Abstract
Adipose tissue inflammation is a major mechanistic link between obesity and chronic disease. To isolate and characterize specific leukocyte populations, e.g. by flow cytometry, tissue needs to be processed to digest the extracellular matrix. We have systematically compared the impact of different commonly used collagenase preparations, digestion times, and normalization strategies on the reproducibility of flow cytometric phenotyping of adipose tissue leukocyte populations. Subcutaneous adipose tissue was obtained from 11 anonymous donors undergoing elective procedures at a plastic surgery clinic in Seattle, WA. We found that collagenase alone consistently produced better cell yields (p=0.007) than when combined with additional proteases such as the commercially available liberases. Moreover, liberase significantly degraded the cell surface expression of CD4 (p<0.001) on T cells and to a lesser extent CD16 (p=0.058) on neutrophils. Extension of the digestion interval from 30 to 120 min did not significantly impact cell viability (p=0.319) or yield (p=0.247). Normalization by either ‘live-gate’, or percentage of CD45pos leukocytes exhibited the lowest coefficient of variation for tissue digests between 60 and 75 min, compared to normalization per gram of tissue, which consistently exhibited the greatest variability. Our data suggest that digestion of adipose tissue using pure collagenase for 60 to 75 min provides the best cell yield and viability, with minimal degradation of cell surface markers used to identify immune cell subpopulations, and best reproducibility independent of the normalization strategy.
Keywords: adipose tissue, leukocytes, enzymatic digestion, normalization
1. Introduction
The global prevalence of obesity has reached epidemic proportions, serving as a harbinger of greater morbidity and mortality. In addition to being strongly associated with the incidence of type 2 diabetes mellitus (T2DM) (Shoelson et al., 2007) and cardiovascular disease (CVD) (Berg and Scherer, 2005), obesity also increases the risk of several common cancers (Calle et al., 2003; McMillan et al., 2006; Pischon et al., 2008). Key components linking these diseases with obesity are metabolic disruptions such as insulin resistance and hyperinsulinemia, as well as the establishment of a chronic low-grade inflammatory state (Greenberg and Obin, 2006; Shoelson et al., 2006; Andersson et al., 2008). This inflammatory state is reflected by elevated concentrations of circulating inflammatory cytokines and acute phase proteins (Yudkin et al., 1999; Clement et al., 2004; Berg and Scherer, 2005; Kahn et al., 2006; Shoelson et al., 2006; Lumeng et al., 2007c).
Systemic inflammation in obesity is thought to be largely caused by adipose tissue infiltration with macrophages as body fat mass expands (Weisberg et al., 2003; Xu et al., 2003; Curat et al., 2006; Zeyda et al., 2007; Zeyda and Stulnig, 2007; Andersson et al., 2008). Importantly, macrophages are known to secrete the pro-inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor α (TNFα) that have been shown to impair insulin action in adipocytes (Uysal et al., 1997; Lumeng et al., 2007c; Tilg and Moschen, 2008). Moreover, in models of diet-induced obesity, pro-inflammatory macrophages increasingly express the integrin CD11c (Lumeng et al., 2007a). Intriguingly, ablation of CD11cpos cells results in normalization of insulin sensitivity and reduced systemic inflammation (Patsouris, 2008). Still, macrophages alone are not the sole drivers of tissue inflammation. Both T and B cells play important roles as both inhibitors and effectors of tissue specific inflammation. Specifically, the typically abundant CD4posFoxp3pos T cells that regulate the innate immune system are markedly reduced in the adipose tissue of insulin-resistant obese mice (Feuerer et al., 2009; Winer et al., 2009). In contrast, CD8pos effector T cells accumulate in adipose tissue in response to diet-induced obesity, in advance of macrophage accumulation. More importantly, depletion of CD8pos T cells lowers subsequent macrophage infiltration, tissue inflammation and systemic insulin resistance (Nishimura et al., 2009). Taken together, the prevailing evidence suggests that adipose tissue inflammation is characterized by the accumulation of classically activated macrophages and cytotoxic T-cells and represents a key etiological factor in the development of systemic inflammation and insulin resistance (Xu et al., 2003; Arkan et al., 2005; Lumeng et al., 2007a).
In order to study individual cell populations within tissues, they must be effectively isolated, intact, while maintaining cellular function. This cell isolation process is highly influenced by the digestive enzymes employed, the target population(s), and the structural variability of the tissues in question (Zaba et al., 2007; Pilgaard et al., 2008). A review of the literature revealed that tissue dissociation protocols essentially use one of two enzymatic preparations: either collagenase alone (Janke et al., 2002; Xu et al., 2002; Xu et al., 2003; Curat et al., 2004; Miranville et al., 2004; Schupp et al., 2005; Curat et al., 2006; Lumeng et al., 2007a; Lumeng et al., 2007b; Lumeng et al., 2007c; Nomiyama et al., 2007; Bourlier et al., 2008; Kintscher et al., 2008; Patsouris, 2008; Duffaut et al., 2009; Feuerer et al., 2009; Nishimura et al., 2009; Winer et al., 2009; Tam et al., 2010; Wang et al., 2010; Wu et al., 2010; Yang et al., 2010; O’Rourke et al., 2011), or a blend of collagenase and other proteases (i.e., liberases) (Weisberg et al., 2003; Khazen et al., 2005; Angel et al., 2006; Zaba et al., 2007; Zeyda et al., 2007; Pilgaard et al., 2008; Shaul et al., 2010; Wentworth et al., 2010).
Recently, these two enzyme preparations were compared directly with respect to their ability to isolate stem cells from adipose tissue. The key finding was that the different enzyme preparations produced marked differences in cell yields, viability, and cell surface antigen expression (Pilgaard et al., 2008). The authors of this study, in concordance with Williams et al. (Williams et al., 1995), concluded that digestion with collagenase alone is “insufficient, as additional protease activity has proven to be essential for optimal tissue digest efficacy and cell yield” (Pilgaard et al., 2008). Unsurprisingly, however, it turns out that characteristics of the target population to be isolated and/or the tissue to be digested are ultimately more important considerations in the selection of digestive enzymes. While collagenase alone may be suboptimal for extraction of stem cells from adipose tissue (Pilgaard et al., 2008), this may not be the case with respect to the isolation of leukocytes from adipose tissue. In the present study, we systematically compared different commercially available collagenase preparations with regard to cell yield and viability of stromavascular cells (SVC) isolated from human adipose tissue, as well as their impact on cell surface markers commonly used to identify and characterize tissue leukocyte populations. In addition, we investigated the impact of different tissue processing and normalization strategies on characterizing and quantifying these leukocyte populations for flow cytometry, with a specific focus on reproducibility.
2. Methods
2.1. Adipose Tissue Collection
Subcutaneous adipose tissue was collected anonymously from 11 donors undergoing elective abdominoplasty (‘tummy tuck’) at a plastic surgery clinic in Seattle, WA. Harvested adipose tissues were placed in PBS with 1% BSA and transported on ice to the Fred Hutchinson Cancer Research Center for processing within one hour of collection. The Fred Hutchinson Cancer Research Center Institutional Review Board approved this study.
2.2. Stromavascular Cell Isolation
Using previously established protocols (Xu et al., 2002; Weisberg et al., 2003; Nomiyama et al., 2007; Zeyda and Stulnig, 2007; Yang et al.) we compared collagenases I and IV individually (Worthington Biochemical Corp., Lakewood, NJ) with blends of collagenases I and II plus a neutral protease, either thermolysin (Liberase Blendzyme 3 and Liberase TM; Roche Diagnostics, Mannheim, Germany) or dispase (Liberase DH; Roche Diagnostics). These collagenase preparations were chosen because they are the most commonly used collagenases in the field. Briefly, 1 g of minced adipose tissue was digested in 5 mL of buffered saline with either 1 mg/mL collagenase or 0.035 mg/mL liberase (concentrations as recommended by the manufacturer) for 30 to 120 min at 37°C on a gently rocking platform. Following incubation, the digestate was passed through a 180 μm filter and washed twice with PBS supplemented with 1% BSA. Contaminating red blood cells were eliminated via a short incubation with 1X lysis solution (BD Biosciences, San Jose, CA). The SVC fraction was then resuspended in PBS with 0.2% BSA plus 0.09% NaN3 and counted using a hemocytometer.
2.3 Immunophenotyping by Flow Cytometry
Following isolation, SVCs were stained for 30 min at 4°C with a combination of up to nine directly conjugated primary antibodies purchased from BD Pharmingen (San Jose, CA) or BioLegend (San Diego, CA). Appropriate isotype controls were used in conjunction with the population defining markers CD45 (leukocytes), CD14 and CD206 (adipose tissue macrophages; ATM), CD15 and CD16 (neutrophils), CD3, CD4 and CD8 (T cells), along with CD11c and CD40 to further characterize individual leukocyte populations. Samples were analyzed immediately following staining using a LSRII flow cytometer (Beckton Dickinson, San Jose, CA) to collect up to 30,000 events in a broad gate defined by forward- and side-scatter attributes. Analysis was conducted with FlowJo version 9.3.3 (TreeStar, Ashland, OR) using histograms and dot plots on live cells. Live cells were defined by fluorescence levels associated with the lower uptake of 4′,6-diamidino-2-phenylindole (DAPI), a reactive dye that binds strongly to A-T rich regions in DNA (Calbiochem, EMD Chemicals, Gibbstown, NJ). Positive staining was determined by comparison to staining with the appropriate isotype controls.
2.4 Statistical Analyses
All variables were assessed for consistency with a normal distribution using normal plots and histograms, and by the Kolmogorov-Smirnov and Shapiro-Wilk tests. Non-normally distributed variables were logarithmically transformed prior to analysis. Results are expressed as the mean ± standard deviation and/or as median (range). Comparisons between groups were carried out using Student’s t-test or analysis of variance (with post-hoc Bonferroni) where appropriate. An α-error of p<0.05 was considered significant. Statistical analyses were performed using SPSS software (version 20.0, SPSS Inc., Chicago, IL).
3. Results
3.1 Stromavascular cell yield and viability
In the first set of experiments, we compared two different collagenases and three different Liberase preparations separately. As outlined above, 1 g portions of minced subcutaneous adipose tissue from the same individual donors were digested in duplicate, for varying periods of time. Collagenase IV generally underperformed relative to collagenase I, both in terms of cell yield (5.5 × 105 ± 3.4 × 105 cells/g of tissue [n=8] vs. 7.8 × 105 ± 4.0 × 105 cells/g of tissue [n=11]; p=0.223) and viability (74.4 ± 6.2% vs. 80.2 ± 4.4%; p=0.028), respectively. Similarly among the liberase blends, Liberase DH produced both lower cell viabilities (67.4 ± 8.3%, n=5; p=0.055) and yields (1.3 × 105 ± 8.3 ×104 cells/g of tissue; p=0.004) relative to Liberase TM (viability= 76.7 ± 6.8%; yield= 5.3 × 105 ± 2.3 × 105 cells/g of tissue; n=6) or Blendzyme 3 (viability= 75.6 ± 4.9%; yield= 3.6 × 105 ± 1.6 × 105 cells/g of tissue, n=9).
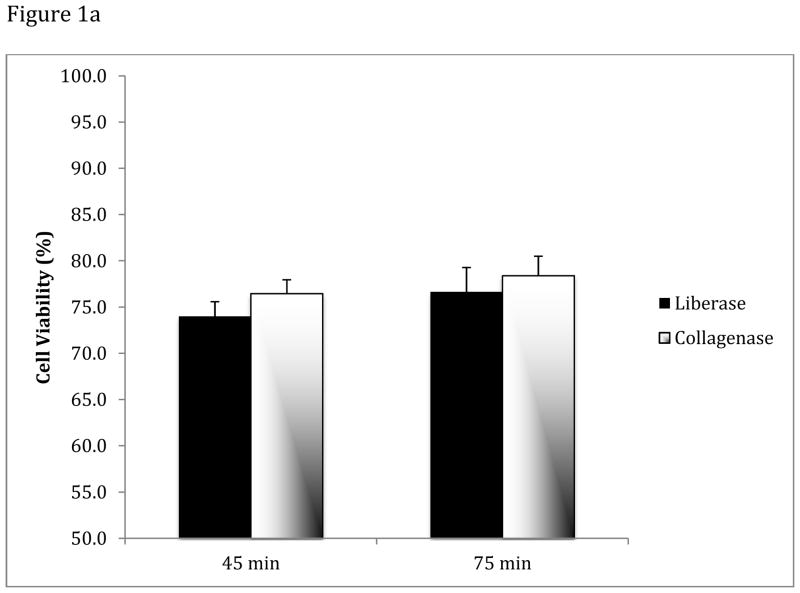
All subsequent experiments were therefore carried out comparing collagenase I with Liberase Blendzyme 3 and Liberase TM grouped as ‘liberase’. These two enzymes were pooled together since they produced comparable results and owing to the fact that Liberase TM is the second-generation replacement product of Liberase Blendzyme 3. We next compared the performance of these two enzymes over time, with respect to yield and viability (Figure 1). Analysis of variance (ANOVA) showed that neither the type of digestive enzyme (liberase vs. collagenase) nor digestion time (45 min vs. 75 min) explained variation in cell viability (p=0.175). However, collagenase produced higher cell yields (6.9 × 105 ± 3.6 × 105 cells/g of tissue) than liberase (4.3 × 105 ± 2.0 × 105 cells/g of tissue) independent of digestion time (ANOVA: p=0.031; post hoc tests: type of enzyme: p=0.007, digestion time: p=0.306).
Figure 1. Stromavascular cell viability and yield from adipose tissue following digestions with either collagenase or Liberase.
(A) Analysis of variance (ANOVA) revealed that neither the type of digestive enzyme used (collagenase or liberase) nor digestion time (45 vs. 75 min) explained variation in cell viability (p=0.175). (B) Cellular yields differed with the enzyme used (ANOVA: p=0.031), with collagenase producing on average higher yields (post hoc: p=0.007) independent of digestion time (post hoc: p=0.306). All tissue digests were carried out in duplicate on 1 g of minced adipose tissue. Data are presented as mean ± S.D. of 6–7 replicate experiments.
3.2 Enzymatic digestion alters cell surface antigen expression
We next examined the effect of the tissue processing protocol (i.e., the enzyme used and digestion duration) on the expression of leukocyte-specific cell surface markers. During our initial series of experiments, collagenase IV appeared to offer greater preservation of cell surface antigen expression as compared to collagenase I. On average, relative mean fluorescence intensity (rMFI) for cells isolated by collagenase IV was higher than among cells isolated by collagenase I. However, these differences were not significant and in general relatively minor (data not shown). In contrast to collagenase alone, all liberases either eliminated or markedly reduced the expression of several important leukocyte cell surface markers (Table 1). Specifically, while CD4 was clearly expressed among a subset of CD45posCD3pos T cells isolated with collagenase (Figure 2A), it was virtually undetectable following digestion with liberase (Figure 2B). The rMFI of CD4 expression among all T cells isolated with collagenase or liberase was 667 ± 390 AU and 90 ± 37 AU, respectively (p<0.001). To a lesser extent, liberase similarly tended to impact CD8 expression (Figures 2C and 2D). Among CD45posCD3pos T cells isolated with collagenase, the rMFI of CD8 was 2,389 ± 1,269 AU compared to 1,584 ± 1,139 AU (p=0.182) following digestion with liberase. Finally, liberase also markedly degraded the Fcγ receptor III (CD16; Figure 2, panels E and F), which is highly expressed on neutrophils. Note the leftward shift in the CD45posCD15posCD16hi neutrophil population isolated with liberase (rMFI= 62,066 ± 43,961 AU) in comparison with collagenase-isolated cells (rMFI= 123,951 ± 75,778 AU, p=0.058; Table 1). Because the degradation of CD4, CD8 and CD16 was consistent irrespective of digestion time (data not shown), the observed effect can be attributed solely to the different enzyme preparations.
Table 1.
The effect of different enzyme preparations on the relative mean fluorescence intensity (rMFI)* of leukocyte surface antigens in human stromavascular cells freshly isolated from adipose tissue following a 45–75 min digestion.
| Cell population | Surface Marker | Collagenase I (1 mg/ml) mean (SD) N=10 |
Liberase (0.035 mg/ml) mean (SD) N=8 |
p value |
|---|---|---|---|---|
| Leukocytes (CD45pos) | CD45 | 3,771 (553) | 3,992 (771) | 0.489 |
|
| ||||
| Neutrophils (CD15posCD16hi) | CD16 | 123,951 (75,778) | 62,066 (43,961) | 0.058 |
|
| ||||
| Macrophages (CD14posCD206pos) | CD14 | 4,237 (1,892) | 4,972 (1,598) | 0.146 |
| CD206 | 6,038 (1,538) | 5,143 (1,476) | 0.397 | |
| CD11c | 310 (141) | 318 (124) | 0.696 | |
| CD40 | 686 (413) | 565 (311) | 0.502 | |
| CD16 | 738 (671) | 1051 (774) | 0.372 | |
|
| ||||
| T lymphocytes (CD3pos) | CD3 | 1,423 (856) | 1,664 (831) | 0.558 |
| CD4 | 667 (390) | 90 (37) | <0.001 | |
| CD8 | 2,389 (1,269) | 1,584 (1,139) | 0.182 | |
|
| ||||
| Lymphocytes | CD16 | 21,117 (21,450) | 18,476 (16,418) | 0.897 |
rMFI was determined by subtracting the MFI of the isotype controls from the MFI for each population identified by a defined gate using specific cell surface markers. MFI itself was obtained using the geometric mean statistic.
Figure 2. Dot plots of surface antigen expression of select cell populations isolated from subcutaneous fat.
Adipose tissue was digested for 60 min with either collagenase I (panels A, C and E) or Liberase (panels B, D and F) at 37°C. Among CD3pos lymphocytes, CD4 expression was preserved following digestion with collagenase (A), but was eliminated by Liberase (B). Similarly, while CD8 expression was preserved in digestions with collagenase (panel C), there was a marked reduction (leftward shift) in CD8 signal intensity following digestion with Liberase (panel D). Comparable results for CD16 expression on CD45posCD15posCD16hi neutrophils were also observed in comparisons between collagenase (E) and Liberase (F) preparations.
3.3 Optimizing the digestion interval
Having established that collagenase I was superior to all other enzyme preparations with regard to both cell viability and yield as well as preservation of important cell surface markers, we then set out to determine the optimal digestion time. Once again, yield, viability and cell surface marker expression were the main outcome variables. For these experiments, 1 g of minced adipose tissue was digested in duplicate, for 30, 60, 90 and 120 min, and replicated using three separate donors. Over this 90 min span, cell viability was not affected by the duration of digestion (Figure 3A; test for trend p=0.171), while cell yield increased over time (Figure 3B; test for trend p=0.05). The expression of several cell surface markers including CD16, CD4, and CD8 was greatly lower in 60 min digests as compared to 30 min (Table 2), with minimal change between 60 and 90 min. Extending digestions beyond 90 min consistently revealed further substantial degradation in the rMFI of almost all markers examined (Table 2). Nevertheless, while most surface antigens exhibited some degree of attenuation after following prolonged digestions the identity of specific cell populations remained readily ascertainable. This suggests that adherence to a strict digestion time is less crucial for subsequent immunophenotyping of immune cells in tissue than is the type of enzyme used to liberate the cells. It does suggest, however, that digestion time should be standardized to minimize variability between tissue digests. Thus, taking the data from Figure 3 and Table 2 into account, extending tissue digestions beyond 30 min leads to some degradation of cell surface antigens, but also provides much greater yields of SVCs from adipose tissue. By contrast, extending the duration from 90 to 120 min did not enhance cell yield while continuing to degrade cell surface antigens. These data suggest that digestion with Collagenase I for 60 to 90 min may provide a good balance between cell yields and preservation of cell surface markers.
Figure 3. Stromavascular cell viability and yield over time.
Adipose tissue was digested in duplicate for up to 120 min with collagenase I (1 mg/ml) at 37°C. Tests for trend revealed no differences in viability (A, p=0.171) but improving yields with longer digestions (B, p=0.05). Data represent the mean ± S.D. of three replicate experiments.
Table 2.
The effect of digestion duration using collagenase I (1 mg/ml) on the relative mean fluorescence intensity (rMFI) of leukocyte surface antigens in human adipose tissue stromavascular cells. Data are from three replicate experiments.
| Cell population | Surface Marker | 30 min mean (SD) | 60 min mean (SD) | 90 min mean (SD) | 120 min mean (SD) | p value test for trend |
|---|---|---|---|---|---|---|
| Leukocytes (CD45pos) | CD45 | 960 (1038) | 873 (977) | 788 (835) | 708 (682) | 0.725 |
|
| ||||||
| Neutrophils (CD15posCD16hi) | CD16 | 69,538 (36,823) | 49,001 (16,948) | 50,243 (20,406) | 47,755 (28,817) | 0.383 |
|
| ||||||
| Macrophages (CD14posCD206pos) | CD14 | 5,961 (1,249) | 5,068 (1083) | 4,886 (873) | 4,013 (1,550) | 0.091 |
| CD206 | 9,240 (1,362) | 9,418 (2,965) | 8,215 (2,336) | 6,211 (2,465) | 0.129 | |
| CD11c | 659 (290) | 628 (242) | 646 (268) | 635 (256) | 0.940 | |
| CD40 | 546 (250) | 532 (209) | 527 (205) | 520 (181) | 0.884 | |
| CD16 | 320 (262) | 225 (121) | 239 (71) | 196 (55) | 0.385 | |
|
| ||||||
| T lymphocytes (CD3pos) | CD3 | 920 (57) | 856 (127) | 828 (163) | 665 (222) | 0.083 |
| CD4 | 1,551 (793) | 1,085 (588) | 921 (333) | 730 (312) | 0.098 | |
| CD8 | 6,878 (10,790) | 5,847 (9,097) | 5,158 (8,060) | 5,551 (8,826) | 0.850 | |
|
| ||||||
| Lymphocytes | CD16 | 7,831 (7,197) | 5,333 (3,102) | 6,318 (4,783) | 6,955 (6,085) | 0.911 |
3.4 Data normalization
Contextually, it is desirable for comparative purposes to normalize flow cytometry data to a predefined reference or standard, particularly when the goal is to quantify a certain leukocyte population or subpopulation. Unfortunately, this is often either not performed, or the method of normalization is unclear. There are several such approaches described in the literature, including normalization as a percent of the SVC (Weisberg et al., 2003; Curat et al., 2004; Miranville et al., 2004; Curat et al., 2006; Bourlier et al., 2008; Patsouris, 2008; Duffaut et al., 2009; Nishimura et al., 2009; O’Rourke et al., 2011), per g of tissue digested (Lumeng et al., 2007a; Zeyda et al., 2007; Bourlier et al., 2008; Duffaut et al., 2009; Shaul et al., 2010; Wu et al., 2010), as a percentage of all leukocytes (CD45pos) (Wu et al., 2010; O’Rourke et al., 2011), or as a percentage of a specific leukocyte population, such as ATM (Shaul et al., 2010; Wentworth et al., 2010). We conducted a series of experiments to assess the impact of different methods of normalization on reproducibility and data interpretation. In these experiments, we compared normalization by live-gate, per g of tissue used, and as percentage of the total CD45pos cell population, for CD14posCD206pos ATM and CD3pos T cells.
As described previously, 1 g of minced fat was digested in duplicate with collagenase I for 30 to 120 min, and repeated with three subjects. We then quantified the CD14posCD206pos ATM and CD3pos T cell populations in the SVCs and normalized them as a percentage of live-gate (defined by negative uptake of DAPI), as a percentage of CD45pos leukocytes, and per g of AT. The coefficient of variation (CV) for each of these three normalization strategies, as a measure of reproducibility, was then calculated based on three replicate experiments. When considering ATM, the lowest CV across all normalization strategies was seen at 60 min with relatively good CV at 75 min (Table 3). Interestingly, normalization per g of tissue consistently exhibited the greatest variability, possibly related to the large variability in cell yields particularly when the tissues were digested for a relatively short period of time (i.e., 30 min). For CD3pos T cells, all three approaches showed the best reproducibility (lowest CV) at 75 min, although normalization by either live-gate or percent CD45pos exhibited comparably little variability at 45 min (Table 3). Of the three normalization strategies examined, percent live-gate and percent of CD45pos cells exhibited the least amount of variability and greatest agreement across a broad range of digestion intervals. In contrast, reporting as per g of adipose tissue was highly variable and only provided good reproducibility at 60 and 75 min digestion intervals.
Table 3.
The influence of duration of digestion on mean coefficient of variation (CV) among different normalization approaches. Data are from three replicate experiments.
| Macrophages (CD14posCD206pos) | T lymphocytes (CD3pos) | ||||||
|---|---|---|---|---|---|---|---|
| Live-gate (%) | CD45pos (%) | per g AT (%) | Live- gate (%) | CD45pos (%) | per g AT (%) | ||
| Digestion Time (min) | 30 | 16 | 11 | 59 | 19 | 20 | 38 |
| 45 | 15 | 17 | 36 | 7 | 8 | 30 | |
| 60 | 12 | 5 | 16 | 14 | 16 | 16 | |
| 75 | 15 | 13 | 21 | 9 | 9 | 14 | |
| 90 | 14 | 13 | 50 | 5 | 7 | 36 | |
| 120 | 4 | 7 | 20 | 10 | 15 | 28 | |
4. Discussion
The isolation of specific cell populations from tissue remains a critical step in the understanding and characterization of many human diseases. Key to such studies is the development of standardized, reproducible approaches for cell isolation and characterization. Variations among existing protocols are likely to produce suboptimal cell isolates, inconsistencies between cell yields, viability, or cell surface marker expression leading to poor reproducibility, and ultimately to unintended biases or mistakes in subsequent data interpretation. We therefore set out to identify an approach that focused on reproducibility and consistency in the isolation of leukocytes from human adipose tissue. Specifically, we assessed the performance of five different commonly used digestive enzymes over an extended interval and assessed cell viability, yield, and cell surface marker expression by flow cytometry.
Our results on enzyme performance contrast markedly with the study by Pilgaard et al. (Pilgaard et al., 2008), which focused on isolating stem cells from adipose tissue. In our study, the isolation of leukocytes from adipose tissue revealed that both collagenase alone or in combination with other proteases appear generally comparable in terms of cell viability. However, with respect to cell yield and surface marker expression, we observed superior performance when using collagenase alone versus liberase. Notably, collagenase typically yielded 60% more cells per g of tissue than liberase (when both are used in the concentrations typically described in the literature). This difference has the potential to significantly impact studies that aim to characterize the relationship between adipose tissue leukocyte populations and phenotypic features such as body mass or insulin resistance (Weisberg et al., 2003; Curat et al., 2004; Curat et al., 2006; Zeyda et al., 2007; Winer et al., 2009; Wentworth et al.; Wu et al., 2010). Moreover, in both human and animal studies alike, live tissue is often difficult to obtain in large quantities, hence maximizing cell yield is important. Finally, an additional important consideration would be potential lot-to-lot variability in commercially available enzyme preparations. While this was not tested formally in the present study, more than one lot of collagenase I and the liberase preparations were used in different experiments, without any clear impact on cell viability or other measured outcomes. It would nevertheless remain advisable to obtain a sufficient amount of a given enzyme lot to complete a specified set of experiments so as to minimize potential variability.
Another aspect where collagenase alone clearly outperformed liberase, not addressed by Pilgaard et al. (Pilgaard et al., 2008), involved maintaining the integrity of cell surface markers. Specifically, the added proteases in liberase blends selectively degraded CD4. This eliminates the possibility of identifying CD4pos T cells, which include TH1, TH2, and regulatory sublineages (such as FoxP3pos). Notably in humans, the relative proportion of TH1 to FoxP3pos T cells in visceral adipose tissue correlates with body mass index (Winer et al., 2009). Combined with the additional revelation that liberase similarly degrades CD8 expression, it would follow that the use of liberase is inappropriate for studies characterizing T cell populations in tissue. As a final confounding factor, the apparent increase in the number of CD4neg cells (Figures 2A and 2B) suggests that liberase may simply eliminate cell surface marker expression without harm to the cells. This could then lead to a significant overestimation (misrepresentation) of individual cell populations, particularly when a small panel of markers is used.
Finally, we set out to address the issue of data normalization as it pertains to flow cytometry. Oftentimes in the published literature, either the method of normalization is not stated or the reference population remains unclear, which creates difficulties in comparing results across studies. As our data show, the digestion protocol and normalization strategy also greatly affect reproducibility of the data yielded from flow cytometry experiments. One of the keys to this process is defining a reference cell population, which is typically defined by a broad gate aimed at encompassing the viable, intact, single cells of the SVC fraction. This ‘gate’, frequently referred to as a ‘live-gate’ is typically drawn by eye based on the assumption that live cells fall within a certain range of size and granularity (i.e. forward and side-scatter attributes). In order to unequivocally define a live cell population, the ‘live-gate’ should be further refined using a cell permeable dye, such as DAPI, propidium iodide or one of the LIVE/DEAD® viability assays in order to remove dead cells from the analysis. This live cell population can then serve as the defining reference population for further normalization approaches discussed above.
Of course, all normalization approaches have inherent limitations. Normalization by live-gate is strongly dependent upon cell viability post digestion, which impacts cell yield. Our data suggest that over- or under-digesting the tissue reduces the ability to reproducibly measure the number of cells in a given amount of tissue. Not surprisingly, normalizing to the number of all CD45pos cells (i.e., all leukocytes) showed less variability across tissue processing conditions, and an overall good reproducibility. A potential limitation of normalizing to live-gate or all CD45pos cells is that in inflammation, the number of total leukocytes and with that also the number of SVCs will dramatically increase in adipose tissue. This is illustrated by the fact that the SVC yield is often several-fold greater if adipose tissue from an obese individual is digested, as compared to tissue obtained from a lean person. Normalizing to live-gate or all CD45pos cells may not be able to detect higher numbers of one specific type of leukocyte if the number of other leukocytes is also higher in adipose tissue from that individual. It does therefore seem important to consider the total number of SVCs isolated from adipose tissue in the normalization strategy, as this may more accurately reflect the actual numbers of specific leukocyte populations. At the same time, because the number of SVCs isolated from adipose tissue depends greatly on the digestion protocol, specifically the type of collagenase and the digestion time, it is important to standardize the tissue processing in all regards when using this normalization approach. Given that each of these approaches has important limitations, it would appear advisable to routinely use and report data using at least two normalization strategies, as this approach would provide the most complete picture of the cellular composition of the tissue and allow for changes in cell populations to be accurately measured and compared.
5. Conclusion
The identification, characterization, and isolation of selected immune cell populations remain a critical step in furthering the understanding of disease etiology. Key to such studies is the development of standardized, reproducible approaches as variations among existing protocols are likely to produce suboptimal cell isolates, inconsistencies between cell yields, viability, or cell surface marker expression, or even incorrectly identified cell populations. Any or all of these may contribute to errors in data interpretation, resulting in misleading or erroneous conclusions. An issue of potential importance not addressed experimentally in this study is that of lot-to-lot variability in enzyme preparations. While all experiments described herein were conducted with individual batches of enzymes, we
Our data suggest that the isolation of SVCs from human adipose tissue, using collagenase I for 60 to 75 min, provides the best cell yield and viability, with the lowest degree of degradation of cell surface markers used to define resident leukocyte populations. It also provides the best reproducibility, independent of the normalization strategy. We further recommend considering the use of two normalization strategies to compensate for the limitations that are inherent within each individual approach.
Acknowledgments
This work was supported by the American Diabetes Association (7-09-CT-36 to M.K.), the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK-17047; University of Washington Diabetes Research Center), and the Fred Hutchinson Cancer Research Center Cancer Center support grant (P30CA015704). A.C. received a Fulbright Research Scholarship at the FHCRC in 2010. We would like to thank the volunteers for their participation in these studies. We are also indebted to the Fred Hutchinson Cancer Research Center Prevention Center staff for their excellent technical assistance.
Abbreviations
- ATM
adipose tissue macrophage
- CV
coefficient of variation
- DAPI
diamidino-2-phenylindole
- rMFI
relative mean fluorescence intensity
- SVC
stromavascular cell
Footnotes
Disclosure Summary: The authors have nothing to disclose.
Author Contributions
D.K.H. and J.N.K. collected and interpreted study data, co-wrote the draft manuscript and reviewed/revised the final manuscript. K.E.F-S. and K.W.M aided in study design, contributed to specimen collection and data interpretation, and reviewed/edited the manuscript. I.L. and J.R.G. recruited study subjects, contributed to specimen collection, and reviewed/edited the draft manuscript. L-Y.K., A.C., and E.G. contributed to data collection and interpretation, and reviewed/edited the manuscript. M.K. designed the study, collected and interpreted study data, co-wrote draft manuscript, and reviewed/revised final draft.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson CX, Gustafson B, Hammarstedt A, Hedjazifar S, Smith U. Inflamed adipose tissue, insulin resistance and vascular injury. Diabetes/metabolism research and reviews. 2008;24:595–603. doi: 10.1002/dmrr.889. [DOI] [PubMed] [Google Scholar]
- Angel CE, George E, Brooks AE, Ostrovsky LL, Brown TL, Dunbar PR. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J Immunol. 2006;176:5730–4. doi: 10.4049/jimmunol.176.10.5730. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nature medicine. 2005;11:191–8. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation research. 2005;96:939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C, Galitzky J, Lafontan M, Karpe F, Frayn KN, Bouloumie A. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–15. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England journal of medicine. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Basdevant A, Stich V, Cancello R, Langin D. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, Bouloumie A. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–92. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, Sengenes C, Lafontan M, Galitzky J, Bouloumie A. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1608–14. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature medicine. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. The American journal of clinical nutrition. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–707. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Zinman B, Haffner SM, O’Neill MC, Kravitz BG, Yu D, Freed MI, Herman WH, Holman RR, Jones NP, Lachin JM, Viberti GC. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006;55:2357–64. doi: 10.2337/db06-0116. [DOI] [PubMed] [Google Scholar]
- Khazen W, M’Bika JP, Tomkiewicz C, Benelli C, Chany C, Achour A, Forest C. Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett. 2005;579:5631–4. doi: 10.1016/j.febslet.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1304–10. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007a;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007b;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. American journal of physiology. 2007c;292:E166–74. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DC, Sattar N, McArdle CS. ABC of obesity. Obesity and cancer. BMJ (Clinical research ed. 2006;333:1109–11. doi: 10.1136/bmj.39042.565035.BE1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–55. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature medicine. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschop MH, Bruemmer D. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. The Journal of clinical investigation. 2007;117:2877–88. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke RW, White AE, Metcalf MD, Olivas AS, Mitra P, Larison WG, Cheang EC, Varlamov O, Corless CL, Roberts CT, Jr, Marks DL. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54:1480–90. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris DL, P-P, Thapar D, Chapman J, Olefsky M, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgaard L, Lund P, Rasmussen JG, Fink T, Zachar V. Comparative analysis of highly defined proteases for the isolation of adipose tissue-derived stem cells. Regen Med. 2008;3:705–15. doi: 10.2217/17460751.3.5.705. [DOI] [PubMed] [Google Scholar]
- Pischon T, Nothlings U, Boeing H. Obesity and cancer. The Proceedings of the Nutrition Society. 2008;67:128–45. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- Schupp M, Clemenz M, Gineste R, Witt H, Janke J, Helleboid S, Hennuyer N, Ruiz P, Unger T, Staels B, Kintscher U. Molecular characterization of new selective peroxisome proliferator-activated receptor gamma modulators with angiotensin receptor blocking activity. Diabetes. 2005;54:3442–52. doi: 10.2337/diabetes.54.12.3442. [DOI] [PubMed] [Google Scholar]
- Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–81. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. The Journal of clinical investigation. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CS, Viardot A, Clement K, Tordjman J, Tonks K, Greenfield JR, Campbell LV, Samocha-Bonet D, Heilbronn LK. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes. 2010;59:2164–70. doi: 10.2337/db10-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Molecular medicine (Cambridge, Mass. 2008;14:222–31. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–4. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Tang L, Charnigo R, de Villiers W, Eckhardt E. T-lymphocyte responses to intestinally absorbed antigens can contribute to adipose tissue inflammation and glucose intolerance during high fat feeding. PLoS One. 2010;5:e13951. doi: 10.1371/journal.pone.0013951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–56. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SK, McKenney S, Jarrell BE. Collagenase lot selection and purification for adipose tissue digestion. Cell Transplant. 1995;4:281–9. doi: 10.1177/096368979500400306. [DOI] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nature medicine. 2009;15:921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Perrard XD, Wang Q, Perrard JL, Polsani VR, Jones PH, Smith CW, Ballantyne CM. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:186–92. doi: 10.1161/ATVBAHA.109.198044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Hirosumi J, Uysal KT, Guler AD, Hotamisligil GS. Exclusive action of transmembrane TNF alpha in adipose tissue leads to reduced adipose mass and local but not systemic insulin resistance. Endocrinology. 2002;143:1502–11. doi: 10.1210/endo.143.4.8715. [DOI] [PubMed] [Google Scholar]
- Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–45. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arteriosclerosis, thrombosis, and vascular biology. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. The Journal of clinical investigation. 2007;117:2517–25. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–8. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112:61–7. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]