Abstract
NKT cell activation with CD1d-binding glycolipid α-galactosylceramide (α-GC) enhances antibody responses to co-administered T-dependent antigen. The efficacy of α-GC relative to other CD1d-binding glycolipids and adjuvants is not known. There is little information on how NKT cells affect antibody production beyond initial booster-stimulated recall responses. We therefore tested the hypothesis that α-GC stimulates induction of plasma cells and antibody responses as effectively as Th1- and Th2-skewing variants of α-GC and several other adjuvants. C57BL/6 and CD1d−/− mice were immunized with nitrophenol-conjugated keyhole limpet hemocyanin (NP-KLH) plus α-GC or NP-KLH plus adjuvants before administration of an NP-KLH booster and assessing antibody responses and plasma cell frequency. α-GC boosted long-term antibody responses as efficiently as all other agents tested and induced plasma cells that were detected in bone marrow 13 weeks after immunization. We then determined whether NKT cells were required in the presence of other adjuvants. CD1d−/− mice had a reduced induction of plasma cells in response to NP-KLH/Alum as compared to C57BL/6 mice. However, NKT cells were not required for the continued presence of those cells that were induced. Although NKT cells are capable of inducing persistent plasma cell responses, they may not play a major role in supporting longevity post-induction.
Keywords: Antibodies, B cells, NKT cells, Transgenic/knockout mice
Introduction
CD1d is a non-polymorphic MHC class I-related protein expressed on APC that activates CD1d-restricted NKT cells [1, 2]. Type I invariant NKT cells are activated by APC presenting glycolipid molecules including α-galactosylceramide (α-GC) and bacterial glycolipids bound to CD1d. α-GC-reactive murine NKT cells have a tightly restricted TCR repertoire expressing the Vα14/Jα281−/− arranged TCR gene [3]. NKT cells can trigger both Th1 and Th2 responses in vivo [4–6].
CD1d-dependent activation of NKT cells induced by immunization with CD1d-binding glycolipids is considered as a viable approach for boosting cell-mediated immune responses. This approach has such promise that the CD1d-binding glycolipid α-GC has rapidly progressed to successful Phase I clinical trials in cancer patients [7, 8].
A recently appreciated aspect of NKT function is their potential to regulate, enhance and perhaps sustain humoral immune responses [9–14]. For example, human peripheral blood NKT cells stimulated Ab production by autologous B cells in response to α-GC [10]. Contact allergen-induced IgM production by murine B-1 B cells was CD1d/NKT dependent [9]. Production of allergen-specific IgE was deficient in Jα281−/− mice and CD1d blocking Ab reduced IgE production in an experimental asthma model [15]. Production of Ab reactive to GPI-anchored circumsporozoite proteins from Plasmodium falciparum was abrogated in CD1d−/− [13]. NKT cells may be important for Ab-mediated protection against pathogenic bacteria and viruses. CD1d−/− mice infected with the spirochete Borrelia hermsii had impaired production of specific Ab and had a higher pathogen burden than CD1d-expressing controls [16, 17]. Production of Ab reactive with polysaccharide Ag from Streptococcus pneumoniae is also CD1d-dependent [18]. α-GC was shown to have an adjuvant effect following intranasal co-administration with influenza HA Ag [19]. We demonstrated that α-GC co-administered s.c. with a T-dependent Ag could induce greater primary Ab responses against the Ag in a CD1d/NKT-dependent manner [20]. These findings are of particular importance when one considers that the most successful vaccines in use in the clinic today stimulate protective and long-lived Ab responses.
The mechanisms by which NKT cells impact humoral immunity are not well understood. Galli and colleagues showed NKT cells were required for sustained serum Ab titers using NKT-deficient Jα281−/− mice [21], but did not assess the persistence of plasma cells (PC) over time. The longevity of bone marrow PC is a major determinant of sustained humoral immunity [22]. Furthermore, the ability of α-GC to stimulate persistent PC responses and compared to other CD1d-binding glycolipids and a range of individual TLR agonists has not been reported.
In the present study, we report that NKT activation with α-GC enhances primary and recall Ab responses as effectively as Alum and several TLR agonists. We also shed light on the mechanisms by which NKT cells enhance and sustain humoral immunity. We show that NKT cells are required for optimal induction of persistent Ag-specific PC responses that are detectable in bone marrow. However, NKT cells do not appear to directly support PC maintenance. We also show that α-GC enhances the induction of persistent PC responses as effectively as Alum and TLR agonists.
Results
NKT activation enhances Ab recall responses in a CD1d/NKT-dependent manner
We reported that NKT activation enhanced primary Ab responses in an Ag-specific and CD1d/NKT-dependent manner [20]. We extended this finding by comparing the adjuvant effects of α-GC to Alum on the anti-nitrophenol-conjugated keyhole limpet hemocyanin (NP-KLH) response (Fig. 1a). α-GC enhanced the primary Ab titer to a lesser, but not statistically significant degree than Alum. When mice were boosted with NP-KLH alone 28 days after an initial immunization, the α-GC-enhanced Ab recall response was 50–60-fold higher on day 33 than before the boost and similar in magnitude to that induced by Alum (Fig. 1b). Furthermore, the enhanced recall response was CD1d/NKT dependent, as evidenced by the lack of a recall response on NP-KLH/α-GC-immunized CD1d−/− mice. Comparison of titers from NP-KLH-immunized C57BL/6 and CD1d−/− mice showed that CD1d expression and/or NKT cells were required for an Ab recall response in the absence of α-GC immunization. However, Alum was able to induce a similar recall Ab titer in C57BL/6 and CD1d−/− mice. We confirmed that the α-GC-enhanced Ab responses were Ag specific, by showing that the anti-NP-KLH sera did not elicit reactivity against OVA or HEL (data not shown). To ensure that differences in Ab titer were not attributable to subtle differences in kinetics of response, we compared titers at several time points during the course of a typical experiment (Fig. 1c). The kinetics of response induced by NP-KLH and NP-KLH plus α-GC were similar, but the magnitude of response was different at every time point. Ag-specific memory B cells are induced in mice as early as 15 days after a primary immunization [23], suggesting that our booster administered on day 28 triggers a genuine recall response. To further confirm that NKT activation on day 0 established such a recall response, we administered booster immunizations at later time points after the primary immunization (Fig. 1d). NP-KLH plus α-GC stimulated a memory response resulting in rapid Ab recall titers 56 days after the initial immunization. This shows that NKT activation via CD1d/α-GC complexes enhances specific Ab recall responses.
Figure 1.
NKT activation enhances primary and recall Ab responses. (a) C57BL/6 and CD1d−/− mice were untreated (naive) or immunized s.c. with 10 µg NP-KLH, NP-KLH adsorbed to Alum or NP-KLH plus 4 µg α-GC. Mice were bled on day 14. (b) Mice were boosted on day 28 with 10 µg NP-KLH and bled on day 33. (c) In a separate experiment, C57BL/6 mice were immunized with NP-KLH or NP-KLH plus α-GC and blood samples drawn at times indicated (left panel). Mice then received a booster (NP-KLH) and blood samples were drawn at times indicated (right panel). (d) C57BL/6 mice were immunized with NP-KLH or NP-KLH plus α-GC and bled on days indicated before receiving an NP-KLH booster on day 56. Blood samples were collected on days 61 and 66 and anti-NP IgG1 titers assessed by ELISA. For (a–d) ELISA were performed to determine the endpoint anti-NP IgG1 Ab titer. Data in (a–d) show the geometric mean titers ±95% confidence intervals for five mice per group. Significantly different titers from 'NP-KLH only' controls are shown in asterisks. (e) Ratios for Ab binding to NP-(3)-BSA versus NP-(30)-BSA-coated plates were assessed. Data show mean±SD ratio for five mice per group. Data are representative of two independent experiments. Significantly different titers from NP-KLH only controls are shown by asterisks.
We then tested the effect of NKT activation on Ab affinity by comparing the ratio of specific Ab binding to ELISA plates coated with BSA with a low versus a high degree of NP substitution to selectively capture high affinity versus total Ab. We observed a greater proportion of high-affinity specific Ab in serum from NP-KLH/Alum or NP-KLH/α-GC-immunized mice than from mice immunized with NP-KLH alone (Fig. 1e). In the CD1d−/− mice, there was a lower proportion of high-affinity Ab in mice immunized with NP-KLH alone than in the C57BL/6 mice, and they were responsive to Alum but not α-GC. This suggests that NKT cells play a role in Ab affinity maturation.
The data presented here focus on IgG1 titers, the predominant isotype produced in response to NP-KLH in combination with Alum or α-GC. IgG2b and IgG2c titers were improved by α-GC, but the adjuvant effect was considerably less than that for IgG1 (data not shown). Whether this is specific to the anti-NP-KLH response, or would differ for other Ag is presently unknown.
In our experiments, α-GC was administered by the s.c. route. Higher doses (2–5 µg), administered i.p. are deleterious to the NKT population [24–26]. The 4 µg α-GC dose administered s.c. in our experiments did not decrease the frequency of splenic or lymph node NKT cells detected with CD1d tetramers and led to a modest enhancement in NKT numbers with our s.c. immunization method (Fig. 2a–c). The ability of NKT cells to release IL-4 and IFN-γ following in vitro re-stimulation with α-GC was not compromised by prior s.c. immunization (Fig. 2d).
Figure 2.
Dose-dependent effects of α-GC in vivo. (a) C57BL/6 mice were immunized s.c. with PBS (naive) or α-GC. After the time indicated, spleens were harvested and analyzed for CD1d tetramer/TCRβ-positive cells, indicated by the box R2. (b) Graph shows effects of dosage and time on the splenic NKT population. Similar results were observed for lymph nodes (not shown) and are representative of at least three independent experiments. (c) Spleen and lymph node were harvested 16 h after immunization and NKT cells enumerated. (d) Splenocytes were cultured with or without α-GC at a 100 ng/mL final concentration. After 20 h, supernatants were collected and concentrations of IL-4 and IFN-γ measured by Bio-Plex assay.
Th1-skewing α-GC molecules enhance short-term Ab recall responses
We compared the effect of α-GC to that of structural variants of the α-GC molecule (Fig. 3). The OCH variant of the α-GC molecule has a truncated sphingosine side chain and induces a predominant Th2 response in vivo [27–29], while the C-glycoside variant has an altered galactose headgroup and induces a Th1 response in vivo [30]. When mice were immunized with NP-KLH mixed with either of these molecules, the adjuvant effect on the primary and recall Ab response was largest with α-GC and the C-glycoside molecule, but moderate with the OCH molecule (Fig. 3). These data suggest that the NKT-derived Th1 cytokines play a significant role in driving NKT-enhanced short-term recall responses. Notably, the C-glycoside or OCH molecules did not stimulate substantial IgG2b or IgG2c responses (data not shown).
Figure 3.
Th1-skewing α-GC enhances short-term Ab recall responses. (a) C57BL/6 mice were bled (naive), then immunized s.c. with 10 µg NP-KLH alone, adsorbed to Alum or mixed with the α-GC molecules indicated. Dosages are detailed in the Materials and methods. Mice were bled on day 14 and ELISA performed to determine the endpoint anti-NP IgG1 Ab titer. (b) Mice were boosted on day 28 with 10 µg NP-KLH and bled on day 33. Data show the geometric mean titer ±95% confidence intervals for five mice per group [with exception of Alum and α-GC in (a) where n=3 and 4, respectively]. Asterisks indicate titers significantly different from NP-KLH only control.
Lack of co-operation between α-GC and TLR ligands for humoral responses
NKT cells are activated via CD1d-dependent and TLR-dependent mechanisms and these mechanisms can co-operate to enhance NKT-driven immune responses [31–33]. We tested several combinations of α-GC with the TLR agonists described here, using the dosages described in methods. At these concentrations there was no synergy between α-GC and the TLR agonists nor was any additive effect observed indicating maximal stimulation via either pathway in vivo (data not shown).
We therefore modified the immunization regimen administering lower doses or each agent, using 8-fold less α-GC and TLR agonist (Fig. 4). We observed that either agent alone had a potent adjuvant effect on recall Ab responses and that the combination of α-GC and TLR3 or TLR7 agonist resulted in a small but non-significant inhibition of Ab production. Interestingly, 0.5 µg α-GC had a minor effect on primary Ab titers but a substantial effect on recall. These results show that α-GC and TLR agonists do not act co-operatively to enhance Ab responses.
Figure 4.
Lack of co-operation between α-GC and TLR ligands in Ab responses. (a) C57BL/6mice were bled (naive), then immunized s.c. with 10 µg NP-KLH alone, or mixed with 0.5 µg α-GC, 6.25 µg poly I:C, 6.25 µg Imiquimod, or a combination of α-GC and TLR agonist. Mice were bled at day 14 and ELISA were performed to determine the endpoint anti-NP IgG1 Ab titer. (b) Mice were boosted on day 28 with 10 µg NP-KLH, and then bled on day 33. Data in (a) and (b) show the geometric mean titer ±95% confidence intervals for five mice per group (n=3 for naive group). Asterisks indicate titers significantly different from NP-KLH only control.
NKT cells are required for induction of PC but not persistent responses
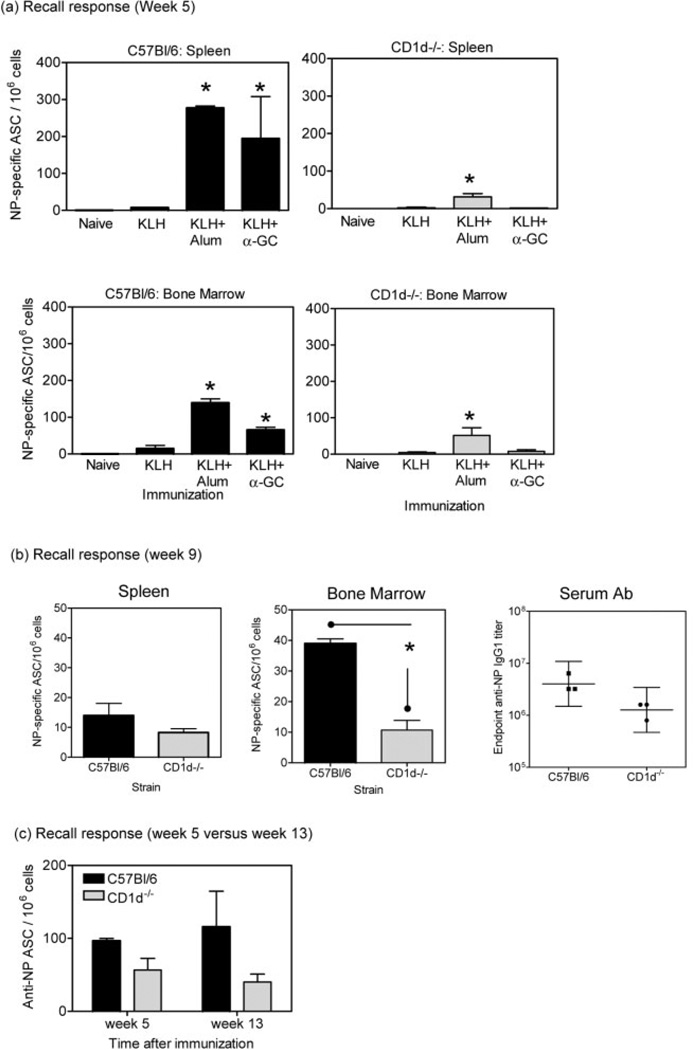
Serum Ab titers are maintained by PC, which can reside in secondary lymphoid organs but are largely found in the bone marrow. To determine if the lower recall Ab titers observed in CD1d−/− mice were due to a reduced number of PC being generated and/or maintained, we performed ELISPOT analyses after secondary Ag boost to enumerate splenic and bone marrow PC specific for the NP hapten (Fig. 5). Adsorption of Ag to Alum enhanced the frequency of PC in C57BL/6 mice as expected and, consistent with the serum recall Ab titers, there was an enhancement that resulted from mixing the Ag with α-GC (Fig. 5a). Similar to the Ab titers, Alum resulted in a moderately higher frequency than α-GC in spleen and lymph node at 1 week post immunization (1 week after the secondary Ag boost). In contrast, CD1d−/− mice displayed a tenfold lower frequency of PC in spleen and threefold lower frequency in bone marrow in response to Alum adsorption of the Ag. Consistent with Ab titers, α-GC did not induce a measurable frequency of NP-specific PC in CD1d−/− mice. As expected, the frequency of cells detected by the ELISPOT assay was very low prior to the secondary Ag boost and consistent with primary Ab titers (data not shown).
Figure 5.
NKT cells are required for induction of PC (antibody secreting cells, ASC) but not persistent responses. C57BL/6 and CD1d−/− mice were immunized as indicated on day 0, and then boosted with NP-KLH on week 4 (day 28). On week 5 (day 35) (a) and week 9 (day 63) (b), mice were killed and splenocytes and bone marrow cells assessed by ELISPOT assay. Data show mean ± SD frequency of NP-specific PC for three mice per group, each sample being performed in triplicate. Data are representative of three separate experiments. In (b), the endpoint anti-NP IgG1 Ab titer is also shown. Asterisks indicate titers significantly different from NP-KLH only control in (a) or between C57BL/6 and CD1d−/− mice in (b). In (c), C57BL/6 and CD1d−/− mice (five per group) were immunized as indicated on day 0, and then boosted with NP-KLH on week 4 (day 28), and bone marrow PC were enumerated on week 5 (day 35) and week 13 (day 91).
At 9 weeks after immunization with NP-KLH adsorbed to Alum (5 weeks after secondary NP-KLH boost), C57BL/6 and CD1d−/− mice were compared by ELISPOT (Fig. 5b). Although the frequency of PC had partially diminished compared to the 5-week time point, the frequency was considerably lower in CD1d−/− mice than in C57BL/6 mice. Serum Ab titers were consistent with the PC frequencies (Fig. 5b).
To determine if NKT cells were required for persistence of the PC response, we performed a further experiment where we compared PC frequency at 5 weeks and 13 weeks in C57BL/6 and CD1d−/− mice (Fig. 5c). Although the difference in induction between C57BL/6 and CD1d−/− mice was less dramatic than in the prior experiment, a higher number of cells were induced in the C57BL/6 mice. The number of PC then increased slightly in C57BL/6 mice and diminished slightly over the next 8 weeks in CD1d−/− mice. These changes were not statistically significant. The data are therefore consistent with NKT cells being required for PC induction, but not playing a major role in supporting their maintenance.
Activation of NKT cells leads to persistent PC responses
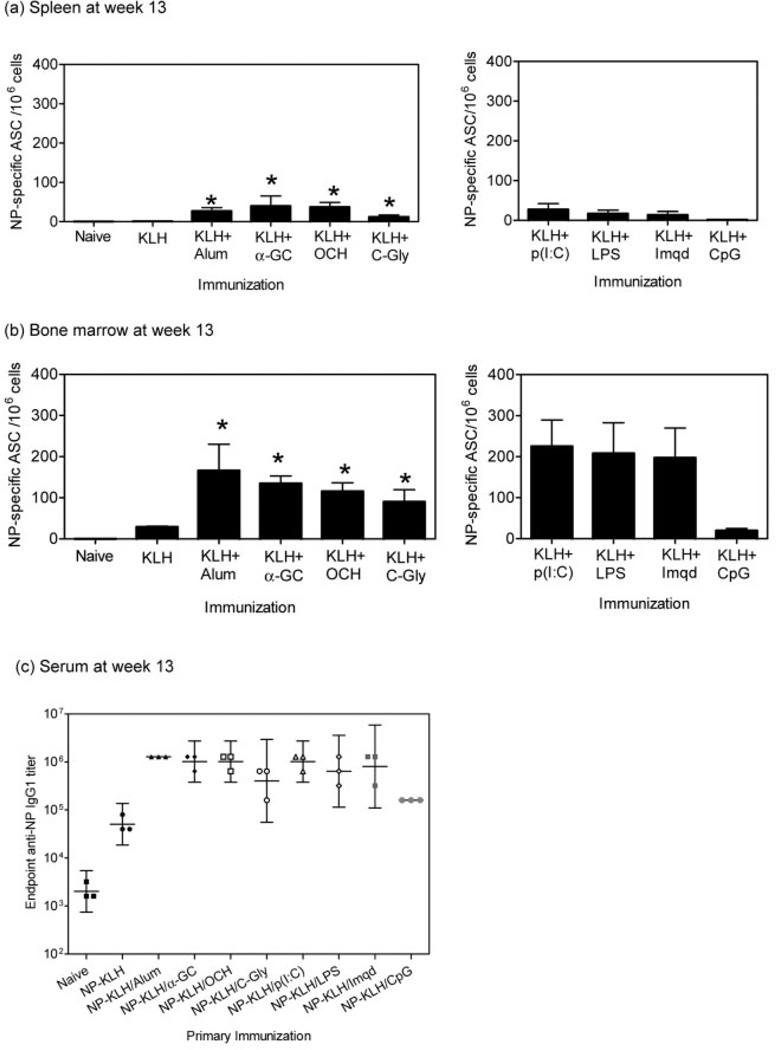
Certain adjuvants improve maintenance of the humoral immune response to T-dependent Ag, and this is evident by the persistence of high frequencies of immunization-induced Ab-secreting PC in secondary lymphoid organs and bone marrow [34]. We therefore enumerated Ab-secreting PC in bone marrow and spleens of mice immunized with NP-KLH alone or in combination with α-GC, the OCH and C-glycoside variants and several TLR agonists. The mice were then boosted with NP-KLH on day 28 and tissues harvested for ELISPOT at later times (Fig. 6). At 13 weeks following the primary immunization (9 weeks after secondary Ag boost), spleens from mice immunized with NP-KLH plus α-GC contained as many Ag-specific PC as those mice immunized with NP-KLH plus TLR3 agonist poly I:C (Fig. 6a). Furthermore, there was a higher frequency in the α-GC-treated mice than OCH-, C-Glycoside, TLR4-, TLR7- and TLR9-agonist-treated mice. These data show that α-GC leads to persistent splenic PC responses that are comparable to that of major TLR agonists.
Figure 6.
NKT activation leads to persistent PC (antibody secreting cells, ASC) responses. C57BL/6 mice were immunized as indicated on day 0 and then boosted with NP-KLH on week 4 (day 28). On week 13 (day 91), mice were killed and splenocytes (a) and bone marrow cells (b) assessed by ELISPOT assay. Data in (a) and (b) show mean ± SD frequency of NP-specific PC for three mice per group, each sample being performed in triplicate. (c) Sera were obtained 13 weeks post immunization and anti-NP IgG1 endpoint titers measured by ELISA. Data show geometric mean titers ±95% confidence intervals for three mice per group.
Bone marrow cells from the same mice were also analyzed by ELISPOT. We observed a higher frequency of Ag-specific PC in mice immunized with NP-KLH plus α-GC than the C-glycoside molecule and a similar frequency to the OCH molecule (Fig. 6b). This is in contrast to the weaker responses to OCH observed at 5 weeks. Mice immunized with TLR3, TLR4 and TLR7 agonists had a moderately higher frequency of Ag-specific PC than from the NP-KLH/α-GC-immunized mice, whereas the mice immunized with the TLR9 agonist had considerably fewer PC. The Ab titers at these times revealed that α-GC sustains Ab titers as efficiently as the other adjuvants tested. These data show that NKT cell activation with α-GC leads to the generation of a persistent PC response in the spleen and bone marrow, which is consistent with serum Ab titers (Fig. 6c).
Discussion
We have demonstrated that α-GC boosts humoral immune responses in a manner that rivals that of Alum and the major TLR agonists. Notably, Alum is the only adjuvant currently approved for use in the clinic, and TLR agonists, LPS in particular, have limited application because of toxicity [35]. Our data show that α-GC is at least as effective as these other agents in stimulating a rapid and sustained Ab recall response following secondary boost with Ag.
Examination of Ab titers in C57BL/6 and CD1d−/− mice before and after administration of a booster vaccine revealed that NKT cell activation enhances not only primary, but also recall Ab responses and in a CD1d/NKT-dependent manner (Fig. 1). Interestingly, the kinetics of response appeared similar between both strains, suggesting that, although NKT cells are innate immune effectors, they do not lead to more rapid humoral immune responses. However, in CD1d−/− mice, the production of high-affinity Ab (in the primary bleed sera) was impaired but affinities were similar in recall sera (data not shown). This indicates that the kinetics of Ab affinity maturation rather than production is regulated by NKT cells. Since affinity maturation takes place in the germinal center [23], NKT cells could contribute to regulation of the germinal center reaction. Further investigation in this area is warranted.
The Th1- and Th2-skewing α-GC molecules OCH and C-Gly had contrasting effects on recall responses with C-Gly stimulating the best early response (5 weeks) (Fig. 3) and OCH stimulating the better late response (13 weeks) (Fig. 6). While this may seem counterintuitive since the Th2 cytokine IL-4 leads to IgG1 production, the NKT-enhanced Ab response is occurring in the context of numerous cytokines from other cellular sources. We cannot yet assign individual NKT-derived cytokines to the NKT-enhanced Ab response. However, it appears that Th1 cytokines may be of greater importance than Th2 cytokines, and the manner in which they shape recall responses over time is likely to be complicated.
Surprisingly, we did not observe additive or synergistic effects on the Ab response when combining α-GC and TLR agonists in the vaccination regimen (Fig. 4). Cerundolo and colleagues [32] reported co-operation between CD1d- and TLR-dependent mechanisms for DC maturation, CTL induction and Ab responses. Although they observed a modest effect of α-GC and LPS on anti-OVA Ab titers, we did not observe any co-operation between α-GC and TLR3, TLR4 or TLR7 agonists. Given that TLR stimulation of DC can induce NKT cells to produce IFN-γ in an IL-12-dependent manner and stimulate immune responses [31], the α-GC/TLR co-operation may be biased towards DC-dependent CTL responses and perhaps this is separable from humoral immune responses. These observations could be important for tailoring different types of immune response such as Ab for infectious disease versus CTL activation for tumor therapy.
Low doses of α-GC did not enhance primary Ab responses (Fig. 4), but did induce good recall responses. It is possible that CD1d presentation of α-GC acts as a 'dial' to fine tune the balance between induction of memory B cell responses and production of Ab-secreting PC. The Noelle laboratory reported that the TLR9 agonist CpG DNA (also used in this study) promoted efficient development of memory B cells with relatively weak induction of PC [36]. Consistent with those experiments, we show that α-GC stimulates a stronger PC response than the TLR9 agonist. Therefore, NKT cells and TLR-dependent responses may differentially affect the development and maintenance of PC. Further investigation is desirable to determine if vaccines containing α-GC can be designed to be efficacious in the event of a pathogenic insult, but without inducing a strong primary response following immunization.
Our results show that NKT activation leads to the generation of higher numbers of PC (Fig. 5). The frequency of NP-specific cells detected at that time by ELISPOT, was considerably lower in CD1d−/− mice than C57BL/6 mice, regardless of Alum adsorption, which is in contrast to the serum Ab titers. Serum Ab reflects an accumulation of Ab, whereas ELISPOT analysis captures a single point in time, and this could explain the discrepancy. Although initial Ab recall titers in CD1d−/− mice appear to be intact, NKT cells were required for the continued Ab production. Interestingly, serum Ab titers are enhanced in Jα281−/− mice and the lack of NKT cells promotes Ab-mediated autoimmunity in aged mice [37, 38]. One might therefore contend that NKT cells are not required for development of an Ab repertoire, although they clearly enhance new Ab responses following vaccination.
NKT cells appear to be required for optimal generation of PC, but do not appear to directly support survival since there is no significant change in frequency of bone marrow PC in C57BL/6 and CD1d−/− mice after induction. However, this does not preclude NKT-mediated effects giving newly induced PC an ability to compete for a survival niche in an otherwise highly occupied bone marrow.
A remaining question is whether the observed persistence of the PC response is due to continued generation of short-lived PC with a rapid turnover or generation of non-dividing true long-lived PC. In future, the BrdU PC labeling strategy, such as that developed by Manz and colleagues [39], will be required to distinguish between PC populations induced by NKT cells.
Since NKT cells can facilitate optimal humoral immunity in the absence as well as the presence of exogenous α-GC, presentation of a self CD1d-binding glycolipid to NKT cells could signal NKT cells to direct humoral immune responses. Although self CD1d Ag have been identified [40], as far as we are aware there is no information on how they may direct humoral immune responses. Alternatively, the mechanism could be CD1d independent and rely on NKT 'steady state' production of factors that drive B cell differentiation into a PC with a high survival capacity. No factor derived from NKT cells that meets this description has yet been identified.
The study described here raises new questions regarding the mechanisms by which activated NKT cells affect the development and lifespan of PC. Presumably NKT cells either directly or indirectly induce the expression of B cell development and survival factors. If survival factors expressed by NKT cells have effects on mature, Ag-experienced B cells, they could stimulate additional PC development as well as prolong their longevity. Answering these questions represents a major and current focus of our laboratory.
Materials and methods
Reagents
NP-KLH was purchased from Biosearch Technologies Inc. (Novato, CA). α-GC was purchased from Axorra Inc. (San Diego, CA). We have previously described the purity, structural integrity and functionality of α-GC [41]. The α-GC structural variants OCH and C-glycoside were obtained from the NIAID tetramer facility (Emory University, Atlanta, GA). A full description of these molecules is reported in [27–30]. TLR agonists [poly I:C, LPS, Imiquimod, and CpG DNA (ODN1826 5′-TCC ATG ACG TTC CTG ACG TT-3′)] were purchased from Invivogen (San Diego, CA). Imject™ Alum adjuvant was purchased from Pierce Biotechnology Inc. (Bedford, IL). HRP-conjugated anti-IgG1, IgG2b and IgG2c were purchased from Southern Biotechnology (Birmingham, AL). Lack of endotoxin contamination of vaccine components was confirmed using a Limulus kit (Cambrex, East Rutherford, NJ).
Mice
C57BL/6 mice were purchased from the National Cancer Institute (Bethesda, MD). CD1d−/− mice on the C57BL/6 genetic background have been described previously [42] and were bred in the Animal Resource Center at the University of Oklahoma Health Sciences Center (OUHSC). CD1d−/− mice were kindly provided by Dr. Mark Exley (Harvard Medical School). All procedures were approved by the Institutional Animal Care and Use Committee at OUHSC.
Immunizations and experimental schedule
Female mice of 6–10 weeks of age were used and five mice per group were immunized unless indicated otherwise. A single s.c. immunization was administered over both flanks on day 0 immediately following collection of pre-bleed sera. Immunizations consisted of 10 µg NP-(19)-KLH in 200 µL sterile endotoxin-free PBS, NP-KLH adsorbed to Alum, or NP-KLH mixed with 4 µg α-GC, 4 µg OCH, 4 µg C-glycoside, 50 µg poly I:C, 5 µg LPS, 100 µg Imiquimod or 100 µg CpG DNA. Ag alone is not generally sufficient to induce optimal Ab responses [43], but limited Ab titers are produced in the absence of adjuvant [44]. This makes the NP-KLH alone condition suitable as a baseline control to assess the adjuvant-like effects of α-GC. Mice were then bled at day 14 post immunization and sera obtained. On day 28 mice were boosted s.c. with 10 µg NP-KLH in PBS and bleed on day 33. On days 35, 63 and 91 mice were killed to obtain bone marrow and spleen.
Retro-orbital eye bleed and serum collection
Mice were anesthetized using a vaporized 4% isofluorane/96% oxygen mixture and 100 µL blood collected by retro-orbital bleed with heparinized microcapillary tubes (Fisher, Hampton, NH). Samples were transferred immediately to polypropylene micro-centrifuge tubes. Blood samples were incubated for 30 min at room temperature then allowed to clot overnight at 4°C, before centrifugation at 13 000 × g for 15 min at 4°C. Sera were withdrawn with a pipette and stored in aliquots at –20°C.
Isolation of splenocytes and bone marrow cells
Spleens were harvested into HBSS buffer and a single-cell suspension obtained by mechanical disruption. Bone marrow cells were flushed from the tibia and femur of mice with media using a 27-gauge needle and 1 mL syringe. Splenocytes and bone marrow cells were then re-suspended gently using a 1 mL pipette. Erythrocytes were removed by incubation with ammonium chloride lysis buffer (0.16 M NH4Cl, 0.17 M Tris-HCl, pH 7.4) for 2 min at 37°C. After washing in culture media, cell viability was confirmed as >98% by trypan blue exclusion. Cells were enumerated using a Beckman Coulter Z2 cell/particle counter (Fullerton, CA).
ELISA
Nunc MaxiSorp ELISA–plates (Nalge Nunc International, Rochester, NY), were coated with NP-(30)-BSA or NP-(3)-BSA at 10 µg/mL in binding buffer (0.1 M Na2HPO4, pH 9.0), overnight at 4°C before washing plates and blocking for 2 h at room temperature with 1.0% BSA in PBS/0.05% Tween 20/0.05 % NaN3. Sera were diluted 100- or 10 000-fold in PBS/0.05% Tween and subjected to 2-fold serial dilutions, before adding to NP-BSA-coated, pre-blocked plates. Plates were incubated overnight at 4°C with diluted sera, before washing four times in PBS/0.05% Tween 20. Plates were incubated for 1 h at room temperature with HRP-conjugated anti-mouse IgG1, IgG2b or IgG2c at a final concentration of 0.2 µg/mL. Plates were washed and developed for 5 min at room temperature using 90 µL ABTS substrate per well (KPL, Gaithersburg, MD). Reactions were stopped by addition of 110 µL 10% SDS. Plates were analyzed using a Dynex MRX Revelation plate reader. Endpoint titers were determined as absorbance (A) <0.01 at 405 nm (equivalent to A of 1:200 dilution of pre-bleed sera). Individual Ab titers were plotted as geometric mean ± 95% confidence intervals using GraphPad Prism software. Differences in Ab titer were assessed for statistical significance using a nonparametric two-tailed Mann-Whitney test.
Cytokine assay
Single-Plex mouse IL-4 and IFN-γ kits were purchased from Bio-Rad (Hercules, CA) and used according to manufacturers instructions in conjunction with Bio-Plex instrumentation (Bio-Rad). IL-4 and IFN-γ concentrations in culture supernatants were measured in duplicate and determined by interpolation from standard curves using the Bio-Plex software.
ELISPOT
Multiscreen HTS 96-well ELISPOT plates (Millipore, Bedford, MA) were pre-wet with 15 µL/well 35% ethanol before washing twice with PBS. Plates were then coated with 100 µL/well NP-30-BSA (10 µg/mL in PBS) overnight at 4°C. Plates were washed twice in PBS before blocking with culture media (200 µL/well) for 2 h at room temperature. After blocking, media was discarded. Splenocytes in culture media were adjusted to a density of 1.2 × 107 cells/mL and 200 µL of cell suspension serially diluted threefold into wells of the ELISPOT plate. All samples were added in triplicate. Cells were incubated for 4.5 h at 37°C in a 5% CO2 incubator. Plates were washed three times with PBS containing 0.05% Tween 20. A solution containing 0.2 µg/mL HRP-goat anti-mouse IgG1 Ab (Southern Biotech, Birmingham, AL) and 5% FCS in PBS was then incubated with the plates overnight at 4°C before washing three times with PBS/0.05% Tween 20 and colorimetric development. The development solution [47.5 mL 0.0075 N acetic acid/0.0175 M sodium acetate/2.5 mL dimethylformamide containing one dissolved 3-amino-9-ethyl-carbazole (AEC) tablet (Sigma Chemical Co., St. Louis, MO) and 0.0005% H2O2] was added to each well (100 µL) and allowed to develop for 10 min before stopping the reaction by washing 12 times with dH20. Spots were enumerated with the aid of a dissecting microscope. A nonparametric two-tailed Mann-Whitney test was used to determine significance of differences between experimental groups.
Acknowledgements
This work was supported by American Lung Association Research Grant RG-21019-N to M.L.L and NIH Grant 5P20RR015564–07 from the National Center for Research Resources. We thank: Dr. Exley (Harvard Medical School, Boston, MA) for providing CD1d−/− mice; the NIAID tetramer facility (Emory University, Atlanta, GA) for supplying the OCH and C-glycoside molecules and CD1d tetramers; Dr. Ira Blader (University of Oklahoma Health Sciences Center, Oklahoma City, OK) for critical review of the manuscript.
Abbreviations
- α-GC
α-galactosylceramide
- KLH
keyhole limpet hemocyanin
- NP
nitrophenol
- PC
plasma cell
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Porcelli SA, Modlin RL. The CD1 system: Antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: What's in a name? Nat. Rev. Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: Facts, functions and fallacies. Immunol. Today. 2000;21:573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Hong S, Scherer DC, Serizawa I, Burdin N, Kronenberg M, Koezuka Y, Van Kaer L. Cutting edge: Activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 1999;163:2373–2377. [PubMed] [Google Scholar]
- 5.Burdin N, Brossay L, Kronenberg M. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J Immunol. 1999;29:2014–2025. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Cui J, Watanabe N, Kawano T, Yamashita M, Kamata T, Shimizu C, Kimura M, et al. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Valpha14 natural killer T cells. J. Exp. Med. 1999;190:783–792. doi: 10.1084/jem.190.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BM, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 8.Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, Iizasa T, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 9.Campos RA, Szczepanik M, Itakura A, Akahira-Azuma M, Sidobre S, Kronenberg M, Askenase PW. Cutaneous immunization rapidly activates liver invariant Valpha14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J. Exp. Med. 2003;198:1785–1796. doi: 10.1084/jem.20021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, Dellabona P, Abrignani S. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J. Exp. Med. 2003;197:1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamura H, Ohta A, Sekimoto M, Sato M, Iwakabe K, Nakui M, Yahata T, et al. alpha-Galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell. Immunol. 2000;199:37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 12.Procopio DO, Almeida IC, Torrecilhas AC, Cardoso JE, Teyton L, Travassos LR, Bendelac A, Gazzinelli RT. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins from Trypanosoma cruzi bind to CD1d but do not elicit dominant innate or adaptive immune responses via the CD1d/NKT cell pathway. J. Immunol. 2002;169:3926–3933. doi: 10.4049/jimmunol.169.7.3926. [DOI] [PubMed] [Google Scholar]
- 13.Schofield L, McConville MJ, Hansen D, Campbell AS, Fraser-Reid B, Grusby MJ, Tachado SD. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimoto T, Min B, Sugimoto T, Hayashi N, Ishikawa Y, Sasaki Y, Hata H, et al. Nonredundant roles for CD1d-restricted natural killer T cells and conventional CD4+ T cells in the induction of immunoglobulin E antibodies in response to interleukin 18 treatment of mice. J. Exp. Med. 2003;197:997–1005. doi: 10.1084/jem.20021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, Fourneau JM, et al. Cutting edge: Invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyper-reactivity in an experimental asthma model. J. Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 16.Belperron AA, Dailey CM, Bockenstedt LK. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 2005;174:5681–5686. doi: 10.4049/jimmunol.174.9.5681. [DOI] [PubMed] [Google Scholar]
- 17.Kumar H, Belperron A, Barthold SW, Bockenstedt LK. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J. Immunol. 2000;165:4797–4801. doi: 10.4049/jimmunol.165.9.4797. [DOI] [PubMed] [Google Scholar]
- 18.Kobrynski LJ, Sousa AO, Nahmias AJ, Lee FK. Cutting edge: Antibody production to pneumococcal polysaccharides requires CD1 molecules and CD8+ T cells. J. Immunol. 2005;174:1787–1790. doi: 10.4049/jimmunol.174.4.1787. [DOI] [PubMed] [Google Scholar]
- 19.Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. Alpha-galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J. Immunol. 2005;175:3309–3317. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 20.Lang GA, Exley MA, Lang ML. The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology. 2006;119:116–125. doi: 10.1111/j.1365-2567.2006.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc. Natl. Acad. Sci. USA. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 23.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu. Rev. Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 24.Ikarashi Y, Iizuka A, Koshidaka Y, Heike Y, Takaue Y, Yoshida M, Kronenberg M, Wakasugi H. Phenotypical and functional alterations during the expansion phase of invariant Valpha14 natural killer T (Valpha14i NKT) cells in mice primed with alpha-galactosylceramide. Immunology. 2005;116:30–37. doi: 10.1111/j.1365-2567.2005.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, Joyce S, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc. Natl. Acad. Sci. USA. 2003;100:10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJ, Hayakawa Y, Sidobre S, Keating R, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J. Immunol. 2003;171:4020–4027. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno M, Masumura M, Tomi C, Chiba A, Oki S, Yamamura T, Miyake S. Synthetic glycolipid OCH prevents insulitis and diabetes in NOD mice. J. Autoimmun. 2004;23:293–300. doi: 10.1016/j.jaut.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Forestier C, Takaki T, Molano A, Im JS, Baine I, Jerud ES, Illarionov P, et al. Improved outcomes in NOD mice treated with a novel Th2 cytokine-biasing NKT cell activator. J. Immunol. 2007;178:1415–1425. doi: 10.4049/jimmunol.178.3.1415. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 30.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-galactosylceramide. J. Exp. Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 32.Hermans IF, Silk JD, Gileadi U, Masri SH, Shepherd D, Farrand KJ, Salio M, Cerundolo V. Dendritic cell function can be modulated through cooperative actions of TLR ligands and invariant NKT cells. J. Immunol. 2007;178:2721–2729. doi: 10.4049/jimmunol.178.5.2721. [DOI] [PubMed] [Google Scholar]
- 33.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 34.Slifka MK, Ahmed R. Long-lived plasma cells: A mechanism for maintaining persistent antibody production. Curr. Opin. Immunol. 1998;10:252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 35.Thompson BS, Chilton PM, Ward JR, Evans JT, Mitchell TC. The low-toxicity versions of LPS, MPL adjuvant and RC529, are efficient adjuvants for CD4+ T cells. J. Leukoc. Biol. 2005;78:1273–1280. doi: 10.1189/jlb.0305172. [DOI] [PubMed] [Google Scholar]
- 36.Raman VS, Lind EF, Benson MJ, Noelle RJ. Strategies for selective priming of memory B cells. Immunol. Lett. 2007;109:93–100. doi: 10.1016/j.imlet.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sireci G, Russo D, Dieli F, Porcelli SA, Taniguchi M, La Manna MP, Di Liberto D, et al. Immunoregulatory role of Jalpha281 T cells in aged mice developing lupus-like nephritis. Eur. J. Immunol. 2007;37:425–433. doi: 10.1002/eji.200636695. [DOI] [PubMed] [Google Scholar]
- 38.Yang JQ, Singh AK, Wilson MT, Satoh M, Stanic AK, Park JJ, Hong S, et al. Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J. Immunol. 2003;171:2142–2153. doi: 10.4049/jimmunol.171.4.2142. [DOI] [PubMed] [Google Scholar]
- 39.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J. Exp. Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 41.Lang GA, Maltsev SD, Besra GS, Lang ML. Presentation of alpha-galactosylceramide by murine CD1d to natural killer T cells is facilitated by plasma membrane glycolipid rafts. Immunology. 2004;112:386–396. doi: 10.1111/j.1365-2567.2004.01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Exley MA, Bigley NJ, Cheng O, Shaulov A, Tahir SM, Carter QL, Garcia J, et al. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology. 2003;110:519–526. doi: 10.1111/j.1365-2567.2003.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 44.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]