Abstract
Trastuzumab has shown positive results in many patients with metastatic HER2-positive breast cancer, but it is less effective for controlling metastases in the CNS, which remains a site of relapse. The poor prognosis for patients with brain metastases is thought to be largely due to the presence of the blood-brain barrier (BBB) that prevents delivery of most drugs to the CNS and to the heterogeneous and limited permeability of the blood-tumor barrier (BTB). Focused ultrasound (FUS) bursts combined with circulating microbubbles can temporarily permeabilize both the BBB and the BTB. This technique has been investigated as a potential noninvasive method for targeted drug delivery in the brain. Here, we investigated whether BBB/BTB permeabilization in the tumor and surrounding brain tissue induced by FUS and microbubbles can slow tumor growth and improve survival in a breast cancer brain metastases model. HER2/neu-positive human breast cancer cells (BT474) were inoculated in the brains of 41 nude (nu/nu) rats. Animals in the treatment group received six weekly treatments of BTB/BBB permeabilization under MRI guidance combined with IV administration of trastuzumab (2 mg/kg). Tumor growth and survival rates were monitored via MRI for seven weeks after sonication. Starting at week seven and continuing through the end of the study, the mean tumor volume of the FUS+trastuzumab group was significantly (P<0.05) less than those of the three control groups (no treatment, FUS alone, trastuzumab alone). Furthermore, in four out of 10 rats treated with FUS+trastuzumab, the tumor appeared to be completely resolved in MRI, an outcome which was not observed in any of the 31 rats in three control groups. Trastuzumab improved median survival by 13% compared to the no treatment group, a difference which was significant (P=0.044). Treatment with FUS+trastuzumab produced the most significant benefit compared to the no-treatment controls (P=0.0084). More than half (6/10) animals survived at the study endpoint, leading to a median survival time greater than 83 days (at least 32% longer than the untreated control group). Overall, this work suggests that BBB/BTB permeabilization induced by FUS and microbubbles can improve outcomes in breast cancer brain metastases.
Keywords: Blood-brain barrier, targeted drug delivery, MRgFUS, breast cancer, trastuzumab, microbubbles
1. Introduction
Among patients with advanced metastatic breast cancer, 10–16% develop metastases in the central nervous system (CNS) [1,2] with a true rate that may be higher based on autopsy data [3]. Breast cancers that overexpress human epidermal growth factor receptor 2 (HER2, HER2/neu, ErbB2, or c-erbB2) have been found to metastasize to the brain at higher frequencies than those that do not [2,4,5]. The rate for CNS metastases in breast cancer patients has appeared to increase in recent years, a change thought to reflect advances in detection and improved survival rates resulting from better management of systemic disease [1,2,6].
The prognosis for patients with advanced breast cancer who have CNS metastases is generally poor, with reported one- and five-year survival rates of 20% and 1.3%, respectively [1,7]. The current standard of care for patients with CNS metastases is treatment with steroids and radiotherapy [8–11], with surgery or stereotactic radiosurgery an option that can improve survival for patients with limited tumor burden [11–14]. Chemotherapy has not generally been considered an effective option for patients with CNS metastases due to the presence of the blood-brain barrier (BBB), a physical and functional barrier that restricts the delivery of most substances from the vasculature and hence to the tumor(s).
While the blood vessels in most brain tumors, including metastases, do not have an intact BBB and are somewhat permeable, infiltrating cancer cells at the tumor margins and small metastatic seeds may be protected by the BBB of the surrounding normal and intact tissue [15]. Furthermore, it is known that tumor vasculature permeability is heterogeneous, and there are additional barriers to drug delivery such as increased interstitial pressures [16]. Indeed, work in mice suggests that the blood-tumor barrier (BTB) is only partially compromised in breast cancer brain metastases, and that toxic concentrations of chemotherapy agents are only achieved in a small subset of metastases that are highly permeable [17].
These barriers, along with the increased aggressiveness of tumors that is thought to exist for breast cancers that metastasize to the CNS [18], pose a challenge for a growing number of patients. As systemic treatments have improved, the number of patients with CNS metastases has increased, and those who face recurrence after radiation therapy currently lack effective treatment options. Indeed, while patients with HER2-positive breast cancer with CNS metastases who receive trastuzumab do show a survival benefit [6,19], this is thought to be largely due to control of systemic disease, with the brain remaining a sanctuary site [20]. Smaller molecule agents such as lapatinib may be more effectively delivered across the BTB and have shown promise in patients with breast cancer brain metastases [21]. However, with its molecular weight of 581 Da, lapatinib also will not effectively cross the intact BBB and may not reach all cancer cells.
To effectively treat metastases in the CNS, drugs will need to get past the BBB and overcome limitations imposed by the BTB. A number of strategies have been developed to get drugs past the BBB that range from invasive interventions such as convection-enhanced delivery [22] and minimally-invasive approaches such as transarterial injection of substances that transiently disrupt the BBB [23,24], to the development of drugs or drug carriers that utilize endogenous transport mechanisms to ferry drugs across the BBB [25,26].
Another approach to get drugs past the BBB that has been investigated in pre-clinical studies is the use of focused ultrasound (FUS), that when combined with circulating microbubbles (ultrasound contrast agents), can induce temporary BBB disruption and has been investigated as a method for targeted drug delivery in the brain [27]. Acoustic waves can be focused deeply into soft tissue, and the mechanical interaction between the ultrasound, the microbubbles, and the vasculature can then induce a transient disassembly of tight junction proteins [28,29] and the stimulation of active transport [30] to allow for a temporary window for drug delivery.
This technique has several advantages over other approaches in that it is completely noninvasive, readily repeatable, targeted only to desired regions in the brain, and compatible with currently-approved drugs. Animal studies have shown that the disruption is not associated with significant tissue damage [31–33]. It has also been shown to enhance delivery of therapeutic agents [34,35], including trastuzumab (Herceptin®) [36], and it can increase the permeability of the BTB [37,38]. Recent work has shown that the method is capable of enhancing delivery of BCNU, improving survival a rat glioma model [39], and delivering antibodies that improved outcomes in a mouse Alzheimer’s disease model [40]. Most studies have shown that the barrier is restored in a few hours [27,28,41].
In this study, we investigated whether temporary permeabilization induced by focused ultrasound (FUS) and microbubbles combined with trastuzumab can improve outcomes in a HER2/neu positive breast cancer brain metastases model. It was designed to demonstrate effective control of tumor progression and survival time with a course of multiple treatments that that approximates the trastuzumab therapy regimen that a patient would receive. It was also chosen because, due to its large molecular weight (~150 kDa), we hypothesized that it would benefit significantly from enhanced local delivery in the brain.
2. Materials and Methods
2.1 Animals and cell culture
All experiments were done in accordance with procedures approved by the Harvard Medical School Institutional Animal Care and Use Committee. A total of 41 male nude (nu/nu) rats (Charles River Laboratories, Boston, MA) weighing 180 – 210 g (six-week old) were used. Animals were anesthetized during all procedures with either 2% isoflurane (for estrogen pellet implantation) administered through a nose cone, or with ketamine (90 mg/kg) and xylazine (10 mg/kg) administered via IP injection (all other procedures). Surgical procedures were performed under aseptic conditions.
BT-474 HER2-positive human breast ductal carcinoma cells (ATCC, Manassas, VA) were cultured in RPMI 1640 medium (Invitrogen, San Diego, CA) containing 10% fetal bovine serum at 37°C in 5% CO2. One week prior to the cancer cell implantation, a 17β estradiol pellet (1.7 mg estrogen/pellet, 90 days release, Innovative Research of America, Sarasota, FL) was subcutaneously implanted near the cervical vertebrae. For tumor implantation, the animals were positioned in a stereotactic positioner, and a linear skin incision (1 cm) was made over the bregma. A 1 mm diameter burr-hole was drilled 1.5 mm anterior to the bregma and 2 mm to the right of sagittal suture. Using a 10 μl gas-tight Hamilton syringe with a 27G needle, 1×106 cells suspended in 2 μl of cell culture medium was injected into the right frontal lobe at a depth of 3.5 mm relative to the dural surface of the brain. In order to minimize cell loss at the injection site, the cell suspension was slowly injected over 5 minutes. Two minutes after the cell injection, the needle was retracted slowly over an additional 5 minutes. After rinsing the wound with 0.9% sodium chloride solution, the burr hole was occluded with sterile bone wax, and the skin was sutured with 5-0 silk. Then, the animal recovered from anesthesia under observation, and antibiotic (Baytril®, Bayer Healthcare, Shawnee Mission, KS, 2.5 mg/kg, IP) and analgesic (Buprenex®, Reckitt Benckiser Pharmaceuticals, Richmond, VA, 0.05 mg/kg SQ every 12 h for two days) were administered. Sutures were removed one week after the surgery. Tumor growth was monitored with MRI until the tumor reached 2–3 mm in diameter (approximately 14 days).
For the FUS treatments, the fur on the head was removed, an IV catheter was placed in the tail vein, and a rectal thermocouple was inserted to monitor the animals’ temperature during the procedure. Body temperature was maintained with a heated water pad.
2.2 Study design
The 41 rats were divided into four experimental groups: 1) control (no treatment), 2) FUS-only (six weekly sessions with FUS with microbubbles to produce BBB/BTB permeabilization), 3) trastuzumab-only (six weekly treatments with trastuzumab), and 4) FUS + trastuzumab (six weekly treatments with trastuzumab following BBB/BTB permeabilization). For groups 3–4, trastuzumab was administered weekly through the tail vein at a dose of 2 mg/kg. For groups 2 and 4, multiple overlapping locations were sonicated to permeabilize the BTB and to disrupt the BBB in a ~1 mm rim surrounding the tumor. For group 4, trastuzumab was injected immediately after confirming the BBB/BTB permeabilization. The sonications were all delivered at a single depth at the center of the tumor; the length of the focal region (see below) was sufficient to cover the thickness of the tumor. The spacing between the sonication targets was 1.5 mm. The number of sonications varied from 1–27, depending on the size of the tumor at the time of treatment. We waited at least 2 min between each sonication to minimize the accumulation of microbubbles.
The tumor volume was monitored weekly with MRI for seven weeks following the last treatment. At the endpoint of the study, surviving animals were deeply anesthetized and then euthanized by transcardiac perfusion with 0.9% sodium chloride solution followed by 10% buffered formalin phosphate solution while under deep anesthesia with a mixture of ketamine and xylazine. For histological examination, the brains were immediately harvested and fixed in the formalin solution. Animals were euthanized before the study endpoint if the tumor size exceeded 13 mm in diameter (i.e. if it reached the width of the brain in the left/right direction), the animal showed excessive weight loss (20% of body weight in a week), or if he showed physiological signs of suffering. The description of experiments with the timeline is shown in Fig. 1.
Fig. 1.
Timeline for the experiments. A total of six weekly treatments were performed, followed by another seven weeks of post-treatment MRI to monitor tumor growth.
Histological examination was performed on two representative examples from each treatment group. Brain samples fixed in 10% buffered formalin phosphate solution were cut in 4 mm tissue blocks using a brain matrix (model RBM-4000DV, ASI Instruments, Warren, MI, USA). Blocks containing the tumor embedded in paraffin, and several (generally four) horizontal sections were stained with hematoxylin and eosin (H&E).
2.3 Ultrasound
An air-backed, single-element, spherically-focused transducer (diameter = 10 cm, f-number = 0.8, frequency = 690 kHz) was used. The transducer’s frequency was chosen to be similar to that used in a clinical system developed for transcranial FUS ablation [42,43]. The transducer was driven by a radio frequency (RF) signal generated by an arbitrary waveform generator (Model 395, Wavetek Inc., San Diego, CA) and amplified by an RF amplifier (Model 240L, ENI Inc., Rochester, NY). Using an external inductor-capacitor tuning network, the electrical impedance of the transducer was matched to the output impedance of the amplifier. The electrical power was monitored with a power meter (Model E4419B, Agilent, Santa Clara, CA). The transducer efficiency was measured with a radiation force balance consisting of an absorbing brush attached to a digital scale. The peak negative pressure amplitude was measured with a calibrated membrane hydrophone (spot diameter 0.5 mm, GEC-Marconi Research Center, Chelmsford, England). The spatial pressure distribution was measured with a 2 mm diameter needle hydrophone; the half width/length of the focal region measured was 2.3/14 mm.
A bolus of a microbubble-based ultrasound contrast agent (Definity®, Lantheus Medical Imaging Inc., N. Billerica, MA) was injected through the tail vein at a dose of 10 μl/kg 10 s prior to each sonication. The agent was diluted 10× in phosphate buffered saline. Sonications were 60 s in duration and consisted of 10 ms bursts applied at a pulse repetition frequency of 1 Hz (1% duty cycle). The sonications were delivered at an acoustic power of 0.32 W, which corresponded to a peak negative pressure amplitude in water of 0.69 MPa. This operating condition was obtained from a prior safety study of multiple BBB disruption sessions in normal (non-tumor-bearing) rats [44].
The experimental setup for MRgFUS treatment is shown in Fig. 2. The focused ultrasound transducer was mounted in a three-dimensional positioning system and submerged in a tank filled with degassed, deionized water. The rat was placed supine on a tray above the water tank. A thin plastic water bag was placed between the tray and water tank for acoustic coupling between the skin on the head and the transducer. A 7.5 cm diameter transmit/receive surface coil (constructed in-house) mounted under the head was used for the MR image acquisition. The initial coordinates of the acoustic focus were determined by visualizing thermal changes induced in a silicone ultrasound phantom using a T1-weighted fast spin echo (FSE) sequence.
Fig. 2.
Experimental setup for MR-guided experiments that used FUS and microbubbles to permeabilize the BTB and disrupt the BBB in a surrounding brain region. A three-dimensional positioning system was used to steer to the transducer to different locations. The components of driving system for the FUS transducer were located outside of the MRI room.
2.4 MRI
A clinical MRI scanner (3T, Signa, GE Healthcare, Milwaukee, WI) was used to guide the FUS treatments, to evaluate the tumor location and volume, and to confirm the BTB/BBB permeabilization. Tumor volume and location were determined in multi-planar T2-weighted FSE images (echo time (TE)/repetition time (TR) = 85/2000 ms, echo train length (ETL) = 8, matrix size = 256 × 256. slice thickness = 1 mm, field of view (FOV) = 80 mm, number of excitations (NEX) = 4, flip angle = 90°) and multi-planar T1-weighted FSE images (TE/TR = 13.3/500 ms, echo train length (ETL) = 4, matrix size = 256 × 256. slice thickness = 1 mm, FOV = 80 mm, number of excitations (NEX) = 4, flip angle = 90°). The T1-weighted images were acquired after IV injection of an MR contrast agent (Gd-DTPA, Magnevist®, Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ; dosage: 0.2 ml/kg) to evaluate BBB/BTB permeabilization. In select animals, pre-treatment contrast-enhanced T1-weighted imaging was acquired after sonication at a location outside the tumor to confirm targeting accuracy. Additional T1-weighted and T2-weighted images were acquired weekly for seven weeks after the last treatment to monitor the tumor volume.
2.5 Data analysis
The tumor volume was evaluated with image analysis software (ImageJ). The visible extent of the tumor (excluding surrounding edema) was manually segmented in the multislice T1-weighted and T2-weighted images, and the volume per image was calculated by multiplying the area of the segment by the image slice thickness (1 mm); the total volume of tumor (V) for each rat was calculated by summing up the segmented volumes for each image.
Tumor volumes of the treated animals were compared to the other groups using a one-tailed t-test. Log-transformed data was used for this analysis. The doubling time of each tumor was obtained from least-squares nonlinear regression analysis of the volume increase as a function of time and compared between groups. The median survival time and hazard rate of each group was determined and compared to the no-treatment control group using Kaplan-Meier analysis. Survival curves were compared using the log-rank test with Yates’ correction. Reported data are mean values ± the standard error (s.e.).
3. Results
BBB and BTB permeabilization induced by FUS and microbubbles was confirmed by a signal intensity increase measured in contrast enhanced T1-weighted images obtained after the sonications. Fig. 3 shows example contrast-enhanced images before (a) and after (b) the sonications. Due to the enhanced delivery of the MR contrast agent across these barriers, higher MR signal intensities were observed throughout the vascular portion of the tumor and a narrow rim of tissue surrounding it.
Fig. 3.

Contrast-enhanced T1-weighted MR images acquired (a) before and (b) and after sonication with microbubbles. The vascular part of the tumor was enhancing before sonication. After sonication, the level of this enhancement increased due to BTB permeabilization, and enhancement was also evident in a surrounding rim of tissue due to BBB disruption. BBB disruption evident in the contralateral hemisphere was the location of a pre-treatment sonication used to verify the targeting.
The tumor volume of each rat was obtained from the MR images acquired immediately before each treatment and weekly afterwards (Fig. 4). The average initial tumor volume was 2.1 ± 0.2 mm3. This initial volume was not significantly different between the different groups. The mean tumor volume for the two control groups who did not receive trastuzumab increased exponentially as a function of time. The mean volume for trastuzumab-only group also increased, but at a slightly slower rate suggesting perhaps a mild treatment effect. The FUS+trastuzumab group in contrast, showed a more substantial reduction in mean tumor volume. The difference in tumor volume between the FUS+trastuzumab group and the three control groups was significant (P<0.05) at week 7, the last session where all animals survived.
Fig. 4.
Tumor volume measurements for each group over 13 weeks. The measurements were obtained in MRI acquired before each of the six treatments and weekly afterwards. (a) Tumor volume vs. time; (b) Measurements normalized to the volume measured at week 1; (c) Individual measurements for all tumors at week 7 (the last week where all animals survived). The mean tumor growth with FUS+trastuzumab was substantially reduced compared to the control groups. Tumor volume was significantly less (P<0.05) than each of the three control groups at week 7. This improvement was largely driven by four animals where the tumor appeared in MRI to be completely resolved; tumor growth for the six “non-responders” was similar to the trastuzumab-only group. At the study endpoint (week 13), only the four “responders” survived. The tumor volume at week 1 was approximately 2 mm3 (mean ± standard error shown).
The improvement evident for the FUS+trastuzumab group was largely due to the strong response that was observed in 4/10 animals (called “responders”). In three animals in this group, no tumor was detected in MRI at weeks 5–13, and in an additional animal, no tumor was detected at weeks 7–13. For the animals whose tumors appeared to be resolved before the sixth treatment, the treatment consisted of BBB disruption in a small brain region where the tumor was observed previously. Example images showing at tumor growth different times are shown in Fig. 5 for one of these four animals and for a non-treated control. Such tumor disappearance was not observed in any of the 31 tumors in the three control groups. The tumor volume increase for the 6/10 “non-responders” in the FUS+trastuzumab group was similar to the trastuzumab-only group (Fig. 4). Fig. 6 shows T1-weighted images acquired before and after FUS for two animals in the FUS+trastuzumab group- one that disappeared in MRI after three treatments, and one that did not have this strong response. In both cases, the enhancing area after sonication was larger than the extent of the tumor before treatment. The difference in initial volume was not significant between the “responders” and the “non-responders”.
Fig. 5.
Example T1-weighted MR images of two rat brains over time from the (a) FUS+trastuzumab and (b) no treatment groups. The example shown in (a) is one of the 4/10 rats treated with FUS+trastuzumab where the tumor appeared to be completely resolved in MRI. In this example, no tumor was detected in MRI at weeks 7–13. Slight differences evident in the imaging planes from week-to-week was due to variability in animal positioning and MRI slice selection.
Fig. 6.
Serial T1-weighted images (T1WI) acquired before and after six treatments with FUS+trastuzumab for an animal where the tumor that disappeared in MRI after four weeks (a) and another that did not show this strong response (b). Post-treatment images were acquired after Gd-DPTA MRI contrast injection. Contours show the areas where the signal intensity was at least 5% above that in non-sonicated regions in the brain. In most of the treatments, the enhancing area was larger than the tumor extent seen in the pre-treatment images. After the tumor disappeared shown in (a) at week four, sonications were targeted in a brain region where the tumor was previously evident. The last treatment in that animal produced only low-level (less than 5%) contrast enhancement. enhancement.
Excluding the four animals whose tumors appeared to be completely resolved in MRI, the volume increase of each tumor was well defined by an exponential (R2 ≥ 0.87). This growth was fit using nonlinear least-squares regression to calculate the doubling time for each tumor. The mean doubling time was 7.1 ± 0.3, 6.7 ± 0.6, and 9.3 ± 0.8 days for the no treatment, FUS-only, and trastuzumab-only groups, respectively. The mean doubling time for the “non-responders” in the FUS+trastuzumab group was 10.3 ± 2.0 days. The doubling time for these animals as well as those in the trastuzumab-only group was significantly (P<0.05) longer than that of the two control groups that did not receive drug.
Survival curves for the different treatment groups are shown in Fig. 7; statistical data after Kaplan-Meier analysis are shown in Table 1. The difference in median survival for the two control groups that did not receive trastuzumab were not significant (P=0.69). Trastuzumab improved median survival by 13% compared to the no treatment group, a difference which was significant (P=0.044). Treatment with FUS+trastuzumab produced the most significant benefit compared to the no-treatment controls (P=0.0084). The difference in survival between the trastuzumab-only and the FUS+trastuzumab were not significant (P>0.05) at week 13. However, more than half (6/10) animals survived at the study endpoint, leading to a median survival time greater than 83 days (at least 17% longer than the trastuzumab-only group and 32% longer than the untreated control group).
Fig. 7.

Survival analysis for the different treatment groups.
Table 1.
Survival analysis
| Treatment Group | Median Survival (days) | Mean Survival (days)* | Surviving Fraction at Study End | P-value** | Hazard Ratio** |
|---|---|---|---|---|---|
| No Treatment | 63 | 65.5 ± 3.5 | 0/11 | --- | --- |
| FUS-Only | 64.5 | 67.8 ± 4.0 | 2/10 | 0.69 | 0.902 |
| Trastuzumab-Only | 71 | 74.8 ± 3.7 | 5/10 | 0.044 | 0.461 |
| FUS+Trastuzumab | >83 | 76.8 ± 3.6 | 6/10 | 0.0084 | 0.416 |
Measured at study endpoint (83–85 days). As some animals survived at the end of the study, this is a minimal value (mean ± s.e.).
Relative to the “no treatment” group.
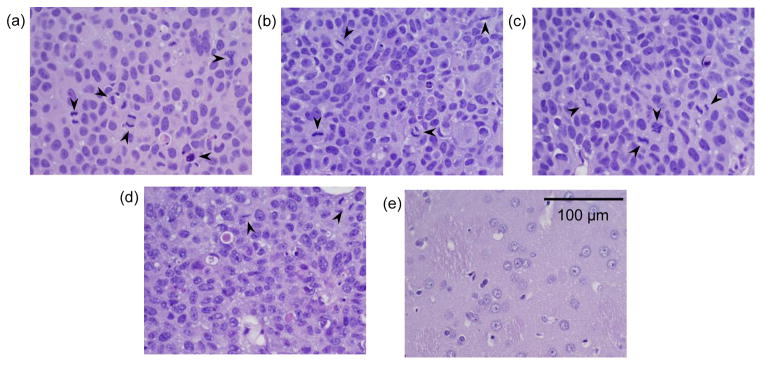
Histological examination was performed on representative examples from each treatment group, with examples shown in Fig. 8. More mitotic activity was evident within the tumors in the three control groups (Fig. 8a–c) compared to the “non-responder” example that was evaluated from the FUS+trastuzumab group (Fig. 8d). Vacuolation was evident in the tumor parenchyma in this example, and a foamy-appearance of the cells was observed. The example shown in Fig. 8e was the animal where no tumor was detected in MRI after seven weeks. The brain region at the treatment depth appeared normal. In this animal, a small cluster (~700 μm) of tumor cells was observed in the cortex (i.e. near the brain surface) that was residual or recurring tumor not seen in MRI.
Fig. 8.
Microphotographs of H&E stained sections showing examples of breast ductal carcinoma inoculated in the rat brain after no treatment (a), and treatment with trastuzumab-only (b), FUS-only (c), and FUS+trastuzumab (d). More mitotic activity (arrows) was evident in three control groups compared to the example in (d) treated with FUS+trastuzumab. The example shown in (e) shows the brain in the treated area after FUS+trastuzumab for a case where no tumor was detected in MRI starting at week seven.
4. Discussion
Since it was approved in 1998, trastuzumab, a monoclonal antibody that has high affinity to the growth-promoting protein HER2/neu, has been used in treating metastatic breast cancers [45–47]. It binds to the extracellular segment of the HER2/neu receptor and suppresses dimerization of the HER2/neu receptor, leading to a disruption in signaling for cell proliferation. In patients with HER2-positive metastatic breast cancer, trastuzumab is used as a first-line treatment in combination with the chemotherapy drug paclitaxel (Taxol®), or it used alone for the patients who have received one or more chemotherapy courses [46]. Trastuzumab has been shown to be effective for reducing the rate of tumor progression and increasing median survival for patients with extracranial metastases. However, it has been ineffective for the treatment of brain metastases [1,7]. This difficulty is thought to be due to the restrictions imposed on drug delivery by the BBB/BTB which lead to poor penetration after systemic administration.
In the present study, we showed that therapeutic effects of a six-week course of trastuzumab on HER2-positive tumors inoculated in the rat brain can be enhanced through permeabilization of the BBB/BTB using focused ultrasound and microbubbles. At the end of the therapy we found a significant reduction in the mean tumor volume and a significant improvement in the survival time. Moreover, tumors in four of 10 rats in this group were undetectable at the resolution of the MRI over the course of treatments without evident recurrence over the course of the study. This strong response was not observed in any of the 31 control animals. Despite this response, differences in survival between the trastuzumab-only and FUS+trastuzumab groups were not significant. We suspect that we would have observed a difference in survival rate if we had monitored the animals for a longer period, since the surviving animals in the FUS+trastuzumab group had no evident tumor at week 13 and were in good health, while the trastuzumab-only animals had substantial tumors that were still growing. However, this needs to be confirmed.
The outcomes – tumor volume, doubling time, and survival – were similar for the six “non-responders” in the FUS+trastuzumab group and the animals who received trastuzumab alone. It is not clear why only some of the tumors responded so strongly. Comparison of the MRI obtained before treatment to the contrast-enhanced images acquired after treatment (such as that shown in Fig. 6) did not show any obvious differences in the treatments between animals that responded strongly to treatment and those that did not. In particular, we did not find that portions of the tumor were missed; the post-sonication enhancement always included the tumor and some adjacent brain tissue. However, due to treatment time constraints, we were only able to obtain limited imaging. If this disparate response was due to the quality of the treatment, detailed comparison of the MRI contrast kinetics in the tumor and surrounding areas before and after each treatment may provide more information that could be predictive of the outcome. It is also possible that the tumor vasculature in the “responders” was more permeable before FUS. More advanced histological examination at different points throughout the treatments may also elucidate differences. In particular, it would be interesting to evaluate the vascular density, through imaging or histological methods, and the distance that trastuzumab can penetrate after BBB/BTB permeabilization, to further understand under which circumstances that a strong therapeutic response can be induced. These detailed analyses were beyond the scope of this work.
Previously it was shown that BBB disruption induced by FUS and microbubbles can deliver trastuzumab antibodies across the BBB in rabbits [36], but it was not known whether the amount delivered was sufficient to have a therapeutic effect in a tumor. This study demonstrates that it can. However, it had several limitations. We had a relatively small sample size, a limited post-treatment observation schedule, and limited investigation in histology of untreated or recurring tumor below the resolution of the MRI and of the effects of repeated sonication and drug delivery to the brain. Future work should expand upon this work to evaluate outcomes in a larger cohort and for a longer time after treatment and with more treatment sessions if needed. More realistic breast cancer breast metastases models, such as using intracarotid tumor cell injections [48], should be explored to better mimic the tumor environment in a patient. Imaging or acoustic monitoring approaches to control the sonications and evaluate the resulting permeabilization may also improve treatment consistency. Finally, the combination of trastuzumab with other anti-cancer agents should be explored.
Despite these limitations, this study is highly encouraging and supportive of this promising approach to overcoming barriers to drug delivery in brain tumors. The use of focused ultrasound has several advantages compared to most other approaches that have been investigated for this delivery problem, which have been invasive, non-targeted, or require the development of new drugs or drug carriers [49]. BBB/BTB permeabilization with focused ultrasound and microbubbles is a completely noninvasive procedure that can target the delivery of drugs to only the desired regions in the brain. The method is readily repeatable and amenable for multiple sessions to match a patient’s drug schedule. Furthermore, it allows the use of already approved drugs, which in many cases (such as metastases), are expected to be effective if could be delivered effectively.
5. Conclusions
This study demonstrates for the first time that combining weekly trastuzumab therapy with BBB/BTB permeabilization induced by FUS and microbubbles can improve outcomes in HER2-postive breast tumors inoculated in the brain. A significant reduction in mean tumor volume and survival were found after six weekly FUS+trastuzumab treatments, and no sequelae were found as the result of the procedure. Moreover, after treatment with ultrasound and trastuzumab, four of the ten tumors disappeared completely in MRI, a result that was not observed in untreated control animals, animals treated with ultrasound-induced BBB/BTB permeabilization only, or in animals who received trastuzumab alone. While additional work is needed to evaluate long-term recurrence rates and other factors not considered here, these results are nevertheless highly encouraging and support the therapeutic potential of this noninvasive technique for targeted drug delivery to the brain.
Acknowledgments
This research was supported by NIH grants P41RR019703, P41EB015898, a grant from CIMIT (Center for Integration of Medicine and Innovative Technology), and a gift from Betty Brudnick.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pienkowski T, Zielinski CC. Trastuzumab treatment in patients with breast cancer and metastatic CNS disease. Ann Oncol. 2010;21:917–924. doi: 10.1093/annonc/mdp353. [DOI] [PubMed] [Google Scholar]
- 2.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27:5278–5286. doi: 10.1200/JCO.2008.19.8481. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52:2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, Abdulkarim B. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24:5658–5663. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 5.Kaal EC, Vecht CJ. CNS complications of breast cancer: current and emerging treatment options. CNS Drugs. 2007;21:559–579. doi: 10.2165/00023210-200721070-00003. [DOI] [PubMed] [Google Scholar]
- 6.Dawood S, Broglio K, Esteva FJ, Ibrahim NK, Kau SW, Islam R, Aldape KD, Yu TK, Hortobagyi GN, Gonzalez-Angulo AM. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242–1248. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 7.Hall WA, Djalilian HR, Nussbaum ES, Cho KH. Long-term survival with metastatic cancer to the brain. Med Oncol. 2000;17:279–286. doi: 10.1007/BF02782192. [DOI] [PubMed] [Google Scholar]
- 8.Melisko ME, Moore DH, Sneed PK, De FJ, Rugo HS. Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J Neurooncol. 2008;88:359–365. doi: 10.1007/s11060-008-9578-5. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud-Ahmed AS, Suh JH, Lee SY, Crownover RL, Barnett GH. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys. 2002;54:810–817. doi: 10.1016/s0360-3016(02)02967-x. [DOI] [PubMed] [Google Scholar]
- 10.Chang EL, Lo S. Diagnosis and management of central nervous system metastases from breast cancer. Oncologist. 2003;8:398–410. doi: 10.1634/theoncologist.8-5-398. [DOI] [PubMed] [Google Scholar]
- 11.Alexander E, III, Moriarty TM, Davis RB, Wen PY, Fine HA, Black PM, Kooy HM, Loeffler JS. Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst. 1995;87:34–40. doi: 10.1093/jnci/87.1.34. [DOI] [PubMed] [Google Scholar]
- 12.Black PM, Johnson MD. Surgical resection for patients with solid brain metastases: current status. J Neurooncol. 2004;69:119–124. doi: 10.1023/b:neon.0000041875.09048.e7. [DOI] [PubMed] [Google Scholar]
- 13.Kondziolka D, Kano H, Harrison GL, Yang HC, Liew DN, Niranjan A, Brufsky AM, Flickinger JC, Lunsford LD. Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. Clinical article. J Neurosurg. 2011;114:792–800. doi: 10.3171/2010.8.JNS10461. [DOI] [PubMed] [Google Scholar]
- 14.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ., Jr Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 15.Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8:344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 17.Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, Huang S, Palmieri D, Steeg PS, Smith QR. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16:5664–5678. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Angulo AM, Hennessy BT, Broglio K, Meric-Bernstam F, Cristofanilli M, Giordano SH, Buchholz TA, Sahin A, Singletary SE, Buzdar AU, Hortobagyi GN. Trends for inflammatory breast cancer: is survival improving? Oncologist. 2007;12:904–912. doi: 10.1634/theoncologist.12-8-904. [DOI] [PubMed] [Google Scholar]
- 19.Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, Tudor IC, Wang LI, Brammer MG, Shing M, Yood MU, Yardley DA. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 20.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 21.Lin NU, Dieras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, Roche H, Liu MC, Greil R, Ciruelos E, Loibl S, Gori S, Wardley A, Yardley D, Brufsky A, Blum JL, Rubin SD, Dharan B, Steplewski K, Zembryki D, Oliva C, Roychowdhury D, Paoletti P, Winer EP. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 22.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doolittle ND, Miner ME, Hall WA, Siegal T, Jerome E, Osztie E, McAllister LD, Bubalo JS, Kraemer DF, Fortin D, Nixon R, Muldoon LL, Neuwelt EA. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000;88:637–647. doi: 10.1002/(sici)1097-0142(20000201)88:3<637::aid-cncr22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Guillaume DJ, Doolittle ND, Gahramanov S, Hedrick NA, Delashaw JB, Neuwelt EA. Intra-arterial chemotherapy with osmotic blood-brain barrier disruption for aggressive oligodendroglial tumors: results of a phase I study. Neurosurgery. 2010;66:48–58. doi: 10.1227/01.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- 26.Pardridge WM. Biopharmaceutical drug targeting to the brain. J Drug Target. 2010;18:157–167. doi: 10.3109/10611860903548354. [DOI] [PubMed] [Google Scholar]
- 27.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 28.Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 2008;34:1093–1104. doi: 10.1016/j.ultrasmedbio.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shang X, Wang P, Liu Y, Zhang Z, Xue Y. Mechanism of low-frequency ultrasound in opening blood-tumor barrier by tight junction. J Mol Neurosci. 2011;43:364–369. doi: 10.1007/s12031-010-9451-9. [DOI] [PubMed] [Google Scholar]
- 30.Sheikov N, McDannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med Biol. 2006;32:1399–1409. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 31.McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol. 2005;31:1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105:445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 33.Baseri B, Choi JJ, Tung YS, Konofagou EE. Multi-modality safety assessment of blood-brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultrasound Med Biol. 2010;36:1445–1459. doi: 10.1016/j.ultrasmedbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 35.Mei J, Cheng Y, Song Y, Yang Y, Wang F, Liu Y, Wang Z. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med. 2009;28:871–880. doi: 10.7863/jum.2009.28.7.871. [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang FY, Lin GL, Horng SC, Chang TK, Wu SY, Wong TT, Wang HE. Pulsed high-intensity focused ultrasound enhances the relative permeability of the blood-tumor barrier in a glioma-bearing rat model. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:964–970. doi: 10.1109/TUFFC.2011.1897. [DOI] [PubMed] [Google Scholar]
- 38.Yang FY, Teng MC, Lu M, Liang HF, Lee YR, Yen CC, Liang ML, Wong TT. Treating glioblastoma multiforme with selective high-dose liposomal doxorubicin chemotherapy induced by repeated focused ultrasound. Int J Nanomedicine. 2012;7:965–974. doi: 10.2147/IJN.S29229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu HL, Hua MY, Chen PY, Chu PC, Pan CH, Yang HW, Huang CY, Wang JJ, Yen TC, Wei KC. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 2010;255:415–425. doi: 10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- 40. Jordao JF, yala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, Hynynen K, Aubert I. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS One. 2010;5:e10549. doi: 10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 42.Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66:858–861. doi: 10.1002/ana.21801. [DOI] [PubMed] [Google Scholar]
- 43.McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66:323–332. doi: 10.1227/01.NEU.0000360379.95800.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pilatou MC, Stewart EA, Maier SE, Fennessy FM, Hynynen K, Tempany CM, McDannold N. MRI-based thermal dosimetry and diffusion-weighted imaging of MRI-guided focused ultrasound thermal ablation of uterine fibroids. J Magn Reson Imaging. 2009;29:404–411. doi: 10.1002/jmri.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genentech. Herceptin. 2012 Aug 3; http://www.herceptin.com/metastatic/what-is/index.jsp.
- 46.National Breast Cancer Coalition. Trastuzumab (Herceptin®) 2012 Mar 8; http://www.knowbreastcancer.org/controversies/herceptin/
- 47.U.S. Food and Drug Administration. Trastuzumab, Genentech Herceptin approval letter. 2012 Mar 8; http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm091360.pdf.
- 48.Wasita B, Kamitani H, Kinoshita Y, Mamun MH, Watanabe T. A rat glioblastoma model with diffuse leptomeningeal gliomatosis induced by intracarotid injection of C6 glioma cells. Neurol Res. 2009;31:453–462. doi: 10.1179/174313209X403904. [DOI] [PubMed] [Google Scholar]
- 49.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]