp Neurol. 1978;181:763–780. doi: 10.1002/cne.901810406. [DOI] [PubMed] [Google Scholar]
- Harnois C, Filion M. Pallidofugal projections to thalamus and midbrain: a quantitative antidromic activation study in monkeys and cats. Exp Brain Res. 1982;47:277–285. doi: 10.1007/BF00239387. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Nakano K, Jayaraman A, Carpenter MB. Projections of the globus pallidus and adjacent structures: an autoradiographic study in the monkey. J Comp Neurol. 1976;169:263–290. doi: 10.1002/cne.901690302. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel WH, Nitsch C, Mugnaini E. Immunocytochemical demonstration of the GABA-ergic neurons in rat globus pallidus and nucleus entopeduncularis and their GABA-ergic innervation. Adv Neurol. 1984;40:91–98. [PubMed] [Google Scholar]
- Parent M, Levesque M, Parent A. Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. J Comp Neurol. 2001;439:162–175. doi: 10.1002/cne.1340. [DOI] [PubMed] [Google Scholar]
- Penney JB, Jr, Young AB. GABA as the pallidothalamic neurotransmitter: implications for basal ganglia function. Brain Res. 1981;207:195–199. doi: 10.1016/0006-8993(81)90693-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Stephenson DT, Li Q, Simmons C, Connell MA, Meglasson MD, Merchant K, Emborg ME. Expression of GAD65 and GAD67 immunoreactivity in MPTP-treated monkeys with or without L-DOPA administration. Neurobiol Dis. 2005;20:347–359. doi: 10.1016/j.nbd.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sutton R, Barto A. Reinforcement Learning. MIT press; 1998. [Google Scholar]
- Vincent SR, Kimura H, McGeer EG. A histochemical study of GABA-transaminase in the efferents of the pallidum. Brain Res. 1982;241:162–165. doi: 10.1016/0006-8993(82)91239-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.