Abstract
The aim of this study was to determine the role of small intestinal (SI) cytochrome P450 (P450) enzymes in the metabolic activation of diclofenac (DCF), a widely used nonsteroidal anti-inflammatory drug, and DCF-induced intestinal toxicity. DCF induces intestinal ulcers in humans and mice, but the underlying mechanisms, including the necessity for drug bioactivation in the target tissues and the sources and identities of reactive intermediates, are not fully understood. We found that the number of DCF-induced (at 50 mg/kg p.o.) intestinal ulcers was significantly smaller in an intestinal epithelium (IE)-specific P450 reductase (CPR) knockout (IE-Cpr-null) mouse model, which has little P450 activity in the IE, than in wild-type (WT) mice, determined at 14 h after DCF administration. The involvement of intestinal P450 enzymes was confirmed by large reductions (>80–90%) in the rates of in vitro formation, in SI microsomal reactions, of hydroxylated DCF metabolites and reactive intermediates, trapped as DCF-glutathione (GSH) conjugates, in the IE-Cpr-null, compared with WT mice. The SI levels of DCF-GSH conjugates (at 4 h after dosing) and DCF-protein adducts (at 14 h after dosing) were significantly lower in IE-Cpr-null than in WT mice. In additional experiments, we found that pretreatment of mice with grapefruit juice, which is known to inhibit SI P450 activity, ameliorated DCF-induced intestinal toxicity in WT mice. Our results not only strongly support the notion that SI P450 enzymes play an important role in DCF-induced intestinal toxicity, but also illustrate the possibility of preventing DCF-induced intestinal toxicity through dietary intervention.
Introduction
Diclofenac (DCF) [2-(2,6-dichloroanilino)-phenylacetic acid], a nonsteroidal anti-inflammatory drug, is widely prescribed for the treatment of osteoarthritis and rheumatoid arthritis (Small 1989). A major adverse effect of the use of DCF is gastrointestinal injury. There is increasing evidence showing that the small intestine (SI), in addition to the stomach, is a major target organ of DCF-induced toxicity (Bjarnason et al., 1993; Davies et al., 2000); typical clinical signs of DCF-induced intestinal toxicity include small intestinal ulceration, bleeding, and inflammation (Allison et al., 1992; Bjarnason et al., 1993; Wolfe et al., 1999). DCF also induces intestinal injury in various experimental animal models, including mice, where DCF induced multiple ulcers and smaller erosions in the small intestinal mucosa (Atchison et al., 2000; Ramirez-Alcantara et al., 2009). Given the reported high proportion (up to 70%) of patients who develop intestinal injury while receiving DCF therapy, and the severity of the clinical consequences of the adverse drug response (including both morbidity and mortality) (Fortun and Hawkey, 2007; Maiden, 2009), it is imperative to identify endogenous and exogenous factors that may influence the risks of developing DCF-induced gastrointestinal injury.
The mechanisms of DCF-induced intestinal injury have been studied intensely (Tang, 2003; Treinen-Moslen and Kanz, 2006; Higuchi et al., 2009). DCF may directly inhibit cyclooxygenase, resulting in the suppression of prostaglandin production; the latter is important in maintaining homeostasis of the gastrointestinal mucosa (Sigthorsson et al., 2002; Tanaka et al., 2002). The toxicity of DCF may also be derived from its reactive metabolites, produced through biotransformation, which readily form conjugates with reduced glutathione (GSH) or attack proteins to form protein adducts (Boelsterli, 2003; Tang, 2003; Treinen-Moslen and Kanz, 2006). Studies have also revealed the important roles of several downstream signaling molecules or pathways (Watanabe et al., 2008; Ramirez-Alcantara et al., 2009).
DCF is metabolized by both phase I [cytochrome P450 (P450)] and phase II [UDP-glucuronosyltransferase (UGT)] biotransformation enzymes. For P450-mediated DCF metabolism, CYP2C and CYP3A are the major active enzymes (Kenny et al., 2004). CYP2C catalyzes the formation of 4′-hydroxylated DCF (4′-OH-DCF), and CYP3A catalyzes the formation of 5-hydroxylated DCF (5-OH-DCF). Those two hydroxylated metabolites are further metabolized to form reactive quinoneimines, which are highly unstable and could react with either GSH or protein. On the other hand, DCF can also be metabolized by UGT enzymes to form reactive DCF acyl-glucuronide (DCF-G) conjugate, which can covalently bind to proteins and form adducts. DCF-induced formation of protein adducts has been proposed to be one of the causal factors for ulcer formation in the intestine (Atchison et al., 2000; Boelsterli, 2003). However, the relative importance of the P450 and the UGT systems in DCF-induced intestinal toxicity has not been clearly defined.
In general, the liver is the major metabolic organ for the clearance of drugs. DCF-G formed in the liver is excreted mainly through the bile. A recent study (LoGuidice et al., 2012) clearly demonstrated that DCF-G is transported from liver to intestine, and the intraluminal release of DCF by bacterial β-glucuronidase is a key factor in the initiation of DCF enteropathy. For orally administered drugs, the SI is the portal-of-entry organ; P450 enzymes in the SI may play an essential role in the first-pass metabolism of absorbed xenobiotics (Zhang et al., 2007, 2009; Zhu et al., 2011). The potential role of target tissue metabolic activation in DCF SI toxicity has also been suggested; however, until now, there has been no direct proof regarding whether intestinal P450 enzymes can transform DCF into reactive intermediates that form protein adducts and GSH conjugates, and, more importantly, whether DCF bioactivation by intestinal P450 enzymes is essential for DCF-induced intestinal toxicity.
In the present study, we have determined whether intestinal P450 enzymes are responsible for DCF-induced toxicity in vivo, using an intestinal epithelium (IE)-specific cytochrome P450 reductase (CPR) knockout (IE-Cpr-null) mouse model. In the IE-Cpr-null mouse, the activities of all microsomal P450s are blocked in the intestinal epithelial cells (Zhang et al., 2009). We examined the impact of the tissue-specific loss of intestinal CPR/P450 activities on the rates of DCF metabolism, including the formation of DCF-GSH conjugates and DCF-protein adducts, both in vitro and in vivo, as well as the impact on DCF-induced ulceration in the SI. We then determined whether pretreatment of mice with grapefruit juice (GFJ), which is known to inhibit SI P450 activity, can ameliorate DCF-induced intestinal toxicity in wild-type (WT) mice and whether GFJ can inhibit the DCF bioactivation catalyzed by mouse and human intestinal microsomes in vitro.
Materials and Methods
Chemicals and Reagents.
DCF sodium salt, reduced β-NADPH, UDP-glucuronic acid (UDPGA) trisodium salt, GSH, and Evans blue dye were purchased from Sigma-Aldrich (St. Louis, MO). Acetaminophen-GSH was purchased from Toronto Research Chemicals Inc. (Toronto, ON, Canada). Formalin (10% buffered) was from Thermo Fisher Scientific (Waltham, MA). DCF-D4 [2-(2,6-dichlorophenyl)amino-(benzene-D4)-acetic acid], 4′-OH-DCF-13C6 [2-(2,6-dichloro-4′-hydroxyphenyl)amino-(benzene-13C6)-acetic acid], 4′-OH-DCF, and 5-OH-DCF were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). DCF antiserum was from Antibodies-Online (Atlanta, GA). Western blotting reagents were from Invitrogen (Carlsbad, CA). All solvents (acetonitrile, methanol, and water) were of high-performance liquid chromatography grade (Thermo Fisher Scientific). Human SI microsomes, prepared from the jejunum of organ donors, were obtained from the International Institute for the Advancement of Medicine (Scranton, PA).
Animals and Treatments.
All studies with mice were approved by the Wadsworth Center Institutional Animal Care and Use Committee (Albany, NY). Adult (3–4 months old) IE-Cpr-null (Zhang et al., 2009) mice and age-matched WT littermates (breeding stocks maintained at the Wadsworth Center) were used. Animals were normally maintained at 22°C with a 12-h on-off light cycle and given food and water ad libitum. Mice were given a bolus dose of DCF, dissolved in water, by oral gavage, for pharmacokinetics (5 or 50 mg/kg) and toxicity (10–50 mg/kg) studies. For studies with GFJ (pure unsweetened Florida GFJ, 4× concentrate; Great Value brand, local Wal-Mart), mice were given GFJ (20 ml/kg; 2× concentrate) via oral gavage 2 h before DCF (50 mg/kg) oral administration.
Examination of Ulcer Formation in the SI.
The examination of ulcer formation in the SI was essentially as described previously (Takeuchi and Satoh, 2010). In brief, at 14 h after DCF administration, mice were injected with 100 μl of Evans blue dye through the tail vein and sacrificed 30 min later. The SI was dissected, flushed with ice-cold phosphate-buffered saline to remove the luminal content, and then cut open longitudinally and fixed in 2% formalin for 10 min. The tissues were examined for ulcers under a microscope (Eclipse 50i; Nikon, Melville, NY). The number and area of ulcers were quantified at 4× magnification by using SPOT imaging software (SPOT Imaging Solutions, Sterling Heights, MI) and assigned to the respective quartiles.
Histopathological Examination.
The SI tissue was coiled, from proximal to distal end, to form a Swiss roll in a tissue cassette, and then soaked in 10% buffered formalin for 24 h. Paraffin-embedded tissue sections (4 μ thick) were stained with hematoxylin and eosin. Tissue sections (five per mouse) of the Swiss roll were obtained for examination of the entire length of the intestine in a single section. Images were obtained by using a Nikon Eclipse model 50i light microscope, fitted with a digital camera, at the Wadsworth Center Light Microscopy Core.
Isolation of Mouse Intestinal Epithelial Cells and Preparation of Microsomes.
Tissues from three to five mice were combined for each microsomal preparation. Epithelial cells from the SI were isolated, and microsomes were prepared as described previously (Zhang et al., 2003). Liver microsomes were prepared essentially as reported previously (Fasco et al., 1993). Microsomes were stored at −80°C until use.
In Vitro Assay for DCF Metabolism.
For P450-mediated metabolism, the method was essentially as described previously (Mankowski et al., 2000), with minor modification. In brief, DCF (100 μM) was incubated with microsomes for 30 min at 37°C in a 200-μl reaction mixture containing 0.1 M potassium phosphate buffer, pH 7.4, 1.0 mM NADPH, and 0.1 mg of microsomal protein. For UGT-mediated metabolism, the method was modified from Harada et al. (2009). DCF (100 μM) was incubated with microsomes for 60 min at 37°C in a 200-μl reaction mixture containing 0.1 M potassium phosphate buffer, pH 7.4, 5.0 mM UDPGA, and 0.2 mg of microsomal protein. The reactions were initiated by the addition of NADPH or UDPGA and terminated by the addition of 400 μl of acetonitrile to the reaction mixture. Control experiments were performed in which NADPH or UDPGA was omitted. DCF-D4 and 4′-OH-DCF-13C6 (4 ng each) were added [in 10 μl of 50% (v/v) methanol in water] as internal standards for monitoring extraction efficiency for DCF-G and hydroxyl-DCF, respectively. After centrifugation at 1500g for 10 min, the organic layer was transferred to a new tube, and then centrifuged at 1500g for another 10 min. Aliquot (5 μl each) of the supernatant were taken for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of 4′-OH-DCF, 5-OH-DCF, and DCF-G. Recovery was >85%.
LC-MS/MS Analysis of DCF, 4′-OH-DCF, 5-OH-DCF, and DCF-G.
The LC-MS/MS system consisted of an Agilent 1200 Series High Performance Liquid Chromatograph (Agilent Technologies, Santa Clara, CA) and an ABI 4000 Q-Trap mass spectrometer (Applied Biosystems, Foster City, CA), with an Atlantis C18 column (100 × 2.1 mm i.d., 3 μm; Waters, Milford, MA). The LC-MS conditions were modified from a method described previously (Sparidans et al., 2008). The solvent system was composed of solvent A [8.5 mM ammonium acetate containing 0.0075% (w/v) formic acid] and solvent B (100% methanol). A 4-min linear gradient from 55 to 95% B was applied at a flow rate of 0.2 ml/min, followed by a 1-min isocratic elution at 95% B and then a 6-min wash at 55% B, before returning to the starting condition. The mass spectrometer was operated in a positive ion mode with an electrospray ionization source. The parameters for the chamber were as follows: curtain gas, 25 psi; heated nebulizer temperature, 400°C; ion spray voltage, 4700 V; nebulizer gas, 43 psi; turbo gas, 50 psi, declustering potential, 50 V; and entrance potential, 10 V. The mass spectrometer was set for the information-dependent acquisition scan mode, using multiple reaction monitoring-dependent enhanced product ion acquisition. DCF (and DCF-G) and the internal standard DCF-D4 were monitored at m/z 296/214 and m/z 302/220, respectively. 4′-OH-DCF (and 5-OH-DCF) and 4′-OH-DCF-13C6 were monitored at m/z 312/230 and m/z 318/236, respectively.
Determination of DCF-GSH Formation In Vitro.
Microsomal metabolic activation of DCF was assayed by determining the rates of formation of DCF-GSH, essentially as described previously (Yan et al., 2005). Reaction mixtures contained 50 mM phosphate buffer, pH 7.4, 100 μM DCF, 10 mM GSH, 0.2 mg of SI microsomal protein, and 1 mM NADPH in a final volume of 0.2 ml. After a 5-min preincubation at 37°C, reactions were initiated by the addition of NADPH. The reaction was carried out at 37°C for 60 min and quenched by the addition of 60 μl of 10% trichloroacetic acid. Acetaminophen-GSH (2 ng) was then added (in 10 μl of methanol) as the internal standard for monitoring extraction efficiency. Recovery was >87%. Samples were centrifuged at 10,000g for 15 min at 4°C to precipitate protein, and aliquots (30 μl each) of the supernatant were used for analysis by LC-MS/MS. In control incubations, the reaction was quenched before the addition of NADPH.
LC-MS/MS Analysis of DCF-GSH Conjugates.
The LC-MS/MS system described above was used. The mass spectrometer was set to the multiple-reaction monitoring mode and was operated with an electrospray ionization source. The parameters for the chamber were as described above. The method for the analysis of DCF-GSH was essentially the same as reported previously (Yan et al., 2005). In brief, the solvent system was composed of solvent A [0.1% (v/v) formic acid] and solvent B (acetonitrile with 0.1% formic acid). A 12-min linear gradient from 5 to 85% B was applied at a flow rate of 0.3 ml/min, and then a 5-min wash at 5% B, before returning to the starting condition. DCF-GSH was simultaneously monitored at three transitions, m/z 617/542, m/z 617/488, and m/z 617/342 (Yan et al., 2005).
Determination of Plasma DCF.
Whole blood (30 μl at each time point) was collected form mouse tail vein at 0.25, 0.5, 1, 2, 4, and 6 h after DCF dosing by using heparin-coated capillaries (Thermo Fisher Scientific). To each aliquot of the plasma (10 μl), DCF-D4 [4 ng in 10 μl of 50% (v/v) methanol in water] was added as an internal standard, and the mixture was extracted with 240 μl of acetonitrile (Sparidans et al., 2008). After centrifugation at 1500g for 10 min, the organic layer was transferred to a new tube, and then centrifuged at 1500g for another 10 min. Aliquots of the supernatant (5 μl each) were injected for LC-MS/MS analysis.
Determination of DCF-GSH Conjugate Formation In Vivo.
Mice were sacrificed 4 h after DCF dosing. The enterocytes were prepared by using the same method as described above for microsomal preparation. The enterocytes were homogenized on ice in four volumes of 0.1 M Tris-acetate buffer, pH 7.4, containing 1 mM EDTA and 150 mM KCl, using a Polytron homogenizer (Kinematica, Littau-Lucerne, Switzerland), at a setting of 3000 rpm for 15 s. Tissue homogenates (200-μl aliquots) were spiked with an internal standard, acetaminophen-GSH (2 ng, added in 10 μl), and then mixed with 400 μl of acetonitrile containing 10% trichloroacetic acid to precipitate protein. The mixtures were centrifuged at 1500g for 10 min, and the supernatant were transferred to a new tube and centrifuged at 1500g for another 10 min. Aliquots of the supernatant (5 μl each) were injected for LC-MS/MS analysis.
DCF Protein Adduct Formation In Vitro and In Vivo.
For in vitro assays, microsomes (1 mg of protein) from mouse SI or human jejunum were incubated with 0.1 M potassium phosphate buffer, pH 7.4, 2 mM NADPH, and 1 mM DCF, in a total volume of 0.2 ml, at 37°C for 4 h. Reactions were stopped by putting samples on ice. To detect DCF-protein adduct formation in vivo, mice were killed at 14 h after DCF dosing, and small intestinal epithelial cell microsomal fractions were prepared as described previously (Zhang et al., 2003). Immunoblot detection of DCF-protein adducts was performed by using NuPAGE Bis-Tris gels (10%) (Invitrogen) and a goat polyclonal antibody against DCF (Antibodies-Online). The secondary antibody, rabbit anti-goat IgG, was detected with an enhanced chemiluminescence kit (Thermo Fisher Scientific). The optical density of detected bands was determined with a Bio-Rad ChemiDoc XRS+ System (Bio-Rad Laboratories, Hercules, CA).
Other Methods.
Fresh GFJ (Simply Orange Juice Company, Apopka, FL) from a local market (50 ml) was extracted with ethyl acetate (150 ml) as described previously (Fukuda et al., 1997). The dried organic residue was weighed and then redissolved in methanol (1 ml) for in vitro assays. Protein concentration was determined by using the bicinchoninic acid protein assay kit (Thermo Fisher Scientific), with bovine serum albumin as the standard. Pharmacokinetic parameters were calculated by using the PK Solver program in Excel (Microsoft, Redmond, WA). Statistical significance of differences between two groups in various parameters was examined by using Student's t test and SigmaStat software (SPSS Inc., Chicago, IL).
Results
Impact of IE CPR Loss on DCF-Induced Ulcer Formation in the SI.
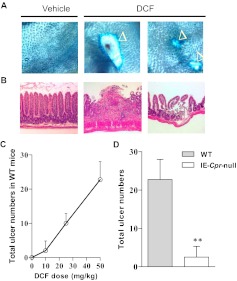
For studying DCF-induced toxicity in the mouse SI, a 50-mg/kg DCF dose was selected; the same dosage had been found in previous studies to reproducibly induce numerous ulcers in the mouse intestine (Atchison et al., 2000). Microscopic examination of the SI from DCF-treated WT mice revealed extensive mucosal damages featuring round or elongated ulcers, when examined at 14 h after dosing (see Fig. 1A for examples). Histopathological examination of the damaged area showed both loss of intestinal villi and compromised integrity of the submucosa, in contrast to the normal epithelial structure seen in vehicle-treated mice (Fig. 1B). The extent of SI ulcer formation (measured as total number of ulcers per animal) was DCF dose-dependent in WT mice (Fig. 1C). It is noteworthy that the number of ulcers detected in the SI was much reduced (by ∼90%) in the IE-Cpr-null mice relative to the WT mice (Fig. 1D) when tested at the DCF dose of 50 mg/kg. The extent of mucosal damage was also measured by the total area of ulcers (mm2) in the entire SI, which was divided into four quartiles of equal length, with the most proximal region being the first quartile. As shown in Table 1, at a DCF dose of 50 mg/kg, ulcers were predominantly located on the third and fourth quartiles of the SI in both WT and the null mice. However, the total area of the ulcers was significantly smaller (by ∼84%) in the IE-Cpr-null mice compared with WT mice (Table 1).
Fig. 1.
Induction of intestinal ulcers by DCF in WT and IE-Cpr-null mice. A and B, microscopic detection (A) and histopathological examination (B) of DCF-induced intestinal ulcers. Examples of ulcers detected in WT mice are shown. Mice were treated with DCF (50 mg/kg) or vehicle via oral gavage and sacrificed ∼14 h later for ulcer detection by using Evans blue dye (A; 40× magnification; ulcers are indicated by ▵) or histopathological analysis of the intestinal mucosa with hematoxylin and eosin staining (B; 40× magnification). The intestinal villi were lost, and the submucosa was severely compromised in the damaged area of DCF-treated mice (typical results are shown). C, a dose-response of DCF-induced ulcer formation, detected at 14 h after DCF treatment, was established by using WT mice (n = 3–4 for each dose), with a DCF dose range of 0 to 50 mg/kg p.o. D, the total number of ulcers detected in the entire SI, determined at 14 h after DCF treatment, was compared between WT and IE-Cpr-null mice treated with DCF at 50 mg/kg, p.o. (**, p < 0.01, compared with WT mice; n = 4; Student's t test). Shaded bar represents WT mice, and open bar represents IE-Cpr-null mice. All mice were 2- to 3-month-old females.
TABLE 1.
Ulcer area in DCF-treated IE-Cpr-null and WT mice
Mice were treated with DCF (50 mg/kg) or vehicle alone as described in the legend to Fig. 1D. Ulcer area was determined as described under Materials and Methods. Values reported represent means ± S.D. (n = 5–6).
| Strain | Treatment | Total Ulcer Area Along the SI |
||||
|---|---|---|---|---|---|---|
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | Combined | ||
| mm2 | ||||||
| WT | Vehicle | 0 | 0 | 0 | 0 | 0 |
| DCF | 0 | 0 | 5.49 ± 0.37 | 11.30 ± 5.20 | 16.79 ± 3.41 | |
| IE-Cpr-null | Vehicle | 0 | 0 | 0 | 0 | 0 |
| DCF | 0 | 0 | 2.27 ± 0.18a | 0.37 ± 0.09a | 2.64 ± 0.06a | |
p < 0.01, compared with the corresponding vehicle-treated group (Student's t test).
Impact of IE CPR Loss on P450-Mediated DCF Metabolism in the SI.
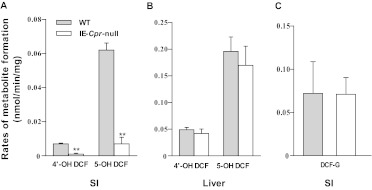
In vitro P450-mediated metabolism of DCF by SI and liver (as a control) microsomes was compared between IE-Cpr-null and WT mice. The rates of formation of the two major DCF metabolites, 4′-OH-DCF and 5-OH-DCF, were reduced by more than 80% in assays for SI microsomes from IE-Cpr-null mice compared with WT mice (Fig. 2A). In contrast, with liver microsomes no difference was detected in the rates of formation of either 4′-OH-DCF or 5-OH-DCF (Fig. 2B). The activity of microsomal UGT enzymes toward DCF was also determined for SI microsomes from IE-Cpr-null and WT mice. As shown in Fig. 2C, there was no difference between the two mouse strains in the rates of formation of DCF-G. Thus, these results suggested that the resistance of IE-Cpr-null mice to DCF-induced intestinal toxicity was related to the loss of P450-mediated, but not UGT-mediated, DCF metabolism.
Fig. 2.
In vitro metabolism of DCF by SI and liver microsomes from IE-Cpr-null and WT mice. A and B, P450-mediated hydroxyl-DCF formation by mouse SI (A) and liver (B) microsomes. Reaction mixtures contained 0.1 M phosphate buffer, pH 7.4, 100 μM DCF, and 0.5 mg/ml microsomal protein, in a final volume of 0.2 ml. Reactions were carried out at 37°C for 30 min in the presence or absence of 1 mM NADPH. C, UGT-mediated DCF-G formation by SI microsomes. Reaction mixtures contained 0.1 M phosphate buffer, pH 7.4, 100 μM DCF, 0.2 mg of microsomal protein, and 10 mM MgCl2 in a final volume of 0.2 ml. Reactions were carried out at 37°C for 60 min in the presence or absence of 5 mM UDPGA. Each microsomal preparation was obtained from pooled SI or liver tissues from two to three adult female mice. The values reported represent means ± S.D. (n = 3). **, p < 0.01; compared with WT mice (Student's t test). Shaded bars represent WT mice, and open bars represent IE-Cpr-null mice.
Impact of IE CPR Loss on First-Pass Clearance of Oral DCF.
The role of intestinal P450 enzymes in the clearance of orally administered DCF was assessed by comparing plasma levels of DCF between WT and IE-Cpr-null mice. When mice were treated with DCF at a low dose of 5 mg/kg via oral gavage, the plasma level of DCF was slightly higher (by ∼50%) in IE-Cpr-null mice than in WT mice at the first time point tested (15 min), but not at any other time points examined; whereas when the dose of oral DCF was increased to 50 mg/kg, there was no difference in plasma DCF level between the two mouse strains at any time points examined (15 min-4 h; data not shown). These results indicated that SI P450 enzymes have very limited contribution to the first-pass clearance of oral DCF, especially at the higher DCF dose tested. Moreover, the data confirm that the levels of circulating DCF were similar between IE-Cpr-null and WT mice at the dose (50 mg/kg) used for the SI toxicity study.
Impact of IE CPR Loss on the Formation of DCF-Reactive Intermediates In Vitro and In Vivo.
The impact of the abrogation of IE CPR expression on microsomal DCF metabolic activation was examined by comparing rates of DCF-GSH conjugate formation between IE-Cpr-null and WT mice. The rates in SI microsomes were reduced by more than 80% in the IE-Cpr-null mice compared with WT mice (Fig. 3A); in contrast, there was no difference between the two mouse strains in the rates of DCF-GSH formation in liver microsomes (Fig. 3B). The in vivo levels of DCF-GSH were also determined for intestinal enterocytes and liver 4 h after DCF treatment. The levels of DCF-GSH in the enterocytes were reduced by 37% in the IE-Cpr-null mice relative to WT mice (Fig. 3C); whereas, in the liver, no difference was detected in the levels of DCF-GSH between the two mouse strains (Fig. 3D). The smaller reduction in vivo compared with the result in vitro is probably caused by the intestinal absorption of DCF-GSH from the bile. These results indicated that the loss of intestinal P450-mediated DCF metabolism led to tissue-specific reductions in the levels of DCF-reactive intermediates in the SI of IE-Cpr-null mice.
Fig. 3.
Formation of DCF-GSH conjugates in vitro and in vivo. A and B, relative rates of in vitro formation of DCF-GSH conjugate were determined for microsomal preparations from mouse SI (A) and liver (B). Reaction mixtures contained 50 mM phosphate buffer, pH 7.4, 100 μM DCF, 10 mM GSH, and 1 mg/ml microsomal protein in a final volume of 0.2 ml. Reactions were carried out at 37°C for 60 min in the presence or absence of 1 mM NADPH. Each microsomal preparation was obtained from pooled liver or SI tissues of two to three adult female mice. The values reported, which are relative rates in arbitrary units (percentages of the rates determined for respective WT microsomes), represent means ± S.D. (n = 3). C and D, relative tissue levels of DCF-GSH conjugates in SI (C) and liver (D). Adult female IE-Cpr-null and age-matched WT mice were given a single dose of DCF, via oral gavage, at 50 mg/kg. The values reported, which are percentages of the levels determined for respective WT mice, represent means ± S.D. (n = 4). **, p < 0.01; compared with WT mice (Student's t test).
Impact of IE CPR Loss on DCF Protein Adduct Formation In Vitro and In Vivo.
DCF protein adducts were detected by immunoblot analysis using an anti-DCF antibody. The antibody had cross-reactivity with nonadducted proteins, which were detected in both complete reaction mixtures and negative controls, in which either DCF or NADPH was omitted. As shown in Fig. 4A, when SI microsomes from WT mice were incubated with 1 mM DCF and NADPH (i.e., a complete reaction), several prominent bands (indicated by arrows) that seemed to be unique to the complete reaction mixture, thus representing putative protein adducts of DCF, were detected, including one at ∼50 kDa, which is similar in size to a mouse liver DCF protein adduct (Pumford et al., 1993) or a DCF protein adduct in rat liver and SI (Ware et al., 1998). It is noteworthy that these putative protein adducts were barely detected in complete reaction mixtures of SI microsomes from IE-Cpr-null mice. Numerous putative DCF protein adducts were also detected in complete reaction mixtures of liver microsomes from WT mice (Fig. 4B), of which the most prominent (indicated by an arrowhead) was ∼50 kDa in size, apparently the same size as the one detected in SI microsomes in Fig. 4A. However, there was no strain difference in the intensity of any of the putative adduct bands.
Fig. 4.
Immunoblot analysis of DCF protein adducts formed in microsomal reactions in vitro. Complete reaction mixtures contained 0.1 M potassium phosphate, pH 7.4, 2 mM NADPH, 1 mM DCF, and 5 mg/ml microsomal protein from intestinal enterocytes (A) or liver (B) of WT or IE-Cpr- null mice in a total volume of 0.2 ml. DCF or NADPH was omitted in control reactions. Reactions were carried out at 37°C for 4 h. Aliquots of the reaction mixture (∼170 μg of total protein) were analyzed, upon termination of reaction, on immunoblots, with use of an anti-DCF antibody. The positions of prominent, putative DCF-protein adducts are indicated by arrows (A) or an arrowhead (B). Typical results are shown.
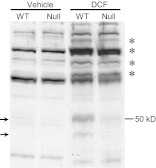
The 50-kDa putative DCF protein adduct was also detected in vivo in mouse intestinal microsomes obtained at 14 h after DCF administration (50 mg/kg p.o.). For the in vivo study, specific bands representing putative DCF protein adducts were revealed by comparing bands detected in DCF-treated mice with bands detected in vehicle-treated mice. As shown in Fig. 5, the putative DCF protein adduct band at ∼50 kDa (arrow) was clearly detected in SI microsomes from WT mice, but it was barely detected in SI microsomes from IE-Cpr-null mice. A weaker band at a lower molecular weight (Fig. 5, arrow) also seemed to be unique for DCF-treated WT mouse, thus also representing a putative P450-dependent DCF protein adduct. Several other bands at higher molecular mass region (indicated by* in Fig. 5), which were not detected in vehicle-treated mice, seem to be either slightly decreased in intensity in the DCF-treated null mice, relative to DCF-treated WT mice, or unchanged. It remains to be determined whether these represent DCF protein adducts, possibly formed by P450-independent pathways. These results further support the notion that intestinal P450-mediated DCF metabolism is important for in situ production of DCF reactive intermediates.
Fig. 5.
Immunoblot analysis of DCF adducts formed with SI microsomal proteins in vivo. WT and IE-Cpr-null mice were treated with DCF (50 mg/kg p.o) or vehicle alone. SI enterocytes were obtained at 14 h after DCF dosing for microsomal preparation. Aliquots of microsomal proteins (200 μg per lane) were analyzed on immunoblots with use of an anti-DCF antibody. The positions of putative P450-dependent DCF-protein adducts are indicated by arrows, whereas other bands that are apparently unique to DCF-treated mice are indicated by *. Typical results are shown.
Effects of GFJ on DCF Metabolism and DCF-Induced Toxicity in Mouse SI.
Our finding that intestinal P450-mediated DCF metabolism is important for DCF-induced intestinal toxicity suggested a possibility of ameliorating DCF-induced intestinal toxicity through the inhibition of SI P450 enzymes. Thus, we next tested the hypothesis that the inhibition of SI P450 enzymes by GFJ can ameliorate DCF-induced intestinal toxicity. As shown in Fig. 6, GFJ extract inhibited the in vitro formation of both 4′-OH-DCF and 5-OH-DCF (Fig. 6A) and DCF-GSH conjugates (Fig. 6B), by SI microsomes from WT mice, in a dose-dependent manner, with estimated IC50 values of ∼0.3 (for 4′-OH-DCF), ∼0.08 (for 5-OH-DCF), and ∼0.01 mg/ml (for DCF-GSH), at a DCF concentration of 100 μM. When GFJ was administered orally (at 20 ml/kg) to WT mice 2 h before DCF dosing, significant decreases in the total number of DCF-induced ulcers were found, compared with the mice that were pretreated only with vehicle (Fig. 6C). In control experiments, pretreatment with GFJ did not reduce the total number of DCF-induced ulcers in IE-Cpr-null mice, confirming that the protective effects of GFJ pretreatment in WT mice were caused by the inhibition of P450-mediated metabolic activation of DCF.
Fig. 6.
Effects of GFJ on DCF metabolism and SI DCF toxicity in mice. A, effects of GFJ on microsomal DCF metabolism. Reaction condition is the same as described in Fig. 2, except with the addition of GFJ extract at various concentrations (0–1.65 mg/ml, added in 5 μl of methanol). The rates reported (means ± S.D.; n = 3) are relative rates in arbitrary units (relative to the no GFJ group). B, effects of GFJ on microsomal formation of DCF-GSH conjugates. Reaction condition is the same as described in Fig. 3, except with the addition of GFJ extract at various concentrations (0–1.65 mg/ml, added in 5 μl of methanol). The values reported (means ± S.D., n = 3) are relative rates in arbitrary units (relative to the no GFJ group). C, effects of GFJ pretreatment on DCF-induced intestinal ulcer formation. WT and IE-Cpr-null mice were treated with GFJ (20 ml/kg) or vehicle alone 2 h before DCF treatment (50 mg/kg p.o). Fourteen hours later, mice were injected with Evans blue for the detection of ulcers. The values reported represent means ± S.D. (n = 4–5). **, p < 0.01; compared with WT mice (Student's t test). Shaded bars represent control group, and open bars represent GFJ-treated group.
Effects of GFJ on DCF Bioactivation by Human SI Microsomes.
To test the potential protective role of GFJ against DCF-induced intestinal toxicity in humans, we determined the effects of GFJ extract on DCF metabolism by human SI microsomes. The basal activity determined in the absence of GFJ varied by ∼5-fold among the samples analyzed (Table 2). It is noteworthy that whereas the rates of formation of 5-OH-DCF (a CYP3A metabolite) by human SI microsomes (0.03–0.12 nmol/min/mg protein) were comparable with those of mouse SI microsomes (∼0.06; Fig. 2A), the rates of formation of 4′-OH-DCF (a CYP2C metabolite) by human SI microsomes (0.07–0.16 nmol/min/mg protein) were much greater than those of mouse SI microsomes (<0.01; Fig. 2A), indicating that CYP2C-mediated metabolism is more dominant in human SI. It is noteworthy that although we were not able to determine the absolute rates of DCF-GSH formation, a direct comparison (data not shown) revealed that the rates of formation of DCF-GSH (representing the overall rates of DCF bioactivation) were almost the same between mouse SI microsomes (Fig. 6) and the SI microsomes from patient 16 (Table 2), which had the highest activity among the five samples examined.
TABLE 2.
Rates of DCF metabolism by individual human SI microsomes
Assays for DCF metabolism were conducted as described in the legend for Figure 7. The values shown, which are for the no-GFJ groups, are averaged results of duplicate assays and represent either actual rates of hydroxyl-DCF formation or relative rates of DCF-GSH formation in arbitrary units (percentages of the sample with the highest rate). Demographic information for the individual microsomal samples studied is also shown.
| Subject ID | Age | Race | Gender | Rate of DCF Metabolite Formation |
||
|---|---|---|---|---|---|---|
| 4′-OH-DCF | 5-OH-DCF | DCF-GSH | ||||
| nmol/min/mg | % | |||||
| 16 | 67 | White | Male | Not determined | Not determined | 100 |
| 27 | 49 | White | Female | 0.158 | 0.119 | 27 |
| 31 | 48 | Unknown | Female | 0.067 | 0.026 | 17 |
| 33 | 52 | White | Male | Not determined | Not determined | 52 |
| 35 | 62 | White | Male | 0.162 | 0.091 | 31 |
As shown in Fig. 7, GFJ extract inhibited P450-mediated formation of both hydroxyl-DCF metabolites (Fig. 7A) and DCF-GSH conjugates (Fig. 7B) by human SI microsomes from multiple individuals in a dose-dependent manner. The estimated IC50 values for the formation of 4′-OH-DCF (0.2–0.5 mg/ml), 5-OH-DCF (0.02–0.1 mg/ml), and DCF-GSH (0.015–0.15 mg/ml) in individual human SI microsomal samples were similar to the mouse values shown earlier, suggesting that GFJ would also be effective in protection against DCF-induced SI toxicity in patients.
Fig. 7.
Effects of GFJ on DCF metabolism by human SI microsomes. A, effects of GFJ addition on microsomal formation of hydroxyl-DCF metabolites. B, effects of GFJ addition on microsomal formation of DCF-GSH conjugates. Components of reaction mixtures and assay conditions were the same as described in the legend for Fig. 6, A and B, except that human jejunum microsomes rather than mouse SI microsomes were used. The values reported (averaged results of duplicate assays) are relative rates in arbitrary units (relative to the no GFJ group) for individual microsomal samples (#16, 27, 31, 33, and 35).
Discussion
We have for the first time provided definitive evidence that intestinal P450 enzymes play a critical role in the intestinal toxicity induced by DCF, a widely used nonsteroidal anti-inflammatory drug that is frequently associated with adverse drug reactions in patients (Bjarnason et al., 1993; Davies et al., 2000). We showed that SI P450 enzymes are capable of bioactivating DCF to form reactive intermediates, which conjugate GSH and adduct proteins, both in vitro and in vivo, and that mice lacking P450 activities specifically in intestinal epithelial cells are largely resistant to DCF-induced intestinal ulceration. These findings suggest that patients harboring high CYP2C and CYP3A activities in the SI, as a result of either genetic polymorphisms or enzyme induction by other drugs or dietary components, are at elevated risks of developing DCF-induced intestinal injury.
Critical to our conclusion regarding the involvement of intestinal P450 enzymes in DCF-induced intestinal toxicity was the utility of the IE-Cpr-null mouse model. The IE-Cpr-null mouse has been used previously in studies on the SI contributions to the first-pass metabolism of a number of oral drugs and in protection against systemic exposure of orally administered environmental contaminants (Zhang et al., 2009; Fang and Zhang, 2010; Zhu et al., 2011); but this is the first application of the IE-Cpr-null mouse model to study the role of intestinal microsomal P450s in chemical toxicity in the SI. The tissue-specific loss of CPR expression in the SI was not associated with any biological phenotypes, despite the loss of all microsomal P450 and hemeoxygenase activities in the enterocytes (Zhang et al., 2009). The loss of CPR expression in the enterocytes of IE-Cpr-null mice has been found recently to lead to changes in the expression of genes related to antigen presentation/processing (D'Agostino et al., 2012), including the major histocompatibility complex class II genes. The levels of the cholesterol precursor farnesyl pyrophosphate and its derivative geranylgeranyl pyrophosphate were also increased, although the level of cholesterol itself was unchanged in the enterocytes of the IE-Cpr-null mice relative to those in WT mice. Nevertheless, none of the aforementioned genomic and metabolomics changes in the enterocytes are known or expected to cause resistance to DCF toxicity. It should also be noted that the loss of heme oxygenase activity was unlikely a contributing factor to the resistance of the IE-Cpr-null mice to DCF-induced SI toxicity, given that heme oxygenase has been reported to inhibit nonsteroidal anti-inflammatory drug-induced toxicity (Higuchi et al., 2009). On the other hand, the conclusion that the loss of P450-mediated DCF bioactivation is the reason for the resistance of the IE-Cpr-null mice to DCF-induced SI injury was supported by both in vitro and in vivo evidence that the rates of SI formation of reactive DCF intermediates, trapped as DCF-GSH conjugates and DCF protein adducts, were significantly reduced in the IE-Cpr-null mice, compared with WT mice. We also confirmed that the UGT-mediated metabolism of DCF in the SI was not changed by the CPR loss, which ruled out possible contributions by a decrease in UGT-mediated reactive metabolite formation to the resistance to DCF-induced intestinal injury in the IE-Cpr-null mice.
The formation of protein adducts, which was suggested to be one of the causal factors for DCF-induced ulcer formation in the intestine, may either directly impair cellular signal transduction cascades or indirectly cause tissue damage by eliciting an immune response (Atchison et al., 2000; Boelsterli, 2003). Several DCF protein adducts were detected previously in mice and rats (Pumford et al., 1993; Hargus et al., 1994; Ware et al., 1998), including adducts with liver plasma membrane proteins (110, 140, and 200 kDa in size), formed presumably through UGT-mediated formation of DCF-G as the proximate toxicant, and an adduct with a liver microsomal protein (50 kDa in size), formed apparently via P450-catalyzed formation of reactive DCF intermediates. In the current study, a 50-kDa protein adduct band was also detected in vitro and in vivo in SI microsomes from WT mice; this adduct was not detected in SI microsomes from the IE-Cpr-null mice, thus supporting the notion that intestinal P450-catalyzed metabolic activation of DCF is responsible for the formation of this protein adduct. Several other putative protein adduct bands were also detected in SI microsomes from DCF-treated WT mice in the current study; however, the identities of all of these intestinal proteins and their potential connection of DCF-induced SI injury remain to be determined.
Our study with DCF provides a paradigm for investigating the potential roles of SI P450 in the intestinal toxicity of numerous other drugs that are P450 substrates. For orally administered drugs, the SI as the first site of contact is exposed to relatively high concentrations of drugs, which present ample opportunity for enterocyte P450 to convert inert drugs to reactive intermediates. For many drugs that undergo entero-hepatic recirculation, such as DCF, the enterocyte P450 would have additional and repeated opportunities to act on the drugs as they pass through the intestinal epithelium, leading to further increased risks of drug-induced toxicity in SI. It is noteworthy that our present finding of a critical role of SI P450 in DCF-induced intestinal injury does not conflict with the role of bacterial β-glucuronidase as a key factor in the initiation of DCF enteropathy, as elegantly demonstrated by LoGuidice et al. (2012), given that the DCF released by luminal bacteria would still require further bioactivation, by SI P450, to cause tissue injury. In that connection, the preferential damage by DCF to the distal parts of SI, despite their lower P450 content than that in the proximal SI (Zhang et al., 2003), is not fully understood, but may be partly caused by higher regional drug exposure resulting from regional differences in bile concentration in the intestinal lumen (Boelsterli and Ramirez-Alcantara, 2011).
Our finding that SI P450 plays a critical role in DCF-induced intestinal injury suggests that it might be possible to prevent or reduce DCF's intestinal toxicity through the direct inhibition of P450 enzymes that are involved in DCF bioactivation in human SI, namely CYP3A4 and CYP2C9. We showed, for the first time, that DCF's intestinal toxicity can be blocked by the oral administration of GFJ in mice and GFJ was equally effective in its inhibition of DCF bioactivation by mouse and human intestinal microsomes. These findings may have direct therapeutic application for patients at elevated risk (or past experience) of adverse intestinal responses to DCF treatment, in that DCF's side effects may be reduced or blocked by concomitant consumption of moderate amounts of GFJ. DCF is not a prodrug, and blocking intestinal P450 activity has little effect on the systemic bioavailability of oral DCF (data not shown). Therefore, oral consumption of GFJ would not affect the systemic therapeutic efficacy of DCF, while reducing DCF's side effects in the gut. Indeed, GFJ has been found to potentiate the anti-inflammatory effects of DCF on carrageenan-induced paw edema in rats (Mahgoub, 2002); that result was proposed by the authors to be caused by the inhibition of P450-mediated DCF clearance by GFJ, although the effect of GFJ on DCF pharmacokinetics was not studied.
Our finding that GFJ extract inhibited the formation of both 4′-OH- and 5-OH-DCF in human SI microsomal reactions is consistent with a previous report that GFJ can inhibit both human CYP3A4 and CYP2C9 in vitro (Girennavar et al., 2007). Oral administration of GFJ is known to inhibit CYP3A-mediated metabolism of many drugs (Bailey et al., 1998) and reduce drug (lovastatin) clearance in patients (Kantola et al., 1998; Mertens-Talcott et al., 2006) and animal models (Zhu et al., 2011). The specific GFJ ingredients that are responsible for the protection against DCF toxicity are not yet known, but they probably include various furanocoumarins, such as bergamottin and dihydroxybergamottin, which are the main GFJ components that cause the inhibition of intestinal P450 (Dresser and Bailey, 2003; Girennavar et al., 2007).
In addition to inhibiting P450, GFJ is known to affect transporters, such as P-glycoprotein (Dresser and Bailey, 2003), and inhibit esterases (Li et al., 2007). Therefore, the protection by GFJ might involve other mechanisms, in addition to inhibiting the formation of DCF reactive metabolites. However, our observation that the protective effect of GFJ pretreatment against DCF-induced SI injury does not occur in the IE-Cpr-null mice confirmed that the protective effects of GFJ seen in WT mice were caused by its specific inhibition of SI P450 enzymes.
In summary, we have determined the in vivo roles of SI P450 enzymes in the metabolic activation of DCF and DCF-induced intestinal toxicity by using the IE-Cpr-null mouse. Our results provide strong support for a crucial role of SI P450 enzymes in DCF-induced toxicity in the SI, illustrate the possibility of preventing DCF-induced intestinal toxicity through dietary intervention, and provide an example for investigating the potential roles of SI P450 in the intestinal toxicity of numerous other drugs that are P450 substrates. Our findings endorse further studies on the association between variations in SI CYP3A4/CYP2C9 activities and interindividual differences in susceptibility to DCF-induced intestinal injury as well as clinical testing of the value of GFJ administration as a preventative measure against DCF-induced intestinal toxicity in at-risk patients.
Acknowledgments
We thank Dr. Xinxin Ding of the Wadsworth Center for helpful discussions and a critical reading of the manuscript; Dr. Xin Zhou for help with LC-MS/MS method development; Dr. Fang Xie for assistance with microscopy; and Weizhu Yang for mouse production. We gratefully acknowledge the use of the Histopathology Core and the Advanced Light Microscopy and Image Analysis Core Facilities of the Wadsworth Center.
This study was supported in part by the National Institutes of Health National Institute of General Medical Sciences [Grant GM082978].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- DCF
- diclofenac
- DCF-D4
- deuterated diclofenac
- 4′-OH-DCF
- 4′-hydroxy-diclofenac
- 5-OH-DCF
- 5-hydroxy-diclofenac
- DCF-G
- diclofenac acyl-glucuronide
- P450
- cytochrome P450
- CPR
- cytochrome P450 reductase
- WT
- wild type
- SI
- small intestine
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- IE
- intestinal epithelium
- GFJ
- grapefruit juice
- GSH
- glutathione
- UGT
- UDP-glucuronosyltransferase
- UDPGA
- UDP-glucuronic acid.
Authorship Contributions
Participated in research design: Zhu and Zhang.
Conducted experiments: Zhu and Zhang.
Performed data analysis: Zhu and Zhang.
Wrote or contributed to the writing of the manuscript: Zhu and Zhang.
References
- Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI. (1992) Gastrointestinal damage associated with the use of nonsteroidal antiinflammtory drugs. N Engl J Med 327:749–754 [DOI] [PubMed] [Google Scholar]
- Atchison CR, West AB, Balakumaran A, Hargus SJ, Pohl LR, Daiker DH, Aronson JF, Hoffmann WE, Shipp BK, Treinen-Moslen M. (2000) Drug enterocyte adducts: possible causal factor for diclofenac enteropathy in rats. Gastroenterology 119:1537–1547 [DOI] [PubMed] [Google Scholar]
- Bailey DG, Malcolm J, Arnold O, Spence JD. (1998) Grapefruit juice-drug interactions. Br J Clin Pharmacol 46:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. (1993) Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 104:1832–1847 [DOI] [PubMed] [Google Scholar]
- Boelsterli UA. (2003) Diclofenac-induced liver injury: a paradigm of idiosyncratic drug toxicity. Toxicol Appl Pharmacol 192:307–322 [DOI] [PubMed] [Google Scholar]
- Boelsterli UA, Ramirez-Alcantara V. (2011) NSAID acyl glucuronides and enteropathy. Curr Drug Metab 12:245–252 [DOI] [PubMed] [Google Scholar]
- D'Agostino J, Ding X, Zhang P, Jia K, Fang C, Zhu Y, Spink DC, Zhang QY. (2012) Potential biological functions of cytochrome P450 reductase-dependent enzymes in small intestine: novel link to expression of major histocompatibility complex class II genes. J Biol Chem 287:17777–17788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NM, Saleh JY, Skjodt NM. (2000) Detection and prevention of NSAID-induced enteropathy. J Pharm Pharm Sci 3:137–155 [PubMed] [Google Scholar]
- Dresser GK, Bailey DG. (2003) The effects of fruit juices on drug disposition: a new model for drug interactions. Eur J Clin Invest 33 (Suppl 2):10–16 [DOI] [PubMed] [Google Scholar]
- Fang C, Zhang QY. (2010) The role of small-intestinal P450 enzymes in protection against systemic exposure of orally administered benzo[a]pyrene. J Pharmacol Exp Ther 334:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasco MJ, Silkworth JB, Dunbar DA, Kaminsky LS. (1993) Rat small intestinal cytochromes P450 probed by warfarin metabolism. Mol Pharmacol 43:226–233 [PubMed] [Google Scholar]
- Fukuda K, Ohta T, Yamazoe Y. (1997) Grapefruit component interacting with rat and human P450 CYP3A: possible involvement of non-flavonoid components in drug interaction. Biol Pharm Bull 20:560–564 [DOI] [PubMed] [Google Scholar]
- Fortun PJ, Hawkey CJ. (2007) Nonsteroidal antiinflammatory drugs and the small intestine. Curr Opin Gastroenterol 23:134–141 [DOI] [PubMed] [Google Scholar]
- Girennavar B, Jayaprakasha GK, Patil BS. (2007) Potent inhibition of human cytochrome P450 3A4, 2D6, and 2C9 isoenzymes by grapefruit juice and its furocoumarins. J Food Sci 72:C417–C421 [DOI] [PubMed] [Google Scholar]
- Harada H, Endo T, Momose Y, Kusama H. (2009) A liquid chromatography/tandem mass spectrometry method for detecting UGT-mediated bioactivation of drugs as their N-acetylcysteine adducts in human liver microsomes. Rapid Commun Mass Spectrom 23:564–570 [DOI] [PubMed] [Google Scholar]
- Hargus SJ, Amouzedeh HR, Pumford NR, Myers TG, McCoy SC, Pohl LR. (1994) Metabolic activation and immunochemical localization of liver protein adducts of the nonsteroidal anti-inflammatory drug diclofenac. Chem Res Toxicol 7:575–582 [DOI] [PubMed] [Google Scholar]
- Higuchi K, Umegaki E, Watanabe T, Yoda Y, Morita E, Murano M, Tokioka S, Arakawa T. (2009) Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol 44:879–888 [DOI] [PubMed] [Google Scholar]
- Kantola T, Kivistö KT, Neuvonen PJ. (1998) Grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid. Clin Pharmacol Ther 63:397–402 [DOI] [PubMed] [Google Scholar]
- Kenny JR, Maggs JL, Meng X, Sinnott D, Clarke SE, Park BK, Stachulski AV. (2004) Syntheses and characterization of the acyl glucuronide and hydroxy metabolites of diclofenac. J Med Chem 47:2816–2825 [DOI] [PubMed] [Google Scholar]
- Li P, Callery PS, Gan LS, Balani SK. (2007) Esterase inhibition by grapefruit juice flavonoids leading to a new drug interaction. Drug Metab Dispos 35:1203–1208 [DOI] [PubMed] [Google Scholar]
- LoGuidice A, Wallace BD, Bendel L, Redinbo MR, Boelsterli UA. (2012) Pharmacologic targeting of bacterial β-glucuronidase alleviates nonsteroidal anti-inflammatory drug-induced enteropathy in mice. J Pharmacol Exp Ther 341:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahgoub AA. (2002) Grapefruit juice potentiates the anti-inflammatory effects of diclofenac on the carrageenan-induced rat's paw oedema. Pharmacol Res 45:1–4 [DOI] [PubMed] [Google Scholar]
- Maiden L. (2009) Capsule endoscopic diagnosis of nonsteroidal antiinflammatory drug-induced enteropathy. J Gastroenterol 44 (Suppl 19):64–71 [DOI] [PubMed] [Google Scholar]
- Mankowski DC, Lawton MP, Ekins S. (2000) Characterization of transgenic mouse strains using six human hepatic cytochrome P450 probe substrates. Xenobiotica 30:745–754 [DOI] [PubMed] [Google Scholar]
- Mertens-Talcott SU, Zadezensky I, De Castro WV, Derendorf H, Butterweck V. (2006) Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol 46:1390–1416 [DOI] [PubMed] [Google Scholar]
- Pumford NR, Myers TG, Davila JC, Highet RJ, Pohl LR. (1993) Immunochemical detection of liver protein adducts of the nonsteroidal anti-inflammatory drug diclofenac. Chem Res Toxicol 6:147–150 [DOI] [PubMed] [Google Scholar]
- Ramirez-Alcantara V, LoGuidice A, Boelsterli UA. (2009) Protection from diclofenac-induced small intestinal injury by the JNK inhibitor SP600125 in a mouse model of NSAID-associated enteropathy. Am J Physiol Gastrointest Liver Physiol 297:G990–G998 [DOI] [PubMed] [Google Scholar]
- Sigthorsson G, Simpson RJ, Walley M, Anthony A, Foster R, Hotz-Behoftsitz C, Palizban A, Pombo J, Watts J, Morham SG, et al. (2002) COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology 122:1913–1923 [DOI] [PubMed] [Google Scholar]
- Small RE. (1989) Diclofenac sodium. Clin Pharm 8:545–558 [PubMed] [Google Scholar]
- Sparidans RW, Lagas JS, Schinkel AH, Schellens JH, Beijnen JH. (2008) Liquid chromatography-tandem mass spectrometric assay for diclofenac and three primary metabolites in mouse plasma. J Chromatogr B Analyt Techol Biomed Life Sci 872:77–82 [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Satoh H. (2010) Measurement of small intestinal damage. Curr Protoc Toxicol Chapter 21:Unit 21 7 [DOI] [PubMed] [Google Scholar]
- Tanaka A, Hase S, Miyazawa T, Takeuchi K. (2002) Up-regulation of cyclooxygenase-2 by inhibition of cyclooxygenase-1: a key to nonsteroidal anti-inflammatory drug-induced intestinal damage. J Pharmacol Exp Ther 300:754–761 [DOI] [PubMed] [Google Scholar]
- Tang W. (2003) The metabolism of diclofenac–enzymology and toxicology perspectives. Curr Drug Metab 4:319–329 [DOI] [PubMed] [Google Scholar]
- Treinen-Moslen M, Kanz MF. (2006) Intestinal tract injury by drugs: importance of metabolite delivery by yellow bile road. Pharmacol Ther 112:649–667 [DOI] [PubMed] [Google Scholar]
- Ware JA, Graf ML, Martin BM, Lustberg LR, Pohl LR. (1998) Immunochemical detection and identification of protein adducts of diclofenac in the small intestine of rats: possible role in allergic reactions. Chem Res Toxicol 11:164–171 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Higuchi K, Kobata A, Nishio H, Tanigawa T, Shiba M, Tominaga K, Fujiwara Y, Oshitani N, Asahara T, et al. (2008) Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut 57:181–187 [DOI] [PubMed] [Google Scholar]
- Wolfe MM, Lichtenstein DR, Singh G. (1999) Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 340:1888–1899 [DOI] [PubMed] [Google Scholar]
- Yan Z, Li J, Huebert N, Caldwell GW, Du Y, Zhong H. (2005) Detection of a novel reactive metabolite of diclofenac: evidence for CYP2C9-mediated bioactivation via arene oxides. Drug Metab Dispos 33:706–713 [DOI] [PubMed] [Google Scholar]
- Zhang QY, Dunbar D, Kaminsky LS. (2003) Characterization of mouse small intestinal cytochrome P450 expression. Drug Metab Dispos 31:1346–1351 [DOI] [PubMed] [Google Scholar]
- Zhang QY, Fang C, Zhang J, Dunbar D, Kaminsky L, Ding X. (2009) An intestinal epithelium-specific cytochrome P450 (P450) reductase-knockout mouse model: direct evidence for a role of intestinal p450s in first-pass clearance of oral nifedipine. Drug Metab Dispos 37:651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QY, Kaminsky LS, Dunbar D, Zhang J, Ding X. (2007) Role of small intestinal cytochromes P450 in the bioavailability of oral nifedipine. Drug Metab Dispos 35:1617–1623 [DOI] [PubMed] [Google Scholar]
- Zhu Y, D'Agostino J, Zhang QY. (2011) Role of intestinal cytochrome P450 (P450) in modulating the bioavailability of oral lovastatin: insights from studies on the intestinal epithelium-specific P450 reductase knockout mouse. Drug Metab Dispos 39:939–943 [DOI] [PMC free article] [PubMed] [Google Scholar]