Abstract
Despite being banned in the U.S., organochlorine toxins such as DDT are frequently detected in human adipose tissue. The main route of exposure is through the consumption of contaminated foods and subsequent intestinal packaging of DDT into chylomicrons. These chylomicrons, which also contain dietary triacylglycerol (TG), are delivered directly to peripheral tissues without first being metabolized by the liver. The physiological process by which these compounds are delivered from chylomicrons to adipose is not well understood, but is clinically relevant since it bypasses first-pass metabolism. Based on its highly lipophilic nature, it has been assumed that DDT is transferred to peripheral tissues similar to TG; however, this has not been measured. Here, we use the lymph fistula rat to isolate chylomicrons containing both DDT and TG. These chylomicrons are the in vivo DDT delivery vehicle. Using 3T3-L1 adipocytes, we investigated the rate at which DDT transfers from chylomicrons to adipocytes, and mediators of this process. This novel approach closely approximates the in vivo DDT exposure route. We show that: 1) DDT repartitions from chylomicrons to adipocytes, 2) this transport does not require hydrolysis of TG within the chylomicron, and is stimulated by the inhibition of LPL, 3) albumin does not inhibit DDT uptake, 4) DDT dissolved in DMSO does not appropriately mimic in vivo DDT transport; and most importantly, 5) DDT uptake from chylomicrons does not mimic the uptake of TG from the same particles. Understanding these factors is important for designing interventions for human populations exposed to DDT.
Keywords: Chylomicrons, Organochlorines, DDT, Xenobiotics, Triacylglycerol, 3T3-L1 adipocytes
1. Introduction
Organochlorine pesticides such as dichlorodiphenyltrichloroethane (DDT) were used for more than 50 years in the United States to combat disease vectors such as mosquitoes. In many parts of the world, especially India and parts of the African continent, DDT is still heavily relied upon to combat the spread of malaria. Although DDT is now banned in North America, DDT persists in areas where it was manufactured and heavily used. DDT has a very long environmental half-life due to the stability of its carbon–chlorine bond. Therefore, these compounds continue to contaminate soil, air, and water where they ascend the food chain to humans. These compounds are also incredibly persistent once they enter the body [1].
Despite their ban in the U.S., organochlorine toxins and DDT in particular are frequently detected in human breast milk and in adipose tissue. The main route of human exposure is through the consumption of contaminated foods [2]. Since they are highly lipophilic, DDT and other organochlorine compounds associate with dietary triacylglycerol (TG) as they pass through the mouth and stomach into the small intestine.
Once in the small intestine, the TG is hydrolyzed by pancreatic lipase to form free fatty acids and monoacylglycerols, which can enter the enterocytes along with small lipophilic molecules such as DDT [3]. Once inside the enterocyte, the fatty acids are then re-esterified and assembled with the DDT into chylomicrons [4]. Chylomicrons are first transported via the intestinal (mesenteric) lymphatic duct and then through the thoracic duct before entering the circulation through the subclavian vein [5]. Upon entering the circulation, the chylomicrons are carried in the blood to extrahepatic tissues so that those tissues can metabolize the chylomicron particle and take up dietary fat. It has been suggested that this is the major route by which adipose tissue is exposed to DDT.
The physiological process by which organochlorine compounds are delivered from chylomicrons to peripheral tissues is an area that is not well understood. Understanding this process is critical however in fully understanding how these compounds exert deleterious effects in the body, especially because this route of delivery avoids metabolism of potentially harmful compounds by the liver.
Based on its highly lipophilic nature, DDT was thought to be found in the core of the chylomicron particle (like cholesterol esters and retinyl esters). Therefore, it was hypothesized that DDT would be slowly transferred in a similar manner from the chylomicron to peripheral tissues. Surprisingly however, we and others have shown that the in vivo rate at which organochlorine compounds transfer out of chylomicrons to peripheral tissues was markedly faster than lipids in the core of chylomicrons [4,6]. DDT disappeared more rapidly than cholesteryl oleate and triacylglycerol. However, the factors influencing these events (such as hydrolysis and the presence of albumin) have not been addressed through these experiments. Moreover, these observations have raised important questions about the way that organochlorine compounds are transported from chylomicrons to peripheral tissues. The fact that these compounds move readily from the chylomicron may have a significant impact on their toxicity profiles.
To address these questions, we have used 3T3-L1 adipocytes incubated with DDT-containing chylomicrons. This novel approach, which utilizes the lymph fistula rat, closely approximates in vivo organo-chlorine exposure since chylomicrons are isolated from lymph after the rats have received a bolus of lipid containing DDT; this mimics the main route of human exposure (ingestion). Lymphatic-derived chylomicrons represent a physiologically relevant DDT vehicle for several reasons, including: 1) these particles are the precise postprandial lymphatic output of the entire intestine, 2) we know that greater than 60% of the ingested DDT in absorbed through the lymphatic route, and 3) peripheral tissues including adipose are directly exposed to these chylomicrons once they enter the circulation [7]. Prior to this study, DMSO (dimethyl sulfoxide), a non-physiological vehicle, was frequently used to study organochlorine metabolism [8].
This model enabled us to determine the rate of DDT uptake from chylomicrons to 3T3-L1 adipocytes, as well as the role of TG hydrolysis and serum albumin in the partitioning of DDT into those cells. We also compared uptake of DDT versus TG from chylomicrons into the cells. Finally, we compared uptake of DDT in chylomicrons versus DDT dissolved in DMSO into cells, in order to determine whether DMSO accurately mimics in vivo transport. We report here that DDT partitions from chylomicrons to adipocytes more quickly than does TG within those chylomicrons, and that this transport does not require hydrolysis of the chylomicron. Additionally, TG and DDT may compete for uptake by adipocytes. The observation that DDT transport from chylomicrons to adipocytes does not mirror TG transport is quite novel and we believe represents an important step towards understanding the way that DDT enters circulation and then interacts with one of its main storage depots. This finding gives us important information about the physiology of DDT transport, with the goal of potentially interfering with this process in the future.
2. Materials and methods
2.1. Lymph fistula rats and lipid infusion
Male Sprague–Dawley rats were fitted with a mesenteric lymph cannula, as described previously [9]. After recovering overnight from the lymph fistula surgery, rats received an intraduodenal infusion of 0.5 mL of olive oil containing 10 μCi (39 μg) of 14C-DDT (DDT [ring-U-14C] 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane, purchased from the Institute of Isotopes Co., Ltd, Budapest, Hungary) and 33 μCi (0.56 μg) of 3H-triolein (Triolein-[9,10-3H(N)], purchased from Perkin Elmer, Waltham, MA). The specific activity of the infused lipid was 0.0717 μCi/mg. Post-infusion lymph was collected on ice for 6 h. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
2.2. Isolation of chylomicrons and chylomicron remnants
To isolate the doubly labeled chylomicrons, lymph from 3 rats was pooled. The pooled lymph was centrifuged at 25,000 g for 60 min at 4 °C in a 50.3 Ti fixed angle rotor (Beckman Instruments, Palo Alto, CA) [10]. Large chylomicrons were harvested by removing the top 1.0 to 1.5 mL fraction. Chylomicron remnants were isolated as described previously, with minor modifications [11]. Briefly, chyle triacylglycerol was digested by mixing 6 ml of lymph with 9 ml 5% fatty acid-free bovine serum albumin and 3 ml of post-heparin plasma as a source of lipoprotein lipase, and incubating for 30 min at 28 °C. Chylomicron remnants were then isolated by adjusting the density to 1.063 g/ml with NaCl and KBr. This solution was overlayered with 1.1% NaCl (d= 1.006 g/ml) and centrifuged at 37,000 g for 100 min at 4 °C in a SW41 swinging bucket rotor (Beckman). The top 1.5 ml was collected, and overlayered again with 1.1% NaCl and centrifuged under the same conditions. Chylomicron and chylomicron remnant TG was assessed using the Randox Triacylglycerol Kit (Randox) and the [3H]-TG and [14C]-DDT activity was assessed by liquid scintillation counting.
2.3. Cell culture and adipocyte differentiation
Undifferentiated 3T3-L1 preadipocytes were cultured at subconfluency in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% (v/v) fetal calf serum at 37 °C in a 5% CO2 incubator. Two days post-confluency, cells were placed in DMEM containing 10% (v/v) fetal bovine serum (FBS), 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 0.25 μM dexamethasone (DEX), and 1 μg/mL insulin for the induction of differentiation. Two days later, the induction medium was replaced with DMEM containing 10% (v/v) FBS and 1 μg/mL insulin. The medium was then replaced again with fresh DMEM containing 10% (v/v) FBS every other day for the following 10–12 days until intracellular lipid droplets were visible. For studies with preadipocytes, cells were kept at sub-confluency prior to the experiment.
2.4. Uptake experiments
3T3-L1 cells (both preadipocytes and differentiated, mature adipocytes) were maintained in culture media (as described above) containing serum prior to and during each experiment. Immediately before each experiment media was removed and media containing 1000 μg of TG in chylomicrons or chylomicron remnants was added to each well in a total volume of 1.5 ml (as indicated in the figure legends). The cells were then incubated for 30, 90, or 180 min at 37 °C in a 5% CO2 incubator. Media was then removed, and the cell monolayer was gently washed 3 times with 2 mL of cold PBS. Cells were then isolated in 100 μL of PBS and assessed for uptake by liquid scintillation counting. Where indicated in the figure legends, 0.1 mg/ml of orlistat (a gift from GlaxoSmithKline, Parsippany New Jersey) or 38.8 mg/ml bovine serum albumin (fatty acid free, Sigma–Aldrich) was added to 1.5 ml of culture media. For the experiments with lipase, the media containing 1000 μg of chylomicron triacylglycerol was incubated with 16 mg/ml pancreatic Lipase (porcine, Sigma–Aldrich) for 30 min prior to the addition of that media to the cells in culture, as described above.
2.5. Statistical analyses
Statistical analyses were performed using GraphPad Prism (version 4.0). Overall statistical significance was determined for the analyses in Figs. 1 and 4 by two-tailed unpaired t-tests; differences were considered significant at P<0.05. For Figs. 2 and 3, overall statistical significance was determined by one-way ANOVA; multiple comparisons were made using a Bonferroni post-test if the overall p-value after ANOVA was p<0.05. All data are presented as the mean±the S.E.
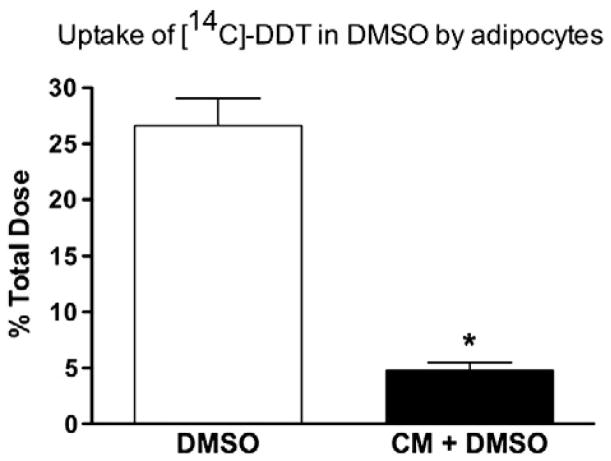
Fig. 1.
Difference in uptake of DDT by adipocytes when delivered in DMSO or chylomicrons (CM). Differentiated 3T3-L1 adipocytes were incubated with [14C]-DDT delivered in either DMSO or in CM for 180 min. Values are means±SE. *P<0.001.
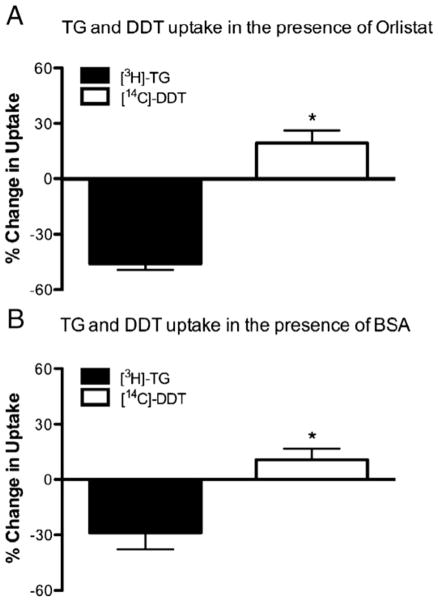
Fig. 4.
Triacylglycerol (TG) and DDT uptake are differentially affected by lipase inhibitor orlistat and by increased BSA concentrations. Differentiated 3T3-L1 adipocytes were incubated with CM containing both [14C]-DDT and [3H]-TG and either (A) lipase inhibitor (orlistat) or (B) bovine serum albumin (BSA). Total uptake of [14C]-DDT and [3H]-TG was assessed after 180 min and percent change in uptake was calculated versus uptake of [14C]-DDT and [3H]-TG from untreated CM. Values are means±SE. *P<0.05 versus TG uptake.
Fig. 2.
Triacylglycerol (TG) and DDT uptake from chylomicrons (CM) to (A) differentiated and (B) undifferentiated pre-adipocytes. For both experiments, adipocytes were incubated with CM containing both [14C]-DDT and [3H]-TG for 30, 90, or 180 min. The cells were isolated and uptake of [14C]-DDT and [3H]-TG was assessed by scintillation counting. Values are means±SE. *P<0.05 versus TG uptake at each time point.
Fig. 3.
Triacylglycerol (TG) and DDT uptake are differentially affected by chylomicron (CM) hydrolysis. Chylomicrons containing [14C]-DDT and [3H-TG] were treated with and without pancreatic lipase for 30 min prior to their addition to differentiated 3T3-L1 adipocytes. (A) Uptake of [14C]-DDT and [3H]-TG was assessed after 30, 90, or 180 min. *P<0.05 versus TG uptake at each time point from CM not treated with lipase. #P<0.05 versus DDT uptake at each time point from CM not treated with lipase. (B) The fold increase in uptake of [14C]-DDT and [3H]-TG from hydrolyzed CM was determined versus uptake of non-hydrolyzed CM, after a 180 min incubation. (C) The fold increase in uptake of [14C]-DDT and [3H]-TG from chylomicron remnants was determined after a 180 min incubation. Values are means±SE. *P<0.05 [14C]-DDT and [3H]-TG uptake at each time point.
3. Results
3.1. DDT dissolved in DMSO does not mimic the in vivo transport process
Adipose tissue represents a major storage depot for DDT in vivo, therefore we used differentiated murine 3T3-L1 adipocytes as a model system in which to explore tissue uptake of DDT from lymphatically derived chylomicrons. Previous studies on organochlorine uptake by adipocytes have used DMSO as a delivery vehicle for the highly lipophilic DDT, since it is not otherwise soluble in culture media [12,13]. While this approach effectively causes differentiated adipocytes to take up DDT from the media, this model does not mirror the in vivo human condition in which DDT from the environment is ingested, packaged in chylomicrons in the gut, and secreted into the lymphatic system prior to traveling to adipose tissue [4]. Many factors, including the presence of lipoproteins or the distribution of DDT within the chylomicron particle, may have an impact on the delivery of DDT to adipose tissue that DMSO does not mimic. As shown in Fig. 1, in order to directly assess the difference between DMSO and chylomicron delivery of DDT to adipocytes, we measured the uptake of [14C]-DDT by differentiated adipocytes incubated with the same amount of [14C]-DDT, delivered to the cells in either chylomicrons or dissolved in DMSO. When dissolved in DMSO, differentiated adipocytes take up nearly 5-fold more [14C]-DDT as compared to cells incubated with chylomicrons containing [14C]-DDT. This observation suggests that there are multiple factors influencing the uptake of DDT from chylomicrons to differentiated adipocytes in vivo that are not mirrored by DMSO delivery.
3.2. DDT rapidly repartitions from chylomicrons to adipocytes
It is not known how DDT is transferred from chylomicrons to adipose tissue. Since DDT is highly lipophilic, we hypothesized that DDT would be dissolved in the core of the chylomicron particle and would be slowly transferred from the chylomicron to adipocytes in a similar manner to TG within the core of the chylomicron [4]. Surprisingly, as shown in Fig. 2A, differentiated adipocytes take up significantly more [14C]-DDT than [3H]-TG at 30, 90, and 180 min, with an average of 8-fold more [14C]-DDT than [3H]-TG taken up throughout the time course.
We further characterized the uptake of DDT from chylomicrons to preadipocytes to see whether this difference in DDT versus TG uptake was dependent on differentiated (mature) adipocytes, perhaps due to the increased expression of lipoprotein lipase (LPL) in mature adipocytes [14]. As expected, there was very little uptake of [3H]-TG by preadipocytes (less than 0.5% the total dose and approximately 13-fold lower than the uptake by differentiated adipocytes) (Fig. 2B). Preadipocytes also take up very small amounts of [14C]-DDT (less than 1% the total dose and approximately 13-fold less than their mature adipocytes counterparts). Despite this loss in uptake of both [3H]-TG and [14C]-DDT by the preadipocytes, the trend is still preserved that higher levels of [14C]-DDT are taken up versus [3H]-TG, suggesting that movement of DDT from the chylomicron to adipocytes is different than the mechanism for TG.
3.3. DDT transport does not require hydrolysis of chylomicron triacylglycerol
Triacylglycerols lie in the core of the chylomicron and require the action of LPL to hydrolyze fatty acids from the glycerol backbone. Once hydrolyzed, both the fatty acids and glycerol can move from the chylomicron into adipocytes. We wondered whether hydrolysis of chylomicron TG would impact the movement of DDT from the chylomicron to differentiated adipocytes. As shown in Fig. 3, treatment with lipase increased both [14C]-DDT and [3H]-TG uptake by the adipocytes when compared to uptake of [14C]-DDT and [3H]-TG from the untreated chylomicrons at 30, 90 and 180 min. [14C]-DDT uptake under these conditions is still significantly greater than [3H]-TG uptake at all time-points (Fig. 3A). As shown in Fig. 3B, lipase treatment increased [3H]-TG uptake by adipocytes by an average of 12-fold over the untreated chylomicrons. On the contrary, [14C]-DDT uptake was only stimulated 1-fold by lipase treatment.
Although pancreatic lipase bears considerable homology to lipoprotein lipase (LPL) and hydrolyzes chylomicron triacylglycerol to form similar hydrolytic products, we wanted to corroborate this data with a more physiological model. Therefore, we isolated chylomicron remnants after exposure to LPL. As shown in Fig. 3C, [3H]-TG uptake from chylomicron remnants was more than 2-fold higher than from intact chylomicrons. Conversely, [14C]-DDT uptake was stimulated by only 1-fold in the presence of chylomicron remnants. This observation supports our conclusion that the behavior and properties of DDT within the chylomicron may differ greatly from triacylglycerol within the same particles.
3.4. DDT may compete with triacylglycerol for adipocyte uptake in the presence of Orlistat and albumin
We inhibited LPL on differentiated adipocytes by incubating them with the LPL-inhibitor orlistat. As expected, orlistat inhibited [3H]-TG uptake from chylomicrons by nearly 50% after 180 min (Fig. 4A, closed bars). However, the inhibition of LPL did not inhibit [14C]-DDT uptake; rather, this treatment stimulated [14C]-DDT uptake by nearly 25% (open bars). This suggests that TG and DDT may compete for transport into adipocytes, and therefore when less TG is taken up more DDT may be able to enter the cell.
Since serum albumin readily binds lipophilic molecules and has already been implicated as a potential DDT carrier protein [13], we hypothesized that adding albumin to the incubation media might increase the amount of DDT being taken up by the adipocytes (since the previous experiment suggested that when TG uptake is inhibited, DDT uptake actually increases). As shown in Fig. 4B, adding albumin to the culture media, in a concentration similar to rat plasma albumin levels [15], causes a 35% decrease in [3H]-TG uptake while concomitantly increasing [14C]-DDT uptake by approximately 14%. This significant difference suggests that DDT uptake may compete with TG uptake in adipocytes, and that increased concentrations of albumin do not sequester DDT from the adipocytes as they do to TG.
4. Discussion
The main route of exposure of humans to lipophilic organochlorine pesticides (such as DDT) is through ingestion. It is known that the route of absorption is a major determinant for the tissue distribution and subsequent metabolism of these compounds [16]. For example, absorption from the intestine directly into the portal vein results in direct transport of the compound to the liver, where detoxification or metabolism may occur prior to delivery of that compound to extrahepatic tissues. Conversely, if the compound is primarily absorbed via the intestinal lymphatics this can result in delivery of the parent compound to extrahepatic tissues prior to any detoxification. This route of absorption is especially important since many organochlorine compounds, including DDT, undergo negligible metabolism in the enterocyte prior to absorption.
It has previously been shown that DDT is primarily absorbed via the lymphatics [16], herein we have made use of the lymph fistula rat model to isolate lymphatic chylomicrons. These particles represent the precise postprandial output of the intestine, and are the vehicle for ingested DDT (since it is known that ingested DDT is absorbed through the lymphatic route) [7].
Both lipids and lipophilic compounds are secreted into the intestinal lymph from the gastrointestinal system after a meal. Lymph is formed when fluid and proteins from the interstitial space that are not reabsorbed by blood capillaries enter the lymphatic capillaries [17]. The lymphatics of the gastrointestinal tract are responsible for the transport of lipids and lipophilic compounds (in chylomicrons) into the circulation. chylomicrons containing dietary lipids and lipophilic compounds are first transported via the intestinal (mesenteric) lymphatic duct and then through the thoracic duct before entering the circulation through the subclavian vein [5].
It is known that in the small intestine, lipophilic organochlorine compounds such as DDT associate with emulsified lipid droplets and specifically with the lipophilic core of bile salt mixed micelles; this occurs during chylomicron formation in the enterocyte. The most lipophilic organochlorine compounds, with log10Pow (octanol-water partition coefficient) >5.0, enter the circulation as a part of intestinal triacylglycerol-rich lipoproteins, called chylomicrons [16]. This entry route bypasses first-pass liver metabolism, therefore the chylomicron components, which include TG and associated organochlorine compounds, are delivered directly to peripheral tissues. Adipose tissue is exposed to DDT in this manner, resulting in its accumulation there [4].
Therefore, direct sampling of the lymph through lymph cannulation is a remarkably powerful tool for studying the mechanism by which DDT is delivered from lymph chylomicrons to adipose tissue [3]. This transport of DDT in chylomicrons to adipose tissue has not been explored. Instead, experiments with DDT have used the vehicle DMSO for its delivery to cells. We directly compared the uptake of DDT in chylomicrons to DMSO and found that DMSO delivery markedly increased the uptake of DDT by the cells (Fig. 1). This observation suggests that the physiological delivery of DDT to adipose tissue is much smaller from chylomicrons and may be influenced by other components of the lymphatic milieu. The composition of chylomicrons (triacylglycerol, cholesterol, phospholipids, apolipoproteins) may inhibit DDT efflux into cells, or perhaps the location of DDT within the chylomicron simply slows the process of transfer. Our data suggest that DMSO may not be the appropriate delivery vehicle to model these important physiological questions.
We cannot be certain, however, that the differences we see between DDT uptake delivered in DMSO and chylomicrons are actually due to inherent differences in DDT uptake from a chylomicron, as opposed to being due to changes that DMSO may cause in the adipocytes (such as causing a change in membrane fluidity or enzymatic activity of surface proteins). We believe that this uncertainty is precisely why DMSO is a poor model for studying the uptake of DDT (or other lipophilic molecules) from chylomicrons by intact cells.
The more rapid cellular uptake of DDT from chylomicrons to either differentiated adipocytes or preadipocytes, as compared to TG uptake, is consistent with earlier in vivo chylomicron disappearance studies in whole animal (Fig. 2) [4,7]. Those studies suggested that DDT is more rapidly removed from circulating chylomicrons than TG. Our findings in both differentiated adipocytes and preadipocytes are consistent with this in vivo data. This finding suggests that since DDT is absorbed lymphatically and avoids first-pass metabolism, and since DDT uptake into adipocytes occurs even faster than TG uptake, that extrahepatic tissues may be exposed to a significantly higher proportion of ingested DDT than might be expected for other lipophilic compounds (which also eventually travel to the liver).
Interestingly, despite an overall lowering of DDT uptake in preadipocytes, there is still more DDT taken up than TG by these cells. Therefore, the differential mechanism of DDT uptake (as compared to TG uptake) appears to require the presence of mature adipocytes. This could be due to an unknown mechanism in the mature adipocyte that is responsible for uptake, or merely due to a lack of storage capacity by the pre-adipocyte.
The addition of lipase accelerated the uptake of both TG and DDT into adipocytes (Fig. 3), although the effect of lipase on DDT uptake was significantly smaller than its effect on TG uptake. As we expected, lipase stimulated the hydrolysis of fatty acids from TG within the chylomicron, which were then taken up by adipocytes. This significant difference in the effect of lipase action (both pancreatic lipase and LPL) suggests that hydrolysis is very important for increasing the uptake of TG from chylomicrons to adipocytes, but is less important for stimulating the uptake of DDT. Therefore, the movement of DDT from chylomicrons to adipocytes does not mirror the movement of triacylglycerol hydrolytic products from those chylomicrons, since we know that hydrolysis increases triacylglycerol uptake by adipose. This observation supports our conclusion that the behavior and properties of DDT within the chylomicron may differ greatly from triacylglycerol within the same particles.
When we inhibited LPL with orlistat, which inhibits the active site of LPL, the transport of TG and fatty acids was significantly inhibited (Fig. 4A), as expected [18,19]. Interestingly, the transport of DDT increased under both lipase treatment and LPL inhibition, suggesting that DDT transport occurs in a manner independent of TG hydrolysis, and does not require LPL. It is possible, however, that the transport of DDT may accompany fatty acid release from the chylomicron during TG hydrolysis.
Given this data suggesting that DDT uptake may be lipase independent, it is interesting to speculate that DDT may enter previously uncharacterized tissue depots, especially where LPL is not abundant. This might include, for example, the adipocytes surrounding lymphatic vessels and lymph nodes [20,21]. Since LPL activity in these depots is minimal, and since they have access to significantly less blood flow and more lymph (where LPL activity is low), it would be possible for TG uptake by these depots to be low but for DDT uptake from chylomicrons to be high. This difference in uptake would lead to a unique adipose depot very rich in DDT (and possibly other organochlorine toxins).
Interestingly, DDT does not seem to partition from chylomicrons into circulating albumin rather than being taken up by adipocytes. We found no evidence that serum concentrations of albumin hindered uptake of DDT delivered in chylomicrons (Fig. 4B). Previous studies have shown apparently conflicting effects of albumin on DDT uptake. Ohmiya and Koga found DDT uptake by cells to be stimulated by albumin, while Gulden and Seibert reported that albumin hindered its uptake [12,13]. DDT was delivered to cells in DMSO in both of these studies. We did, however, see a decrease in TG uptake when albumin was added to the media as expected. This observation suggests, along with our experiments with lipase and orlistat, that DDT is transported in a manner independent from the TG within the chylomicron.
Our data are consistent with a model in which a significant portion of DDT in the chylomicron is distributed in the phospholipid monolayer that encapsulates the lipophilic core of the chylomicron (where the TG is found). The relatively small size of the DDT molecule allows it to readily intercalate into the fatty acid moieties of a phospholipid monolayer or bilayer [16]. This distribution of DDT has been suggested by reports of altered fluidity of phospholipid liposomes and of native membranes in the presence of DDT [22,23]. TG can also be part of the phospholipid surface of the chylomicron, but its concentration in the monolayer is extremely low; for example, in a chylomicron-like emulsion of triolein, cholesterol, lecithin, and water, only 2–4% of the surface of that particle was triolein at 22–24 °C [24]. This TG in the monolayer is available for lipase-catalyzed hydrolysis and subsequent transport of the fatty acids to tissues such as adipose. The data presented here show that DDT uptake is greater than TG, and support the idea that DDT is likely on the surface of the chylomicron where it can be rapidly taken up by adipose tissue.
This conclusion is consistent with several experimental and theoretical calculations of DDT distribution on chylomicron particles. It is known that phospholipids at the surface of rat chylomicrons account for 5–11% of the lipid mass, whereas TG accounts for 79–90% [25]. If DDT distributes uniformly among these lipids of the chylomicron, then theoretically 5–11% of the DDT would reside in the fatty acid chains of the phospholipids near the surface of the chylomicron. A similar calculation predicts that less than 0.5% of the triacylglycerol will reside in the surface monolayer. Pocock and Vost measured the distribution of DDT between membrane and core TG in chylomicrons and estimated 3–4% of the DDT to be in the membrane [7]. Again, the greater distribution of DDT on the chylomicron surface (compared with TG on the surface) could cause significantly higher levels of DDT to be taken up by adipocytes compared to TG. Our data suggest that the relatively small DDT molecule rapidly diffuses from the surface phospholipid monolayer of the chylomicron into the recipient adipocytes. During the metabolism of the chylomicron particle, we hypothesize that this could be followed by diffusion of DDT from the core of the chylomicron to the surface layer.
In summary, we show here for the first time that DDT carried by chylomicrons is available for delivery to adipocytes and that this process may be independent of TG transport. This novel finding has important implications because it shows that using chylomicrons to deliver DDT to tissues may be a more physiologically accurate model in which to understand and mediate DDT toxicity in humans. The finding that DDT movement does not mimic the movement of TG is also novel, and we believe that this represents an important step towards understanding the way that this lipophilic toxin enters circulation and then interacts with one of its main storage depots (adipose tissue). We are believe that the data presented here will represent a major step forward in the ability to model the movement of DDT, and possibly other lymphatically absorbed lipophilic toxins, with the future goal of being able to describe the mechanism by which DDT moves out of the chylomicron and how we may be able to influence this process.
Acknowledgments
We are extremely grateful to the National Institutes of Health (NIH) for the support of this study. This work was supported by the National Institutes of Health Grants ES014464 and DK-076928 (to Dr. Tso), DK-059630 (University of Cincinnati Mouse Metabolic Phenotyping Center), and NIH F32-091173-01 (to Dr. Kohan).
Abbreviations
- CM
chylomicrons
- DDT
dichlorodiphenyltrichloroethane
- TG
triacylglycerol
- LPL
lipoprotein lipase
References
- 1.Woodruff T, Wolff MS, Davis DL, Hayward D. Organochlorine exposure estimation in the study of cancer etiology. Environ Res. 1994;65(1):132–144. doi: 10.1006/enrs.1994.1026. [DOI] [PubMed] [Google Scholar]
- 2.Schafer KS, Kegley SE. Persistent toxic chemicals in the US food supply. J Epidemiol Community Health. 2002;56(11):813–817. doi: 10.1136/jech.56.11.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohan AB, Yoder SM, Tso P. Using the lymphatics to study nutrient absorption and the secretion of gastrointestinal hormones. Physiol Behav. 2011;105(1):82–88. doi: 10.1016/j.physbeh.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trevaskis NL, Tso P, Rider T, Charman WN, Porter CJ, Jandacek R. Tissue uptake of DDT is independent of chylomicron metabolism. Arch Toxicol. 2006;80(4):196–200. doi: 10.1007/s00204-005-0028-2. [DOI] [PubMed] [Google Scholar]
- 5.Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6(3–4):109–122. doi: 10.1089/lrb.2008.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vost A, Maclean N. Hydrocarbon transport in chylomicrons and high-density lipoproteins in rat. Lipids. 1984;19(6):423–435. doi: 10.1007/BF02537404. [DOI] [PubMed] [Google Scholar]
- 7.Pocock DE, Vost A. DDT absorption and chylomicron transport in rat. Lipids. 1974;9(6):374–381. doi: 10.1007/BF02532054. [DOI] [PubMed] [Google Scholar]
- 8.Slim R, Toborek M, Robertson LW, Hennig B. Antioxidant protection against PCB-mediated endothelial cell activation. Toxicol Sci. 1999;52(2):232–239. doi: 10.1093/toxsci/52.2.232. [DOI] [PubMed] [Google Scholar]
- 9.Tso P, Karlstad MD, Bistrian BR, DeMichele SJ. Intestinal digestion, absorption, and transport of structured triglycerides and cholesterol in rats. Am J Physiol. 1995;268(4 Pt 1):G568–G577. doi: 10.1152/ajpgi.1995.268.4.G568. [DOI] [PubMed] [Google Scholar]
- 10.Floren CH, Nilsson A. Degradation of chylomicron remnant cholesteryl ester by rat hepatocyte monolayers. Inhibition by chloroquine and colchicine. Biochem Biophys Res Commun. 1977;74(2):520–528. doi: 10.1016/0006-291x(77)90335-7. [DOI] [PubMed] [Google Scholar]
- 11.Kohan AB, Qing Y, Cyphert HA, Tso P, Salati LM. Chylomicron remnants and nonesterified fatty acids differ in their ability to inhibit genes involved in lipogenesis in rats. J Nutr. 2011;141(2):171–176. doi: 10.3945/jn.110.129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulden M, Morchel S, Tahan S, Seibert H. Impact of protein binding on the availability and cytotoxic potency of organochlorine pesticides and chlorophenols in vitro. Toxicology. 2002;175(1–3):201–213. doi: 10.1016/s0300-483x(02)00085-9. [DOI] [PubMed] [Google Scholar]
- 13.Ohmiya Y, Koga K. Protein-mediated transfer of DDT to adipose tissue. J Toxicol Sci. 1978;3(1):31–37. doi: 10.2131/jts.3.31. [DOI] [PubMed] [Google Scholar]
- 14.Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130(12):3122S–3126S. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- 15.Liao WS, Jefferson LS, Taylor JM. Changes in plasma albumin concentration, synthesis rate, and mRNA level during acute inflammation. Am J Physiol. 1986;251(6 Pt 1):C928–C934. doi: 10.1152/ajpcell.1986.251.6.C928. [DOI] [PubMed] [Google Scholar]
- 16.Jandacek RJ, Rider T, Yang Q, Woollett LA, Tso P. Lymphatic and portal vein absorption of organochlorine compounds in rats. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):G226–G234. doi: 10.1152/ajpgi.90517.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50(1–2):3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 18.Eckel RH, Fujimoto WY, Brunzell JD. Development of lipoprotein lipase in cultured 3T3-L1 cells. Biochem Biophys Res Commun. 1977;78(1):288–293. doi: 10.1016/0006-291x(77)91252-9. [DOI] [PubMed] [Google Scholar]
- 19.Lookene A, Skottova N, Olivecrona G. Interactions of lipoprotein lipase with the active-site inhibitor tetrahydrolipstatin (Orlistat) Eur J Biochem. 1994;222(2):395–403. doi: 10.1111/j.1432-1033.1994.tb18878.x. [DOI] [PubMed] [Google Scholar]
- 20.Pond CM. Adipose tissue and the immune system. Prostaglandins Leukot Essent Fatty Acids. 2005;73(1):17–30. doi: 10.1016/j.plefa.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Mattacks CA, Sadler D, Pond CM. The effects of dietary lipids on dendritic cells in perinodal adipose tissue during chronic mild inflammation. Br J Nutr. 2004;91(6):883–892. doi: 10.1079/BJN20041147. [DOI] [PubMed] [Google Scholar]
- 22.Antunes-Madeira MC, Madeira VM. Membrane fluidity as affected by the insecticide lindane. Biochim Biophys Acta. 1989;982(1):161–166. doi: 10.1016/0005-2736(89)90187-9. [DOI] [PubMed] [Google Scholar]
- 23.Antunes-Madeira MC, Almeida LM, Madeira VM. Depth-dependent effects of DDT and lindane on the fluidity of native membranes and extracted lipids. Implications for mechanisms of toxicity. Bull Environ Contam Toxicol. 1993;51(6):787–794. doi: 10.1007/BF00198271. [DOI] [PubMed] [Google Scholar]
- 24.Miller KW, Small DM. The phase-behavior of triolein, cholesterol, and lecithin emulsions. J Colloid Interface Sci. 1982;89(2):466–478. [Google Scholar]
- 25.Kalogeris TJ, Story JA. Lymph chylomicron composition and size are modified by level of intestinally infused cholesterol and triglyceride source in rats. J Nutr. 1992;122(5):1045–1055. doi: 10.1093/jn/122.5.1045. [DOI] [PubMed] [Google Scholar]