Abstract
Deep vein thrombosis (DVT) is a major health problem that requires improved prophylaxis and treatment. Inflammatory conditions such as infection, cancer and autoimmune diseases are risk factors for DVT. We and others have recently shown that extracellular DNA fibers produced in inflammation and known as neutrophil extracellular traps (NETs) contribute to experimental DVT. NETs stimulate thrombus formation and coagulation and are abundant in thrombi in animal models of DVT. It appears that, in addition to fibrin and VWF, NETs represent a third thrombus scaffold. Here we review how NETs stimulate thrombosis and discuss known and potential interactions of NETs with endothelium, platelets, red blood cells, coagulation factors and how NETs could influence thrombolysis. We propose that drugs which inhibit NET formation or facilitate NET degradation may prevent or treat DVT.
Keywords: deep vein thrombosis, inflammation, mouse model, NETs, DNase
Deep vein thrombosis: a major health problem
Deep vein thrombosis (DVT) is a debilitating disease that may be complicated by pulmonary embolism (PE)1. Together DVT and PE are designated as venous thromboembolism (VTE). In the US, VTE develops in an estimated 900,000 patients each year and PE is responsible for about 300,000 deaths, which exceeds the mortality from myocardial infarction or stroke2, 3. DVT complications, in addition to PE, include post-thrombotic syndrome, caused by chronic venous stasis even in the absence of active thrombosis4.
DVT prophylaxis and treatment include anti-coagulation by heparin, vitamin K antagonists and thrombin and Factor Xa inhibitors5, 6. Surgical intervention for DVT treatment includes thrombectomy or catheter-based thrombolysis using urokinase, streptokinase or tPA7. The success rate of pharmacologic catheter-directed thrombolysis ranges from 59 to 100%7. Morbidity from VTE has not substantially changed within the last two decades1 and contemporary prophylaxis is not always efficient8.
DVT is epidemiologically associated with inflammatory diseases such as infection, autoimmune disorders and cancer. Acute infections9, 10 and autoantibodies against prothrombin11 and phospholipids12 are risk factors for DVT and PE. Coincidence of venous thrombosis and malignancy was documented in the 19th century13. Recently, the risk of VTE and death was associated with elevated neutrophil counts in cancer patients undergoing chemotherapy14. Neutrophils are the most abundant inflammatory cells and recent animal studies emphasize their importance in DVT (Figure 1)15–17.
Figure 1. NETs in the timeline of DVT: a model.
(A) DVT is initiated by local hypoxia and activation of endothelial cells (EC) as a result of flow restriction/disturbances. Activated endothelium releases ultra-large von Willebrand factor (ULVWF) and P-selectin from Weibel-Palade bodies (WPB) which mediate platelet and neutrophil adhesion. Activated platelets recruit tissue factor (TF)- containing microparticles that enhance thrombin generation in the growing thrombus. (B) Activated platelets and endothelium or other stimulus induce NET formation in adherent neutrophils. NETs provide an additional scaffold for platelet and RBC adhesion, promote fibrin formation, and exacerbate platelet and endothelial activation. (C) Plasmin, ADAMTS13 and DNase mediate thrombolysis by degrading fibrin, ULVWF and DNA, respectively. Monocytes/macrophages (MØ) release an additional source of DNase and generate plasmin and promote restoration of blood flow.
Pathogenesis of DVT: lessons from animal models
DVT can be triggered by disturbances in venous blood flow, activation or dysfunction of the vascular endothelium and hypercoagulability. Immobilization from long-haul flight, bed-ridden position or limb paresis may also result in flow disturbances and stagnant blood flow in veins leading to DVT6. Flow restriction can be modeled experimentally in mice or rats by complete18 or partial17, 19 ligation of the inferior vena cava (IVC). These models represent different degrees of severity of blood flow distortion, although the mechanisms of thrombus development in complete and partial IVC closure may not be identical. In both human and murine DVT, thrombi are rich in fibrin and consist of red (rich in RBCs) and white (rich in platelets) parts19, 20. Similar to human DVT, venous thrombosis in mice is induced in the absence of overt endothelial denudation20 and can be prevented by low molecular weight heparin17.
In contrast to arterial thrombosis, which usually results from atherosclerotic plaque erosion or rupture, the mechanisms of DVT development in intact vessels are poorly understood. Venous thrombus development in humans often starts in the valve sinus where, due to complex fluid mechanics in the valve pockets, the blood flow pattern becomes abnormal leading to endothelial dysfunction. Stagnant blood flow (stasis) in the linear section of the blood vessel may have similar consequences21, 22. Stasis leads to hypoxia23, which in turn may contribute to thrombogenesis and locally activate endothelium. We have recently demonstrated that six hours after IVC stenosis in mice, von Willebrand factor (VWF) is released from Weibel-Palade bodies (WPB) and mediates platelet recruitment to endothelium (Figure 1A)19. This represents a key step in the initiation of DVT as neither VWF-deficient nor platelet-depleted mice form a thrombus17, 19. Lack of GPIbα, a platelet receptor for VWF, prevents murine venous thrombosis17 and corroborates the importance of platelet adhesion in DVT. In addition to platelets, neutrophils are crucial for experimental DVT16, 17, 24. P-selectin, exposed on activated endothelium or platelets, mediates initial recruitment of neutrophils (Figure 1A). Depletion of neutrophils or deficiency in P-selectin prevents DVT in mice17. In baboons, pharmacological inhibition of P-selectin accelerated thrombolysis and restoration of blood flow in thrombosed veins25.
Hypercoagulability of blood is another important mechanism which contributes to DVT. Tissue factor (TF) is considered the central coagulation-triggering molecule in DVT26 (Figure 1A, B). TF initiates the extrinsic coagulation pathway and is released in inflammation on microparticles originating from activated leukocytes. In murine DVT, flow restriction-induced fibrinogen deposition in the IVC of transgenic mice expressing low levels of human TF (< 1%) and no mouse TF, was substantially impaired in comparison to mice expressing normal levels of human TF17. This finding indicates that TF is indispensable for thrombus growth. Another potential coagulation-dependent mechanism playing a role in DVT is that blood pooled in large veins (with relatively low endothelial surface-to-blood volume ratio) cannot be efficiently anti-coagulated by the vessel wall as it would be in microcirculation where surface-to-volume ratio is much higher27. Thus, the mechanisms of flow disturbance-triggered DVT involve endothelial activation, pro-coagulant shift in blood and recruitment of platelets and leukocytes. We and others have recently uncovered that neutrophils are recruited and release NETs in experimental DVT (Figure 1B). NETs may be a new target for therapeutic development and their implications for DVT will be the main topic of this review.
Neutrophil extracellular traps (NETs)
Neutrophils are the first leukocytes recruited to sites of infection, where they phagocytose invading bacteria28. Within the phagosome, microbes are killed by locally high concentrations of antimicrobial proteins and reactive oxygen species (ROS). NETs are produced to allow neutrophils to trap and disarm microbes in the extracellular environment29. NETs are scaffolds of intact chromatin fibers with antimicrobial proteins, ideal to retain large quantities of microbes (Figure 2A). Therefore some pathogenic bacteria have evolved to express an extracellular DNase, which dismantles NETs and promotes virulence30, 31.
Figure 2. NET formation and function.
A) Electron micrograph of NETs with trapped Salmonella typhimurium; Bar: 1µm. Courtesy of Dr. Volker Brinkmann, Max Planck Institute for Infection Biology, Berlin, Germany. (B) Electron micrograph of NETs with adherent platelets. Bar: 1µm. Reproduced and modified from reference 15. (C) Scheme of NET formation (NETosis). Enzymes from granules (red) translocate to the nucleus (blue) and facilitate chromatin decondensation. Internal membranes break down and cytolysis releases NETs.
Extracellular traps (ETs) are formed in humans, animals and even plants32 indicating that NETs provide an evolutionary conserved protective mechanism. Indeed, ET formation is not restricted to neutrophils33–35 and different cell types employ different cellular mechanisms to release ET. One mechanism used by human neutrophils is NETosis36. NETosis is a multi-step cell death program (Figure 2C). Upon activation, certain enzymes translocate from the granules to the nucleus37. Histones are degraded by neutrophil elastase (NE)37 and citrullinated by peptidylarginine deiminase 4 (PAD4)38 to unwind chromatin. Further hallmarks are the breakdown of granular and nuclear membranes and cytolysis as the final step in NETosis36. NETosis involves signaling pathways which lead to ROS production36, 39 and up-regulation of anti-apoptotic proteins40. It is distinct from apoptosis, where nuclear condensation and DNA fragmentation occur, and necrosis, where the plasma membrane breaks before the nuclear envelope36. In vitro, the kinetics of NETosis varies from less than 30 min to 240 min, likely depending on the type and concentration of the stimulus, isolation procedure of neutrophils and sensitivity of the detection method36, 41. Alternatively, NETs may be released from viable neutrophils by ejecting mitochondrial DNA42, nuclear contents43 or DNA-containing vesicles44, but the underlying signaling pathways of these processes are not well defined.
NETs link innate immunity with thrombosis
NETs provide a new link between innate immunity and thrombosis. NETs stimulate platelet adhesion (Figure 2B)15 and coagulation17, 45 and are abundant in experimental deep vein thrombi in baboons15 and mice16, 17, where they co-localize with VWF15, 16. Treatment of mice with DNase1 prevents thrombus formation16, 17 underscoring the importance of NETs for DVT. NETs interact with endothelium, platelets, and coagulation factors and may be able to influence thrombolysis.
NETs and endothelium
Activation of endothelium and WPB release play a crucial role in the initiation of DVT. Co-cultures of activated endothelial cells and neutrophils promote NET formation, which is dependent on platelets43 or interleukin-8 and ROS released from endothelium46. NETs in turn induce endothelial cell death43, 46, 47, an effect likely mediated by NET-associated proteases or cationic proteins such as defensins and histones47, 48. Histones display a high affinity for phospholipids and their binding to membranes results in pore formation and an influx of ions49–51. Interactions of histones with endothelium could promote thrombosis by exacerbating endothelial activation and WPB release through an increase of intracellular calcium levels52. We have observed that plasma VWF increases and DVT is aggravated in mice infused with purified histones16.
NETs and platelets
NETs can also contribute to thrombus formation through interaction with platelets (Figure 1B, 2B). NETs are very large structures and may promote thrombus stability similarly to VWF and fibrin53. When perfused with blood, NETs bind platelets and support their aggregation, indicating that they are a substrate for platelet adhesion and also provide a stimulus for platelet activation15. Platelets may bind to NETs both directly and indirectly. Purified histones associate with the platelet surface in vitro54, presumably via electrostatic interactions with phospholipids49 or carbohydrates55 or via Toll-like receptors on platelets56. Platelets also bind double and single stranded DNA in vitro57, 58. Interestingly, platelets from patients with systemic lupus erythematosus (SLE) have immune complexes of DNA and anti-DNA-antibodies on their surface, which can be released by incubation with DNase59. NET degradation is impaired in SLE patients due to a reduced DNase1 activity in serum60 and future studies may address whether the inability to degrade NETs correlates with the increased risk for venous thrombosis in these patients61. Platelet-NET interactions could also be mediated by adhesion molecules such as VWF, fibrinogen or fibronectin15. These molecules bind to NETs presumably because of their affinity for histones or DNA62–64. Activation of platelets by NETs might be triggered by histones or neutrophil proteases in NETs. Purified histones stimulate influx of calcium into platelets and promote activation and aggregation in vitro54, 56. When infused into mice, histones co-localize with platelets and induce thrombocytopenia and thrombosis16, 51, 54. NETs contain enzymatically active NE and cathepsin G29 and these proteases potentiate platelet aggregation through proteolytically activating platelet receptors65, 66. Interactions of NETs with platelets may result in a vicious cycle of NET formation and platelet activation, because platelets prestimulated with LPS or collagen trigger neutrophils to release NETs 43, 45.
NETs and red blood cells
Red thrombi are typical for DVT. But unlike platelets, the role of RBCs in thrombus formation is not well defined and they are frequently considered passively entrapped. However, RBCs may promote coagulation by exposing phosphatidylserine and altering blood viscosity67. We found that in addition to platelets, RBCs avidly bind to NETs after perfusion of whole blood15. Activated neutrophils or platelets can also recruit RBCs at very low venous shear in vitro68. Similar to platelets, RBC may interact with NETs directly or indirectly. DNA was eluted from the surface of isolated RBCs from cancer patients69, indicating that RBCs can bind DNA. Interestingly, in experimental DVT in mice, NETs are predominantly found in the red, RBC-rich part of the thrombus16, suggesting that NETs could be important for RBC recruitment to venous thrombi (Figure 1B).
NETs and coagulation
Fibrin is abundantly present in venous thrombi. In vitro, NETs stimulate fibrin formation and deposition and fibrin co-localizes with NETs in blood clots15, 17, 45. NETs stimulate both the extrinsic and intrinsic coagulation pathway17, 45. NE is known to cleave tissue factor pathway inhibitor (TFPI) and enhance Factor Xa activity70. NETs contain NE and bind TFPI and therefore facilitate proteolytic inactivation of TFPI by NE45. NETs also bind Factor XII and stimulate fibrin formation via the intrinsic coagulation pathway17. DNA and histones in NETs may play an important role in stimulating coagulation as well. Nucleic acids enhance the activity of coagulation serine proteases71 and histones promote coagulation indirectly by activating platelets and stimulating release of pro-coagulant polyphosphates from platelet granules56, 72. In addition, histones inhibit anticoagulants in plasma. Histones interact with thrombomodulin (TM) and protein C and inhibit TM-mediated protein C activation73. As a consequence, histones dose-dependently increase plasma thrombin generation in vitro. Histones in NETs may exhibit similar functions and thus promote fibrin deposition in DVT (Figure 1B).
Implications of NETs in thrombolysis
In order to degrade and solubilize thrombi to restore blood flow, fibrin and VWF as the main scaffolds need to be proteolytically fragmented by the proteases plasmin and ADAMTS13, respectively. NETs are a newly recognized third scaffold that needs to be undone during thrombolysis (Figure 1C).
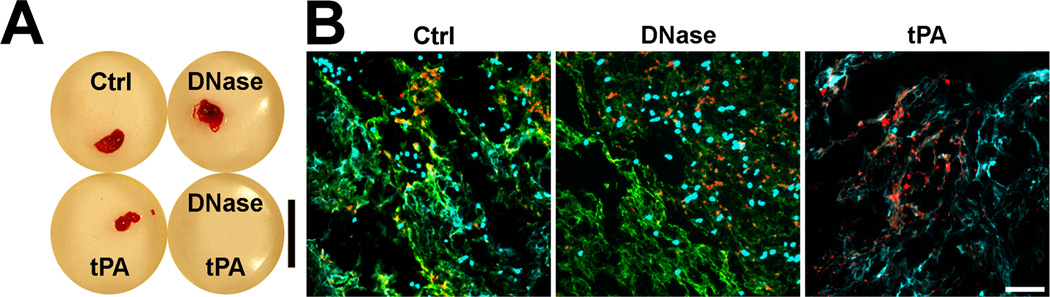
NETs were seen to co-localize with fibrin in clots15 and with VWF in venous thrombi15, 16. In vitro, we could show that NETs provide a scaffold for blood clots that is resistant to tissue plasminogen activator (tPA)-induced thrombolysis15. We incubated re-calcified blood with neutrophils which were pre-stimulated to release NETs. As shown in Figure 3, after filtration, blood clots appeared in control samples and tPA- or DNase-treated blood but not in blood treated with the combination of tPA and DNase (Figure 3A). Immunostainings revealed that in the presence of tPA, blood clots lacked fibrin and were held together by a scaffold of extracellular DNA (Figure 3B).
Figure 3. NETs are a tPA-resistant scaffold of blood clots.
(A) Photographs of blood clots. Citrated blood is mixed with neutrophils, which are pre-stimulated to release NETs. Blood is supplemented with DNase and/or tPA as indicated and clotting is induced by re-calcification. Controls (Ctrl) do not receive DNase or tPA. tPA in combination with DNase dissolves blood clot. Bar: 1cm. (B) Fluorescence images of DNA (light blue), fibrinogen (green) and VWF (red) in blood clots shown in panel A. Extracellular DNA co-localizes with fibrin in untreated blood clots (Ctrl). DNase degrades extracellular DNA but not nuclei and fibrin in blood clots. tPA induces degradation of fibrin but not extracellular DNA. The remaining extracellular DNA scaffold is sufficient to retain RBCs and platelets (indicated by punctuate VWF staining). In this in vitro experiment, ultra large VWF multimers were not present and their role in the thrombus scaffold could not be evaluated. Bar: 50µm. Reproduced and modified from reference 15.
DNase1 is the predominant nuclease in plasma. Interestingly, the plasminogen system cooperates with DNase1 during chromatin degradation74. DNase1 has only limited activity to degrade chromatin as it preferentially degrades protein-free DNA. Plasminogen, activated by either tPA or urokinase-type plasminogen activator (uPA), degrades histones and therefore allows for degradation of DNA by DNase174. Monocytes/macrophages may also support the DNA degradation because their lysosomes contains DNase2, which is important for the removal of apoptotic cells (Figure 1C)75. NETs and fibrin degradation by plasmin and DNase could result in the simultaneous release of DNA and fibrin fragments. In baboon DVT, plasma DNA increases15 with similar kinetics to the fibrin degradation product D-dimers76. Recently, in collaboration with Thomas Wakefield’s group, we found increased levels of DNA in plasma from patients with DVT compared to healthy controls and symptomatic patients who did not have DVT. Here also plasma DNA concentrations correlated with D-dimers (Diaz JA and Fuchs TA et al., unpublished data, 2012). Therefore it is plausible that circulating DNA may reflect the degradation of NETs within a thrombus.
NETs may also promote thrombolysis. In vitro studies have shown that NE and cathepsin G can degrade fibrin77 and these proteases are present on NETs and could enhance fibrinolysis in DVT. In addition, NETs may also recruit plasminogen from the plasma. Histone H2B can serve as a receptor for plasminogen on the surface of human monocytes/macrophages78 and perhaps could do so in NETs.
NETs in DVT: Questions and Challenges for the future
Now that a new polymeric scaffold, NETs, has been identified in thrombi of deep veins15, what should be explored to make the best use of this observation to progress towards generation of new approaches to prevention and treatment of DVT? First, it will be important to learn more about what triggers NET formation in DVT. If the trigger(s) were identified, their generation could be inhibited. The trigger could be the interaction of neutrophils with activated cells such as platelets or endothelium43, 45, 46. This could be confirmed if the neutrophil surface receptors involved were identified and their inhibition tested in DVT. Alternatively, environmental factors such as hypoxia, ROS, cytokines or possibly coagulation proteases generated early in thrombosis could induce NETosis. In vitro, ROS are a common denominator of ET formation used by different types of leukocytes34–36. Platelets may potentiate ROS production in platelet-neutrophil complexes79 and endothelial ROS were shown to trigger NET formation by neutrophils in vitro46. Protease activated receptors are present on neutrophils80 but whether or how they respond in thrombosis is an open question. Perhaps, studies should move from purified neutrophils to more complex in vitro systems, which may better model DVT and involve endothelium, platelets and neutrophils under hypoxic and other experimental conditions.
After the neutrophil is triggered to NETosis, much needs to be clarified about the cell biology of NETs formation. Again, if the cellular processes leading to nuclear dissolution and NET ejection were elucidated, their inhibition in DVT could be envisioned. At least two enzymes: neutrophil elastase37 and PAD438 are implicated in chromatin decondensation and NET generation. Their sequence of action, substrates, and processes through which they access the nucleus are not clear. These enzymes’ inhibition effects on DVT should be evaluated using available inhibitors and knockout mice. Whether the final discharge of NETs (Figure 2C) from the neutrophil is produced by cellular lysis36 or a secretory process42, 43 that could be inhibited is also an important question.
The time of onset and duration of NET formation in venous thrombogenesis needs to be determined to better understand their function in the process. The challenge will be to capture single neutrophils forming NETs in DVT by employing intravital microscopy in mice. If NETs are produced rapidly after flow restriction, they may facilitate thrombus initiation. This is supported by the absence of visible thrombi in most DNase-treated mice16, 17. The observation that NETs are located at the interface between thrombus and vascular wall15 may suggest that they help anchor the thrombus to the vessel. NETs were observed in mature thrombi; but whether these were remnants or whether NETs are formed continuously during venous thrombosis is not known. In an aged thrombus, NETs may participate in thrombus remodeling, for example by recruiting endothelial progenitor cells for neovessel formation. Whether NETs may recruit tissue factor-containing microparticles that are an important component of DVT is also not known.
A very important challenge will be to determine NETs’ effect on thrombolysis. In vitro and in vivo observations indicate that chromatin, fibrin and VWF form a colocalized network within the thrombus that is similar to extracellular matrix15–17. It is likely that each of the components will need to be cleaved by their own appropriate enzyme (Figure 1C) and also that the presence of one component may influence the degradation or stability of the other. Fibrin is crosslinked/stabilized by FXIII transglutaminase. Whether NET components provide a substrate(s) to FXIII and could be crosslinked to fibrin is not known. PAD4 is eventually secreted from neutrophils during NET formation and was shown to citrullinate fibrin in rheumatoid arthritis81. Whether this occurs in DVT and how it may affect fibrin degradation by plasmin is unknown. As part of thrombolysis monocytes are recruited to the thrombus. Interestingly, they are equipped to degrade both fibrin and DNA75, 78. Whether NETs promote or interfere with monocyte recruitment to the thrombus has not been investigated.
In summary, in vitro and in vivo observations indicate that NETs could influence initiation, growth and resolution of DVT. NETs may nucleate thrombus formation by enabling locally high concentrations of platelets, RBCs and coagulation factors. NETs together with fibrin and VWF could cooperatively provide thrombus stability. Future studies should determine whether NETs formation in DVT can be prevented and most importantly from a clinical perspective whether DNase, could be developed as a new, hopefully safer, therapeutic drug for thrombolysis.
Acknowledgments
The authors thank Thomas Wakefield and Kimberly Martinod for their advice, Lesley Cowan for editing and assistance in preparing the manuscript, and Grace Thomas, Julian Borissoff and the anonymous reviewers for critical reading of the manuscript.
Sources of Funding
This study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health grants R01 HL102101, R01 HL041002 and R01 HL095091 (to D.D.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics-- 2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 3.Raskob GE, Silverstein R, Bratzler DW, Heit JA, White RH. Surveillance for deep vein thrombosis and pulmonary embolism: recommendations from a national workshop. Am J Prev Med. 2010;38:S502–S509. doi: 10.1016/j.amepre.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3:1611–1617. doi: 10.1111/j.1538-7836.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 5.Buller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J, Verhamme P, Wells P, Agnelli G, Cohen A, Berkowitz SD, Bounameaux H, Davidson BL, Misselwitz F, Gallus AS, Raskob GE, Schellong S, Segers A. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 6.Kyrle PA, Eichinger S. Deep vein thrombosis. Lancet. 2005;365:1163–1174. doi: 10.1016/S0140-6736(05)71880-8. [DOI] [PubMed] [Google Scholar]
- 7.Casey ET, Murad MH, Zumaeta-Garcia M, Elamin MB, Shi Q, Erwin PJ, Montori VM, Gloviczki P, Meissner M. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012;55:1463–1473. doi: 10.1016/j.jvs.2011.12.082. [DOI] [PubMed] [Google Scholar]
- 8.Januel JM, Chen G, Ruffieux C, Quan H, Douketis JD, Crowther MA, Colin C, Ghali WA, Burnand B. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA. 2012;307:294–303. doi: 10.1001/jama.2011.2029. [DOI] [PubMed] [Google Scholar]
- 9.Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367:1075–1079. doi: 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- 10.Rogers MAM, Levine DA, Blumberg N, Flanders SA, Chopra V, Langa KM. Triggers of hospitalization for venous thrombosembolism. Circulation. 2012;125:2092–2099. doi: 10.1161/CIRCULATIONAHA.111.084467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikura K, Wada H, Kamikura Y, Hattori K, Fukuzawa T, Yamada N, Nakamura M, Nobori T, Nakano T. High prevalence of anti-prothrombin antibody in patients with deep vein thrombosis. Am J Hematol. 2004;76:338–342. doi: 10.1002/ajh.20124. [DOI] [PubMed] [Google Scholar]
- 12.Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood. 2003;101:1827–1832. doi: 10.1182/blood-2002-02-0441. [DOI] [PubMed] [Google Scholar]
- 13.Trousseau A. Lectures on Clinical Medicine. Delivered at the Hotel-Dieu, Paris. In: Cormack JR, editor. (translated from the edition of 1868) London: The New Sydenham Society; 1872. [Google Scholar]
- 14.Connolly GC, Khorana AA, Kuderer NM, Culakova E, Francis CW, Lyman GH. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res. 2010;126:113–118. doi: 10.1016/j.thromres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Downing LJ, Strieter RM, Kadell AM, Wilke CA, Greenfield LJ, Wakefield TW. Low-dose low-molecular-weight heparin is anti-inflammatory during venous thrombosis. J Vasc Surg. 1998;28:848–854. doi: 10.1016/s0741-5214(98)70060-6. [DOI] [PubMed] [Google Scholar]
- 19.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, Wakefield TW, Lammle B, Massberg S, Wagner DD. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sevitt S. The structure and growth of valve-pocket thrombi in femoral veins. J Clin Pathol. 1974;27:517–528. doi: 10.1136/jcp.27.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karino T, Motomiya M. Flow through a venous valve and its implication for thrombus formation. Thromb Res. 1984;36:245–257. doi: 10.1016/0049-3848(84)90224-x. [DOI] [PubMed] [Google Scholar]
- 22.Bovill EG, van der Vliet A. Venous valvular stasis-associated hypoxia and thrombosis: what is the link? Annu Rev Physiol. 2011;73:527–545. doi: 10.1146/annurev-physiol-012110-142305. [DOI] [PubMed] [Google Scholar]
- 23.Hamer JD, Malone PC, Silver IA. The PO2 in venous valve pockets: its possible bearing on thrombogenesis. Br J Surg. 1981;68:166–170. doi: 10.1002/bjs.1800680308. [DOI] [PubMed] [Google Scholar]
- 24.Downing LJ, Strieter RM, Kadell AM, Wilke CA, Brown SL, Wrobleski SK, Burdick MD, Hulin MS, Fowlkes JB, Greenfield LJ, Wakefield TW. Neutrophils are the initial cell type identified in deep venous thrombosis induced vein wall inflammation. ASAIO J. 1996;42:M677–M682. doi: 10.1097/00002480-199609000-00073. [DOI] [PubMed] [Google Scholar]
- 25.Ramacciotti E, Myers DD, Jr, Wrobleski SK, Deatrick KB, Londy FJ, Rectenwald JE, Henke PK, Schaub RG, Wakefield TW. P-selectin/ PSGL-1 inhibitors versus enoxaparin in the resolution of venous thrombosis: a meta-analysis. Thromb Res. 2010;125:e138–e142. doi: 10.1016/j.thromres.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manly DA, Boles J, Mackman N. Role of tissue factor in venous thrombosis. Annu Rev Physiol. 2011;73:515–525. doi: 10.1146/annurev-physiol-042210-121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esmon CT, Esmon NL. The link between vascular features and thrombosis. Annu Rev Physiol. 2011;73:503–514. doi: 10.1146/annurev-physiol-012110-142300. [DOI] [PubMed] [Google Scholar]
- 28.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 29.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 30.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 31.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 32.Wen F, White GJ, VanEtten HD, Xiong Z, Hawes MC. Extracellular DNA is required for root tip resistance to fungal infection. Plant Physiol. 2009;151:820–829. doi: 10.1104/pp.109.142067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 35.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 41.von Kockritz-Blickwede M, Chow OA, Ghochani M, Nizet V. Visualization and functional evaluation of phagocyte extracellular trap. In: Kabelitz D, Kaufman SHE, editors. Methods in Microbiology Volume 37. London: Academic Press, Elsevier; 2011. [Google Scholar]
- 42.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 43.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 44.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 45.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 46.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okrent DG, Lichtenstein AK, Ganz T. Direct cytotoxicity of polymorphonuclear leukocyte granule proteins to human lung-derived cells and endothelial cells. Am Rev Respir Dis. 1990;141:179–185. doi: 10.1164/ajrccm/141.1.179. [DOI] [PubMed] [Google Scholar]
- 49.Pereira LF, Marco FM, Boimorto R, Caturla A, Bustos A, De la Concha EG, Subiza JL. Histones interact with anionic phospholipids with high avidity; its relevance for the binding of histone-antihistone immune complexes. Clin Exp Immunol. 1994;97:175–180. doi: 10.1111/j.1365-2249.1994.tb06064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleine TJ, Gladfelter A, Lewis PN, Lewis SA. Histone-induced damage of a mammalian epithelium: the conductive effect. Am J Physiol. 1995;268:C1114–C1125. doi: 10.1152/ajpcell.1995.268.5.C1114. [DOI] [PubMed] [Google Scholar]
- 51.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sporn LA, Marder VJ, Wagner DD. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986;46:185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- 53.Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson K, Gooderham NJ, Davies DS, Edwards RJ. Nucleosomes bind to cell surface proteoglycans. J Biol Chem. 1999;274:21707–21713. doi: 10.1074/jbc.274.31.21707. [DOI] [PubMed] [Google Scholar]
- 56.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clejan L, Menahem H. Binding of deoxyribonucleic acid to the surface of human platelets. Acta Haematol. 1977;58:84–88. doi: 10.1159/000207812. [DOI] [PubMed] [Google Scholar]
- 58.Dorsch CA. Binding of single-strand DNA to human platelets. Thromb Res. 1981;24:119–129. doi: 10.1016/0049-3848(81)90037-2. [DOI] [PubMed] [Google Scholar]
- 59.Frampton G, Perl S, Bennett A, Cameron JS. Platelet-associated DNA and anti- DNA antibody in systemic lupus erythematosus with nephritis. Clin Exp Immunol. 1986;63:621–628. [PMC free article] [PubMed] [Google Scholar]
- 60.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zoller B, Li X, Sundquist J, Sundquist K. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012;379:244–249. doi: 10.1016/S0140-6736(11)61306-8. [DOI] [PubMed] [Google Scholar]
- 62.Pande H, Calaycay J, Hawke D, Ben-Avram CM, Shively JE. Primary structure of a glycosylated DNA-binding domain in human plasma fibronectin. J Biol Chem. 1985;260:2301–2306. [PubMed] [Google Scholar]
- 63.Gonias SL, Pasqua JJ, Greenberg C, Pizzo SV. Precipitation of fibrinogen, fibrinogen degradation products and fibrin monomer by histone H3. Thromb Res. 1985;39:97–116. doi: 10.1016/0049-3848(85)90125-2. [DOI] [PubMed] [Google Scholar]
- 64.Ward CM, Tetaz TJ, Andrews RK, Berndt MC. Binding of the von Willebrand factor A1 domain to histone. Thromb Res. 1997;86:469–477. doi: 10.1016/s0049-3848(97)00096-0. [DOI] [PubMed] [Google Scholar]
- 65.Renesto P, Chignard M. Enhancement of cathepsin G-induced platelet activation by leukocyte elastase: consequence for the neutrophil-mediated platelet activation. Blood. 1993;82:139–144. [PubMed] [Google Scholar]
- 66.Si-Tahar M, Pidard D, Balloy V, Moniatte M, Kieffer N, Van Dorsselaer A, Chignard M. Human neutrophil elastase proteolytically activates the platelet integrin alphaIIbbeta3 through cleavage of the carboxyl terminus of the alphaIIb subunit heavy chain. Involvement in the potentiation of platelet aggregation. J Biol Chem. 1997;272:11636–11647. doi: 10.1074/jbc.272.17.11636. [DOI] [PubMed] [Google Scholar]
- 67.Andrews DA, Low PS. Role of red blood cells in thrombosis. Curr Opin Hematol. 1999;6:76–82. doi: 10.1097/00062752-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Goel MS, Diamond SL. Adhesion of normal erythrocytes at depressed venous shear rates to activated neutrophils, activated platelets, and fibrin polymerized from plasma. Blood. 2002;100:3797–3803. doi: 10.1182/blood-2002-03-0712. [DOI] [PubMed] [Google Scholar]
- 69.Laktionov PP, Tamkovich SN, Rykova EY, Bryzgunova OE, Starikov AV, Kuznetsova NP, Vlassov VV. Cell-surface-bound nucleic acids: Free and cell-surface-bound nucleic acids in blood of healthy donors and breast cancer patients. Ann N Y Acad Sci. 2004;1022:221–227. doi: 10.1196/annals.1318.034. [DOI] [PubMed] [Google Scholar]
- 70.Higuchi DA, Wun TC, Likert KM, Broze GJ., Jr The effect of leukocyte elastase on tissue factor pathway inhibitor. Blood. 1992;79:1712–1719. [PubMed] [Google Scholar]
- 71.Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, Song Y, Tzima E, Kennerknecht E, Niepmann M, von Bruehl ML, Sedding D, Massberg S, Gunther A, Engelmann B, Preissner KT. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9:1795–1803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 74.Napirei M, Wulf S, Mannherz HG. Chromatin breakdown during necrosis by serum Dnase1 and the plasminogen system. Arthritis Rheum. 2004;50:1873–1883. doi: 10.1002/art.20267. [DOI] [PubMed] [Google Scholar]
- 75.Odaka C, Mizuochi T. Role of macrophage lysosomal enzymes in the degradation of nucleosomes of apoptotic cells. J Immunol. 1999;163:5346–5352. [PubMed] [Google Scholar]
- 76.Meier TR, Myers DD, Jr, Wrobleski SK, Zajkowski PJ, Hawley AE, Bedard PW, Ballard NE, Londy FJ, Kaila N, Vlasuk GP, Schaub RG, Wakefield TW. Prophylactic P-selectin inhibition with PSI-421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost. 2008;99:343–351. doi: 10.1160/TH07-10-0608. [DOI] [PubMed] [Google Scholar]
- 77.Plow EF. The major fibrinolytic proteases of human leukocytes. Biochim Biophys Acta. 1980;630:47–56. doi: 10.1016/0304-4165(80)90136-1. [DOI] [PubMed] [Google Scholar]
- 78.Das R, Burke T, Plow EF. Histone H2B as a functionally important plasminogen receptor on macrophages. Blood. 2007;110:3763–3772. doi: 10.1182/blood-2007-03-079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haselmayer P, Grosse-Hovest L, von Landenberg P, Schild H, Radsak MP. TREM-1 ligand expression on platelets enhances neutrophil activation. Blood. 2007;110:1029–1035. doi: 10.1182/blood-2007-01-069195. [DOI] [PubMed] [Google Scholar]
- 80.Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–1760. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 81.Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, Serre G. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001;166:4177–4184. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]