Background: Human aminopeptidase N (hAPN) is a dimeric cell surface protease involved in peptide processing, cell adhesion, endocytosis, and signal transduction.
Results: Crystal structures of peptide and inhibitor complexes were determined.
Conclusion: Unlike other family members, hAPN shows substrate-dependent loop ordering and a novel dimer structure.
Significance: A model for catalysis and conformational changes provides mechanistic insights into how hAPN mediates its functional roles.
Keywords: Aminopeptidase, Metalloprotease, Protease Inhibitor, Renin Angiotensin System, X-ray Crystallography, CD13
Abstract
Human aminopeptidase N (hAPN/hCD13) is a dimeric membrane protein and a member of the M1 family of zinc metallopeptidases. Within the rennin-angiotensin system, its enzymatic activity is responsible for processing peptide hormones angiotensin III and IV. In addition, hAPN is also involved in cell adhesion, endocytosis, and signal transduction and it is an important target for cancer therapy. Reported here are the high resolution x-ray crystal structures of the dimeric ectodomain of hAPN and its complexes with angiotensin IV and the peptidomimetic inhibitors, amastatin and bestatin. Each monomer of the dimer is found in what has been termed the closed form in other M1 enzymes and each monomer is characterized by an internal cavity surrounding the catalytic site as well as a unique substrate/inhibitor-dependent loop ordering, which in the case of the bestatin complex suggests a new route to inhibitor design. The hAPN structure provides the first example of a dimeric M1 family member and the observed structural features, in conjunction with a model for the open form, provide novel insights into the mechanism of peptide processing and signal transduction.
Introduction
Aminopeptidase N (APN)2 (also known as CD13) is a cell surface membrane protein that plays important roles in a wide range of normal physiological functions including the processing of peptide hormones, such as angiotensin III and IV (1), neuropeptides important in pain (2), and chemokines involved in inflammation and angiogenesis (3). APN is also known to mediate cell adhesion and endocytosis (4–7), it is involved in cancer progression (1, 8–10), and it serves as receptor for various mammalian coronaviruses (11, 12). Although typically described as a cell surface dimer, APN is also found as monomers on the cell surface (13) and a soluble form of the ectodomain constitutes a major component of its activity in blood (14).
APN functions in the rennin-angiotensin system to remove the N-terminal arginine residue from the peptide hormone angiotensin III (AngIII; RVYIHPF) to generate angiotensin IV (AngIV; VYIHPF). It can further degrade AngIV into smaller peptides although the physiological role, if any, of these degraded forms is unknown (7). AngIII is the main effector in the brain rennin-angiotensin system for vasopressin release (15), whereas AngIV has been shown to cause vasodilatation, hypertrophy, and activation of NF-κB, and it is involved in memory (16–18). The rennin-angiotensin system is a multicomponent system of peptide hormones and signaling receptors important in blood pressure regulation and electrolyte balance and there is now much evidence to support the fact that it is dysregulated during malignancy (1). Direct support for the role of APN in cancer stems from work done with the APN knock-out mouse and cancer models showing that up-regulation of APN promotes angiogenesis, tumor growth, and metastasis (8–10). Because of its overexpression on tumor cells (19–21), human APN (hAPN) has been targeted for the development of anti-cancer therapeutics (22). The hAPN inhibitor bestatin, for example, has been shown to increase the survival rates of post-operative cancer patients (23) and hAPN-specific cyclic peptides containing the Asn-Gly-Arg (NGR) motif are being developed as a means of targeting tumor cells (24–28).
hAPN (EC 3.4.11.2) is a member of the M1 family of aminopeptidases, zinc metallopeptidases represented in all kingdoms of life. Members of the family fall into two structural categories with either a three- or four-domain organization and in all cases domain II possesses the thermolysin-fold (29). They are characterized by conserved HEXXHX18E zinc-binding and GXMEN catalytic motifs and all share mechanistic features with thermolysin. Typically these M1 enzymes possess relatively broad specificity for the N-terminal amino acid (P1) of their peptide substrates and members of the M1 family possess an internal cavity surrounding the catalytic site that has been argued to confer on these enzymes specificity for small peptide substrates (30). Although the means by which substrates gain access to the catalytic site and products are released has been the subject of some debate (30–32), human endoplasmic reticulum aminopeptidase (ERAP) 1 (33, 34) and archeal tricorn interacting factor F3 (35) can exist in an open form where the cavity and catalytic site are exposed to bulk solvent. In the case of the plasmodium M1, the tricorn interacting factor F3, and the bacterial PepN, the enzymes are thought to degrade small peptides to amino acids (30, 32, 35). This is to be contrasted with mammalian APN and ERAP1 and -2, enzymes that can generate defined peptide products (33, 34, 36) such as AngIV and peptides trimmed for presentation by major histocompatibility complex class I proteins. Interestingly, APN is also thought to be involved in peptide degradation in the renal proximal tube and the small intestine (37, 38) and it too has been found to have a relatively broad substrate specificity at the P1 position with preference for the removal of small hydrophobic or basic amino acids (39). To shed light on the ability of hAPN to process various peptide substrates including AngIII and AngIV we determined its x-ray crystal structure in the presence of AngIV and two peptidomimetic inhibitors, amastatin and bestatin. The structure represents the first example of a dimeric M1 enzyme and has provided novel insights into the mechanism of peptide processing and signal transduction.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
A soluble form of hAPN (residue 66–967) was expressed and purified from a stably transfected HEK 293S GnT1− cell line (40) essentially as previously described (41). Cells were grown in DMEM/F-12 supplemented with 3% FBS (Invitrogen), 1× penicillin-streptomycin (Invitrogen), 1 mg/liter of doxycycline (Sigma), and 1 mg/liter of aprotinin (Bioshop Canada). The harvested medium was concentrated 10-fold and the fusion protein was purified by IgG-Sepharose affinity chromatography. The protein A tag was removed by on-column tobacco etch virus protease digestion and the liberated hAPN was further purified by Q-Sepharose ion exchange chromatography. The resultant hAPN was treated with endo-β-N-acetylglucosaminidase A (42) in 10 mm HEPES, 50 mm NaCl, pH 7.5, at 37 °C, followed by jack bean α-mannosidase (Prozyme) in 50 mm MES, 0.4 mm ZnSO4, pH 5.5. The deglycosylated hAPN was then purified by Q-Sepharose ion exchange chromatography and Superdex 200 gel filtration chromatography in 10 mm HEPES, 50 mm NaCl, pH 7.4, and concentrated to 20 mg/ml. The selenomethionine-labeled protein was expressed by supplementing methionine-free media with 30 mg/liter of seleomethionine as previously described (41).
Protein Crystallization
The deglycosylated native and seleomethionine-labeled hAPN was crystallized by the hanging drop method. Protein stock solutions at 20 mg/ml in 10 mm HEPES, pH 7.5, and 50 mm NaCl were mixed 1:1 with well solution containing 2 m (NH4)2SO4, 10% glycerol, and 100 mm sodium acetate, pH 5.0. Crystals were cryoprotected with well solution containing 25% glycerol. For crystallization of the AngIV complex, hAPN was preincubated for 3 days with 2.5 mm EDTA, and AngIII (Anaspec) was then added at 300 μm. Crystals were grown for approximately 1 week before cryoprotection and data collection. Complexes of bestatin (300 μm) (Bioshop Canada) and amastatin (300 μm) (Bioshop Canada) with the zinc-bound native enzyme were obtained by co-crystallization.
Data Collection, Structure Determination, and Refinement
Data were collected at the Canadian Light Source, Saskatoon (Beamline CMCF-08ID-1). A single-wavelength anomalous dispersion experiment was performed at the peak (0.9795 Å) of the selenium absorption edge. Diffraction images were processed and scaled using HKL2000 (43); 5% of each dataset was flagged for the calculation of Rfree. A summary of statistics is provided in Table 1. The SHELX (44) program suite was used to determine the selenium atom positions and to determine phases. Automated model building using ARP/wARP resulted in a model that was 95% complete. Alternate rounds of manual rebuilding using COOT (45) and automated refinement using REFMAC (46) and Phenix (47) were performed. Geometric parameters for bestatin and amastatin were obtained from the Ligand Expo database. Ramachandran analysis of all four structures (native, AngIV, bestatin, and amastatin complex) showed that 92% of the residues are in the most favored region, with 8% in the additionally allowed region. All of the residues in the substrate/inhibitor structured loop also fall in the most favored and additionally allowed regions of Ramachandran space. Figures were generated using the program PyMOL. Interface calculations were done using the PISA server.
TABLE 1.
Data collection and refinement statistics
| APN (SeSAD) | APN-native | APN-AngIV | APN-amastatin | APN-bestatin | |
|---|---|---|---|---|---|
| Data collection | |||||
| Space group | P64 | P64 | P64 | P64 | P64 |
| Cell dimensions | |||||
| a, b, c (Å)a | 158.1, 158.1, 115.2 | 157.9, 157.9, 115.0 | 157.9, 157.9, 115.3 | 157.5, 157.5, 115.1 | 158.1, 158.1, 114.8 |
| α, β, γ (°) | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 |
| Wavelength (Å) | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 |
| Resolution (Å) | 50–1.95 (1.98–1.95) | 50–1.9 (1.93–1.90) | 35–2.0 (2.03–2.00) | 50–1.85 (1.88–1.85) | 50–1.9 (1.93–1.90) |
| Rsym | 0.10 (0.32) | 0.07 (0.27) | 0.12 (0.35) | 0.10 (0.34) | 0.08 (0.35) |
| I/σI | 24.1 (4.8) | 28.5 (5.6) | 34.8 (8.7) | 56.0 (5.7) | 36.5 (5.8) |
| Completeness (%) | 100 (100) | 99.9 (100) | 96.4 (99.0) | 99.9 (98.6) | 100 (100) |
| Redundancy | 7.0 (6.8) | 7.1 (7.0) | 7.4 (8.1) | 10.6 (5.7) | 9.5 (9.0) |
| Refinement | |||||
| Resolution (Å) | 27.5–1.9 | 27.8–2.0 | 27.3–1.85 | 27.5–1.9 | |
| No. reflections | 121327 | 97974 | 130639 | 126908 | |
| Rwork/Rfree | 0.17/0.19 | 0.17/0.20 | 0.17/0.18 | 0.17/0.19 | |
| No. atoms | |||||
| Protein | 7284 | 7414 | 7346 | 7351 | |
| Ligand/ion | 211 | 192 | 225 | 242 | |
| Water | 693 | 875 | 667 | 950 | |
| B-factors (Å2) | |||||
| Protein | 19.3 | 25.8 | 19.4 | 20.2 | |
| Ligand/ion | 38.7 | 49.6 | 38.6 | 39.6 | |
| Water | 27.5 | 36.6 | 26.8 | 31.8 | |
| R.m.s. deviations | |||||
| Bond lengths (Å) | 0.011 | 0.012 | 0.010 | 0.011 | |
| Bond angles (°) | 1.323 | 1.421 | 1.299 | 1.329 | |
a Values in parentheses are for the highest resolution shell.
Surface Plasmon Resonance Analysis, Analytical Ultracentrifugation, and Enzyme Kinetics
The ectodomain was used without deglycosylation for the C18 HPLC-based kinetics assay, the sedimentation equilibrium analysis, the surface plasmon resonance peptide binding assay, and the colorimetric enzyme assay. hAPN enzymatic activity was assayed using l-leucine-ρ-nitroanalide (Sigma) in 10 mm MES, pH 6.5. Initial velocities were obtained at 298 K over a range of substrate concentrations at an enzyme concentration of 10 nm. The generation of ρ-nitroanalide was monitored at 405 nm. Kinetic analysis of the removal of the first amino acid from AngIII, AngIV, and the peptides, VVYIHPF and RYIHPF, was performed by measuring the loss of the substrate using a C18 reverse phase HPLC assay. Various concentrations of peptides were mixed with hAPN (0.5 nm) in 10 mm MES, pH 6.5, at 298 K, and the digest was stopped with 5% phosphoric acid at various time points to obtain initial velocities. Each stopped reaction was loaded onto a C18 column (Vydac 218TP) and eluted isocratically with 85 mm phosphoric acid, adjusted to pH 3 with triethanolamine, containing 15 (VVYIHPF) or 17% (the others) acetonitrile. Peptides were quantitated at 195 nm based on a standard curve generated with known peptide concentrations. AngIII, AngIV, AngI/II(4–8), VVYIHPF, and RYIHPF are all baseline separated. Sedimentation equilibrium analysis of hAPN at concentrations of 0.1, 0.25, and 0.5 mg/ml were performed at speeds of 7000 and 9000 rpm at 277 K. Surface plasmon resonance was performed on CM-5 chips coupled with hAPN that had been preincubated with 2.5 mm EDTA (1 day) and analysis was performed in buffer containing 2.5 mm EDTA. Binding plateau values as a function of AngIII and AngIV concentration were used to compute the dissociation constants assuming a 1:1 binding model.
RESULTS
Overall Structure of the hAPN Dimer
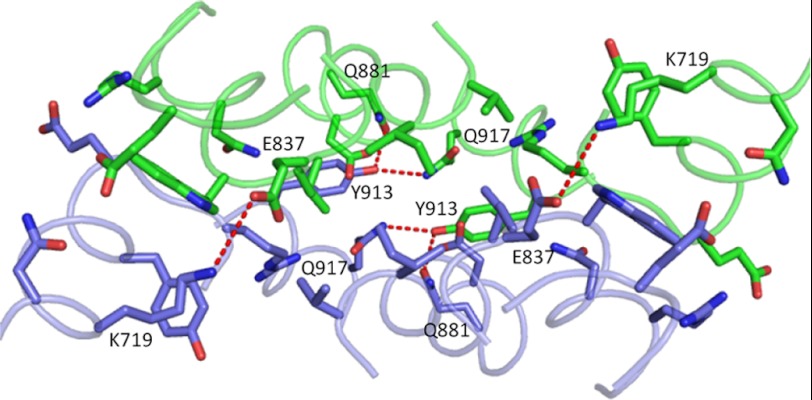
hAPN is a 967-residue type-2 membrane glycoprotein as shown in Fig. 1a. The ectodomain was expressed and shown to possess a Km of 0.3 ± 0.05 mm for the hydrolysis of leucine-ρ-nitroanilide, a value similar to that obtained for the rabbit and porcine enzymes purified from tissue (48). The intact membrane protein exists as dimers and monomers on the cell surface in rabbit (13) and using analytical ultracentrifugation we have determined that the ectodomain expressed alone dimerizes with a KD of 0.8 μm. The ectodomain is also dimeric in the crystal (data collection and refinement statistics in Table 1). As shown in Fig. 1b, each monomer possesses the four-domain structure (domains I-IV) characteristic of the four-domain M1 metallopeptidases whose structures have been determined to date (30–36, 49). Domain II possesses the thermolysin-fold and contains both the zinc binding site and the catalytic site, as well as the characteristic consensus motifs, 388HEXXHX18E411 and 352GXMEN356. The dimer interface is mediated by hydrophobic interactions and a hydrogen bond and salt-bridge network, and it buries ∼840 Å2 of surface area on each monomer (Fig. 2). In each monomer, the catalytic site is exposed to a large internal cavity (∼2800 Å3), which is inaccessible to the bulk solvent. The native, peptide bound, and inhibitor bound structures are very similar (root mean square deviation over all protein atoms of 0.13–0.17 Å) except for a flexible loop in domain IV that is structured by substrate and inhibitor binding as discussed below. Fig. 1b shows a model for the orientation of hAPN on the cell surface. The dimer possesses dimensions of 131 Å × 62 Å in projection, values very close to those measured by negative stain electron microscopy (135 Å × 55 Å) for intact porcine APN in reconstituted lipid vesicles (50).
FIGURE 1.
Structure of the hAPN ectodomain dimer. a, domain organization of the hAPN polypeptide chain. The cytoplasmic domain is white; the transmembrane segment is black; the Ser/Thr rich stalk is gray; and the remaining domains, domain I, II, III, and IV are colored in blue, green, magenta, and yellow, respectively. Numbers indicate domain boundaries. b, ribbon diagram of the hAPN dimer depicted in its likely orientation with respect to the plasma membrane. Colored according to domains (as described above). Yellow spheres indicate the location of the active site zinc ions. N-Linked oligosaccharides are shown in stick representation.
FIGURE 2.
Ribbon diagram of the hAPN dimeric interface. The two monomers are colored green and purple. The side chains of interacting residues are shown with oxygen and nitrogen atoms colored red and blue, respectively. Hydrogen bonds and salt bridges are shown as red dashed lines.
Peptide Binding and the Catalytic Site
To shed light on the structural basis for substrate binding and catalysis we determined the x-ray crystal structure of hAPN in its native form and in complex with peptide substrate. The latter was obtained by the co-crystallization of zinc-depleted hAPN with 300 μm AngIII (RVYIHPF), although the clearly defined electron density of the first three residues shows that only AngIV (VYIHPF) is bound in the catalytic site (Fig. 3a). Zinc-depleted hAPN binds both AngIII (5 ± 0.4 μm) and AngIV (15 ± 4 μm) with similar affinity (Table 2 and supplemental Fig. S1). Fig. 3b shows an overlay of the zinc-bound native enzyme with that of the zinc-free AngIV complex in the vicinity of the zinc binding site. In the native enzyme the zinc ion is coordinated by His388, His392, and Glu411 (of the 388HEXXHX18E411 motif) and both oxygen atoms of an acetate molecule from the crystallization buffer (supplemental Table S1). In the AngIV complex the N-terminal Val residue is deeply buried, an observation consistent with the fact that these M1 enzymes are exopeptidases. The carbonyl oxygen atom of the valine occupies the same position as that of the OD1 acetate oxygen atom in the native structure (there is no bound acetate in the peptide complex) and at the same time it accepts a hydrogen bond from Tyr477, a residue proposed to stabilize the oxyanion generated in the transition state (51). In addition, the α-amino group of the Val residue is hydrogen bonded to Glu355 (of the 352GXMEN356 motif), Glu411 and Gln213 (a cis peptide conserved among members of the family), residues all implicated in substrate binding and/or transition state stabilization (51). Taken together, it is clear that the bound peptide is poised for catalysis and represents a substrate complex. Further support for this suggestion stems from the fact that the scissile bond (between the Val (P1) and Tyr (P1′)) straddles the side chain of Glu389, a residue proposed to shuttle a proton from the hydrolytic water molecule to the amide nitrogen of the scissile bond (51). Indeed, the side chain of Glu389 forms a hydrogen bond to the more weakly coordinated OD2 acetate oxygen atom (supplemental Table S1), an atom whose position approximates that of the hydrolytic water molecule prior to nucleophilic attack. Because the zinc ion is thought to both activate the hydrolytic water molecule and coordinate the oxyanion in the transition state (supplemental Fig. S2), we propose that our native acetate-bound zinc structure, in conjunction with the zinc-free peptide complex, serves as a good model for that of the Michaelis complex.
FIGURE 3.
Substrate binding and catalytic site of hAPN. a, AngIV electron density. The AngIV peptide carbons are colored gray, and nitrogen and oxygen atoms are in blue and red, respectively. The electron density corresponds to an Fo − Fc omit map contoured at 2.0 σ. b, overlay of the native and AngIV bound catalytic sites. hAPN carbons are colored green (AngIV complex) and magenta (native), and nitrogen and oxygen atoms are in blue and red, respectively. The zinc ion is shown in yellow, acetate carbon atoms are in pink. Red dashed lines represent hydrogen bonds and black dashed lines represent zinc coordination bonds. Residues of AngIV beyond P1′ are omitted for clarity and denoted by an arrow. c, stereoview of the AngIV binding site. The carbon atoms are colored by domains as described in the legend to Fig. 1. The backbone of the structured loop is colored red.
TABLE 2.
Summary of kinetics and binding data
| P1 | P1′ | P2′ | P3′ | P4′ | P5′ | P6′ | Vmax | Km | KD,apo | kcat | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| μmol/min/mg | μm | μm | s−1 | ||||||||
| Arg | Val | Tyr | Ile | His | Pro | Phe | AngIII | 29.8 ± 4.8 | 6.2 ± 2.0 | 5 ± 0.4 | 51 |
| Val | Val | Tyr | Ile | His | Pro | Phe | 9.4 ± 0.4 | 5.7 ± 1.9 | NDa | 17 | |
| Val | Tyr | Ile | His | Pro | Phe | AngIV | 9.7 ± 1.6 | 2.1 ± 0.3 | 15 ± 4.0 | 16 | |
| Arg | Tyr | Ile | His | Pro | Phe | 52.1 ± 4.1 | 2.5 ± 0.5 | ND | 89 |
a ND, not determined.
Peptide Binding, Loop Ordering, and Specificity
Both the electron density and temperature factors show that the first three amino acids (residue positions P1-P1′-P2′) of the bound AngIV peptide are the most well defined, whereas the last three (residue positions P3′-P5′) are increasingly disordered (Fig. 3a). The binding of AngIV buries ∼470 Å2 of hAPN, and the binding site is mainly composed of residues from domain II (336Å2) and domain IV (134Å2) (Fig. 3c). In addition, comparison of the peptide complex with that of the native enzyme shows that peptide binding leads to the ordering of an eight-residue flexible loop (891YGGGSFSF898) that is not observed in the electron density maps of the native structure. The loop further buries the bound substrate and although the interactions between the loop and the bound peptide are not that extensive, the electron density describing it is strong and its temperature factors (25–35 Å2) are comparable with that of non-loop residues in the vicinity (supplemental Fig. S3a).
The Val (P1) and Tyr (P1′) residues make extensive interactions with hAPN and both are completely buried (Fig. 3, b and c). In addition to the key interactions between the Val amino group and its carbonyl oxygen atom (as discussed above), its side chain sits in an apolar pocket formed by Gln211, Gln213, Ala351, Met354, and Phe472 and the side chain of loop residue Phe896, which serves to cap the pocket (supplemental Fig. S4a). Notably, the observed loop conformation would not be able to accommodate the bulkier N-terminal Arg residue found at substrate position P1 in AngIII (supplemental Fig. S4b). With regard to the Tyr at position P1′, its amide nitrogen donates a hydrogen bond to the carbonyl oxygen of Ala353 and its carbonyl oxygen atom accepts a hydrogen bond from Gly352, residues found in the 352GXMEN356 motif. The Tyr side chain also makes stacking interactions with the side chains of hAPN residues Val385 and His388, of the S1 pocket, and its hydroxyl group makes a water-mediated hydrogen bond to the carboxyl group of Glu418. In contrast to that observed for the first two amino acids, the main chain atoms of the remaining peptide residues are not hydrogen bonded to hAPN and their interaction with hAPN lacks structural/chemical complementarity as reflected in a buried surface calculation that shows that on complex formation 715 Å2 of surface area is buried on the peptide, whereas only 470 Å2 is buried on the surface of hAPN.
In addition to the interactions that the structured loop makes with the bound peptide it also makes interactions with domain II. Loop residue Phe896 makes a stacking interaction with Phe472, and loop residues Gly894 and Ser895 make hydrogen bonds with Ser469 and Asn350, respectively. As a result, a total surface area of 402 Å2 is buried between the loop and domain II.
Inhibitor Binding and an Alternate Loop Conformation
To provide further insight into how hAPN might bind different peptide substrates we determined the structure of native zinc-bound hAPN in complex with amastatin (LeuβN[αOH]-Val-Val-Asp) and bestatin (PheβN[αOH]-Leu) (52), peptidomimetic inhibitors each possessing a nonhydrolyzable α-hydroxyl-β-amino acid at its N terminus (Fig. 4a). Comparison of the amastatin complex (Fig. 4, b and c) with that of the native enzyme and the AngIV complex shows that the LeuβN[αOH] moiety makes essentially the same interactions as that of the N-terminal Val residue (P1) of the AngIV substrate complex (supplemental Table S1). The α-hydroxyl group of this moiety also coordinates the zinc ion and as such provides an additional model for activation of the hydrolytic water molecule. The second residue (Val) of amastatin makes backbone interactions with hAPN similar to those of the second residue (Tyr, P1′) of AngIV in the AngIV complex, and the side chains of the second (Val) and third (Val) residues of amastatin occupy the same sites as those in the AngIV complex. The electron density for the fourth amino acid (Asp) of amastatin is weak. The interactions between hAPN and the first two residues of amastatin are very similar to those observed in the bestatin complexes of other M1 enzymes (30–34, 53). Amastatin binding also structures the flexible loop (average temperature factors are 25–30 Å2) around the side chain of the LeuβN[αOH] moiety in a fashion very similar to that seen for Val (P1) in the AngIV complex (supplemental Fig. S3b and S4c). In addition, the hydrogen bonding pattern between the loop and other hAPN residues is essentially the same as that observed in the AngIV complex.
FIGURE 4.
Substrate binding and catalytic site of the bestatin and amastatin complexes. a, chemical structure of amastatin and bestatin. The rectangular box encloses the α-hydroxyl group. b, Fo − Fc omit maps contoured at 2.0 σ for amastatin and bestatin. Carbons, oxygens, and nitrogens are colored gray, red, and blue, respectively. c, stereoview of the amastatin binding site. hAPN carbon atoms are colored purple. The zinc ion is shown as a green sphere. The substrate-induced loop is colored red. d, stereoview of the bestatin binding site. hAPN carbon atoms are colored teal. The zinc ion is shown as a green sphere and water molecules as red spheres. Selected hydrogen bonds are shown as red dashed lines. The substrate-induced loop is colored red.
In contrast to that observed for the amastatin complex, the interactions between bestatin and hAPN do not correspond to that of a substrate complex (Fig. 4, b and d). The PheβN[αOH] moiety of bestatin is pushed deeply into the S1 pocket that accommodates the Val (P1) side chain in the AngIV complex and its amino group, carbonyl oxygen atom, and α-hydroxyl group make only water-mediated hydrogen bonds to hAPN. The carboxyl group of the C-terminal Leu residue coordinates the zinc ion in a fashion similar to that of acetate in the native complex (supplemental Table S1), and a tetrahedrally coordinated water molecule, also observed in the native structure, occupies the pocket filled by the Val (P1) α-amino group in the AngIV complex. The side chain of the C-terminal Leu residue occupies the S1′ pocket that accommodates the Tyr (P1′) side chain of AngIV and the Val (P1′) side chain of amastatin, in their respective complexes. Despite the differences in binding geometry, both bestatin and amastatin would block substrate binding, an observation consistent with the fact that they are both competitive inhibitors.
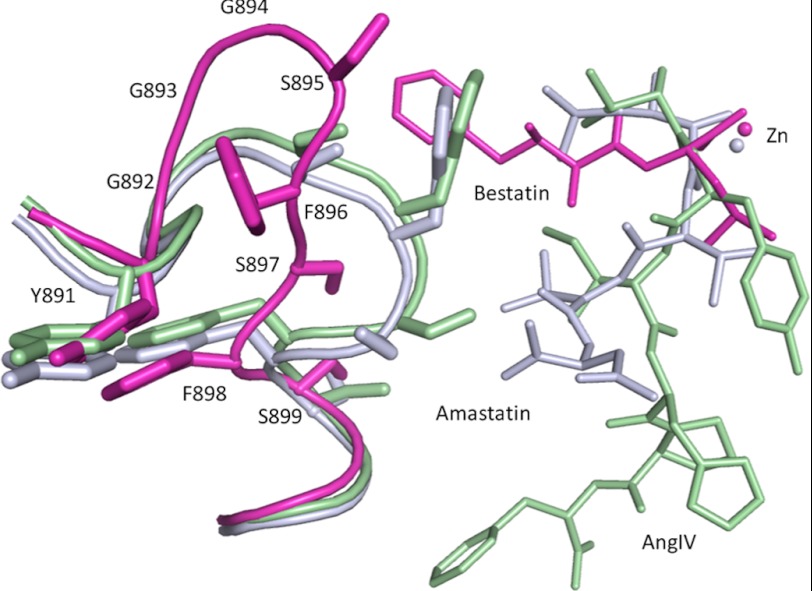
The flexible loop in the bestatin complex is well ordered (average temperature factors are 30–40 Å2) (supplemental Fig. S3c) but now found to assume a conformation very different from that observed in the AngIV and amastatin complexes (Fig. 5). Loop residue Phe896, which formerly capped the S1 pocket has been repositioned to accommodate the Phe side chain of the PheβN[αOH] moiety (supplemental Fig. S4d) and the interactions involving loop residues Gly894 and Ser895, observed in the AngIV and amastatin complexes, are replaced by both direct and water-mediated interactions between loop residue Ser895 and non-loop residues Asp189, Leu190, Asp216, and Gln213 in domain II. The Phe side chain sits in a pocket formed by both loop and non-loop residues. Although the bestatin binding geometry does not mimic a true substrate complex, the loop conformation observed does provide a model for how substrates with bulky side chains at the P1 position might be accommodated (supplemental Fig. S4e).
FIGURE 5.
Conformation of the structured loop in the AngIV, amastatin, and bestatin complexes. Overlay of the AngIV (light green), amastatin (light blue), and bestatin (magenta) complexes. Residues are only labeled in the bestatin complex. Zinc ions are represented as spheres.
Kinetic Analysis of Angiotensin III and IV and Related Peptides
To gain insight into the basis for the ability of hAPN to processes both AngIII and AngIV we measured the kinetic parameters for the removal of the first amino acid from AngIII, AngIV, and two related peptides where the first amino acids of AngIII and AngIV were swapped for that of the other (Table 2 and supplemental Fig. S5). The data show that although kcat for the removal of Arg from AngIII is about 3-fold higher than that of Val from AngIV, a compensatory increase in Km leads to similar catalytic efficiencies. Comparison of the control peptide pairs which differ only in the N-terminal amino acid shows that kcat for the removal of Arg is 3–5-fold higher than that of Val and that the nature of the N-terminal amino acid does not significantly affect Km.
Access to the Catalytic Site and a Model for the Open Conformation
As discussed above, the catalytic site of hAPN is exposed to a large internal cavity that is not connected to bulk solvent by an appreciable channel or opening (Fig. 6a). Moreover, structural alignment shows that both monomers of the hAPN dimer correspond to that of ERAP1 in its closed conformation (33, 34). To explore the possibility that hAPN might be able to access the ERAP1 open conformation, domains (I + II) and (III + IV) of hAPN were treated as rigid bodies and superimposed on domains I and IV of ERAP1 in the open conformation. Each monomer in the open conformation was then superimposed on the hAPN dimer through domain IV. The resultant models are free of steric clashes and with both monomers in the open conformation, the dimer takes on an S-shaped configuration, each lobe of which corresponds to a monomer whose internal cavity and catalytic site are exposed to bulk solvent (Fig. 6b). Although a large protein interface between domain (I + II) and IV is broken on conversion from the closed to the open form, the interface possesses a large percentage of polar residues. Conversion to the open form pulls the catalytic residues in domain II away from both the loop and non-loop residues in domain IV (see Fig. 3c), in this way pulling apart the residues that serve to sandwich the peptide in the binding site. In the open form of ERAP1 the equivalent of Tyr477 in hAPN is rotated away from its catalytically active conformation, a perturbation thought to render the open form catalytically inactive (33–35) and it is likely that the same would occur in hAPN.
FIGURE 6.
hAPN and the modeled open form. a, open-book view of a surface representation of hAPN sectioned to illustrate the internal cavity and catalytic site. The structured loop is colored red and the AngIV peptide is shown in yellow stick representation. b, open-book view of hAPN with both monomers modeled in the open form. c, surface representation of hAPN viewed from the membrane. d, surface representation of hAPN with both monomers modeled in the open form viewed from the membrane. e, overlay of hAPN in c (green) with that of the model in d (blue) viewed parallel to the membrane surface. The N-terminal amino acids that connect to the transmembrane region of each monomer are colored black. Numerical values refer to the distance between the N-terminal amino acid of each monomer.
DISCUSSION
The work reported here has provided much insight into the basis for peptide recognition and catalysis by hAPN. Significantly, we have found that an eight-residue loop, structured only on substrate or inhibitor binding, forms a cap over the side chain of the N-terminal Val of AngIV in the hAPN-AngIV complex. The loop is structured in a very similar way in the amastatin complex but assumes a fundamentally different conformation in the bestatin complex. Moreover, the loop conformation observed in the hAPN-AngIV complex would not be able to accommodate the bulky N-terminal Arg residue found in AngIII, whereas that observed in the bestatin inhibitor complex would (supplemental Fig. S4, b and e). It follows that substrate-dependent loop ordering and the observed plasticity may reflect a requirement for broad specificity at the P1 position of the bound substrate. Notably, differences in the conformation of a single side chain in the S1 site have served to accommodate different N-terminal amino acids in other M1 family members (31, 32, 54). In addition to its role in processing AngIII and AngIV, hAPN has a number of other physiological peptide substrates and it shows relatively broad specificity when assayed with amino acid analogues (39). Our kinetics data show that the identity of the amino acid in the P1 position does not profoundly affect Km or kcat when measured in the context of AngIII, AngIV, and related control peptides (Table 2). The ability of hAPN to process a range of peptide substrates is also reflected in the fact that interactions with the side chains at peptide substrate positions, P1′ and P2′, are such that a range of amino acid types could be accommodated and that beyond the third amino acid there is little chemical and steric complementarity between the enzyme and the substrate. These structural features are consistent with previous enzyme kinetic analysis that suggested that the active site of rat APN is composed of subsites recognizing the three N-terminal residues (55). This is to be contrasted with that of ERAP1 where recognition of the C-terminal end of the substrate is thought to ensure the production of a peptide product of defined length (33, 34, 56). Finally, the suggestion that hAPN is catalytically active only in the closed form supports a model where hAPN, like other members of the M1 family, is designed to limit its specificity to peptides, substrates that can be accommodated by the internal cavity formed in the closed form.
Comparison of the hAPN-AngIV complex with that of the native enzyme has provided new insight into the mechanism of peptide hydrolysis by M1 family members. Because the geometry of the hAPN-AngIV complex observed is sterically compatible with the presence of a bound zinc ion, it follows that peptide binding, as observed, might lead directly to the transition state with no requirement for a significant change in atomic positions. On peptide binding the zinc-bound water molecule (observed in the absence of acetate (57)), would in a concerted process be displaced by the substrate carbonyl oxygen atom and re-positioned for in-line nucleophilic attack and hydrolysis. The OD2 atom of the bound acetate in the native structure and the α-hydroxyl group in the amastatin complex provide models for the position of the water molecule prior to the transition state. This concerted process is to be contrasted with the two-step model stemming from work on the only other peptide complex of an M1 enzyme available (58). Using a catalytically inactive leukotriene A4 hydrolase mutant, it was found that the deprotonated form of the α-amino group of the bound substrate was found to coordinate the zinc ion in what was assumed to be an initial binding event.
Although amastatin is found to bind hAPN in a fashion very similar to that of AngIV, the binding geometry observed for bestatin does not correspond to that of a substrate complex or that observed in other known bestatin complexes (30–33). In those complexes, the bestatin carbonyl oxygen atom and the α-hydroxyl group are found to coordinate the zinc ion as seen in our amastatin complex. In the hAPN-bestatin complex these groups make only water-mediated interactions with hAPN and the C-terminal carboxyl group of bestatin coordinates the zinc ion in a fashion similar to that observed for the acetate ion in our native structure. Similar zinc-carboxylate interactions have been observed in the structures of many other zinc-dependent proteases (59). As shown in supplemental Fig. S4f, the Phe side chain of the bestatin PheβN[αOH] moiety clashes with the side chain of loop residue Phe896 when modeled with the binding geometry and loop conformation observed in the amastatin complex. The novel binding geometry observed presumably reflects the fact that in all of the M1-bestatin complexes determined, to date, the loop in those structures does not block bestatin from binding in the canonical fashion either because it is too short or it differs in sequence and conformation from that observed in the AngIV and amastatin complexes of hAPN (supplemental Fig. S6). Given the novel binding geometry and the importance of the loop, the hAPN-bestatin complex would be expected to facilitate the development of specific hAPN inhibitors for use as anti-cancer agents and analgesics for pain management (2, 22). Building on the key interactions with the bestatin phenyl and carboxyl groups, while at the same time eliminating the α-hydroxyl and carbonyl functional groups, might provide a means of generating specific inhibitors that do not inhibit other human M1 family members.
Analysis of the hAPN-AngIV complex shows that residues in domains II and IV serve to sandwich the peptide substrate in the closed form (Fig. 3c) and that conversion to the open form would be required for both AngIII binding and AngIV release. Because interactions between the substrate-structured loop and residues in domains II and IV would also be expected to stabilize the closed form, a change in loop conformation or loop ordering, on peptide bond cleavage, might promote conversion to the open form and product release. At the same time, we see no structural reason to exclude the possibility that a peptide product might rebind for another round of cleavage, while the enzyme is still in the closed conformation. Both ordered and disordered loop conformations, as well as peptide-bound and peptide-free structures were obtained in the closed form and the volume required to allow the cleaved peptide to rebind in a shifted register is certainly available. In this way, the internal cavity would also serve to provide a means of trapping peptides for their processive degradation to amino acids or very small peptides. Indeed, a recent molecular dynamics simulation of the plasmodium M1 enzyme has provided evidence of processive peptide degradation in the closed conformation (60).
The hAPN dimer provides the first example of an M1 metallopeptidase in dimeric form and its structure provides further insights into how hAPN mediates its many functions. In addition to its roles in peptide processing, hAPN is also involved in cell adhesion, endocytosis, and signal transduction (4–7, 61), processes often associated with changes in conformation and/or oligomeric state. As shown in Fig. 1b, the dimer interface is made up exclusively of residues from the C-terminal domain (domain IV) of each monomer. Given that hAPN is a type-2 membrane glycoprotein and as such possesses an N-terminal membrane anchor, this arrangement leads to an arch-like structure on the cell surface. This architecture is of particular significance given the suggestion that each monomer can also assume both an open and a closed conformation. As shown in Fig. 6, c–e, conversion from the open/open dimer to the closed/closed dimer leads to a large conformational change that could form the basis of a signal transduction event. Notably, this conversion results in a large change (∼50 Å) in the distance between the membrane anchoring N termini of the two monomers of the dimer. Bradykinin is a known competitive inhibitor of hAPN (62) and its binding would be expected to stabilize the closed form, a conformation that might in turn be responsible for its ability to mediate uptake of the bradykinin receptor complex (6). Given that the membrane-bound dimer possesses two points of membrane attachment it is also conceivable that the rate of interconversion between the open and closed forms, and hence the catalytic activity of hAPN, would be different in the membrane-bound dimer from what it is in either the membrane-bound monomer or soluble forms of the ectodomain. Our structures suggest that a rapid interconversion between the open and closed forms would promote the efficient binding and release of AngIII and AngIV, whereas a slower rate of interconversion might lead to processive degradation. Taken together the novel dimeric structure of hAPN and the resulting models for catalysis and signal transduction are expected to stimulate new research directions.
Acknowledgments
We thank Shaun Labiuk and the Canadian Light Source CMCF 08ID-1 beamline staff for providing assistance during data collection.
This work was supported by the Canadian Institutes of Health Research.

This article contains supplemental Figs. S1–S6 and Table S1.
The atomic coordinates and structure factors (codes 4FYQ, 4FYR, 4FYS, and 4FYT) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- APN
- aminopeptidase N
- AngIII
- angiotensin III
- AngIV
- angiotensin IV
- NGR
- Asn-Gly-Arg
- ERAP
- endoplasmic reticulum aminopeptidase
- GnT1
- N-acetylglucosaminyltransferase 1.
REFERENCES
- 1. George A. J., Thomas W. G., Hannan R. D. (2010) The renin-angiotensin system and cancer. Old dog, new tricks. Nat. Rev. Cancer 10, 745–759 [DOI] [PubMed] [Google Scholar]
- 2. Roques B. P., Fournié-Zaluski M. C., Wurm M. (2012) Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain. Nat. Rev. Drug. Discov. 11, 292–310 [DOI] [PubMed] [Google Scholar]
- 3. Proost P., Mortier A., Loos T., Vandercappellen J., Gouwy M., Ronsse I., Schutyser E., Put W., Parmentier M., Struyf S., Van Damme J. (2007) Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood 110, 37–44 [DOI] [PubMed] [Google Scholar]
- 4. Ghosh M., McAuliffe B., Subramani J., Basu S., Shapiro L. H. (2012) CD13 regulates dendritic cell cross-presentation and T cell responses by inhibiting receptor-mediated antigen uptake. J. Immunol. 188, 5489–5499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mina-Osorio P., Winnicka B., O'Conor C., Grant C. L., Vogel L. K., Rodriguez-Pinto D., Holmes K. V., Ortega E., Shapiro L. H. (2008) CD13 is a novel mediator of monocytic/endothelial cell adhesion. J. Leukocyte Biol. 84, 448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petrovic N., Schacke W., Gahagan J. R., O'Conor C. A., Winnicka B., Conway R. E., Mina-Osorio P., Shapiro L. H. (2007) CD13/APN regulates endothelial invasion and filopodia formation. Blood 110, 142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mina-Osorio P. (2008) The moonlighting enzyme CD13. Old and new functions to target. Trends Mol. Med. 14, 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guzman-Rojas L., Rangel R., Salameh A., Edwards J. K., Dondossola E., Kim Y. G., Saghatelian A., Giordano R. J., Kolonin M. G., Staquicini F. I., Koivunen E., Sidman R. L., Arap W., Pasqualini R. (2012) Cooperative effects of aminopeptidase N (CD13) expressed by nonmalignant and cancer cells within the tumor microenvironment. Proc. Natl. Acad. Sci. U.S.A. 109, 1637–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhagwat S. V., Petrovic N., Okamoto Y., Shapiro L. H. (2003) The angiogenic regulator CD13/APN is a transcriptional target of Ras signaling pathways in endothelial morphogenesis. Blood 101, 1818–1826 [DOI] [PubMed] [Google Scholar]
- 10. Rangel R., Sun Y., Guzman-Rojas L., Ozawa M. G., Sun J., Giordano R. J., Van Pelt C. S., Tinkey P. T., Behringer R. R., Sidman R. L., Arap W., Pasqualini R. (2007) Impaired angiogenesis in aminopeptidase N-null mice. Proc. Natl. Acad. Sci. U.S.A. 104, 4588–4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delmas B., Gelfi J., L'Haridon R., Vogel L. K., Sjöström H., Norén O., Laude H. (1992) Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature 357, 417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeager C. L., Ashmun R. A., Williams R. K., Cardellichio C. B., Shapiro L. H., Look A. T., Holmes K. V. (1992) Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 357, 420–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gorvel J. P., Mishal Z., Liegey F., Rigal A., Maroux S. (1989) Conformational change of rabbit aminopeptidase N into enterocyte plasma membrane domains analyzed by flow cytometry fluorescence energy transfer. J. Cell Biol. 108, 2193–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Favaloro E. J., Browning T., Facey D. (1993) CD13 (GP150; aminopeptidase-N). Predominant functional activity in blood is localized to plasma and is not cell-surface associated. Exp. Hematol. 21, 1695–1701 [PubMed] [Google Scholar]
- 15. Zini S., Fournie-Zaluski M. C., Chauvel E., Roques B. P., Corvol P., Llorens-Cortes C. (1996) Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors. Predominant role of angiotensin III in the control of vasopressin release. Proc. Natl. Acad. Sci. U.S.A. 93, 11968–11973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kehoe P. G., Miners S., Love S. (2009) Angiotensins in Alzheimer disease. Friend or foe? Trends Neurosci 32, 619–628 [DOI] [PubMed] [Google Scholar]
- 17. Ruiz-Ortega M., Esteban V., Egido J. (2007) The regulation of the inflammatory response through nuclear factor-κB pathway by angiotensin IV extends the role of the renin angiotensin system in cardiovascular diseases. Trends Cardiovasc. Med. 17, 19–25 [DOI] [PubMed] [Google Scholar]
- 18. Chai S. Y., Yeatman H. R., Parker M. W., Ascher D. B., Thompson P. E., Mulvey H. T., Albiston A. L. (2008) Development of cognitive enhancers based on inhibition of insulin-regulated aminopeptidase. BMC Neurosci. 9, S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhagwat S. V., Lahdenranta J., Giordano R., Arap W., Pasqualini R., Shapiro L. H. (2001) CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 97, 652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ashmun R. A., Look A. T. (1990) Metalloprotease activity of CD13/aminopeptidase N on the surface of human myeloid cells. Blood 75, 462–469 [PubMed] [Google Scholar]
- 21. Kehlen A., Lendeckel U., Dralle H., Langner J., Hoang-Vu C. (2003) Biological significance of aminopeptidase N/CD13 in thyroid carcinomas. Cancer Res. 63, 8500–8506 [PubMed] [Google Scholar]
- 22. Bauvois B., Dauzonne D. (2006) Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors. Chemistry, biological evaluations, and therapeutic prospects. Med. Res. Rev. 26, 88–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ichinose Y., Genka K., Koike T., Kato H., Watanabe Y., Mori T., Iioka S., Sakuma A., Ohta M., and NK421 Lung Cancer Surgery Group (2003) Randomized double-blind placebo-controlled trial of bestatin in patients with resected stage I squamous-cell lung carcinoma. J. Natl. Cancer Inst. 95, 605–610 [DOI] [PubMed] [Google Scholar]
- 24. Arap W., Pasqualini R., Ruoslahti E. (1998) Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279, 377–380 [DOI] [PubMed] [Google Scholar]
- 25. Pasqualini R., Koivunen E., Kain R., Lahdenranta J., Sakamoto M., Stryhn A., Ashmun R. A., Shapiro L. H., Arap W., Ruoslahti E. (2000) Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 60, 722–727 [PMC free article] [PubMed] [Google Scholar]
- 26. Curnis F., Sacchi A., Borgna L., Magni F., Gasparri A., Corti A. (2000) Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13). Nat. Biotechnol. 18, 1185–1190 [DOI] [PubMed] [Google Scholar]
- 27. Corti A., Curnis F., Arap W., Pasqualini R. (2008) The neovasculature homing motif NGR. More than meets the eye. Blood 112, 2628–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balkwill F. (2009) Tumor necrosis factor and cancer. Nat. Rev. Cancer 9, 361–371 [DOI] [PubMed] [Google Scholar]
- 29. Holmes M. A., Matthews B. W. (1981) Binding of hydroxamic acid inhibitors to crystalline thermolysin suggests a pentacoordinate zinc intermediate in catalysis. Biochemistry 20, 6912–6920 [DOI] [PubMed] [Google Scholar]
- 30. Addlagatta A., Gay L., Matthews B. W. (2006) Structure of aminopeptidase N from Escherichia coli suggests a compartmentalized, gated active site. Proc. Natl. Acad. Sci. U.S.A. 103, 13339–13344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ito K., Nakajima Y., Onohara Y., Takeo M., Nakashima K., Matsubara F., Ito T., Yoshimoto T. (2006) Crystal structure of aminopeptidase N (proteobacteria alanyl aminopeptidase) from Escherichia coli and conformational change of methionine 260 involved in substrate recognition. J. Biol. Chem. 281, 33664–33676 [DOI] [PubMed] [Google Scholar]
- 32. McGowan S., Porter C. J., Lowther J., Stack C. M., Golding S. J., Skinner-Adams T. S., Trenholme K. R., Teuscher F., Donnelly S. M., Grembecka J., Mucha A., Kafarski P., Degori R., Buckle A. M., Gardiner D. L., Whisstock J. C., Dalton J. P. (2009) Structural basis for the inhibition of the essential Plasmodium falciparum M1 neutral aminopeptidase. Proc. Natl. Acad. Sci. U.S.A. 106, 2537–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kochan G., Krojer T., Harvey D., Fischer R., Chen L., Vollmar M., von Delft F., Kavanagh K. L., Brown M. A., Bowness P., Wordsworth P., Kessler B. M., Oppermann U. (2011) Crystal structures of the endoplasmic reticulum aminopeptidase-1 (ERAP1) reveal the molecular basis for N-terminal peptide trimming. Proc. Natl. Acad. Sci. U.S.A. 108, 7745–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen T. T., Chang S. C., Evnouchidou I., York I. A., Zikos C., Rock K. L., Goldberg A. L., Stratikos E., Stern L. J. (2011) Structural basis for antigenic peptide precursor processing by the endoplasmic reticulum aminopeptidase ERAP1. Nat. Struct. Mol. Biol. 18, 604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kyrieleis O. J., Goettig P., Kiefersauer R., Huber R., Brandstetter H. (2005) Crystal structures of the tricorn interacting factor F3 from Thermoplasma acidophilum, a zinc aminopeptidase in three different conformations. J. Mol. Biol. 349, 787–800 [DOI] [PubMed] [Google Scholar]
- 36. Birtley J. R., Saridakis E., Stratikos E., Mavridis I. M. (2011) The crystal structure of human endoplasmic reticulum aminopeptidase 2 reveals the atomic basis for distinct roles in antigen processing. Biochemistry [DOI] [PubMed] [Google Scholar]
- 37. Riemann D., Kehlen A., Langner J. (1999) CD13–not just a marker in leukemia typing. Immunol Today 20, 83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shipp M. A., Look A. T. (1993) Hematopoietic differentiation antigens that are membrane-associated enzymes. Cutting is the key! Blood 82, 1052–1070 [PubMed] [Google Scholar]
- 39. Drag M., Bogyo M., Ellman J. A., Salvesen G. S. (2010) Aminopeptidase fingerprints, an integrated approach for identification of good substrates and optimal inhibitors. J. Biol. Chem. 285, 3310–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reeves P. J., Callewaert N., Contreras R., Khorana H. G. (2002) Structure and function in rhodopsin. High-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. U.S.A. 99, 13419–13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pak J. E., Rini J. M. (2006) X-ray crystal structure determination of mammalian glycosyltransferases. Methods Enzymol. 416, 30–48 [DOI] [PubMed] [Google Scholar]
- 42. Fujita K., Tanaka N., Sano M., Kato I., Asada Y., Takegawa K. (2000) Synthesis of neoglycoenzymes with homogeneous N-linked oligosaccharides using immobilized endo-β-N-acetylglucosaminidase A. Biochem. Biophys. Res. Commun. 267, 134–138 [DOI] [PubMed] [Google Scholar]
- 43. Otwinowski Z., Minor W. (eds) (1997) in Processing of X-ray Diffraction Data Collected in Oscillation Mode, pp. 307–326, Academic Press, New York: [DOI] [PubMed] [Google Scholar]
- 44. Sheldrick G. M. (2008) A short history of SHELX. Acta Crystallogr. A 64, 112–122 [DOI] [PubMed] [Google Scholar]
- 45. Emsley P., Cowtan K. (2004) COOT. Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 46. Potterton E., Briggs P., Turkenburg M., Dodson E. (2003) A graphical user interface to the CCP4 program suite. Acta Crystallogr. D Biol. Crystallogr. 59, 1131–1137 [DOI] [PubMed] [Google Scholar]
- 47. Adams P. D., Mustyakimov M., Afonine P. V., Langan P. (2009) Generalized x-ray and neutron crystallographic analysis. More accurate and complete structures for biological macromolecules. Acta Crystallogr. D Biol. Crystallogr. 65, 567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feracci H., Benajiba A., Gorvel J. P., Doumeng C., Maroux S. (1981) Enzymatic and immunological properties of the protease form of aminopeptidase N and A from pig and rabbit intestinal brush border. Biochim. Biophys. Acta 658, 148–157 [DOI] [PubMed] [Google Scholar]
- 49. Nocek B., Mulligan R., Bargassa M., Collart F., Joachimiak A. (2008) Crystal structure of aminopeptidase N from human pathogen Neisseria meningitidis. Proteins 70, 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hussain M. M., Tranum-Jensen J., Norén O., Sjöström H., Christiansen K. (1981) Reconstitution of purified amphiphilic pig intestinal microvillus aminopeptidase. Mode of membrane insertion and morphology. Biochem. J. 199, 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luciani N., Marie-Claire C., Ruffet E., Beaumont A., Roques B. P., Fournié-Zaluski M. C. (1998) Characterization of Glu350 as a critical residue involved in the N-terminal amine binding site of aminopeptidase N (EC 3.4.11.2). Insights into its mechanism of action. Biochemistry 37, 686–692 [DOI] [PubMed] [Google Scholar]
- 52. Rich D. H., Moon B. J., Harbeson S. (1984) Inhibition of aminopeptidases by amastatin and bestatin derivatives. Effect of inhibitor structure on slow-binding processes. J. Med. Chem. 27, 417–422 [DOI] [PubMed] [Google Scholar]
- 53. Thunnissen M. M., Nordlund P., Haeggström J. Z. (2001) Crystal structure of human leukotriene A4 hydrolase, a bifunctional enzyme in inflammation. Nat. Struct. Biol. 8, 131–135 [DOI] [PubMed] [Google Scholar]
- 54. Helgstrand C., Hasan M., Uysal H., Haeggström J. Z., Thunnissen M. M. (2011) A leukotriene A4 hydrolase-related aminopeptidase from yeast undergoes induced fit upon inhibitor binding. J. Mol. Biol. 406, 120–134 [DOI] [PubMed] [Google Scholar]
- 55. Kania R. K., Santiago N. A., Gray G. M. (1977) Intestinal surface amino-oligopeptidases. II. Substrate kinetics and topography of the active site. J. Biol. Chem. 252, 4929–4934 [PubMed] [Google Scholar]
- 56. Gandhi A., Lakshminarasimhan D., Sun Y., Guo H. C. (2011) Structural insights into the molecular ruler mechanism of the endoplasmic reticulum aminopeptidase ERAP1. Sci. Rep. 1, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vallee B. L., Auld D. S. (1990) Active-site zinc ligands and activated H2O of zinc enzymes. Proc. Natl. Acad. Sci. U.S.A. 87, 220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tholander F., Muroya A., Roques B. P., Fournié-Zaluski M. C., Thunnissen M. M., Haeggström J. Z. (2008) Structure-based dissection of the active site chemistry of leukotriene A4 hydrolase. Implications for M1 aminopeptidases and inhibitor design. Chem. Biol. 15, 920–929 [DOI] [PubMed] [Google Scholar]
- 59. Jacobsen F. E., Lewis J. A., Cohen S. M. (2007) The design of inhibitors for medicinally relevant metalloproteins. ChemMedChem 2, 152–171 [DOI] [PubMed] [Google Scholar]
- 60. Jones P. M., Robinson M. W., Dalton J. P., George A. M. (2011) The Plasmodium falciparum malaria M1 alanyl aminopeptidase (PfA-M1). Insights of catalytic mechanism and function from MD simulations. PLoS One 6, e28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Santos A. N., Langner J., Herrmann M., Riemann D. (2000) Aminopeptidase N/CD13 is directly linked to signal transduction pathways in monocytes. Cell. Immunol. 201, 22–32 [DOI] [PubMed] [Google Scholar]
- 62. Xu Y., Wellner D., Scheinberg D. A. (1995) Substance P and bradykinin are natural inhibitors of CD13/aminopeptidase N. Biochem. Biophys. Res. Commun. 208, 664–674 [DOI] [PubMed] [Google Scholar]