Abstract
Directional movement is a property common to all cell types during development and is critical to tissue remodelling and regeneration after damage1–3. In migrating cells, calcium plays a multifunctional role in directional sensing, cytoskeleton redistribution, traction force generation, and relocation of focal adhesions1, 4–7. Here we visualise, for the first time, high-calcium microdomains (“calcium flickers”), and their patterned activation in migrating fibroblasts. Calcium flicker activity is dually coupled to membrane tension (via TRPM7, a stretch-activated Ca2+-permeant channel of the transient receptor potential superfamily8) and chemoattractant signal transduction (via type 2 inositol 1,4,5-trisphosphate receptors). Interestingly, calcium flickers are most active at the leading lamella of migrating cells, displaying a 4:1 front-to-rear polarisation opposite to the global calcium gradient6. When exposed to a PDGF gradient perpendicular to cell movement, asymmetric calcium flicker activity develops across the lamella and promotes the turning of migrating fibroblasts. These findings illustrate how the exquisite spatiotemporal organisation of calcium microdomains can orchestrate complex cellular processes such as cell migration.
In addition to extracellular chemoattractant stimuli, directional cell movement depends on an intracellular calcium signal that is well-organised in space, time and concentration6, 7, 9. Over a decade ago, Fay and colleagues made the groundbreaking finding that intracellular calcium displays a rear-to-front gradient, with the lowest concentration at the front of a migrating cell6. However, this observation appears to be paradoxical, because the leading lamella, the signalling and motility centre of a migrating cell, contains numerous effector proteins that require high levels of calcium for activation10–13. Although transient increases of calcium concentration have recently been observed in migrating cells, they are infrequent and mainly localised to the tail of the cell, and are thought to facilitate intermittent rear retraction7. Biochemical studies suggest that calcium entry is required to maintain ruffling structure, actin polymerisation, and the phosphatidylinositol-3,4,5-trisphosphate (PIP3) signalling at the leading edge of macrophages14. To date, it remains perplexing how calcium regulates lamella dynamics during cell migration.
Using human embryonic lung fibroblasts (WI-38) as a model, we characterised the spatiotemporal organisation of intracellular calcium signals with the aid of real-time confocal microscopy. In migrating WI-38 fibroblasts that overtly displayed leading and trailing edges, we detected a shallow decreasing gradient of global calcium concentration (indexed by the fluo-4 to fura-red fluorescence ratio) that ran from the rear to the front (Fig. 1c, d), in agreement with previous findings6, 9. Surprisingly, we found that discrete, local and short-lived high-calcium microdomains or “calcium flickers”, analogous to calcium sparks and puffs15, occurred against a quiescent background (supplementary video). High resolution linescan imaging revealed that the flickers occurred at a nearly constant rate of 1.92 ± 0.21 Hz/100 µm linescan (n = 18) (Fig. 1c). Individual events rapidly rose to about double the fluo-4 fluorescence (ΔF/Fo = 1.16 ± 0.02, n = 1,071), lasting variably from 10 ms to 4 s, and were confined to an area 5.27 ± 0.05 µm in diameter (Fig. 1c). Importantly, calcium flickers were abundant at the leading lamella (Fig. 1a), including in motile lamellipodia (Fig. 1b), but were sharply reduced elsewhere, resulting in an approximately 4:1 front-to-rear polarisation (Fig. 1d) that was opposite to the aforementioned global calcium gradient. Polarisation of flicker activity was common to fibroblasts undergoing migration, but it was not seen in stationary fibroblasts lacking morphological polarity and displaying a lower flicker incidence (0.57 ± 0.10 Hz/100 µm linescan, n = 12, p < 0.05 vs. migrating cells) (supplementary Fig. 1). Thus, flickers represent a distinctive, heretofore unappreciated modality of calcium signalling in migrating fibroblasts. Similar flickers were also evident in rat neonatal cardiac fibroblasts and 3T3-Swiss albino mouse embryonic fibroblasts (supplementary Fig. 2).
Figure 1. Calcium flickers in migrating fibroblasts.
a. Calcium flickers. In a polarised WI-38 fibroblast (insert), local calcium increases (ΣΔF) were summed over 30 consecutive images acquired at 6 s intervals. “N” marks the nucleus. Scale bar: 15 µm. b. Calcium flickers (colour overlay) in motile lamellipodia (box in a). Scale bar: 5 µm. c. Polarisation of calcium flicker activity. The image consists of 10000 front-to-rear linescans, expressed as the ratio between fluo-4 and fura-red fluorescence (R). d. Opposing gradients of calcium flicker activity (ΔR) and global calcium (R, inclusive of flicker activity). Similar results were observed in eight cells.
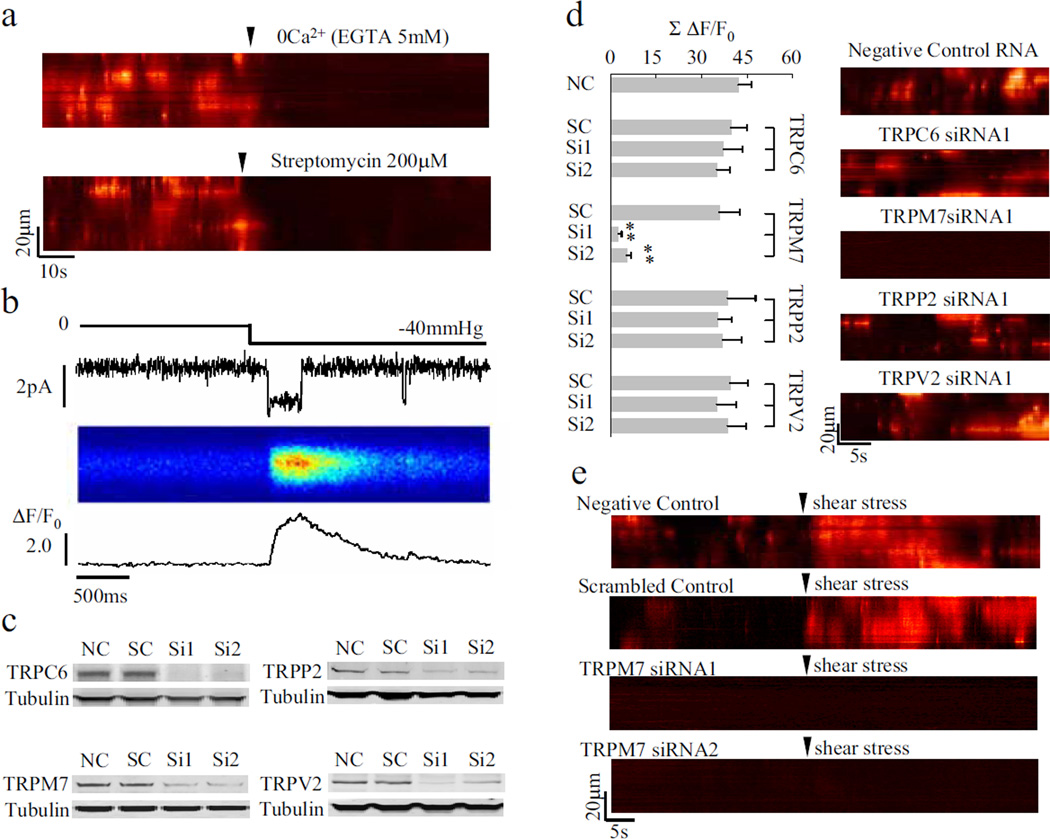
In search of the molecular basis of calcium flickers, we showed that calcium influx through the stretch-activated cation channel (SACC) was obligatory. Application of Ca2+-free medium containing 5 mM EGTA or streptomycin (200 µM), a SACC blocker16, immediately abolished flicker activity in WI-38 fibroblasts (Fig. 2a). On average, the signal mass of calcium flickers (space-time integral of the flicker signal) decreased by 98.3 ± 0.8% (EGTA; n = 9, p < 0.01 vs. control) or 93.1 ± 1.3% (streptomycin; n = 6, p < 0.01 vs. control). Likewise, Gd3+ (200 µM), a non-specific SACC blocker16, diminished the signal mass by 91.9 ± 1.2% (n = 6, p < 0.01 vs. control). To determine whether mechanical forces can directly trigger flickers, we showed that shear stress applied to the front of migrating fibroblasts immediately evoked a flurry of flicker activity (Fig. 2e). In a different approach, relaxing or stretching the cell by pushing or pulling the flexible substrate (with a needle tip) suppressed or enhanced the flicker activity, respectively (supplementary Fig. 3). Under cell-attached patch clamp conditions17, sudden application of negative pressure (~40 mm Hg) elicited bursting single-channel activity, while simultaneous confocal imaging visualised corresponding flicker-like events beneath the patch membrane (Fig. 2b). These results indicate that calcium flickers are triggered by calcium influx through SACCs.
Figure 2. Triggering calcium flickers by TRPM7.
a. Abolition of calcium flickers by streptomycin or removal of external calcium. b. Visualisation of calcium entry through single SACCs. From top to bottom: suction through the patch pipette, single-channel currents, and linescan image and line plot of local calcium transients. c. RNA interference knockdown of TRPC6, TRPM7, TRPV2 and TRPP2 assayed by Western blotting. NC, negative control RNA; SC: scrambled control RNA; Si1 and Si2, different siRNA sequences (see supplementary Table 2). d. Knockdown of TRPM7, but not TRPC6, TRPV2 or TRPP2, abolished calcium flickers. Data are expressed as mean±s.e.m.; n =12–21 cells in each group, **p < 0.01 vs. respective SC. e. TRPM7 knockdown prevented shear stress-induced calcium flickers.
SACCs belong to the transient receptor potential (TRP) ion channel superfamily. In mammals, about eight TRP channels in four subfamilies are thought to be sensitive to mechanical forces while being calcium-permeable8. Of these, TRPM7, TRPC6, TRPV2 and TRPP2 were expressed at relatively high mRNA levels in WI-38 fibroblasts (supplementary Fig. 4). Using RNA interference, we found that calcium flickers were virtually abolished by ~75% knockdown of TRPM7, but not that of the other three TRP channels (Fig. 2c, d, supplementary Fig. 5). More importantly, similar shear stress was unable to evoke flickers in TRPM7 knockdown cells, which displayed rare basal flicker activity (Fig. 2e). These data pinpointed TRPM7 as the specific SACC responsible for transducing mechanical signals into calcium flickers. A hallmark of TRPM7 is its sensitivity to inhibition by Mg2+ in addition to Gd3+ [18]. Raising extracellular Mg2+ from 1.0 to 10 mM largely abolished calcium flickers, while removing it enhanced flicker production (supplementary Fig. 6). Thus, TRPM7 acts as the mechanical sensor, the calcium flicker igniter, and the mechanochemical transducer in fibroblasts, revealing a novel role of this SACC in the regulation of cell migration (see below).
Since calcium release from the endoplasmic reticulum (ER) amplifies calcium influxes via the calcium-induced calcium release mechanism15, we next investigated whether ER calcium release participates in calcium flicker production. Inhibition of ER calcium recycling with the Ca2+ ATPase inhibitor thapsigargin (5 µM, 20 min incubation, after recession of the initial calcium transient) halved flicker amplitude without affecting flicker probability (Fig. 3a, c). Similar results were obtained by inhibiting the IP3 receptor (IP3R) with xestospongin C (5 µM), whereas IP3-BM (2 µM), a membrane-permeable ester precursor of IP3[19], enhanced the flicker amplitude and ryanodine receptor inhibition (ryanodine, 25 µM) had no significant effect (Fig. 3a, c). Quantitative real-time PCR results showed that type 2 IP3R (IP3R2) and type 3 IP3R (IP3R3) are the primary IP3R isoforms expressed in WI-38 fibroblasts (supplementary Fig. 7). In contrast to TRPM7, RNA interference knockdown of IP3R2 (~80%), but not IP3R3 (~60%), significantly decreased flicker amplitude but failed to alter flicker probability (Fig. 3b, c). This IP3R isoform specificity is consistent with the fact that IP3R2 is more sensitive to IP3 and displays little calcium-dependent inactivation20. Taking together, we concluded that calcium entry via TRPM7 is locally amplified by calcium release through IP3R2 in the event of a calcium flicker. Coupling IP3R2 to TRPM7 would enable flicker activity to decode IP3-linked chemoattractant signal transduction.
Figure 3. Amplifying TRMP7 calcium flickers by store calcium release through type 2 IP3 receptors.
a. Typical calcium flicker responses to thapsigargin (TG), xestospongin C (Xec) or IP3-BM. Linescan data of ΔF/F0 are rendered as surface plots. b. Western blotting of IP3Rs. See supplementary Table 2 for sequences of negative control (NC), respective scrambled control (SC), Si1 and Si2. Data are expressed as mean±s.e.m. (n=3–4). **p < 0.01 vs. respective SC. c. Flicker amplitude and probability under various experimental conditions. Pf: fractional time spent in calcium flickers; HBSS: Hepes-buffered saline solution; Rya: ryanodine (25µM, 15 min); n=10–29; *p < 0.05; **p < 0.01 vs. HBSS; ††p < 0.01 vs. respective SC. Note that perturbing the ER calcium release mainly altered flicker amplitude without affecting flicker probability.
Given the role of TRPM7 as a mechanical sensor and a calcium flicker igniter, we anticipated that flicker activity would be coupled to the migration-associated traction force. Indeed, the map of flicker ignition sites at the front of a cell largely overlapped, though with subtle differences, the matrix of focal adhesions (FAs) (Fig. 4a, supplementary Fig. 8), where traction force is created and transmitted21. Rapid local application of RGDS (2 mM, 1 min), which contains the RGD sequence that is recognized by integrins22, enhanced the flicker activity, while the control peptide RGES was ineffective (Fig. 4b, c), consistent with the finding that RGDS stimulates calcium transients in neuronal filopodia and growth cones23. Disruption of protrusion by transient frontal application of cytochalasin D, inhibition of myosin ATPase by 2,3-butanedione monoxime or (−)blebbistatin24 all inhibited lamella flicker production (Fig. 4b, c). These lines of evidence support the idea that decoding local membrane tension by flicker activity depends on cytoskeletal and morphological integrity.
Figure 4. Traction force generation and calcium flicker activity.
a. Maps for calcium flicker ignition sites (red dots) and FAs (green). FAs were visualized by immunostaining for integrin α5 after calcium flicker acquisition. Enlarged views of calcium flickers, FAs, and their overlay are shown to the right. “N” denotes the nucleus. Scale bar: 8 µm. b. Lamella flicker prior to (left) and after (right) application of compounds that affect traction force-generating elements. c. Statistics of calcium flicker amplitude and probability (Pf). HBSS, Hepes-buffered saline solution; BDM, 2,3-butanedione monoxime (10 mM); (−)BB, (−)blebbistatin (100 µm); cytoD, cytochalasin D. Error bars represent s.e.m.; n = 10–19 in each group. **p < 0.01 vs. RGES; ††p < 0.01 vs. HBSS. Note that flicker probability rather than amplitude was preferentially altered by varying the traction force, in contrast to the situation shown in Fig. 3.
Despite the low global calcium concentration, high-calcium microdomains created by mechanical stress in the leading lamella may activate a multitude of local calcium-dependent events critical to cell polarization and movement, including the PIP3 signalling cascade25, 26, a parallel phospholipase A2-mediated signalling mechanism27, cytoskeleton dynamic such as actin remodelling10, FA detachment and relocation, and actin-myosin contraction. Next, we sought to determine the physiological role of calcium flickers in regulating cell migration, particularly turning behaviour that is almost entirely carried out within the leading lamella.
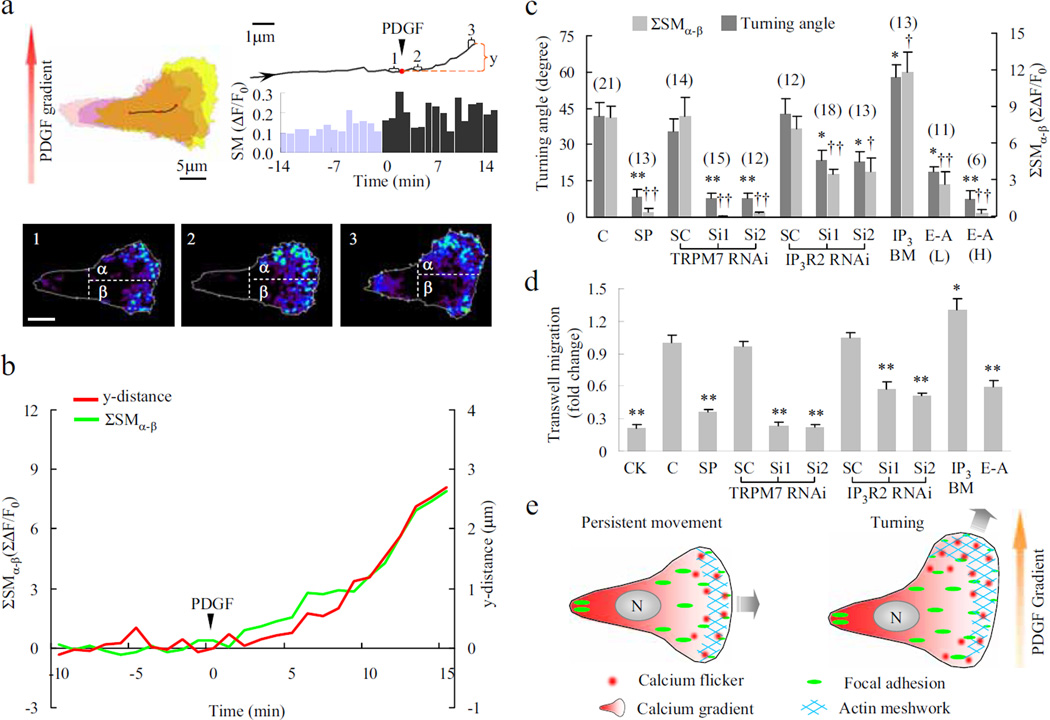
Platelet derived growth factor (PDGF) is a well-known chemoattractant that stimulates fibroblast migration during wound healing3. Its intracellular signalling pathways include generating traction force by Rac-dependent protrusion28 and activation of the phospholipase C-PIP2-IP3 signaling cascade29, both convergent on flicker production. When migrating fibroblasts were exposed to uniform PDGF (0.8 nM), increases in both flicker amplitude and probability were accompanied by a decrease in directional persistence (supplementary Fig. 9), the latter suggesting an enhanced propensity for turning of the cell30. When a PDGF gradient was applied in the direction perpendicular to cell movement, migrating fibroblasts no longer moved persistently along the original path; rather, they turned towards the higher PDGF concentration (Fig. 5a). Time-lapse imaging revealed a rapid increase in lamella flicker activity and an accentuated front-to-rear polarisation (Fig. 5a). More importantly, a greater flicker signal mass was found in the portion facing the PDGF source (SMα) than in the portion farther away from the source (SMβ), indicating the development of an asymmetry of flicker activity within the lamella (Fig. 5a). To demonstrate a linkage between lamella flicker asymmetry and turning behaviour, we examined the cumulative difference between SMα and SMβ (ΣSMα-β) and found that the time course of ΣSMα-β nearly overlapped with that for the distance travelled in the direction of the PDGF gradient (y-distance) (Fig. 5b). On average, the correlation coefficient between ΣSMα-β and y-distance was 0.72 in 25 migrating fibroblasts. Hence, patterned flicker activation in the leading lamella may translate directional sense and steer the cell to turn in response to PDGF gradients.
Figure 5. Calcium flickers steer fibroblast turning.
a. Asymmetrical lamella flicker activity induced by a PDGF gradient. Upper left, contours of the cell border at -14 (pink), 0 (purple) and 15 min (yellow). Upper right, trajectory of the centre of the cell and time course of calcium flicker production (bar graph). “SM”: signal mass of calcium flickers within the lamella. Lower panels: overlays of calcium flickers in 1-min windows (labelled 1–3 in the trajectory above). Dashed lines bisect the leading lamella into upper (α, facing the PDGF source) and lower portions (β) b. Correlation between cumulative asymmetric flicker activity (ΣSMα-β) and displacement along the PDGF gradient (y-distance). c. Relationship between turning angle and ΣSMα-β (at 15 min). C, control with no treatment; SP, streptomycin 200 µM; Si1, Si2 and SC: two siRNA constructs and a scrambled control; IP3-BM 2 µM; E-A, EGTA-AM, L, 2 µM, H, 20 µM. Data are expressed as mean±s.e.m.; “n” values are shown in parentheses. *p < 0.05; **p < 0.01 vs. turning angle of control or respective SC; †p < 0.05; ††p < 0.01 vs. ΣSMα-β of control or respective SC. d. Chemotaxis of WI-38 fibroblasts. CK, chemokinesis assay with the same concentration of PDGF-BB on both sides of the well; E-A, EGTA-AM (2 µM). n=4–10, *p < 0.05; **p < 0.01 vs. control or respective SC. e. Cartoons of patterned calcium flicker activation in persistently moving (left) and turning fibroblasts (right). Calcium flicker activity during persistent movement displays a front-to-rear polarisation, opposing a rear-to-front global calcium gradient. During turning, calcium flicker activity becomes asymmetric across the leading lamella, in addition to enhanced flicker frequency and accentuated front-to-rear polarisation.
To further test the above hypothesis, we showed that impaired PDGF-induced lamella flicker asymmetry or diminishment of ΣSMα-β by streptomycin and TRPM7 or IP3R2 knockdown was accompanied by dwindling of the turning angle in a roughly proportional manner (Fig. 5c). Likewise, a robust match between the flicker activity and population chemotaxis was revealed by various pharmacological and molecular interventions including TRPM7 and IP3R2 knockdown, and SACC blockade (Fig. 5d, supplementary Fig. 10). Furthermore, loading EGTA ester to disturb the flicker signal (supplementary Fig. 11) compromised the turning and chemotaxis abilities (Fig. 5c, d), while flicker activation by IP3-BM enhanced both of them (Fig. 5c, d), suggesting a causal link between calcium flicker activity and fibroblast turning and chemotaxis.
In summary, we have demonstrated that calcium flickers arising from TRPM7 and IP3R2 play an essential role in steering migrating fibroblasts. Despite that the global calcium gradient is opposite to the direction of cell migration, high calcium flicker activity would enable activation of calcium signalling cascades amidst a low calcium background at the leading edge, such that spatiotemporally patterned calcium flicker activity can orchestrate the complex turning behaviour of migrating cells (Fig. 5e). The coupling of TRPM7-mediated force-transducing calcium influx and local IP3-induced calcium release would make this an ideal system for locomotion in response to chemoattractants (Fig. 5e). The present finding may have general ramifications because growth cones of neurons turn away from the side which filopodia displays higher local calcium signals23 and calcium influx is essential to maintaining the leading-edge structure and activity in macrophages14. As such, unveiling calcium flickers in migrating cells opens a new avenue to investigate how local calcium signals orchestrate diverse biochemical pathways in the guidance of directional movement.
Methods
Cell Culture
Human lung embryonic WI-38 fibroblasts (21 population doublings, PDs) obtained from the American Type Culture Collection were maintained and subcultured to 28 PDs in MEM (Gibco) supplemented with 10% FBS (Hyclone), 2 mM glutamine and 200 units/ml penicillin in a 37°C, 5% CO2 incubator. For functional experiments, cells were plated at a density of 1×104/cm2 and cultured for 10 h on coverslips coated with 5 µg/ml fibronectin (Sigma).
Calcium Imaging
WI-38 cells were loaded with fluo-4 AM (5 µM) alone or in combination with fura-red AM (5 µM) for 6 min at 37°C, rinsed twice, and then bathed in Hepes-buffered saline solution containing (in mM): 134 NaCl, 5.4 KCl, 1.0 MgSO4, 1.0 NaH2PO4, 1.8 CaCl2, 20 Hepes, and 5 D-glucose (pH 7.4) with 1% FBS, unless otherwise specified. Cells were placed in a 37°C heated chamber (Zeiss S-Type incubator) and imaged on a Zeiss LSM 510 confocal microscope with a 40× oil objective (NA 1.3) at radial and axial resolutions of 0.4 and 1.0 µm, respectively. For ratiometric imaging, cells were excited at 488 nm, emission was detected at 505–550 nm (fluo-4 signal) and >633 nm (fura-red signal), and DIC transmission image was acquired simultaneously. For migration path analysis and calcium flicker signal mass measurement, 300–600 time-lapse images were acquired at 6 s intervals. High resolution linescan imaging of calcium flickers was performed at 3 ms per linescan.
PCR
Total RNA was isolated from 28PDs WI-38 fibroblasts with TRI Reagent (Sigma) and converted to cDNA by using M-MLV reverse transcriptase (Promega). Quantitative RT-PCR reactions were carried out using these cDNAs in an iQ5 real-time PCR detection system (BioRad). Results were read out using iQ5 optical system software. All samples showing primer dimer formation or spurious, non-specific peaks, as indicated by the dissociation curve, were excluded from analysis. The primers are shown in supplementary Table 1.
RNA Interference
RNAi sequences for IP3R isoforms and TRP channels were designed using RNAi Designer (http://www.invitrogen.com/rnai) (supplementary Table 2). Each scrambled control was designed corresponding to first duplex of siRNA. Briefly, corresponding siRNA duplexes were synthesized (GenePharma, Shanghai or Invitrogen, USA) and transfected into cells with Lipofectamine RNAiMax (Invitrogen) according to the manufacturer’s recommendations. Western blotting or functional studies were carried out 72 hours after transfection.
Western Blotting
Total protein extracted from WI-38 cells with siRNA treatment was separated on 4–12% NuPAGE Novex Bis-Tris gels (Invitrogen) and transferred to PVDF membranes (Millipore). After blocking for 1 h with 5% nonfat dry milk, the PVDF membrane was probed with primary antibody (anti-IP3R2 is the gift of Ju Chen at UCSD; anti-IP3R3 from Santa Cruz; anti-tubulin from Sigma; anti-TRPC6 from Millipore; anti-TRPV2 from ABR; anti-TRPP2 and anti-TRPM7 from Abcam) for 2 h at room temperature, and then secondary antibody (IRDye-conjugated anti-mouse, anti-rabbit and anti-goat IgG from LI-COR) for 1 h at room temperature. Immunoblots were detected using the Odyssey imaging system.
Cell Migration Analysis
Fibroblasts with an overt leading lamella and a thin trailing edge were selected for migration analysis. The outer boundary of the cell was extracted from the respective fluorescence image for calculation of its centre of gravity. The centres of consecutive images (6 s apart) defined the trajectory of cell movement. Migration speed was calculated as the average displacement per min during 30 min. Directional persistence (D/T ratio) was calculated as the ratio between the linear displacement and the total length of the trajectory during 30 min.
To establish a PDGF-BB (PeproTech) gradient perpendicular to the long axis of a polarized migrating fibroblast, a 5 µm internal diameter, PDGF BB-containing (3 nM) micropipette was placed ~150 µm away from one side of the cell. By visualisation of sulfurhodamine fluorescence under similar conditions, we estimated an average PDGF concentration of 1 nM and an edge-to-edge difference of 0.4 nM across the leading lamella (~40 µm).
Chemotaxis Assay
24–well Transwell plates with inserts containing 8 µm pores in a polycarbonate membrane (Corning) were used for chemotaxis assays. Briefly, the outer wells contained 600 µL MEM medium containing 1% FBS with PDGF-BB (0.8 nM) as chemoattractant. Approximately 8×103 overnight-starved (1% FBS) WI-38 fibroblasts in 100 µL PDGF-BB free MEM medium containing1% FBS and designated drug were added to each insert. In chemokinesis control group, PDGF-BB (0.8 nM) was also added to the insert to abolish the concentration gradient. Transwell plate was then incubated for 12 hours in a 37°C, 5% CO2 incubator before assay.
For assay, the inserts were loaded with 5 µM calcein AM for 10 min and then fixed immediately with 3% formaldehyde for 10 min. Cells in inserts were cleared and those under the lower surface of the polycarbonate membrane were imaged and analysed.
Application of Mechanical Forces
Shear stress was locally applied by a gentle jet flow (4 cm H2O pressure) via a patch pipette (10 µm internal diameter) ~80 µm away from the front of migrating fibroblasts. Note that jet flow used in local drug delivery (1 cm H2O pressure, ~120 µm placement, pipette with 5 µm internal diameter) did not alter calcium flicker activity (n=4).
Recording SACC Currents and Imaging Local Calcium Influx
Cell-attached patch-clamp technique, using an EPC-7 amplifier (Germany), was applied to fibroblasts preloaded with the calcium indicator, fluo-4 AM. The patch pipette (2–3 MΩ) solution contained (in mM): NaCl 140, KCl 5.4, MgCl2 1.0, Hepes 20, and CaCl2 1.8 (pH 7.4, adjusted with NaOH). To activate SACCs, mechanical suction of ~40 mm Hg was applied via a syringe connected to the patch pipette while the patch membrane was held 80 mV more negative than the resting membrane potential to enhance Ca2+ entry. The single-channel currents were filtered at 3 kHz and digitised at 5 kHz with pClamp 6.0 software. Linescan images of local calcium immediately beneath the patch membrane were acquired simultaneously at 3 ms resolution.
Data Analysis
Digital image processing used IDL software (Research Systems) and custom-devised computer algorithms. Statistical data are expressed as mean ± s.e.m. Student's t-test and paired t-test were applied when appropriate. A P value less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
We thank IC Bruce, X Zhu, JJ Ma, HQ Cao, XD Fu and RP Xiao for helpful discussions; ZC Liang, Q Du, CM Cao, H Huang, XM Lan, N Lin, YY Wang, RS Song, and Y Zhang for technical supports. Special thanks to XD Fu for making his lab facility available to us. This work was supported by Major State Basic Research Development Program of China (2007CB512100), and National Natural Science Foundation of China (30630021) and NIH grant (HL090905).
References
- 1.Ridley AJ, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 3.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 4.Van Haastert PJ, Devreotes PN. Chemotaxis: signalling the way forward. Nat. Rev. Mol. Cell Biol. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- 5.Pettit EJ, Fay FS. Cytosolic free calcium and the cytoskeleton in the control of leukocyte chemotaxis. Physiol Rev. 1998;78:949–967. doi: 10.1152/physrev.1998.78.4.949. [DOI] [PubMed] [Google Scholar]
- 6.Brundage RA, Fogarty KE, Tuft RA, Fay FS. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science. 1991;254:703–706. doi: 10.1126/science.1948048. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 1999;400:382–386. doi: 10.1038/22578. [DOI] [PubMed] [Google Scholar]
- 8.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 9.Hahn K, DeBiasio R, Taylor DL. Patterns of elevated free calcium and calmodulin activation in living cells. Nature. 1992;359:736–738. doi: 10.1038/359736a0. [DOI] [PubMed] [Google Scholar]
- 10.Stossel TP, Fenteany G, Hartwig JH. Cell surface actin remodeling. J. Cell Sci. 2006;119:3261–3264. doi: 10.1242/jcs.02994. [DOI] [PubMed] [Google Scholar]
- 11.Robinson RC, et al. Domain movement in gelsolin: a calcium-activated switch. Science. 1999;286:1939–1942. doi: 10.1126/science.286.5446.1939. [DOI] [PubMed] [Google Scholar]
- 12.Chew TL, Wolf WA, Gallagher PJ, Matsumura F, Chisholm RL. A fluorescent resonant energy transfer-based biosensor reveals transient and regional myosin light chain kinase activation in lamella and cleavage furrows. J. Cell Biol. 2002;156:543–553. doi: 10.1083/jcb.200110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco SJ, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 14.Evans JH, Falke JJ. Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16176–16181. doi: 10.1073/pnas.0707719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng H, Lederer WJ. Calcium Sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 16.Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacol. Rev. 1996;48:231–252. [PubMed] [Google Scholar]
- 17.Zou H, Lifshitz LM, Tuft RA, Fogarty KE, Singer JJ. Visualization of Ca2+ entry through single stretch-activated cation channels. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6404–6409. doi: 10.1073/pnas.092654999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am. J. Physiol Cell Physiol. 2007;292:C460–C467. doi: 10.1152/ajpcell.00367.2006. [DOI] [PubMed] [Google Scholar]
- 19.Thomas D, et al. Microscopic properties of elementary Ca2+ release sites in non-excitable cells. Curr. Biol. 2000;10:8–15. doi: 10.1016/s0960-9822(99)00258-4. [DOI] [PubMed] [Google Scholar]
- 20.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz AR, Parsons JT. Cell migration--movin' on. Science. 1999;286:1102–1103. doi: 10.1126/science.286.5442.1102. [DOI] [PubMed] [Google Scholar]
- 22.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 23.Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- 24.Shu S, Liu X, Korn ED. Blebbistatin and blebbistatin-inactivated myosin II inhibit myosin II-independent processes in Dictyostelium. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1472–1477. doi: 10.1073/pnas.0409528102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price LS, et al. Calcium signaling regulates translocation and activation of Rac. J. Biol. Chem. 2003;278:39413–39421. doi: 10.1074/jbc.M302083200. [DOI] [PubMed] [Google Scholar]
- 26.Weiner OD, et al. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Haastert PJ, Keizer-Gunnink I, Kortholt A. Essential role of PI3-kinase and phospholipase A2 in Dictyostelium discoideum chemotaxis. J. Cell Biol. 2007;177:809–816. doi: 10.1083/jcb.200701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawkins PT, et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr. Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 29.Claesson-Welsh L. Platelet-derived growth factor receptor signals. J. Biol. Chem. 1994;269:32023–32026. [PubMed] [Google Scholar]
- 30.Ware MF, Wells A, Lauffenburger DA. Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in a matrix-dependent manner. J. Cell Sci. 1998;111(Pt 16):2423–2432. doi: 10.1242/jcs.111.16.2423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.