Abstract
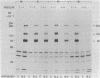
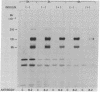
We have measured the turnover rate of the polypeptide subunits of the insulin receptor in cultured human lymphocytes (IM-9 line) and have investigated the mechanism of insulin-induced receptor loss. To estimate the rate of receptor degradation, lymphocytes were either pulse-labeled with [35S]methionine or surface labeled with Na125I and lactoperoxidase. The insulin receptor was isolated by immunoprecipitation with anti-receptor antibody, and the rate of loss of radioactivity from each receptor subunit was determined after sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Two major (Mr 135,000 and 95,000) and one minor (Mr 210,000) subunits were found. By both labeling methods, the half-lives of the major insulin receptor subunits were 9--12 hr in normal media. When the cells were cultured in media containing 1 microM insulin the turnover was accelerated 2.5- to 3.5-fold (half-life approximately 3 hr). The increase in degradation rate was dependent on the insulin concentration and correlated well with the ability to "down-regulate" the receptor. Guinea pig insulin was about 2% as active as porcine insulin in accelerating degradation, and human growth hormone was without effect. The acceleration of receptor degradation induced by insulin was partially blocked by 100 microM cycloheximide. The rate of biosynthesis of the insulin receptor did not appear to be altered in the presence of 1 microM insulin after correction for the change in degradation rate. In conclusion, these data demonstrate that insulin-induced receptor loss in cultured lymphocytes is due to accelerated receptor degradation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Goldstein J. L. Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell. 1975 Nov;6(3):307–316. doi: 10.1016/0092-8674(75)90182-8. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B., Angus C. W., Adams R. N., Michelson J. D., Hoffman G. J. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med. 1978 May 18;298(20):1116–1122. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]
- Epstein D., Elias-Bishko S., Hershko A. Requirement for protein synthesis in the regulation of protein breakdown in cultured hepatoma cells. Biochemistry. 1975 Nov 18;14(23):5199–5204. doi: 10.1021/bi00694a028. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Freychet P. O., Orci L. Internalization of polypeptide hormones: mechanism, intracellular localization and significance. Diabetologia. 1980 Apr;18(4):263–274. doi: 10.1007/BF00251003. [DOI] [PubMed] [Google Scholar]
- Harmon J. T., Kahn C. R., Kempner E. S., Schlegel W. Characterization of the insulin receptor in its membrane environment by radiation inactivation. J Biol Chem. 1980 Apr 25;255(8):3412–3419. [PubMed] [Google Scholar]
- Harrison L. C., Flier J. S., Roth J., Karlsson F. A., Kahn C. R. Immunoprecipitation of the insulin receptor: a sensitive assay for receptor antibodies and a specific technique for receptor purification. J Clin Endocrinol Metab. 1979 Jan;48(1):59–65. doi: 10.1210/jcem-48-1-59. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Flier J., Itin A., Kahn C. R., Roth J. Radioimmunoassay of the insulin receptor: a new probe of receptor structure and function. Science. 1979 Feb 9;203(4380):544–547. doi: 10.1126/science.83675. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Harrison L. C., Roth J. Binding of insulin receptors to lectins: evidence for common carbohydrate determinants on several membrane receptors. Biochemistry. 1981 Jun 9;20(12):3385–3393. doi: 10.1021/bi00515a013. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Kasuga M., Van Obberghen E., Roth J., Kahn C. R. Direct demonstration of glycosylation of insulin receptor subunits by biosynthetic and external labeling: evidence for heterogeneity. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4791–4795. doi: 10.1073/pnas.78.8.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Flier J. S., Bar R. S., Archer J. A., Gorden P., Martin M. M., Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976 Apr 1;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- Kasuga M., van Obberghen E., Yamada K. M., Harrison L. C. Autoantibodies against the insulin receptor recognize the insulin binding subunits of an oligomeric receptor. Diabetes. 1981 Apr;30(4):354–357. doi: 10.2337/diab.30.4.354. [DOI] [PubMed] [Google Scholar]
- Kosmakos F. C., Roth J. Insulin-induced loss of the insulin receptor in IM-9 lymphocytes. A biological process mediated through the insulin receptor. J Biol Chem. 1980 Oct 25;255(20):9860–9869. [PubMed] [Google Scholar]
- Krupp M., Lane M. D. On the mechanism of ligand-induced down-regulation of insulin receptor level in the liver cell. J Biol Chem. 1981 Feb 25;256(4):1689–1694. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang U., Kahn C. R., Harrison L. C. Subunit structure of the insulin receptor of the human lymphocyte. Biochemistry. 1980 Jan 8;19(1):64–70. doi: 10.1021/bi00542a010. [DOI] [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Hollenberg M. D. Insulin receptor: interaction with nonreceptor glycoprotein from liver cell membranes. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3070–3074. doi: 10.1073/pnas.75.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. The insulin receptor: its role in insulin resistance of obesity and diabetes. Diabetes. 1976 Dec;25(12):1154–1162. doi: 10.2337/diab.25.12.1154. [DOI] [PubMed] [Google Scholar]
- Reed B. C., Lane M. D. Insulin receptor synthesis and turnover in differentiating 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):285–289. doi: 10.1073/pnas.77.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Chia G. H., Fung C., Rubin C. S. Tunicamycin-mediated depletion of insulin receptors in 3T3-L1 adipocytes. J Cell Physiol. 1979 Apr;99(1):37–42. doi: 10.1002/jcp.1040990106. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Methods for analysis of enzyme synthesis and degradation in animal tissues. Methods Enzymol. 1975;40:241–266. doi: 10.1016/s0076-6879(75)40020-9. [DOI] [PubMed] [Google Scholar]
- Soli A. H., Kahn C. R., Neville D. M., Jr, Roth J. Insulin receptor deficiency in genetic and acquired obesity. J Clin Invest. 1975 Oct;56(4):769–780. doi: 10.1172/JCI108155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tell G. P., Haour F., Saez J. M. Hormonal regulation of membrane receptors and cell responsiveness: a review. Metabolism. 1978 Oct;27(10):1566–1592. doi: 10.1016/s0026-0495(78)80029-8. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Ksauga M., Le Cam A., Hedo J. A., Itin A., Harrison L. C. Biosynthetic labeling of insulin receptor: studies of subunits in cultured human IM-9 lymphocytes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1052–1056. doi: 10.1073/pnas.78.2.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor proteins of liver plasma membrane preparations. Biochemistry. 1980 Jan 8;19(1):70–76. doi: 10.1021/bi00542a011. [DOI] [PubMed] [Google Scholar]