Abstract
These experiments were completed as part of an NIH-NINDS contract entitled “Facilities of Research Excellence-Spinal Cord Injury (FORE-SCI)—Replication”. Our goal was to replicate data from a paper published by Dr. Lloyd Guth and colleagues in which combined injections of lipopolysaccharide, indomethacin and pregnenolone (referred to herein as LIP therapy) conferred marked neuroprotection in a pre-clinical model of spinal cord injury (SCI). Specifically, post-injury injection of the combination LIP therapy was found to significantly reduce tissue damage at/nearby the site of injury and significantly improve recovery of locomotor function. In this report, we confirm the primary observations made by Guth et al., however, the effects of LIP treatment were modest. Specifically, LIP treatment improved myelin and axon sparing, axonal sprouting while reducing lesion cavitation. However, spontaneous recovery of locomotion, as assessed using historical (Tarlov scoring) and more current rating scales (i.e., BBB scoring), was not affected by LIP treatment. Instead, more refined parameters of functional recovery (paw placement accuracy during grid walk) revealed a significant effect of treatment. Possible explanations for the neuroprotective effects of LIP therapy are described along with reasons why the magnitude of neuroprotection may have differed between this study and that of Guth and colleagues.
Keywords: Neuroinflammation, LPS, Steroids, Spinal cord injury, Replication
Introduction
More than 60 years ago, a serendipitous discovery provided insight to a potentially robust mechanism for promoting recovery after spinal cord injury (SCI). While attempting to study neural centers of temperature regulation, Windle and colleagues discovered that systemic injection of Piromen, a crude pyrogen used to induce fever, augmented recovery of sensory function in spinalized cats (Windle and Chambers, 1950). This same group later showed in monkeys and cats that Piromen enhanced axonal growth which was accompanied by marked intraspinal leukocytosis, comprised mostly of macrophages, and “loosening” of the astroglial scar (Clemente and Windle, 1954).
In the early 1990s, Lloyd Guth and colleagues extended these seminal observations. Since the principal component of Piromen is lipopolysaccharide (LPS), Guth tested the hypothesis that macrophages that were primed by lesion-derived factors would become competent secretory cells with the capacity to promote CNS repair once they were activated with LPS (Guth et al., 1994a, 1994b). He also predicted that any benefit achieved by activating macrophages would be augmented if parallel mechanisms responsible for tissue necrosis were simultaneously blocked or attenuated. Accordingly, Guth compared the histological and behavioral effects of injecting LPS, either alone or in combination with indomethacin, a potent inhibitor of prostaglandin synthesis in a rat model of forceps compression SCI. Alone, LPS minimized lesion cavitation while promoting axon/neurite growth into the lesion site. These effects were markedly enhanced when LPS was combined with indomethacin. Together, LPS and indomethacin marginally improved recovery of motor function in a weight-drop model of spinal contusion injury (Guth et al., 1994b).
In an attempt to further improve anatomical and functional recovery, Guth et al. later tested different combinations of LPS and indomethacin with dehydroepiandrosterone sulfate (DHEAS) or pregnenolone (PREG), two pleotrophic steroids (Guth et al., 1994a). In total, eight different experimental groups were tested using a forceps compression SCI. A modified Tarlov scale was used to define spontaneous locomotor recovery and quantitative morphometric analyses of the lesion were completed. Overall, rats treated with LIP had significantly improved motor function 7–21 days post-injury with >60% of LIP-treated rats achieving “near-normal” locomotor patterns by 3–4 weeks post-injury. Enhanced recovery was associated with reduced lesion cavitation (~17% smaller than in controls) and remarkable preservation of ventral white matter (~17-fold increase in sparing) (Guth et al., 1994a).
Such robust functional and anatomical effects are rarely observed in pre-clinical models of spinal compression or contusion injury. Why then have these data lay dormant for the past 27 years? The commentary by Dr. Guth that accompanies this article explains the background and history of how he came to test this novel combination therapy and why follow-up work was never completed.
To assess the robustness and reproducibility of pre-clinical therapies for SCI, the NINDS created the “Facilities of Research Excellence-Spinal Cord Injury” (FORE-SCI) contracts (see Introductory manuscript this issue). As part of that initiative, we attempted to replicate the neuroprotective effects of the triple combination of LPS, PREG and indomethacin (referred to throughout the remainder of the text as LIP therapy/treatment) as originally described by Guth et al. 17 years ago (Guth et al., 1994a). As shown herein and consistent with the original observations made by Guth et al., LIP treatment is neuroprotective, improves some indices of motor function and enhances intralesional axon growth/sprouting; however, in our hands the benefits of LIP treatment were modest relative to those originally described by Guth et al. Possible explanations for the neuroprotective effects of LIP therapy are described along with reasons why the magnitude of neuroprotection may have differed between this study and that of Guth et al.
Materials and methods
These replication studies compared two experimental groups as described in Table 2 of Guth et al. (1994a), i.e., spinal cord injured rats treated with (1) vehicle or (2) a combination of LPS/PREG/indomethacin (LIP). Although themandate of the FORE-SCI contract is to attempt replication experiments only after attempts have been made to consult with the original author(s), Dr. Guth had retired long ago. Eventually, one of Dr. Guth's former colleagues (Dr. Oswald Steward) provided contact information that allowed one of us (PGP) to speak with Dr. Guth. The background and rationale for his experiments were discussed but a detailed recollection of specific methodological details or reagents was not practical. Thus, formal replication was completed using only information provided in the manuscript targeted for replication and earlier papers from Dr. Guth's lab (Guth et al., 1994b).
Table 2.
Modified Tarlov to BBB Conversions.
| Modified Tarlov Descriptors | Score | BBB Equivalent | Score |
|---|---|---|---|
| Complete hind limb (HL) paralysis | 0 | Paralysis | 0 |
| Barely perceptible HL movement | 1 | Slight movement of most joints | 1–4 |
| Clear-cut HL movements which do not produce locomotion or support weight of body | 2 | Extensive joint movements, sweeping, plantar placing no support | 5–8 |
| Uses limbs to crawl or take a few steps. Can briefly support body weight. | 3 | Plantar placing with weight support and occasional stepping | 9–10 |
| Can walk and support body weight but with a clear-cut deficit | 4 | Frequent to consistent stepping, coordination and paw rotation or dragging throughout the step | 11–15 |
| Walking ability appears almost normal | 5 | Rotation at lift off with or without toe drags | 16–21 |
Surgical procedures
Female Sprague–Dawley (SD) rats (~200 g; Harlan, Indianapolis, IN) were anesthetized with a ketamine/xylazine cocktail (80/10 mg/kg, i.p.). Guth et al. used 4% chloral hydrate as an anesthetic but OSU veterinarians advised against using this drug because it can cause ileus (Fleischman et al., 1977). The skin overlying the thoracic spinal cord was shaved then swabbed with a sequence of betadine scrub, 80% ethanol then betadine solution. Body temperature was maintained at 36–37 °C using a homeothermic blanket (World Precision Instruments Model ATC1000). The thoracic vertebrae from T6–T10 were exposed via incision and a laminectomy was performed at T8. The tips of modified Dumont No. 5 jewelers forceps were placed on either side of the spinal cord between the dura and bone. The forceps were closed for 2 seconds, ensuring that the tips closed completely then were quickly released. Muscle was sutured and the skin incision closed with wound clips. Animals were placed in cages on top of 37 °C warming plates during recovery. Animals received Gentocin (5 mg/kg; s.c.) and 2–5 ml 0.9% saline (s.c.) post-operatively for 6 days. Bladders were expressed manually 2 ×/day until spontaneous voiding was re-established.
Pilot studies were done using n = 12 rats to best replicate the lesions produced by Guth et al. For the formal replication, n = 22 female SD rats were entered into the study protocol. To minimize variability, a single surgeon performed all spinal cord injuries. Prior to surgery, animals were randomly assigned (using GraphPad's QuickCalcs software) to one of two groups: (1) control (vehicle: n = 11) or (2) LPS/Indomethacin/Pregnenolone (LIP: n = 10). One animal died during recovery from surgery in the LIP group.
Preparation and delivery of study compounds
Immediately after SCI, pregnenolone (PREG) was delivered by placing an implantable pellet (SP-111 Innovative Research of America, FL) subcutaneously in a pocket rostral to the laminectomy incision. Although Guth et al. terminated their experiments at 21 days post-injury (dpi), we wanted the option of evaluating rats for longer post-injury survival times. Therefore, to avoid replacing 21 day pellets (50 mg/21 days = 2.38 mg/day) as used by Guth et al. and risk introducing additional surgical variables or changes in circulating and tissue levels of drug, we implanted a 60 day/150 mg pellet (150 mg/60 days = 2.5 mg/day). Animals in the control (vehicle) group received placebo pellets (#SC-111, Innovative Research), which were blank pellets comprised of the same matrix as the PREG pellets. Guth et al. also used time-release pellets prepared by Innovative Research.
Lipopolysaccharide (LPS) from Salmonella enteritidis (Sigma #L6761; 4 mg/ml) was dissolved in sterile pyrogen free water. Guth et al. used an identical species of LPS that was also obtained from Sigma. LPS was delivered via i.p. injection (0.2 mg in 0.1 ml) followed immediately by an i.p. injection of indomethacin (0.16 mg dissolved in 0.09 ml carbonate buffer). The indomethacin used by Dr. Guth was in an intravenous (i.v.) injectable form (Indocin© IV; indomethacin sodium trihydrate: C19H15ClNNaO4•3H2O). We were not able to obtain Indocin© IV. Instead we used indomethacin from Sigma (# I-7378: C19H16ClNO4). To solubilize a stable preparation of indomethacin, the drug was prepared using the technique described by Curry et al. (1982). Briefly, 16.5 mg of indomethacin was slurried with 2 ml of 0.9% NaCl, followed by slow addition with vortexing of 0.022 M Na2CO3 made in 0.9% NaCl. Solution was vortexed until dissolved and then further diluted with 5 ml of 0.9% NaCl. The final solution was filter sterilized and used within 1.5 hours of preparation. The active amount (molar equivalent) of indomethacin used in this study was identical to that used in Guth et al. Rats in the vehicle (control) group received a placebo pellet (above) along with i.p. injections of 0.1 ml sterile pyrogen free water (control for LPS) or 0.09 ml carbonate buffer (control for indomethacin).
Behavioral analysis
Two individuals who were blind to group assignment performed open-field locomotor analysis at 1, 3, 7, 10, 14, 21 and 28 days post-injury using the Basso–Beattie–Bresnahan locomotor rating scale (BBB). Guth et al. reported modified Tarlov scores only at days 10 and 21 post-injury. To facilitate comparison of locomotor recovery scores reported by Guth et al., BBB scores were extrapolated to three different published modifications of the Tarlov scoring system (Table 1).
Table 1.
Evolution of the Modified Tarlov scale.
| Original Tarlov Scale (Tarlov and Klinger, 1954) |
| 0 = no voluntary movement |
| 1 = perceptible movement at joints |
| 2 = good movement at joints but inability to stand |
| 3 = ability to stand and walk |
| 4 = complete recovery |
| * There is no 5 in the original Tarlov scale |
| Wrathall modification of Tarlov scale (Wrathall et al., 1985) |
| 0 = no movement of hind limb; no weight bearing |
| 1 = barely perceptible movement of hind limb; no weight bearing |
| 2 = frequent and/or vigorous movement of hind limb; no weight bearing |
| 3 = can support weight on hind limb; may take one or two steps |
| 4 = walks with only mild deficit |
| 5 = normal walking |
| Guth modifications of Wrathall's modification of Tarlov |
| (Guth et al., 1994a) |
| 0 = complete hind limb paralysis |
| 1 = barely perceptible hind limb movements |
| 2 = clearcut hind limb movements not resulting in weight bearing or locomotion; |
| 3 = hind limbs used to crawl but not to support weight of body; |
| 4 = hindlimbs used to support weight of body for standing and walking, but with clearcut disability; |
| 5 = posture and walking ability nearly normal. |
| (Guth et al., 1994b) |
| 0 = complete paralysis of both hind limbs |
| 1 = barely perceptible hind limb movements |
| 2 = clear-cut hind limb movements which do not produce locomotion or support weight of body |
| 3 = uses limbs to crawl or take a few steps; can briefly support the weight of the body |
| 4 = can walk and support body weight but with a clear-cut deficit |
| 5 = walking ability appears to be almost normal |
For all rats, BBB subscores were derived to quantify recovery of toe clearance, paw position, trunk stability and tail use independent of forelimb–hindlimb coordination. Each rat could achieve a maximum subscore of 13 using the following scale: toe clearance → max score = 6 (3/HL)–[none = 0; occasional = 1; frequent = 2; consistent = 3]; paw position → max score = 4 (2/HL scored at initial contact/lift off) [rotated/rotated = 0; parallel/rotated = 1; parallel/parallel = 2]; trunk → max score = 1; [trunk stable = 1; trunk unstable = 0]; tail use → max score = 2; [tail always down = 0; tail up and down = 1; tail always up = 2].
To provide a non-subjective quantitative analysis of hindlimb function, rats also were tested on a gridwalk task at 28 dpi. Rats crossed a grid (1 in. × 1 in. squares, 36 cm × 28 cm area) in both directions for ~6 minutes. Trials were recorded using a Sony HDR-SR11 HD 1080 video camera. An individual rater unaware of treatment evaluated each hindlimb separately from videos displayed at half speed. Successful hindlimb placements were quantified only when the trunk was moving forward during limb advancement and successful placement occurred when the hind paw was placed on a rung with weight support. Guth et al. did not evaluate precision locomotor function on a grid.
Tissue preparation and histology/immunohistochemistry
Animals were anesthetized with ketamine/xylazine and then transcardially perfused with cold 0.1 M PBS pH 7.4, followed by fixation with 4% paraformaldehyde in 0.1 M PBS, pH 7.4. Spinal cords were removed, post-fixed for 2 hours then placed at 4 °C in 0.2 M PBS pH 7.4 overnight and then cryoprotected in 30% sucrose until the tissue sank. A 20 mm length of spinal cord tissue centered at the injury epicenter was embedded in OCT compound (Thermo Shandon, Pittsburgh, PA), frozen on dry ice and stored at −80 °C. Spinal cords were serially sectioned in the coronal plane using a Microm cryostat at 10 µm. Adjacent sets of cross-sections spaced 100 µm apart were collected on Superfrost slides. A series of sections spanning the entire rostro-caudal extent of the lesion were stained using antibodies specific for neurofilament (NF; 1:2000; RT97 clone; Developmental Hybridoma Studies Bank) and were subsequently stained with eriochrome cyanine (Sigma) to label spared or “intact” myelin. Guth et al. analyzed paraffin-embedded spinal cords prepared at 21 dpi. From these samples, longitudinal sections were cut in either the coronal or sagittal plane then were stained with hematoxylin/eosin for cellular detail or were impregnated with Protargol (silver stain) to visualize axons.
To illustrate representative cellular components at the lesion site using additional contemporary methods, a subset of frozen sections from both groups were prepared for confocal microscopic analyses using a triple immunofluorescent labeling technique. Antibodies specific for Iba-1 (microglia; 1:1500, Wako, 019-19741), NF (see above) and GFAP (astrocytes; Aves; 1:500) were applied to sections overnight at 4 °C then for an additional 2 hrs at room temperature. Slides were rinsed with PBS followed by a wash in high salt PBS (23.38 g NaCl2/1 L of PBS) to reduce non-specific ionic interactions. Primary antibodies were visualized using species-specific directly conjugated secondary antibodies: Iba-1 (AlexaFluor 488 @ 1:500; goat anti-rabbit IgG; Molecular Probes, A11034); NF (AlexaFluor 546 @ 1:1000; goat anti-mouse IgG ; Molecular Probes, A11030); GFAP (AlexaFluor 633 @ 1:500; goat anti-chicken; Molecular Probes, A21103). Sections were incubated with secondary antibodies for 1 hour at room temperature after which sections were rinsed with PBS then coverslipped with Immu-Mount (Thermo, 9990402). All antibodies were diluted in a solution of 4%BSA/0.1%Triton x-100/PBS.
Quantitative morphometric analysis of injured spinal cord sections
Guth et al. prepared sagittal sections of the spinal cord for analysis of lesion volume. Specifically, using individual sections that spanned a width of 360 µm centered on the central canal, Guth et al. quantified changes in lesion pathology. Based on our previous experience and expectation that measurement of lesion volume and cavitation should be independent of plane of section, we cut all specimens in the transverse plane. This allowed unequivocal visualization of cellular changes in gray and white matter while still allowing for quantitative analysis of lesion length and volume.
First, to match the analysis of the region of interest defined in sagittal sections by Guth et al., we placed a rectangular sample box 360 µm wide spanning the dorso-ventral axis of the spinal cord. The sample box was centered on the spinal cord midline of equally spaced cross-sections spanning the rostro-caudal extent of the lesion. The area of lesioned tissue (injured tissue) or cavity was quantified (see Fig. 2). Cavitation was distinguished from histological artifact by microscopically comparing continuity of tissue adjacent to the suspected area of cavitation. Cavities that were not bounded by obvious glial scars or that did not contain inflammatory cells were considered artifact. Using these criteria, the magnitude of cavitation was likely underrepresented in our analyses. However, all tissues were treated identically so changes within and between groups are comparable.
Fig. 2.
Morphometric analysis of midline regions of injured spinal cord. A rectangular sample box (inset) of width 360 µm was centered on the area of the central canal on serial cross-sections spanning the rostral–caudal extent of the lesion. A comparison of spinal cord from vehicle vs. LIP-treated rats revealed no significant differences in the area of spinal cord that was occupied by injured/lesioned tissue (A) or cavity (B). There was a trend for reduced lesion length in LIP-treated rats (C; p = 0.08). Each data point represents average value from a single rat. Representative spinal cord sections from vehicle (D, F) and LIP-treated (E, G) stained with eriochrome cyanine (EC: myelin) and RT-97 (neurofilament: axons) antibodies. In D, E, dark labeling in dorsal spinal cord is a result of extensive Schwann cell infiltration and subsequent EC + staining. These regions (when present) were not included in myelin sparing (or neurofilament - see Fig. 3) analyses. Note mild EC labeling in ventral white matter of section from LIP-treated rat (G) and presence of spared NF + axons (punctate labeling near edge of spinal cord). In both vehicle (F) and LIP-treated (G) sections, axonal sprouts co-localize with inflammatory cells (see also Fig. 4). Scale = 50 µm (F, G) and 200 µm (D, E). Note: Box in D illustrates region of photomicrographs shown in Fig. 4.
Next, instead of limiting our quantitative analyses to a 360-µm width of spinal cord, we quantified the area of spared myelin (defined by normal appearing eriochrome cyanine staining) and lesion cavitation (defined as areas devoid of myelin, neurofilament or background labeling) throughout the entire cross-section using an unbiased point-counting technique (Cavalieri's method) (Gundersen and Jensen, 1987). Mean area was quantified from 5 evenly spaced sections spanning the rostral–caudal length of the lesion containing obvious lesion cavitation.
Finally, as we have described previously (Kigerl et al., 2007), densitometric thresholding and image analysis was used to quantify spared or growing neurofilament-positive (NF+) axons, both within the region confined by the 360-µm sample box or across the entire cross-section. One specimen was damaged during processing leaving n = 10 animals/group for quantitative morphometric analyses.
Statistics
Repeated measures ANOVA was used to compare BBB or Tarlov scores (time vs. treatment) with t-tests for post-hoc comparisons (Scheff et al., 2002). Accuracy of hindlimb placement on the grid was analyzed using χ2 test. Pearson product moment correlation test analyzed the relationship between success on the grid and BBB subscores. Histological analyses were analyzed using 2-way ANOVA or unpaired t-tests.
Results
Pilot studies: calibrating the forceps compression injury and Tarlov/BBB conversions
Experimental spinal cord compression injuries are easily produced using forceps; however the primary trauma varies as a function of compression rate and duration. The magnitude of primary trauma also is expected to vary as a function of the rostro-caudal length of spinal cord that is compressed (determined by the width of the forceps blades) and the magnitude of compression across the ventral-most part of the spinal cord. After reviewing several manuscripts by Guth et al., it became clear that in order to approximate the magnitude of pathology and extent of functional recovery in SCI control rats as described by Guth et al., pilot studies would be needed to calibrate the forceps compression model.
In the manuscript targeted for replication, Guth used a #5 Dumont jewelers forceps to compress the thoracic (T8) spinal cord for 2 seconds. No additional details about the injuries were provided. In other studies Guth et al. described using “thinned and blunted” forceps or jeweler's forceps in which the “tips had been ground to 0.3 mm in width” (Guth et al., 1994b; Zhang and Guth, 1997). Because we did not know which forceps to use for replication, we compared histological and behavioral outcome in rats subjected to SCI with one of two modified pairs of #5 Dumont jeweler's forceps. Forcep tips were ground such that the thickness of the forcep blade in contact with the spinal cord increased from the tip (ventral spinal cord) to the shaft over a range of 0.3–0.5 mm (“thin”) or 0.3–0.8 mm (“thick”). Based on the formula for an area of trapezoid, which is the approximate shape of the forceps in contact with spinal cord, the thick forceps are in contact with ~30% more surface area of spinal cord than the thin forceps. This difference in tip size significantly affected tissue pathology and functional recovery.
Acute (4 days) histological preparations revealed even spinal cord compression across the dorso-ventral axis with both forceps (not shown); however, the thick forceps caused more severe injuries with limited or no functional recovery over the first 5 dpi (average BBB scores = 0 or 1). By 21 dpi, n = 1/8 rats achieved a BBB score of 4.5 (average of both hindlimbs) but the remaining subjects achieved only slight movement of one or two hindlimb joints (BBB scores = 1 or 2). For comparison, these scores correspond to 0–1 on the modified Tarlov scale, which is more severe than the Tarlov scores achieved at 21dpi by control rats in Guth et al. (~2.4 or hindlimb movement but without weight support). Histology at 21 dpi confirmed that the injuries were more severe than those published by Guth et al. (not shown).
“Thin” forceps were then tested on additional rats (n = 4). By 5 dpi, some recovery of function was evident in all rats culminating with weight-supported stepping at 21 dpi. This level of recovery was superior to the control groups described by Guth et al. (Tarlov scores of ~2–3; clear-cut HL movements but without weight-supported stepping) but was much closer than the functional recovery achieved by rats injured with “thick” forceps. Consequently, “thin” forceps were used for the formal replication experiment.
It was also necessary to calibrate techniques for behavioral scoring. All behavioral data from Guth et al. were generated using modifications of the Tarlov scale, citing Wrathall et al. (1985) as the source of these modifications. However, across the different publications from Dr. Guth's lab, inconsistencies were found in the reported methods (see Table 1). Therefore, we applied the more widely used BBB open-field locomotor rating scale then extrapolated those data to different Tarlov equivalents (Table 2 and Fig. 1).
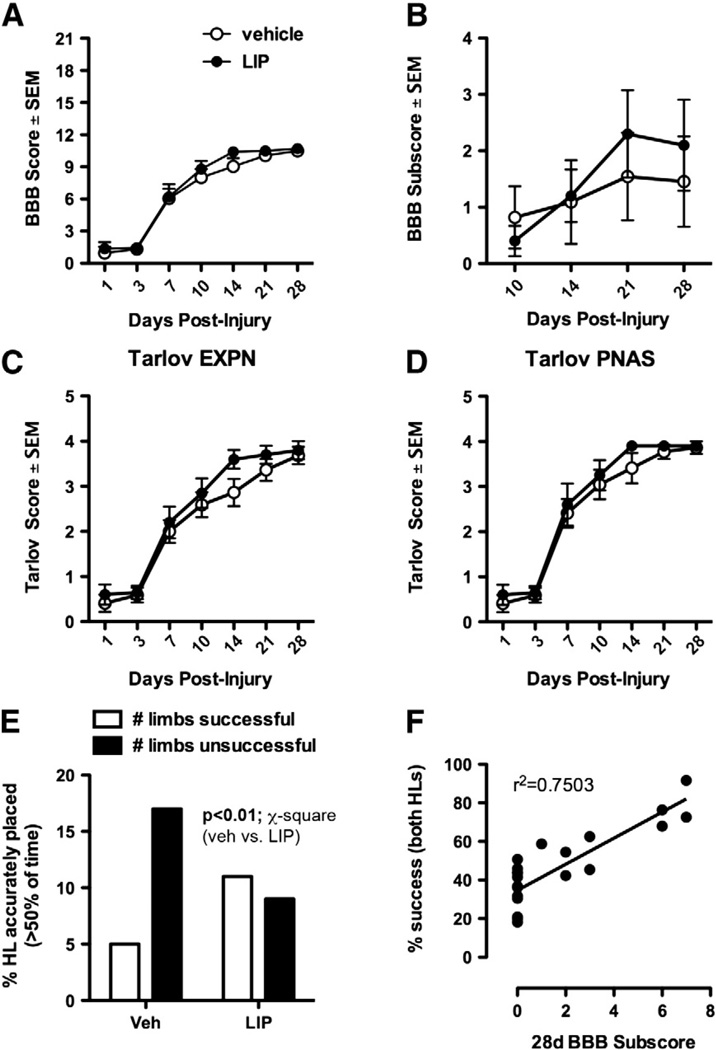
Fig. 1.
Behavioral analysis of vehicle and LIP-treated SCI rats. Analysis of open-field locomotion using the Basso–Beattie–Bresnahan (BBB) rating scale (A), BBB subscores (B), or two modifications of the Tarlov scale as described by Guth et al. (C and D) failed to reveal an effect of treatment on spontaneous recovery of motor function. EXPN and PNAS refer to the different journals in which Guth's two different Tarlov scales were published. However, with each task there was a tendency for LIP-treated rats to achieve frequent or consistent plantar stepping after two weeks. This subtle increase in functional recovery was obvious on a grid walking task, where LIP treatment significantly increased paw placement accuracy (E; p<0.01 LIP vs. vehicle; χ2 analysis). Paw placement success was significantly correlated with higher BBB subscores (F; p<0.05).
Formal replication experiment
Guth et al. quantified spontaneous recovery of motor function every day for 21 dpi using a modified Tarlov scale. Only average scores at days 10 and 21 were reported (see Tables 1 and 2 in Guth et al., 1994a). We scored recovery of motor function from 1 to 28 dpi using the individual criteria defined in 3 different modifications of the original Tarlov scale (see Table 1) and the BBB locomotor rating scale.
In all rats, forceps compression injury caused significant locomotor impairment with some spontaneous functional recovery evident over the course of 28 days; however, there were no differences between vehicle and LIP-treated animals (p > 0.05) (Fig. 1A). Similar patterns of recovery were noted when BBB scores were converted to modified Tarlov scores (Table 2; Fig. 1C and D). Signs of a treatment effect were evident using BBB subscore analyses. BBB subscores showed that most rats in the control group failed to achieve a milestone of frequent stepping and had greater deficits in HL use during stepping. This resulted in a median subscore of 0 at 10 (n = 9), 14 (n = 9) and 21 dpi (n = 7) (Fig. 1B). In contrast, 50% of LIP-treated rats stepped frequently or consistently by 14 dpi, increasing to 70% of the group at 21dpi with a median subscore of 2 (Fig. 1B).
Although group differences in BBB subscores were underwhelming in terms of total score, they were predictive of improved hindlimb function on a grid-walking task (Fig. 1D, E). Indeed, a significant treatment effect was observed when analyzing paw placement accuracy during grid walking. The lowest performers in both groups accurately placed their HLs on 18–36% of their steps. Most of these rats were control animals. Rats with intermediate HL placement accuracy achieved accurate placement with 41–50% of their steps. An equal number of control and LIP-treated rats were classified as “intermediate” performers. High performers were 54–91% accurate during grid walking and were mostly from the LIP-treated group. Using a χ2 analysis, a significant effect of treatment was detected with more LIP-treated rats being able to accurately place > 50% of the time (p< 0.01; LIP vs. vehicle) (Fig. 1E). Grid performance correlated with BBB subscores (r2 = 0.72, p < 0.001) indicating that rats with greater accuracy on the grid had improved recovery of toe clearance and paw position (Fig. 1E).
Histological analysis
Guth et al. evaluated lesion area and spared ventral white matter (VWM) using paraffin-embedded tissue sections cut in the sagittal plane. To ensure that observations were made at the same level in all samples, those analyses were completed only on sections in which portions of the central canal were visible in proximity to the lesion (mid-sagittal section). In total, a width of 360 µm of spinal cord centered on the central canal was analyzed across the entire dorsoventral axis of the spinal cord for each animal at 21 dpi. To ensure that the same regions of spinal cord were analyzed in cross-sections, we quantified areas of lesion (damaged tissue) and cavity (devoid of tissue) within a rectangular box of width 360 µm centered on the spinal cord midline (Fig. 2, inset). Using this approach, no differences were detected in lesion area, cavity area or lesion length; however, cavity size and lesion length were more consistent and tended to be reduced in spinal cords from LIP-treated rats (Fig. 2A–C).
We also analyzed the mean area of spared white matter or lesion cavity throughout the entire cross section of up to 5 tissue sections spanning ~1.2 cm of spinal cord. This length of tissue corresponded with the area in which lesion cavities were grossly obvious in both groups. Using stereological point counting techniques, significantly more spared white matter was detected in LIP-treated spinal cords as compared to vehicle controls (Fig. 3A). Although lesion cavities were not different between groups, there was a trend for smaller cavities in spinal cords of LIP-treated rats (Fig. 3B; p = 0.06 vs. vehicle).
Fig. 3.
Morphometric analysis of injured spinal cord cross-sections using stereological point-counting (A and B) or image analysis (C and D). The cross-sectional area of spared white matter and lesion cavity was quantified in evenly spaced sections spanning the average length of spinal cord containing obvious lesion cavities (1.2 mm). Significantly more white matter sparing was evident in LIP-treated rats and there was a trend for reduced lesion cavity size (p = 0.07; LIP vs. vehicle). Threshold detection and quantification of all neurofilament-positive axons in these same sections revealed a significant increase in LIP-treated rats (C). Within the lesion center, where macrophages predominate, axon growth/sprouting was significantly enhanced in sections from LIP-treated rats (D). *p<0.05; **0.01; ***0.001 vs. vehicle.
During blinded analyses of these sections, marked differences in axonal sparing and/or sprouting were noted between sections. Quantitative analyses confirmed that axonal density (quantified within a rectangular box of width 360 µm centered on the central canal) was significantly greater in LIP-treated rats, both in regions of spared white matter and within the center of the lesion (Fig. 3C, D). Using Protargol (silver stain) to visualize axons, Guth et al. described “remarkable injury-attenuating effects” of LIP in midsagittal plane at 21 dpi with a “modest number of nerve fibers characteristic of regenerating axons coursing within the lesion”. In the present study, triple immunofluorescent labeling and confocal microscopy was used to visualize these cellular responses. In sections that were taken from specimens that were representative of mean neurofilament-labeling in the lesion center of vehicle or LIP-treated rats, a modest but consistent increase in axonal sprouting was co-localized with a robust macrophage response, especially in sections from LIP-treated subjects (Fig. 4). Similar patterns were noted in vehicle-treated rats but fewer axon profiles were detected and the morphology of the macrophage reaction was distinct; i.e., the cells were smaller and exhibited a more ramified morphology. GFAP-immunoreactivity also appeared to be decreased in sections from LIP-treated rats (Fig. 4). Detailed quantitative analyses were not completed since Guth's observations also were qualitative.
Fig. 4.
Triple immunofluorescent confocal images demonstrating LIP-dependent increase in axonal density within the center of the spinal cord (see Fig. 2D). Dense NF + axons co-localize with large accumulations of Iba-1 + microglia/macrophages. Images are representative of two different animals from vehicle (A and B) and LIP (C and D) treatment groups. Although not quantified, there appeared to be more GFAP (astrocytes; blue) immunolabeling in vehicle-treated groups. Scale = 100 µm.
Discussion
It is widely believed by the SCI research community that successful repair of the injured spinal cord will require a combination therapy. More than 20 years ago, Guth and colleagues stated that “a single therapy is not likely to be effective” (Guth et al., 1994a). Instead, they believed that optimal repair could be achieved by coordinating interactions between neuronal and non-neuronal cells, specifically by balancing the growth promoting and inhibitory effects of inflammatory cascades.
Because LPS is the principal component of Piromen, a drug that was found to be effective in promoting axon regeneration in dogs and cats >60 years ago (Windle and Chambers, 1950) and because at the time of his studies, LPS was known to elicit secretions from microglia, macrophages and astrocytes, Guth tested LPS in a model of SCI. When injected alone, LPS reduced the size of lesion cavities and enhanced axon growth into the lesion along cellular bridges (Guth et al., 1994b). Today, LPS is still widely used to activate microglia and macrophages in experimental models (in vitro and in vivo) and LPS pre-conditioning (via systemic injection) is neuroprotective in models of cerebral ischemia/stroke and SCI (Ahmed et al., 2000; Davis et al., 2005).Unbeknownst to Guth at the time was the fact that LPS exerts its effects through activation of toll-like receptor 4 (TLR4), an evolutionarily conserved pattern recognition receptor that stimulates pro-inflammatory cascades in immune cells. TLR4 is also expressed by resident glia (e.g., microglia and astrocytes), neurons and neuronal precursor/stem cells (Hanisch et al., 2008; Rolls et al., 2007). Within the CNS, a number of putative TLR4 agonists exist and their ability to bind TLR4 increases after injury (Kigerl and Popovich, 2009; Rock et al., 2010). Thus, some level of TLR4 signaling may be important for coordinating endogenous CNS repair. Indeed, disruption of TLR4 signaling after SCI impairs spontaneous recovery of function and exacerbates gliosis and intraspinal inflammation (Kigerl et al., 2007). Moreover, a number of TLR agonists and other proinflammatory stimuli can elicit macrophage-mediated axon regeneration (Gensel et al., 2009). However, excess TLR4 activation, whether by LPS or endogenous TLR4 ligands (e.g., HMGB1), can cause pathology and exacerbate neurological impairment (Lehnardt et al., 2003; Yang et al., 2010). This Janus-faced property of TLR4 activation may be why, when used alone, LPS is inferior to the combined effects of injecting LPS, indomethacin and pregnenolone (PREG).
Indomethacin is a widely used non-steroidal anti-inflammatory (NSAID) drug. Guth used it because of its ability to inhibit the synthesis of prostaglandins, presumed effectors of tissue necrosis after SCI. When injected alone after SCI, Guth found that indomethacin had little or no effect on recovery of function nor did it improve anatomical repair. More recently, indomethacin and ibuprofen, another NSAID, were found to improve axon regeneration and reduce the accumulation of inflammatory cells after experimental SCI, in part by suppressing cellular RhoA activation (Fu et al., 2007; Schwab et al., 2004). PREG was later added to the combination simply because LPS and indomethacin did not markedly improve behavioral recovery and it was believed that context dependent metabolism of PREG would enhance neuroprotection. In retrospect, these studies were ahead of their time. Indeed, today there is much controversy surrounding the benefits and detriments of manipulating intraspinal inflammation. The simultaneous use of a potent inflammogen (LPS) with two different types of anti-inflammatory agents (indomethacin and PREG) illustrates that Guth had some insight to the divergent functional consequences of neuroinflammation long ago. Despite the fact that Guth's original observations have been confirmed in this report, the effects that we describe are much less dramatic. The remainder of the discussion will focus on possible explanations for the discrepancy.
An essential component of a formal replication study is communication with the authors of the original manuscript. Critical experimental details are often taken for granted or are unintentionally omitted from the Methods section. Moreover, many high-impact journals (e.g., PNAS, the site of publication for the study that we replicated) limit text length, which further restricts methodological details. Our efforts to replicate Guth's original work were made significantly more difficult by the fact that Dr. Guth is retired. Had we been able to discuss methodological details, we may have a better explanation for why the LIP treatment was less effective in our hands. Therefore, some speculation is needed.
One possibility is that the rats did not receive the same concentrations of LPS or indomethacin. In Guth's first paper describing the use of LPS (Guth et al., 1994b), the methods state that LPS was given twice daily (0.2 mg/injection) but in the paper that was the focus of this replication study, LPS was injected once daily. Whether the dosing was intentionally changed between papers is not known. If not, then it is possible that insufficient LPS was injected in our experiments. The indomethacin used by Dr. Guth was provided to him in an intravenous (i.v.) injectable form (Indocin© IV; indomethacin sodium trihydrate). Presumably, this drug has the same biological activity as the indomethacin that we used but the chemical formulas are distinct (e.g., Indocin© IV is C19H15ClNNaO4•3H2O whereas we used powdered indomethacin from Sigma; C19H16ClNO4). Therefore, solubility and tissue concentrations of indomethacin could be different between our studies and those of Guth et al.
It is also apparent that different approaches were used to evaluate locomotor recovery; even within different publications from the Guth laboratory, subtle but potentially important variations exist in how the Tarlov scale was applied. This is understandable since the Tarlov scale is not linked to strict operational definitions of recovery. Accordingly, differences in subjective interpretation were introduced into the scale making it difficult to apply the same technique within and between laboratories. As an example, compare scores of 2 and 3 between the two Guth et al. manuscripts in Table 1. On the one hand, it is difficult to know exactly how “clear-cut hind limb movements not resulting in weight bearing or locomotion” were distinguished from “hind limbs used to crawl but not to support body weight”. However, in a separate report the difference between a score of 2 and a score of 3 was reaching a threshold of weight-support. This could simply be attributed to an error in copying methods between manuscripts or it could represent a subtle difference in scoring. The point being that when we applied the modified Tarlov, we may have interpreted things differently from Dr. Guth, especially since our group traditionally uses the BBB scale.
Similar to another study described in this issue (Popovich et al., in press), it is possible that differences in injury severity obscured the ability of LIP treatment to improve gross locomotor function. For example, despite our best efforts to replicate the forceps compression injury used by Guth et al., their injuries appear to be more severe. Based on Tarlov scores at 10 dpi, most rats in the Guth study displayed “barely perceptible hind limb movements” whereas our rats were “crawling” or “using their hind limbs extensively” (using Tarlov definitions). On the other hand, if one considers that a combination of LPS + indomethacin was effective after moderate but not severe spinal contusion injury in rats (Guth et al., 1994b), then it is not easy to reconcile discrepancies based on injury severity.
As noted above, tissue preparation, sectioning and staining techniques were different between the two studies. Guth cut paraffin sections, mostly in the sagittal plane then evaluated lesion morphometry and axon labeling using hematoxylin/eosin or protargol, respectively. We rationalized that an anatomical effect as robust as the one that he described should be evident regardless of how tissues were prepared. Although our tissues were processed for immunohistochemistry (using paraformaldehyde-fixed frozen cross-sections), the same regions of the spinal cord that were evaluated by Guth et al. also were analyzed in the present study. The biggest difference was our inability to detect an effect of LIP treatment on ventral white matter (VWM) sparing. This is where Guth described the most remarkable neuroprotection in LIP-treated rats. In our analysis, often times the cross-sections collapsed in the dorso-ventral axis or the tissue sections were severely distorted near the site of trauma—a problem that paraffin embedding prevents. These sections often had to be excluded from our analyses, especially using the 360 µm box/midline analyses described for Fig. 2, and this may have obscured our ability to detect sparing in VWM. Why this spinal cord region was preferentially affected is not clear but it is likely a function of the injury model and the fact that the VWM is susceptible to secondary degenerative cascades (Rosenberg et al., 1999). Guth noted that forceps compression injury “damages only the dorsal 80% of the spinal cord, sparing the ventral most axons” (Guth et al., 1994b). Moreover, the importance of VWM in recovery of function has been documented (Loy et al., 2002). Thus, despite the positive effects that we observed with LIP treatment, the effects may have more closely resembled Guth's had we been able to more precisely mimic the lateral forceps compression injury and subsequent damage to VWM.
Ultimately, therapeutic benefit of any drug or cell-based therapy may vary as a function of the location, type and severity of trauma and not all therapies, whether applied in pre-clinical models or human subjects, should be expected to exert equivalent effects. While each of these biological and technical variables make replication experiments difficult, the fact that a modicum of replication was achieved in the present report may speak to the robustness of effect of the LIP combination therapy. At a minimum, additional research is needed to identify the cellular mechanisms of these drug interactions in order to devise more robust, safe and effective neuroprotective therapies targeting the complex inflammatory response.
Acknowledgments
Thank you to Drs. Dana McTigue, John Buford and Sandra Kostyk and the technical staff and trainees of the Center for Brain and Spinal Cord Repair for the discussions related to manuscript selection and experimental design. Sponsored by NIH-NINDS contract HHSN271200800040C to P.G.P.
Abbreviations
- LPS
lipopolysaccharide
- PREG
prednenolone
- SCI
spinal cord injury
- dpi
days post-injury
- BBB
Basso–Beattie–Bresnahan
- LIP
LPS/indomethacin/PREG
References
- Ahmed SH, He YY, Nassief A, Xu J, Xu XM, Hsu CY, Faraci FM. Effects of lipopolysaccharide priming on acute ischemic brain injury. Stroke. 2000;31(1):193–199. doi: 10.1161/01.str.31.1.193. [DOI] [PubMed] [Google Scholar]
- Clemente CD, Windle WF. Regeneration of severed nerve fibers in the spinal cord of the adult cat. J. Comp. Neurol. 1954;101(3):691–731. doi: 10.1002/cne.901010304. [DOI] [PubMed] [Google Scholar]
- Curry SH, Brown EA, Kuck H, Cassin S. Preparation and stability of indomethacin solutions. Can. J. Physiol. Pharmacol. 1982;60(7):988–992. doi: 10.1139/y82-139. [DOI] [PubMed] [Google Scholar]
- Davis AEM, Campbell SJ, Wilainam P, Anthony DC. Post-conditioning with lipopolysaccharide reduces the inflammatory infiltrate to the injured brain and spinal cord: a potential neuroprotective treatment. Eur. J. Neurosci. 2005;22(10):2441–2450. doi: 10.1111/j.1460-9568.2005.04447.x. [DOI] [PubMed] [Google Scholar]
- Fleischman RW, McCracken D, Forbes W. Adynamic ileus in the rat induced by chloral hydrate. Lab. Anim. Sci. 1977;27(2):238–243. [PubMed] [Google Scholar]
- Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J. Neurosci. 2007;27(15):4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J. Neurosci. 2009;29(12):3956–3968. doi: 10.1523/JNEUROSCI.3992-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling stereology and its prediction. J. Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Guth L, Zhang Z, Roberts E. Key role for pregnenolone in combination therapy that promotes recovery after spinal cord injury. PNAS (USA) 1994a;91(25):12308–12312. doi: 10.1073/pnas.91.25.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L, Zhang Z, DiProspero NA, Joubin K, Fitch MT. Spinal cord injury in the rat: treatment with bacterial lipopolysaccharide and indomethacin enhances cellular repair and locomotor function. Exp. Neurol. 1994b;126(1):76–87. doi: 10.1006/exnr.1994.1043. [DOI] [PubMed] [Google Scholar]
- Hanisch U, Johnson T, Kipnis J. Toll-like receptors: roles in neuroprotection. Trends Neurosci. 2008;31(4):176–182. doi: 10.1016/j.tins.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Popovich PG. Toll-like receptors in spinal cord injury. Curr. Top. Microbiol. Immunol. 2009;336:121–136. doi: 10.1007/978-3-642-00549-7_7. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J. Neurochem. 2007;102(1):37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. PNAS (USA) 2003;100(14):8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Magnuson DSK, Zhang YP, Onifer SM, Mills MD, Cao Q-L, Darnall JB, et al. Functional redundancy of ventral spinal locomotor pathways. J. Neurosci. 2002;22(1):315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Lemeshow S, Gensel JC, Tovar CA. Independent evaluation of the effects of glibenclamide on reducing progressive hemorrhagic necrosis after cervical spinal cord injury. Exp Neurol. doi: 10.1016/j.expneurol.2010.11.016. >in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu. Rev. Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 2007;9(9):1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. Effects of the sodium channel blocker tetrodotoxin on acute white matter pathology after experimental contusive spinal cord injury. J. Neurosci. 1999;19(14):6122–6133. doi: 10.1523/JNEUROSCI.19-14-06122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Saucier DA, Cain ME. A statistical method for analyzing rating scale data: the BBB locomotor score. J. Neurotrauma. 2002;19(10):1251–1260. doi: 10.1089/08977150260338038. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Conrad S, Elbert T, Trautmann K, Meyermann R, Schluesener HJ. Lesional RhoA+ cell numbers are suppressed by anti-inflammatory, cyclooxygenase-inhibiting treatment following subacute spinal cord injury. Glia. 2004;47(4):377–386. doi: 10.1002/glia.20031. [DOI] [PubMed] [Google Scholar]
- Tarlov IM, Klinger H. Spinal cord compression studies. II. Time limits for recovery after acute compression in dogs. A. M. A. Arch. Neurol. Psychiatry. 1954;71(3):271–290. [PubMed] [Google Scholar]
- Windle WF, Chambers WW. Regeneration in the spinal cord of the cat and dog. J. Comp. Neurol. 1950;93(2):241–257. doi: 10.1002/cne.900930206. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Pettegrew RK, Harvey F. Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp. Neurol. 1985;88(1):108–122. doi: 10.1016/0014-4886(85)90117-7. [DOI] [PubMed] [Google Scholar]
- Yang Q-W, Lu F-L, Zhou Y, Wang L, Zhong Q, Lin S, Xiang J, et al. HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J. Cereb. Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Guth L. Experimental spinal cord injury: Wallerian degeneration in the dorsal column is followed by revascularization, glial proliferation, and nerve regeneration. Exp. Neurol. 1997;147(1):159–171. doi: 10.1006/exnr.1997.6590. [DOI] [PubMed] [Google Scholar]