Abstract
Animals exhibit a rapid and sustained anorexia when fed a diet that is deficient in a single indispensable amino acid (IAA). The chemosensor for IAA deficiency resides within the anterior piriform cortex (APC). Although the cellular and molecular mechanisms by which the APC detects IAA deficiency are well established, the efferent neural pathways that reduce feeding in response to an IAA-deficient diet remain to be fully characterized. In the present work, we investigated whether 1) central melanocortin signaling is involved in IAA deficiency-induced anorexia (IAADA) and 2) IAADA engages other key appetite-regulating neuronal populations in the hypothalamus. Rats and mice that consumed a valine-deficient diet (VDD) for 2–3 wk exhibited marked reductions in food intake, body weight, fat and lean body mass, body temperature, and white adipose tissue leptin gene expression, as well as a paradoxical increase in brown adipose tissue uncoupling protein-1 mRNA. Animals consuming the VDD had altered hypothalamic gene expression, typical of starvation. Pharmacological and genetic blockade of central melanocortin signaling failed to increase long-term food intake in this model. Chronic IAA deficiency was associated with a marked upregulation of corticotropin-releasing hormone expression in the lateral hypothalamus, particularly in the parasubthalamic nucleus, an area heavily innervated by efferent projections from the APC. Our observations indicate that the hypothalamic melanocortin system plays a minor role in acute, but not chronic, IAADA and suggest that the restraint on feeding is analogous to that observed after chronic dehydration.
Keywords: essential amino acid, valine, melanocortin, corticotropin-releasing hormone, parasubthalamic nucleus
laboratory animals that are fed an indispensable amino acid (IAA)-deficient diet develop a rapid and sustained anorexia as part of a coordinated adaptive response to maintain a steady supply of IAA from body stores (24, 26). IAA deficiency-induced anorexia (IAADA) is mediated by the central nervous system and is not dependent upon olfaction, taste, or other peripheral sensory systems (25). IAADA necessitates the detection of the IAA deficiency, transduction of the signal, and activation of neural circuits that control feeding behavior (15, 16, 26, 33). The chemosensor for IAA deficiency resides within the anterior piriform cortex (APC). Animals with APC lesions fail to reduce their intake of IAA-deficient diets (14, 26, 31), and infusion of nanomole doses of the limiting IAA into the APC selectively reverses IAADA (2, 28, 35). Furthermore, recent in vitro studies demonstrated that the APC is a direct sensor of IAA deficiency, requiring no afferent input from sensory organs or other brain structures (34). However, it is still unknown how efferent signals from the APC ultimately lead to an inhibition of feeding, where the signals are received, or how the signals are translated into reduced food intake.
The data presented here, as well as data from other groups, indicate that animals with IAADA exhibit hormonal and hypothalamic responses that are identical to or more robust than those found in pair-fed (PF) control animals, reflecting a normal sensing of negative energy balance. This is analogous to animals rendered anorexic secondary to drinking hypertonic saline, in which a feeding inhibitory circuit involving corticotropin-releasing hormone (CRH)-expressing neurons in the lateral hypothalamic area (LHA) was described (41, 44).
In the present study, we sought to identify the neural pathways that bridge the sensing of IAA deficiency in the APC with the control of feeding behavior. We demonstrate that the central melanocortin system plays only a minor role in the acute response to IAA deficiency and no obvious chronic role in IAADA. We also provide evidence for a novel circuit between the APC and CRH-expressing neurons in the parasubthalamic nucleus (PSTN), supporting the idea that IAADA has analogies with the mechanisms underlying dehydration-induced anorexia. Our data offer novel insights into IAADA and indicate that there are endogenous neural systems that can overcome orexigenic drive, thereby providing a novel avenue for obesity therapy.
MATERIALS AND METHODS
Experimental Animals and Diets
Male Sprague Dawley rats (7–8 wk old) were purchased from Charles River Laboratories (Wilmington, MA). Male and female melanocortin receptor 4 (MC4R) knockout (MC4R-KO) and proopiomelanocortin (POMC)-enhanced green fluorescent protein (eGFP) mice (8–10 wk old) were generated as previously described (10, 23). Age-matched C57BL/6J wild-type (WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Rats and mice were individually housed in rooms with controlled temperature (22 ± 2°C) and illumination (12:12-h light-dark cycle). All animals were allowed ad libitum access to food and water unless otherwise stated and were allowed to acclimate for at least 3 days before being used in procedures. All studies were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University. No mortality was observed in any of these studies.
All animals were initially fed standard laboratory rodent chow (Rodent Diet 5001; Purina Mills, St. Louis, MO). Experimental diets were obtained from Harlan Teklad Laboratories (Indianapolis, IN). The control diet (CD, catalog no. TD. 99366) was balanced in amino acids and contained 15.4% protein, 65.0% carbohydrate, and 8.0% fat by weight. The valine-deficient diet (VDD, catalog no. TD. 08578), an isonitrogenous modification of the CD, was deficient in valine and enriched in alanine, aspartic acid, glutamic acid, and glycine but was otherwise identical in nutrient composition to the CD.
One day before each experiment commenced, the animals were weighed and divided into treatment groups such that the mean body weights of each group were similar. During the experiments, food intake and body weight were measured at the same time each day, unless otherwise noted.
Short- and Long-Term Feeding Studies in Rats
All rats were switched from standard chow to the CD for at least 3 days. Rats that continued to gain weight on the CD were divided into the following dietary groups: CD, VDD, or PF (n = 5–6/group). PF rats were given the same amount of the CD as the mean amount of VDD consumed on the previous day by the VDD group. Body weight and food intake were measured daily for 3 (short-term study) or 21 (long-term study) consecutive days. In the long-term study, the VDD rats began to shred their food after 3 days on the VDD. Thereafter, the shredded food was carefully collected, weighed, and then replaced by the same weight of fresh food pellets each day.
Long-Term Feeding Study in Mice
Male WT and MC4R-KO mice were fed the CD for at least 3 days and then were divided into the following treatment groups: WT/CD, WT/VDD, WT/PF, MC4R-KO/CD, MC4R-KO/VDD, and MC4R-KO/PF (n = 7–8/group). Mice in the PF groups were given the same amount of the CD as the mean amount of VDD consumed on the previous day by the VDD mice of the same genotype. Body weight and food intake were measured for 14 consecutive days. Damaged food pellets were first seen in the VDD groups' cages after 6 days of consuming the VDD. For the remainder of the study, the food pellets were replaced each day as described above.
Acute Nocturnal Mouse Feeding Studies
Male WT and MC4R-KO mice were fed the CD for 10 days before initiating study diets. The mice were then divided into the following four groups: WT/CD (n = 15), WT/VDD (n = 24), MC4R-KO/CD (n = 14), and MC4R-KO/VDD (n = 21). Two hours before the onset of the dark phase on the day of the experiment, all animals were placed in clean individual cages without food. Just before lights off, preweighed CD or VDD pellets were placed in each cage. For the next 12 h, food weights were measured hourly under red light illumination. Care was taken to minimize stress to the animals during the frequent food measurements.
In a separate study, female WT (n = 8) and MC4R-KO (n = 7) mice were fed the CD for 10 days. On the day of the experiment, both groups of mice were switched to the VDD. Food intake was recorded hourly throughout the 12-h dark phase using the same procedure described above. We observed identical results in female mice as we observed in male mice (data not shown).
Melanocortin Receptor Antagonism Study
Cannulation and intracerebroventricular infusion.
Rats were implanted with 28-day duration Alzet miniosmotic pumps (model 2004; Alzet, Cupertino, CA) and brain infusion kits (Brain Infusion Kit 2; Alzet). On the day before the surgical procedure, the minipumps, catheters, and brain infusion kits were filled with artificial cerebrospinal fluid (aCSF; Harvard Apparatus, Holliston, MA) or the melanocortin 3/4 (MC3/4R) antagonist SHU-9119 (1 nmol/day; Phoenix Pharmaceuticals, Burlingame, CA) according to the manufacturer's instructions. The filled minipumps with attached brain infusion assemblies were primed in sterile saline at 37°C overnight.
Rats were fed the CD for 1 wk and were then divided into the following two groups: VDD/aCSF (n = 7) and VDD/SHU-9119 (n = 7). Animals were anesthetized with 4% isoflurane, placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA), and implanted with sterile cannulas from the brain infusion kits into the lateral cerebral ventricle. The following coordinates used were: 1.0 mm caudal to bregma, 1.5 mm lateral to the midline, and 4.0 mm below the surface of the skull. Stainless steel screws were anchored to the skull, the cannulas were fixed in place with dental cement, and the attached miniosmotic pumps were implanted subcutaneously in the back. Immediately after the surgeries, all rats were fed the VDD for 21 days. Food intake and body weight were measured daily. The rats were killed after 21 days, at which time any remaining SHU-9119 in the brain infusion systems was drawn out and delivered via intracerebroventricular injection to a separate group of CD-fed rats to confirm bioactivity of the SHU-9119 at the end of the experiment.
Body temperature.
Body temperature was measured using implantable telemetric transponders and external receivers (MiniMitter, Bend, OR) that were implanted 2 days before cannulation and drug administration to establish a baseline for each animal. Rats were anesthetized with 4% isoflurane, a small midline incision was made just anterior to the interscapular brown adipose tissue (BAT), and transponders were implanted beneath the BAT such that innervation was unperturbed. Temperature was recorded at 5-min intervals using the Vital View Data Acquisition System (MiniMitter).
Body Composition
Body composition (fat and lean body mass) was measured using magnetic resonance relaxometry (EchoMRI 4-in-1 Live Animal Composition Analyzer; Echo Medical System, Houston, TX).
Tissue Collection
At the end of each long-term experiment, the animals were killed by decapitation under isoflurane anesthesia. Blood was collected for hormone analysis via cardiac puncture or decapitation into EDTA blood collection tubes, and protease inhibitor (Complete, Roche, Germany) was added according to the manufacturer's instructions. Plasma was isolated and stored at −80°C until used. Brains were immediately removed, and hypothalamic blocks were dissected as described previously (37). Interscapular BAT and epididymal white adipose tissue (WAT) were also isolated. Hypothalami, WAT, and BAT were stabilized in RNAlater at 4°C for subsequent RNA extraction.
RNA Preparation and Quantitative PCR
Total RNA preparation and real-time PCR were performed as described previously (37). RNA was extracted using RNeasy kits (Qiagen, Valencia, CA). Reverse transcription and quantitative PCR reagents were obtained from Life Technologies (Carlsbad, CA). 18S or GAPDH RNA were used as endogenous controls. Gene expression is reported as fold change relative to the CD group using the 2−ΔΔCt method. Statistical analyses were performed on the ΔCt values.
Plasma Leptin, Insulin, Insulin-Like Growth Factor-I, Corticosterone, and Ghrelin Quantification
Concentrations of plasma leptin and insulin-like growth factor-I (IGF-I) in mice and rats were determined using enzyme-linked immunosorbent assay (ELISA) kits [mouse leptin and rodent IGF-I ELISA kits (R&D Systems, Minneapolis, MN) and rat leptin ELISA kit (Millipore, Billerica, MA)]. Concentrations of plasma insulin in mice and rats (Millipore) and plasma corticosterone (MP Biomedicals, Santa Ana, CA) and acyl-ghrelin (Millipore) in rats were quantified with appropriate RIA kits. All assays were performed according to the manufacturers' instructions.
Anterograde Tract-Tracing Study
Male rats (280–320 g; n = 10) were used in this study. Animals received a single iontophoretic injection of phaseolus vulgaris leucoagglutinin (PHA-L; Vector Laboratories, Burlingame, CA) within the APC. Each animal was anesthetized with 4% isoflurane, and a glass micropipette (tip diameter = 10–15 μm) filled with 2.5% PHA-L solution was stereotaxically positioned into the APC. The coordinates used were as follows: x = ± 3.7, y = 2.4, z = −6.3; x = ± 3.2, y = 2.7, z = −6.8; or x = ± 3.6, y = 3.0, z = 5.8 (mm, x = lateral to midline, y = rostral to bregma, z = below surface of skull). A +4.5-μA current pulsed at 7-s intervals was applied for 20 min using an iontophoresis pump (BAB-501; Kation Scientific, Minneapolis, MN). After a survival time of 14–16 days, animals were transcardially perfused with 4% paraformaldehyde in 0.01 M PBS, and brains were processed for immunohistochemistry (IHC). Six series of 30-μm coronal sections were cut and collected from the APC through the hypothalamus. Sections were processed for IHC with anti-PHA-L primary antibody (1:1,000; Vector Laboratories) and secondary antibody conjugated with Alexa Fluor 594 or 488 (1:500; Invitrogen, Carlsbad, CA) for visualization. One complete series of sections from the APC through the hypothalamus was stained for confirmation of injection sites and transporting fibers. Only animals with injection sites centered within the morphological APC borders as well as visible PHA-L-labeled fibers in sections were included in the analysis. To observe connections between PHA-L-labeled fibers and CRH-, somatostatin (SS)-, melanin-concentrating hormone (MCH)-, or orexin-immunoreactive neurons in the hypothalamus, a series of hypothalamic sections was processed for double- or triple-label IHC. Floating sections were incubated for 48 h with primary antibodies against PHA-L (1:1,000), CRH (1:4,000; Bachem, San Carlos, CA), SS (1:100; GeneTex, Irvine, CA), MCH (1:1,500; Santa Cruz Biotechnology), and orexin (1:1,500; Santa Cruz Biotechnology), followed by incubation in secondary antibodies conjugated with Alexa Fluor 488, 594, and 647 for visualization. Sections were viewed under a fluorescence microscope, and a rat brain atlas (32) was used to verify cytoarchitecture. Confocal photomicrographs were taken using a Zeiss LSM710 microscope (Carl Zeiss, Oberkochen, Germany) under identical microscope settings. Images were then processed using Imaris software (Imaris x64 7.3.1; Bitplane).
In Situ Hybridization
After receiving either CD, VDD, or being PF for 5 days, rats were killed, and brains were removed, snap-frozen, stored at −80°C, and processed for in situ hybridization as previously described (20), with the following modifications. Hypothalamic coronal sections (20 μm) were cut on a cryostat, collected in a 1:6 series, and stored at −80°C. Antisense 33P-labeled rat CRH riboprobe (corresponding to bases 344–751 of rat CRH; GenBank accession no. NM_031019.1; 0.15 pmol/ml), SS riboprobe (corresponding to bases 14–447 of rat SS; GenBank accession no. NM_012659; 0.01 pmol/ml), or MCH riboprobe (corresponding to bases 122–449 of rat promelanin-concentrating hormone; GenBank accession no. NM_012625.1; 0.005 pmol/ml) were denatured, dissolved in hybridization buffer containing tRNA (1.7 mg/ml), and applied to slides. Slides were covered with glass cover slips, placed in a humid chamber, and incubated overnight at 55°C. The following day, slides were treated with RNase A and washed under conditions of increasing stringency. Slides were dipped in 100% ethanol, air-dried, and then dipped in NTB-2 liquid emulsion (Eastman Kodak, Rochester, NY). Slides were developed and coverslipped 1 day (SS and MCH) or 7 days (CRH) later. Blinded counts of the number of silver grain clusters (corresponding to radiolabeled CRH, SS, or MCH mRNA) as well as the number of silver grains/cell were made using Grains 2.0.b software (University of Washington, Seattle, WA). After counts were analyzed, all slides were decoded, and representative slides were chosen for Nissl counterstaining to verify important anatomical landmarks and cytoarchitecture.
Nuclear c-Fos Analysis in the PSTN
Male rats were individually housed and fed the CD for 7 days before the experimental day. Two hours before the onset of the dark phase on the experimental day, the rats were placed in clean cages without food. Just before lights off, preweighed CD or VDD pellets were placed in each cage (n = 3 rats/group). Ninety minutes after feeding commenced, the remaining CD and VDD pellets were weighed for confirmation of reduced food intake in the VDD rats. The rats were then deeply anesthetized with a ketamine-xylazine-acepromazine cocktail and transcardially perfused with 0.01 M PBS followed by ice-cold 4% paraformaldehyde in 0.01 M PBS. Brains were removed, postfixed for 2–4 h, cryopreserved overnight in 20% sucrose, frozen on dry ice, and stored at −80°C.
Coronal hypothalamic sections were cut at 30 μm on a freezing microtome. Six sets of sections were collected from the diagonal band of Broca caudally through the mammillary bodies. Free-floating sections were stored in 0.01 M PBS at 4°C. The sections were incubated for 1 h at room temperature in blocking reagent (5% normal donkey serum in 0.01 M PBS and 0.1% Triton X-100; PBST). After the initial blocking step, the sections were incubated in rabbit anti-c-Fos antibody (PC38; EMD Biosciences, San Diego, CA) diluted 1:50,000 and rabbit anti-c-Fos antibody (SC-52; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:25,000 in blocking reagent for 72 h at 4°C. The sections were then incubated in donkey anti-rabbit Alexa Fluor 594 (1:500; Invitrogen) in PBST for 2 h at room temperature. Between each step, the sections were washed thoroughly with 0.01 M PBS. Some sections were incubated in the absence of primary antibodies to confirm specificity of the secondary antibody. Sections were mounted on gelatin-coated slides and coverslipped using Vectashield mounting media (Vector Laboratories). The number of c-Fos-immunoreactive cells was manually counted in sections containing the PSTN using a fluorescence microscope (Leica 4000 DM; Leica Microsystems, Bannockburn, IL) by an investigator blinded to the treatment groups.
Nuclear c-Fos Analysis in POMC Neurons
To ensure robust simultaneous activation of POMC neurons in the experimental groups, we utilized a validated food-entrained mouse model (21). Male POMC-eGFP mice (n = 40) were allowed access to the CD from 0900–1100 each day for 8 days to establish strong and predictable food anticipatory behaviors. Mice were divided into the following nine treatment groups (n = 3–5/group): 3 diet treatments (CD, VDD, and fasted) × 3 time points (1.5, 2, and 3 h of feeding time). On day 9 at 0900, the CD and VDD mice were fed their respective diets for 2 h. Food was withheld from the fasted groups. At 1.5, 2, or 3 h after feeding commenced, mice were deeply anesthetized with a ketamine-xylazine-acepromazine cocktail and transcardially perfused with 0.01 M PBS followed by ice-cold 4% paraformaldehyde in 0.01 M PBS. Brains were processed, sectioned, and used for c-Fos IHC as described above. The number of c-Fos-immunoreactive green fluorescent protein-expressing cells was manually counted in sections containing the arcuate nucleus (ARC) by an investigator blinded to the treatment groups.
Statistical Analysis
All data are presented as means ± SE for each group. Statistical analyses were performed using GraphPad Prism 5 (La Jolla, CA). Comparisons between two groups at a single time point were performed using two-tailed Student's t-tests. Comparisons between three or more groups were performed by one-way ANOVA followed by post hoc analysis using Bonferroni-corrected t-tests. All comparisons involving two variables or multiple time points were performed using two-way ANOVA followed by post hoc analysis using Bonferroni-corrected t-tests. P < 0.05 was considered statistically significant.
RESULTS
Dietary Valine Deficiency Reduces Food Intake and Body Weight in Rats
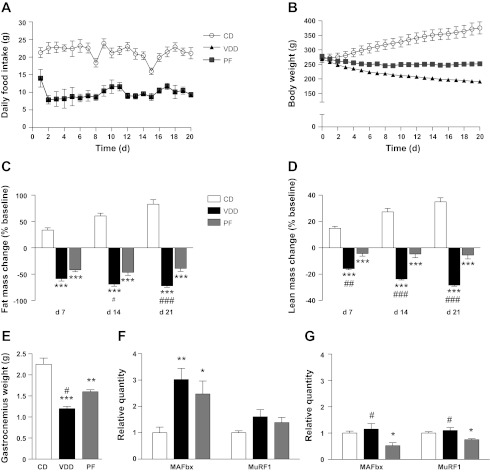
We first evaluated the consequences of chronic dietary valine deficiency on food intake and body weight in rats. In rats fed the experimental diets for 21 days, daily food intake was significantly decreased in the VDD group compared with the CD group during the entire experimental period (P < 0.001; Fig. 1A). Whereas the CD group continued to gain weight over the course of the experiment, the VDD group weighed significantly less than the CD group as early as 4 days after introduction of the VDD and continued to lose weight for the duration of the experiment (P < 0.01; Fig. 1B). In contrast to the VDD group, the initial loss of body weight in the PF group was followed by an extended period of stable weight, typical of calorically restricted animals. The VDD rats weighed significantly less than the PF rats during the last 8 days of the experiment (P < 0.05; Fig. 1B).
Fig. 1.
Chronic dietary valine deficiency reduces feeding and alters body composition in rats. Daily food intake (A), body weight (B), change in fat mass (C), change in lean mass (D), and gastrocnemius weight (E) in rats that were fed the control diet (open circles, n = 5), valine-deficient diet (black triangles, n = 5), or were pair-fed (gray squares, n = 5) for 21 days (d). MAFbx and MuRF1 gene expression in the gastrocnemius muscles of rats that consumed the control diet (n = 5–6), valine-deficient diet (n = 5–6), or were pair-fed (n = 5–6) for 3 days (F) or 21 days (G). CD, control diet; VDD, valine-deficient diet; PF, pair fed. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. CD. #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. PF.
Dietary Valine Deficiency Reduces Fat and Lean Body Mass in Rats
Whereas the CD group gradually gained fat and lean body mass over the course of the 21-day experiment, the VDD rats lost fat and lean body mass after only 7 days on the VDD (Fig. 1, C and D). The PF group also lost a significant amount of fat and lean body mass after 7 days but retained more fat and lean mass than the VDD group on days 14 and 21. Indeed, after 7 days, the PF group did not lose any further lean mass, whereas the VDD group exhibited a steady loss of lean body mass throughout the study. At the end of the experiment, gastrocnemius weights were significantly reduced in the VDD rats compared with the CD or PF animals (Fig. 1E). Furthermore, the expression of the muscle catabolic genes, MAFbx and MuRF1, was significantly elevated in rats that were fed the VDD for 3 days (compared with CD animals; Fig. 1F) or 21 days (compared with PF rats; Fig. 1G), indicating that VDD consumption activates an atrophy transcriptional program in skeletal muscle. Finally, the percentage of lean mass that is comprised of water was the same between all groups after 21 days of VDD exposure, suggesting that dehydration was not present in these animals (CD: 79.7 ± 0.3%, VDD: 82.2 ± 1.0%, PF: 82.7 ± 0.9%; P > 0.05).
Pharmacological Melanocortin Receptor Blockade Does Not Reverse Chronic Anorexia Induced by Dietary Valine Deficiency
We next investigated whether pharmacological blockade of central MC3/4R signaling reverses the effects of VDD consumption on food intake, body weight, and body composition. After 2 days on the CD (days −2–0), rats were switched to the VDD and were chronically infused with aCSF or SHU-9119 (1 nmol/day) for 21 days. For the first 3 days of treatment (days 1–3), the rats that were infused with SHU-9119 exhibited significantly greater daily food intake than the aCSF-treated animals (Fig. 2A). After the 3rd day of treatment, food intake declined in the SHU-9119-treated rats and no longer differed between the two groups for the remainder of the experiment. Similarly, the SHU-9119-treated animals lost less body weight than the aCSF-treated rats during the first 3 days of treatment but not on days 3–21 (Fig. 2, B and C). At the end of the 21-day treatment period, both groups had lost fat and lean body mass compared with pretreatment levels (Fig. 2D). Whereas the aCSF group lost 73% of their baseline fat mass, the SHU-9119 group only lost 49% of their baseline fat mass, indicating that body fat was preserved by SHU-9119 treatment. There was no difference in lean body mass loss between the two treatment groups.
Fig. 2.
Pharmacological melanocortin 3/4 receptor (MC3/4R) antagonism attenuates acute, but not chronic, anorexia induced by dietary valine deficiency. Daily food intake (A), body weight (B), change in body weight (C), change in fat and lean body mass (D), and body temperature (E) in rats that consumed the valine-deficient diet while being centrally infused with artificial cerebrospinal fluid (aCSF) (open circles, n = 5–7) or SHU-9119 (1 nmol/day, black triangles, n = 5–7) for 21 days. All rats were fed the control diet for 2 days before drug administration and introduction of the valine-deficient diet. Shaded bars in E denote the dark phase of the light-dark cycle. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. aCSF-treated rats.
Body temperature gradually decreased in both treatment groups during sustained VDD consumption (Fig. 2E). Notably, the daily nadir was reduced by more than 2°C compared with when the rats consumed the CD (days −2–0). As a result, the difference between daily peak and nadir temperatures gradually increased with prolonged VDD consumption, regardless of drug treatment.
To confirm the bioactivity of SHU-9119 at the end of this experiment, a separate group of animals was injected intracerebroventricularly with the residual drug remaining in the infusion pumps at the end of 21 days. This treatment significantly increased food intake and body weight in this cohort, confirming ongoing bioactivity of the SHU-9119 (data not shown).
Genetic MC4R Deletion Does Not Reverse Anorexia Induced by Chronic Dietary Valine Deficiency
We used MC4R-KO mice to further examine the role of melanocortin signaling in VDD-induced anorexia. WT and MC4R-KO mice that consumed the VDD for 14 days ate significantly less food than their respective CD groups (P < 0.001; Fig. 3A). Unexpectedly, the MC4R-KO/VDD mice reduced their food intake to a greater extent than the WT/VDD mice. When normalized to body weight, cumulative food intake in the MC4R-KO/VDD mice was significantly less than in the WT/VDD animals (WT: 1.12 ± 0.05 g/g and MC4R-KO: 0.85 ± 0.06 g/g; P < 0.01).
Fig. 3.
Melanocortin receptor 4 (MC4R) null mice exhibit chronic anorexia in response to dietary valine deficiency. Daily food intake (A), body weight (B), change in fat mass (C), and change in lean mass (D) in wild-type (circles, n = 7–8/group) and MC4R-knockout (KO) (triangles, n = 7–8/group) mice that were fed the control diet (open symbols), valine-deficient diet (black symbols), or were pair-fed (gray symbols) for 14 days. Note that, in A, the PF group symbols are not visible because they are identical to their respective VDD group. WT, wild type. #P < 0.05 vs. WT/VDD mice.
The WT/VDD and MC4R-KO/VDD groups both exhibited an aggressive, sustained loss of body weight compared with the CD and PF mice of the same genotype (P <0.001; Fig. 3B). Initially, weight loss did not differ between the two VDD groups, but there was a gradual trend over time for exaggerated weight loss in the MC4R-KO mice in both the PF and VDD groups, achieving statistical significance (P < 0.05) after 10 days of diet manipulation.
Both the WT and MC4R-KO mice fed the VDD lost a significant amount of fat and lean body mass compared with their respective CD and PF groups (Fig. 3, C and D). The MC4R-KO/VDD mice demonstrated a relative sparing of fat mass but lost more lean body mass than the WT/VDD mice.
Genetic MC4R Deletion Modestly Attenuates Anorexia Induced by Acute Dietary Valine Deficiency
To investigate acute changes in nocturnal food intake, we divided the 12-h dark phase into three periods (1900–2300, 2300–0300, and 0300–0700) and compared food intake in WT and MC4R-KO mice fed the CD or VDD. Although cumulative food intake did not differ between the WT/CD and MC4R-KO/CD mice during the first 4 h (1900–2300) of diet consumption, cumulative food intake was significantly elevated in the MC4R-KO/VDD mice compared with the WT/VDD mice during this same time period (Fig. 4). Food intake did not differ between the WT/VDD and MC4R-KO/VDD groups for the remainder of the dark phase. The MC4R-KO/CD mice consumed more food during the final 4 h (0300–0700) of the dark phase than the other three groups of mice because of the occurrence of two early morning feeding peaks in the MC4R-KO/CD mice that were absent in the MC4R-KO/VDD mice.
Fig. 4.
Genetic MC4R deletion attenuates acute anorexia in response to a valine-deficient diet. Overnight food intake in wild-type and MC4R-KO mice that were fed a control (wild type: n = 15, MC4R-KO: n = 14) or valine-deficient (wild type: n = 24, MC4R-KO: n = 21) diet. Nocturnal food intake was measured in 4-h intervals. *P < 0.05 and ***P < 0.001 vs. WT mice consuming the same diet.
VDD Activates BAT Uncoupling Protein-1 Expression Via a Melanocortin-Independent Mechanism
Compared with animals in the CD or PF groups, uncoupling protein-1 (UCP-1) mRNA was significantly increased in the BAT of rats and mice after chronic VDD exposure (Fig. 5, A and B). There was a trend toward increased UCP-1 gene expression in rats that were fed the VDD for 3 days, but the difference did not reach statistical significance (Fig. 5A). Central infusion of SHU-9119 for 21 days did not alter UCP-1 gene expression in VDD-fed rats (data not shown), and the induction of UCP-1 mRNA was enhanced in MC4R-KO/VDD mice compared with WT/VDD mice (Fig. 5B). Leptin is a key regulator of central melanocortin tone. We therefore collected WAT to determine how VDD affects leptin gene expression in this tissue. Leptin mRNA expression was dramatically decreased in the WAT of rats fed the VDD for 3 days compared with rats that consumed the CD (Fig. 5C). Rats that consumed the VDD for 21 days had lower WAT leptin mRNA expression than either CD or PF rats.
Fig. 5.
Dietary valine deficiency induces adipose tissue uncoupling protein-1 (UCP1) gene expression. A: UCP1 mRNA expression in brown adipose tissue of rats that were fed the control diet, valine-deficient diet, or were pair-fed for 3 days (n = 6/group) or 21 days (n = 5/group). B: brown adipose tissue UCP1 gene expression in wild-type and MC4R-KO mice that were fed the control diet, valine-deficient diet, or were pair-fed for 14 days (n = 7–8/group). C: leptin mRNA expression in white adipose tissue of rats that were fed the control diet, valine-deficient diet, or were pair-fed for 3 days (n = 6/group) or 21 days (n = 5/group). Gene expression was normalized to 18S RNA. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. CD. #P < 0.05 and ##P < 0.01 vs. PF. §§§P < 0.001 vs. WT/VDD mice.
Changes in Plasma Concentrations of Leptin, Insulin, IGF-I, Ghrelin, and Corticosterone in VDD Rats is Consistent With Severe Energy Deficit Relative to Caloric Restriction Alone
Consistent with leptin mRNA levels in WAT, the plasma concentration of leptin was dramatically decreased in both mice and rats fed long term with the VDD compared with CD or PF animals (Table 1). Similarly, plasma concentrations of insulin and IGF-I were significantly decreased in VDD animals compared with CD or PF animals (Table 1). At the 3-day time point, leptin, insulin, and IGF-I were equally suppressed in VDD and PF rats. In rats exposed to VDD for 21 days, leptin, insulin, and IGF-I continued to fall relative to the 3-day values, whereas PF animals demonstrated an increase in these hormones on day 21 relative to day 3. Ghrelin levels were increased in both the VDD and PF rats relative to CD rats on day 3 (Table 1), but there was no difference between the VDD and PF groups. Ghrelin levels remained elevated in VDD rats after 21 days, although the differences between groups on this experimental day did not reach statistical significance. Plasma corticosterone concentrations did not differ between rats after 3 days (CD: 263 ± 79 ng/ml, VDD: 179 ± 50 ng/ml, PF: 316 ± 106 ng/ml; P > 0.05) or 21 days (CD: 152 ± 25 ng/ml, VDD: 113 ± 37 ng/ml, PF: 231 ± 87 ng/mL; P > 0.05). We also measured a basic electrolyte panel in the rats after 3 and 21 days of VDD exposure. No differences between CD, VDD, or PF groups were found in glucose, creatinine, sodium, potassium, chloride, or bicarbonate at either time point (data not shown), again suggesting that these animals were not dehydrated. We did observe a small but statistically significant increase in plasma blood-urea-nitrogen in the VDD group at 21 days relative to CD and PF groups (CD: 11.2 ± 0.7 mg/dl, VDD: 17.6 ± 1.7 mg/dl, PF: 10.4 ± 1.1 mg/dl; ANOVA P < 0.01), likely reflecting the increased muscle catabolism (and subsequent nitrogen load) observed in this group.
Table 1.
Plasma leptin, insulin, insulin-like growth factor-I, and ghrelin levels in control, valine-deficient diet, and pair-fed animals
| Group (n) | Leptin, pg/ml | Insulin, pg/ml | IGF-I, ng/ml | Ghrelin, pg/ml | |
|---|---|---|---|---|---|
| Rats | |||||
| 3 Days | CD (6) | 5,744 ± 534 | 1,639 ± 333 | 1286 ± 46 | 1,708 ± 146 |
| VDD (6) | 335 ± 56*** | 351 ± 60** | 739 ± 30*** | 2,805 ± 357* | |
| PF (6) | 595 ± 117*** | 281 ± 63*** | 789 ± 24*** | 2,650 ± 176* | |
| 21 Days | CD (5) | 8,578 ± 1250 | 2,914 ± 170 | 1,153 ± 72 | 1,467 ± 98 |
| VDD (5) | 199 ± 96*** | 241 ± 30*** † | 397 ± 15*** ††† | 2,206 ± 395 | |
| PF (5) | 2,028 ± 494*** | 828 ± 111*** | 821 ± 47** | 1,987 ± 255 | |
| Mice | |||||
| WT (14 days) | CD (6–7) | 16,239 ± 1,960 | 1,641 ± 465 | 261 ± 18 | Not assayed |
| VDD (7–8) | 963 ± 196*** † | 315 ± 58** | 152 ± 4*** ††† | Not assayed | |
| PF (7–8) | 6,160 ± 951*** | 430 ± 82** | 225 ± 11 | Not assayed | |
| MC4R-KO (14 days) | CD (5–7) | 28,607 ± 2,930 | 2,226 ± 427 | 290 ± 9 | Not assayed |
| VDD (5–8) | 3,072 ± 190*** † | 379 ± 65*** | 190 ± 15*** †† | Not assayed | |
| PF (5) | 12,247 ± 1,847*** | 667 ± 136** | 267 ± 18 | Not assayed |
Values are means ± SE; n, no. of animals. CD, control diet; VDD, valine-deficient diet; PF, pair fed; WT, wild type; MC4R-KO, melanocortin receptor 4 knockout.
P < 0.05,
P < 0.01, and
P < 0.001 vs. CD.
P < 0.05,
P < 0.01, and
P < 0.001 vs. PF.
VDD-Induced Anorexia is not due to Altered Expression of Appetite-Regulating Neuropeptides or Cytokines in the Hypothalamus
To further explore the role of hypothalamic neuropeptide systems in the anorexic response to the VDD, we measured the hypothalamic gene expression of neuropeptides that modulate feeding behavior. In rats fed the experimental diets for 3 days, agouti-related peptide (AgRP) gene expression was significantly upregulated in the VDD and PF groups (Fig. 6A). POMC and neuropeptide Y (NPY) gene expression did not differ between the groups. Rats that consumed the VDD for 21 days exhibited reduced POMC mRNA and increased AgRP and NPY mRNAs compared with either the CD or PF groups (Fig. 6B). There was no difference in MC4R mRNA expression between the three groups (data not shown). In mice fed the VDD for 14 days, WT animals showed similar changes in gene expression as observed in the 21-day rat study (data not shown). However, there was no difference in the expression of any of these genes between WT and MC4R-KO mice, regardless of diet (data not shown).
Fig. 6.
Dietary valine deficiency alters the hypothalamic expression of metabolic and inflammatory genes. Hypothalamic mRNA expression in rats that consumed the control diet (n = 5–6), valine-deficient diet (n = 5–6), or were pair-fed (n = 5–6) for 3 days (A) or 21 days (B). POMC, proopiomelanocortin; AgRP, agouti-related peptide; NPY, neuropeptide Y; IL-1β, interleukin-1β; IL-1R1, IL-1 type I receptor; TNF-α, tumor necrosis factor-α. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. CD. #P < 0.05 and ##P < 0.01 vs. PF.
To rule out the possibility that VDD-induced anorexia occurs secondary to central inflammation, we measured hypothalamic interleukin (IL)-1β, IL-1 type I receptor, and tumor necrosis factor-α mRNA expression. In rats that were fed the experimental diets for 3 days, the expression of all three proinflammatory genes was similarly suppressed in the VDD and PF groups (Fig. 6A); however, long-term VDD consumption did not significantly alter the expression of these genes in rats or mice (data not shown).
Previous studies demonstrated an increase in hypothalamic SS gene expression in mice that consumed a VDD for 3 days (30). Our quantitative PCR data from mice on the VDD for 14 days are consistent with this observation, with a significant increase in SS expression in VDD animals relative to both the CD and PF groups (relative quantity for CD: 1.00 ± 0.07, VDD: 1.31 ± 0.06, PF: 0.95 ± 0.02; P < 0.01). However, we did not observe an increase in SS mRNA in rats that consumed the VDD for 21 days (Fig. 6B). Finally, we did not detect any global hypothalamic changes in CRH, MCH, or orexin gene expression in VDD rats or mice by quantitative PCR (Fig. 6B and data not shown).
In Situ Hybridization Analysis Reveals Novel Induction of Hypothalamic CRH, but not SS or MCH mRNAs, in Response to the VDD
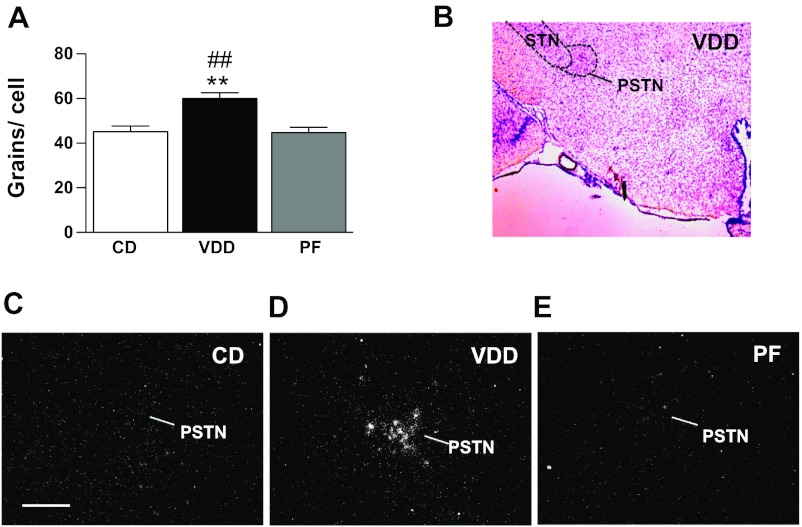
Some neuropeptides implicated in the complex neuroendocrine response to IAA deficiency or dehydration have widespread expression in the brain, with only small neuroanatomically distinct subsets of cells demonstrating transcriptional responses to these challenges (41, 43, 44). We therefore used in situ hybridization analysis to investigate gene expression within specific hypothalamic nuclei. CRH is an anorexigenic neuropeptide that is expressed in neuroendocrine cells in the parvocellular paraventricular nucleus (PVN), with inducible expression in a distinct population of cells in the LHA in response to dehydration. We observed a small amount of CRH mRNA expression in the LHA of all rats, and there was a small but significant increase in grains per cell in the LHA of VDD animals as a whole (Fig. 7A). However, it was obvious that there was a marked induction of CRH mRNA in a distinct population of neurons located in the PSTN (Fig. 7B), found only in the VDD group (Fig. 7, C–E). Because there were no grain clusters in this nucleus in either the CD or PF groups, we did not perform grain-counting analysis on this distinct population of CRH-expressing cells.
Fig. 7.
Dietary valine deficiency induces corticotropin-releasing hormone (CRH) expression in the lateral hypothalamus and parasubthalamic nucleus (PSTN). A: in situ hybridization analysis of CRH mRNA expression in the lateral hypothalamus of rats that consumed the control diet (n = 4), valine-deficient diet (n = 4), or were pair-fed (n = 4) for 5 days. B: Nissl-stained rat brain section illustrating the location of the PSTN in a rat fed the valine-deficient diet. C–E: CRH mRNA-expressing cells in the PSTN of rats that consumed the control diet, valine-deficient diet, or were pair-fed for 5 days. STN, subthalamic nucleus. **P < 0.01 vs. CD. ##P < 0.01 vs. PF. Scale bar = 200 μm.
We performed a similar analysis of MCH expression and saw no differences between groups when observed across the entire LHA or when the dorsomedial nucleus and perifornical area were analyzed separately (data not shown).
Finally, analysis of SS expression in the ARC and PVN revealed no differences in the number of SS-expressing cells (ARC: CD 363 ± 14, VDD 336 ± 34, PF 374 ± 49, P > 0.05; PVN: CD 314 ± 20, VDD 272 ± 14, PF 282 ± 30, P > 0.05) or grains/cell (ARC: CD 101 ± 2, VDD 106 ± 2, PF 111 ± 5, P > 0.05; PVN: CD 191 ± 5, VDD 170 ± 5, PF 171 ± 11, P > 0.05) in either nucleus.
APC Efferent Fibers Project to the PSTN and Form Close Contacts with CRH Neurons in This Nucleus
To provide a neuroanatomical substrate for the regulation of hypothalamic feeding centers by the APC, we traced efferent projections from the APC using anterograde PHA-L tracing. Rats were iontophoretically injected in an area of the brain matching the coordinates of the APC. After sectioning and immunohistochemical processing, we found that 2 of 10 animals had injection sites outside of the APC, and 1 other animal had no visible axonal transport. In the remaining seven animals, the efferent projections from the APC presented a consistent pattern. In each animal, we observed a number of PHA-L-labeled cells and fibers within the APC with a large majority projecting ipsilaterally along and within the cortex to a widespread but anatomically distinct distribution throughout the thalamus, hypothalamus, and limbic area. Within the amygdala, extensive innervation was observed. Within the hypothalamus, we observed extensive PHA-L labeling within the LHA and PSTN, with the projections having a beaded appearance typical of en passant synaptic varicosities (Fig. 8A). In a few cases, fibers were seen projecting dorsally in the dorsal hypothalamic area, posterior hypothalamic area, and the mediodorsal thalamic area. In triple-label immunohistochemical analysis, we observed PHA-L-labeled axonal fibers in close contact with CRH-positive cell bodies within the LHA and PSTN (Fig. 8B). Although we did not seek direct evidence for synaptic contact between PHA-L fibers and CRH cell bodies, we did observe numerous instances of close overlap within the 1-μm confocal field. There was no obvious anatomical relationship between PHA-L-labeled fibers and SS-labeled (Fig. 8B), MCH-labeled (data not shown), or orexin-labeled (data not shown) cell bodies in the LHA or elsewhere. We did not observe any difference in PVN CRH labeling between the three groups (data not shown).
Fig. 8.
Efferent projections from the anterior piriform cortex to the PSTN and lateral hypothalamic area (LHA), and PSTN cFos analysis, in the rat. A: phaseolus vulgaris leucoagglutinin (PHA-L)-labeled fibers (red) in the PSTN following injection of PHA-L in the anterior piriform cortex. B: PHA-L-labeled fibers (green), CRH-expressing cells (red), and somatostatin-expressing cells (blue) in the lateral hypothalamus. Representative images of c-Fos expression in the PSTN of rats fed the control (C and E) or valine-deficient (D and F) diet. The boxes in C and D are magnified in E and F. Scale bar = 200 μm (A, C, and D) and 50 μm (B, E, and F). G: nuclear c-Fos expression in the PSTN (CD = 3, VDD = 3). **P <0.01 vs. CD.
c-Fos Immunoreactivity Demonstrates Activation of PSTN Neurons by VDD Exposure
When we analyzed c-Fos expression in response to introduction of VDD vs. CD, we observed extensive induction of c-Fos in the PSTN in VDD rats (Fig. 8, C–F). The VDD rats had significantly greater c-Fos expression in the PSTN than the CD animals (Fig. 8G). We also observed c-Fos expression in the VDD rats in areas previously shown to express c-Fos after introduction of a threonine-deficient diet, including the infralimbic cortex, dorsomedial hypothalamic nucleus, and central amygdala (data not shown) (40).
ARC POMC Neurons Do Not Have Exaggerated c-Fos Responses After Acute VDD Exposure
In all of our studies, animals begin to reject the VDD within hours of introduction, and we did observe some attenuation of acute anorexia with melanocortin blockade. Therefore, we evaluated neuronal activation in ARC POMC neurons after acute (1.5, 2, or 3 h) exposure to the CD or VDD in food-entrained POMC-eGFP mice to explore the possibility that rejection of the VDD is secondary to enhanced activation of POMC neurons. Compared with fasted mice, introduction of either the VDD or CD evoked significant increases in the number of ARC POMC cells displaying c-Fos immunoreactivity at all three time points (Fig. 9). However, there were no differences between the CD and VDD groups except for a less robust increase in c-Fos immunoreactivity in the VDD mice at the 3-h time point.
Fig. 9.
Activation of proopiomelanocortin (POMC) neurons in response to acute dietary valine deficiency. Nuclear c-Fos expression in POMC-expressing cells in the arcuate nucleus (ARC) of mice that were fasted or fed the control or valine-deficient diets for 1.5, 2, or 3 h before being killed (n = 3–5/group at each time point). *P < 0.05 and ***P < 0.001 vs. fasted. ##P < 0.01 vs. CD.
DISCUSSION
The depression of food intake that results from consuming an amino acid imbalanced diet provides significant health and survival benefits, and sensing of dietary amino acids is thought to be critical for maintaining a balance of complementary IAA profiles in the diet (4, 5, 29). A full discussion of the evolution of this system is beyond the scope of this discussion, but this topic has been the focus of numerous excellent recent reviews (17, 18). The APC comprises the sensory limb for dietary IAA, and the initial molecular mechanisms for sensing IAA deficiency have been described (17). Pathways for initial sensing of dietary IAA deficiency have been defined and links to hypothalamic feeding centers established, but the neural substrates for this powerful behavioral pathway remain unknown. The hypothalamus, hippocampus, and amygdala receive neuronal projections from the APC and control the motivational and motor aspects of feeding (1, 28, 40), but it is unknown whether they are involved in IAADA. Our study and others demonstrate normal hormonal and hypothalamic responses to energy deficit in this model. Furthermore, there is a rapid reinstatement of feeding after returning animals to normal chow (19). The hypothalamic gene expression profile (increased AgRP and NPY expression, decreased POMC expression) is appropriately reflective of the marked hypoleptinemia and hypoinsulinemia found in this model and would be expected to produce a substantial orexigenic drive from the hypothalamus. This is not consistent with the hypothesis that inputs from the APC engage these circuits to produce anorexia but rather implies an active inhibitory brake on food intake that overrides the orexigenic drive from the hypothalamus, a situation analogous to that found in dehydration-induced anorexia (44).
VDD consumption in both rats and mice produced a rapid reduction in food intake and sustained weight loss compared with PF animals. Weight loss due to IAA deficiency is characterized by a steady loss of lean mass over time, which is not typical of starvation but is similar to the effects of chronic inflammation (i.e., cachexia). Given that the anorexia, increased BAT activation, and loss of lean body mass found in chronic disease are largely attenuated by melanocortin blockade, we reasoned that the metabolic and behavioral response to IAA deficiency would also be melanocortin dependent (27, 36, 45). As discussed further below, we found that melanocortin blockade does not preserve lean mass in the setting of dietary valine deficiency. This argues against a melanocortin-dependent anorexia, since animals lacking melanocortin signaling preserve lean mass in the face of external anorexigenic stimuli (27, 36). We observed an initial decrease in fat mass and leptin in all groups, as expected with modest starvation. However, prolonged VDD exposure revealed important differences. First, fat loss stabilized in PF groups, consistent with metabolic adaptation to nutritional restriction, whereas the VDD groups lost fat throughout the study. Second, lean mass loss in the VDD groups continued with prolonged diet exposure. IAA deficiency consistently produces enhanced fat loss relative to PF controls (6, 7). However, excessive loss of lean mass was not previously demonstrated, perhaps because of the shorter duration of exposure to IAA deficiency. Lean mass loss is likely secondary to increased energy expenditure and lack of downregulation of muscle catabolism. Thus, VDD produces sustained pressure on stored fuel utilization, particularly in the lean mass compartment, and is not subject to significant behavioral and metabolic compensation.
The hypothalamic melanocortin system plays an important role in the regulation of energy homeostasis and is a candidate for mediating IAADA (12). Neurons expressing MC3R and/or MC4R are downstream targets of POMC and NPY/AgRP neurons (9). Administration of MC3/4R antagonists or genetic deletion of MC4R increases food intake and energy storage, whereas administration of melanocortin agonists decreases both (12, 23). Continuous intracerebroventricular infusion of SHU-9119 attenuated the reduced food intake during the first 3 days of VDD consumption but failed to alter food intake thereafter. A recent study demonstrated that a single daytime intracerebroventricular injection of 2 nmol AgRP did not improve 2-h food intake in rats fed a VDD for 5 days (19). These contradictory results may be because of different methodologies and/or different timing of VDD feeding and drug treatment. Nevertheless, our data strongly support the idea that central melanocortin receptor antagonism is ineffective at restoring feeding in long-term IAADA. This implies that the brake on food intake is either coincident with, or downstream of, melanocortin receptors.
To further study the role of melanocortins in IAADA, we used MC4R-KO mice. Acutely, IAADA was delayed in the MC4R-KO mice but was apparent during the latter part of the dark phase. In the 14-day study, MC4R-KO/VDD mice demonstrated this initial attenuation of IAADA but eventually exhibited a greater reduction in food intake and body weight than WT/VDD mice. Anorexigenic hormones that activate central melanocortin signaling (e.g., leptin and insulin) are acutely elevated (1 h) following IAA-deficient diet exposure (13), and their anorexic effect is likely attenuated with MC4R deletion. On the other hand, the long-term feeding studies and exaggerated lean mass loss in MC4R-KO mice again argue that the site of feeding inhibitory input is downstream of the MC4R.
We observed an increase in UCP-1 expression in BAT after prolonged VDD consumption. This is consistent with a previous report of BAT UCP-1 expression in mice exposed to 7 days of leucine-deficient diet (6), as well as a study of mice exposed to VDD for 7 days (but lacking a PF control group) (11). Several studies provide evidence of a link between central melanocortin signaling and activation of BAT (3, 39). However, our data are not consistent with melanocortin-dependent regulation of BAT UCP-1 expression in animals exposed to VDD. Previous studies demonstrated a decrease in BAT UCP-1 expression in rats exposed to SHU-9119 for 6 days (38), but we observed no change in UCP-1 expression when SHU-9119 was given during VDD exposure. Furthermore, UCP-1 expression was upregulated in both WT and MC4R-KO mice, with a greater increase in the MC4R-KO than in WT animals. This is consistent with enhanced upregulation of UCP-1 in BAT from MC4R-KO relative to WT mice after prolonged cold exposure (39) and supports the idea that VDD exposure activates central sympathetic outflow at a site coincident or downstream of the MC4R.
The endocrine and neuroendocrine responses to prolonged VDD exposure are typical of negative energy balance and should produce a marked orexigenic and anabolic drive. Indeed, leptin, insulin, and IGF-I levels in the VDD groups were lower than PF groups, indicating a lack of adaptation to caloric deficiency. The neuroendocrine response is reflective of the peripheral response, with lower POMC, and higher NPY and AgRP expression observed in the VDD groups relative to PF animals. Our data on subacute (3 days) diet exposure demonstrate an increase in AgRP expression in the VDD and PF groups, consistent with a previous report of NPY and POMC expression in mice, and likely reflecting the mild energy deficit (30). Also consistent with this report, we observed an increase in hypothalamic SS expression in VDD animals. This finding likely reflects the enhanced somatostatinergic tone underlying the decrease in growth hormone in starved animals (22). Cumulatively, these data show that IAADA does not result from a disordered response to negative energy balance but rather to increased activity of inhibitory networks functionally similar to those found in dehydration-induced anorexia (42).
To investigate the neural substrate of the inhibitory network active in IAADA, we pursued a combination of neural tract tracing and c-Fos expression. We noted dense innervation by APC efferent fibers in the lateral part of the hypothalamus, particularly the PSTN. Upon acute exposure to VDD, we observed extensive c-Fos expression in the thalamus and PSTN, areas that had minimal nuclear c-Fos in CD animals. We also analyzed c-Fos expression in hypothalamic POMC neurons and did not observe enhanced c-Fos expression after VDD exposure, consistent with the lack of APC efferent projections in the ARC. Thus, our data argue that acute rejection of VDD is not secondary to activation of POMC neurons but rather due to activation of distinct neuronal populations within the diencephalon that receive efferent projections from the APC (including the PSTN). The PSTN serves as an important conduit between the hypothalamus and brain stem feeding centers, including the nucleus of the solitary tract, suggesting that the latter structures may be involved in overcoming the orexigenic drive from the hypothalamus in this model (8). The PSTN is also among the nuclei activated in memory-dependent conditioned taste aversion, further strengthening the importance of this structure in restraining food intake in certain situations (46). Whether structures elsewhere in the caudal hypothalamus and brain stem are involved in this circuitry will require further investigation.
Our observations are remarkably similar to dehydration-induced anorexia (41, 44). Watts and colleagues revealed a role for neurons in the lateral PVN and the perifornical LHA in regulating feeding during dehydration-induced anorexia and demonstrated increased expression of CRH in a novel population of CRH neurons in the LHA. Our studies revealed induction of CRH mRNA in the PSTN, with undetectable CRH expression in this nucleus in control groups. Total hypothalamic CRH expression did not differ between groups, likely because most CRH expression is found in the PVN, an area where we did not observe changes in expression. This contrasts with another study demonstrating increased PVN CRH expression in mice exposed to 7 days of leucine-deficient diet (7), but methodological differences preclude direct comparison with our study. Specifically, in addition to differences in the type and duration of IAA-deficient diet exposure, the previous observation of increased CRH immunostaining in the PVN of leucine-deficient mice cannot distinguish between increased biosynthesis or decreased axonal transport and release of this peptide. Indeed, our own data do not demonstrate marked differences in plasma corticosterone in VDD animals relative to PF control animals, suggesting that neuroendocrine CRH release is not enhanced in this model. However, this study did demonstrate that central immunoneutralization of CRH attenuated body fat loss due to leucine-deficient diet, suggesting that central CRH signaling is important for this physiological response. Our study did not rigorously demonstrate synaptic connection between the APC efferent fibers and CRH neurons in the PSTN, nor did we demonstrate functional significance of CRH induction in this nucleus. In fact, it remains possible that this nucleus is not involved in the physiological response to VDD. However, the data presented here, combined with that from the established dehydration-induced anorexia models, lay the groundwork for studies investigating the mechanism for feeding inhibition and weight loss due to dietary IAA deficiency.
GRANTS
This work was funded by the National Institute of Diabetes and Digestive and Kidney Disease Grant DK-070333.
DISCLOSURES
No conflicts of interest are declared by the authors.
AUTHOR CONTRIBUTIONS
X.Z, Q.R.C, P.R.L., T.P.B. and A.J.G performed experiments; X.Z., S.M.K., Q.R.C, T.P.B., A.J.G and D.L.M. analyzed data; X.Z., S.M.K., and D.L.M. interpreted results of experiments; X.Z., Q.R.C, S.M.K., and D.L.M. prepared figures; X.Z., S.M.K., and D.L.M. drafted manuscript; X.Z., S.M.K., and D.L.M. edited and revised manuscript; X.Z., S.M.K., Q.R.C., P.R.L., T.P.B., A.J.G. and D.L.M. approved final version of manuscript; X.Z., D.L.M conception and design of research.
REFERENCES
- 1. Bellinger LL, Evans JF, Gietzen DW. Dorsomedial hypothalamic lesions alter intake of an imbalanced amino acid diet in rats. J Nutr 128: 1213–1217, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Beverly JL, Hrupka BJ, Gietzen DW, Rogers QR. Timing and dose of amino acids injected into prepyriform cortex influence food intake. Physiol Behav 53: 899–903, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148: 5339–5347, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Carpenter KJ. A short history of nutritional science: part 1 (1785–1885). J Nutr 133: 638–645, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Carpenter KJ. A short history of nutritional science: part 2 (1885–1912). J Nutr 133: 975–984, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, Xiao F, Guo F. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 59: 17–25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Y, Zhang Q, Meng Q, Xia T, Huang Z, Wang C, Liu B, Chen S, Xiao F, Du Y, Guo F. Leucine deprivation stimulates fat loss via increasing CRH expression in the hypothalamus and activating the sympathetic nervous system. Mol Endocrinol 25: 1624–1635, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ciriello J, Solano-Flores LP, Rosas-Arellano MP, Kirouac GJ, Babic T. Medullary pathways mediating the parasubthalamic nucleus depressor response. Am J Physiol Regul Integr Comp Physiol 294: R1276–R1284, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Cowley MA, Smart JL, Cerdan M, Rubinstein M, Diano C, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 24: 155–163, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Du Y, Meng Q, Zhang Q, Guo F. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT. Amino Acids 43: 725–734, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385: 165–168, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Feurte S, Nicolaidis S, Gerozissis K. Is the early increase in leptinemia one of the anorectic signals induced by an essential amino acid-deficient diet in the rat? Endocrinology 141: 3916–3919, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Firman JD, Kuenzel WJ. Neuroanatomical regions of the chick brain involved in monitoring amino acid deficient diets. Brain Res Bull 21: 637–642, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Gietzen DW, Erecius LF, Rogers QR. Neurochemical changes after imbalanced diets suggest a brain circuit mediating anorectic responses to amino acid deficiency in rats. J Nutr 128: 771–781, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Gietzen DW, Hammer VA, Beverly JL, Rogers QR. The role of serotonin (5-HT) in feeding responses to amino acids. Adv Exp Med Biol 294: 389–404, 1991 [DOI] [PubMed] [Google Scholar]
- 17. Gietzen DW, Hao S, Anthony TG. Mechanisms of food intake repression in indispensable amino acid deficiency. Annu Rev Nutr 27: 63–78, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Gietzen DW, Rogers QR. Nutritional homeostasis and indispensable amino acid sensing: a new solution to an old puzzle. Trends Neurosci 29: 91–99, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Goto S, Nagao K, Bannai M, Takahashi M, Nakahara K, Kangawa K, Murakami N. Anorexia in rats caused by a valine-deficient diet is not ameliorated by systemic ghrelin treatment. Neuroscience 166: 333–340, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Grossberg AJ, Scarlett JM, Zhu X, Bowe DD, Batra AK, Braun TP, Marks DL. Arcuate nucleus proopiomelanocortin neurons mediate the acute anorectic actions of leukemia inhibitory factor via gp130. Endocrinology 151: 606–616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grossberg AJ, Zhu X, Leinninger GM, Levasseur PR, Braun TP, Myers MG, Jr, Marks DL. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J Neurosci 31: 11376–11386, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hugues JN, Enjalbert A, Moyse E, Shu C, Voirol MJ, Sebaoun J, Epelbaum J. Differential effects of passive immunization with somatostatin antiserum on adenohypophysial hormone secretions in starved rats. J Endocrinol 109: 169–174, 1986 [DOI] [PubMed] [Google Scholar]
- 23. Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Koehnle TJ, Russell MC, Gietzen DW. Rats rapidly reject diets deficient in essential amino acids. J Nutr 133: 2331–2335, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Leung PM, Larson DM, Rogers QR. Food intake and preference of olfactory bulbectomized rats fed amino acid imbalanced or deficient diets. Physiol Behav 9: 553–557, 1972 [DOI] [PubMed] [Google Scholar]
- 26. Leung PM, Rogers QR. Importance of prepyriform cortex in food-intake response of rats to amino acids. Am J Physiol 221: 929–935, 1971 [DOI] [PubMed] [Google Scholar]
- 27. Marks DL, Ling N, Cone RD. Role of the central melanocortin system in cachexia. Cancer Res 61: 1432–1438, 2001 [PubMed] [Google Scholar]
- 28. Monda M, Sullo A, De Luca V, Pellicano MP, Viggiano A. l-Threonine injection into PPC modifies food intake, lateral hypothalamic activity, and sympathetic discharge. Am J Physiol Regul Integr Comp Physiol 273: R554–R559, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Murphy ME, Pearcy SD. Dietary amino acid complementation as a foraging strategy for wild birds. Physiol Behav 53: 689–698, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Nakahara K, Takata S, Ishii A, Nagao K, Bannai M, Takahashi M, Murakami N. Somatostatin is involved in anorexia in mice fed a valine-deficient diet. Amino Acids 42: 1397–1404, 2012 [DOI] [PubMed] [Google Scholar]
- 31. Noda K, Chikamori K. Effect of ammonia via prepyriform cortex on regulation of food intake in the rat. Am J Physiol 231: 1263–1266, 1976 [DOI] [PubMed] [Google Scholar]
- 32. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed). New York, NY: Academic, 1998 [Google Scholar]
- 33. Rogers QR, Leung PM. The influence of amino acids on the neuroregulation of food intake. Fed Proc 32: 1709–1719, 1973 [PubMed] [Google Scholar]
- 34. Rudell JB, Rechs AJ, Kelman TJ, Ross-Inta CM, Hao S, Gietzen DW. The anterior piriform cortex is sufficient for detecting depletion of an indispensable amino acid, showing independent cortical sensory function. J Neurosci 31: 1583–1590, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Russell MC, Koehnle TJ, Barrett JA, Blevins JE, Gietzen DW. The rapid anorectic response to a threonine imbalanced diet is decreased by injection of threonine into the anterior piriform cortex of rats. Nutr Neurosci 6: 247–251, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Scarlett JM, Bowe DD, Zhu X, Batra AK, Grant WF, Marks DL. Genetic and pharmacologic blockade of central melanocortin signaling attenuates cardiac cachexia in rodent models of heart failure. J Endocrinol 206: 121–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scarlett JM, Zhu X, Enriori PJ, Bowe DD, Batra AK, Levasseur PR, Grant WF, Meguid MM, Cowley MA, Marks DL. Regulation of agouti-related protein messenger ribonucleic acid transcription and peptide secretion by acute and chronic inflammation. Endocrinology 149: 4837–4845, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verty AN, Allen AM, Oldfield BJ. The endogenous actions of hypothalamic peptides on brown adipose tissue thermogenesis in the rat. Endocrinology 151: 4236–4246, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148: 1550–1560, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Cummings SL, Gietzen DW. Temporal-spatial pattern of c-fos expression in the rat brain in response to indispensable amino acid deficiency. I. The initial recognition phase. Brain Res Mol Brain Res 40: 27–34, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Watts AG. Osmotic stimulation differentially affects cellular levels of corticotropin-releasing hormone and neurotensin/neuromedin N mRNAs in the lateral hypothalamic area and central nucleus of the amygdala. Brain Res 581: 208–216, 1992 [DOI] [PubMed] [Google Scholar]
- 42. Watts AG, Boyle CN. The functional architecture of dehydration-anorexia. Physiol Behav 100: 472–477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watts AG, Kelly AB, Sanchez-Watts G. Neuropeptides and thirst: the temporal response of corticotropin-releasing hormone and neurotensin/neuromedin N gene expression in rat limbic forebrain neurons to drinking hypertonic saline. Behav Neurosci 109: 1146–1157, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Watts AG, Sanchez-Watts G, Kelly AB. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia. J Neurosci 19: 6111–6121, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wisse BE, Frayo RS, Schwartz MW, Cummings DE. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology 142: 3292–3301, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Yasoshima Y, Scott TR, Yamamoto T. Memory-dependent c-Fos expression in the nucleus accumbens and extended amygdala following the expression of a conditioned taste aversive in the rat. Neuroscience 141: 35–45, 2006 [DOI] [PubMed] [Google Scholar]