Synopsis
Activation of phosphoinositide 3-kinase (PI3K) is a shared response to engagement of diverse types of transmembrane receptors. Depending on the cell type and stimulus, PI3K activation can promote different fates including proliferation, survival, migration and differentiation. The diverse roles of PI3K signaling are well illustrated by studies of lymphocytes, the cells that mediate adaptive immunity. Genetic and pharmacological experiments have shown that PI3K activation regulates many steps in the development, activation and differentiation of both B and T cells. These findings have prompted the development of PI3K inhibitors for the treatment of autoimmunity and inflammatory diseases. However, PI3K activation has both positive and negative roles in immune system activation. Consequently, while PI3K suppression can attenuate immune responses it can also enhance inflammation, disrupt peripheral tolerance and promote autoimmunity. An exciting discovery is that a selective inhibitor of the p110δ catalytic isoform of PI3K, CAL-101, achieves impressive clinical efficacy in certain B cell malignancies. A model is emerging in which p110δ inhibition disrupts signals from the lymphoid microenvironment, leading to release of leukemia and lymphoma cells from their protective niche. These encouraging findings have given further momentum to PI3K drug development efforts in both cancer and immune diseases.
Introduction
The adaptive immune system is crucial for protection from recurring infections by various pathogens. T and B lymphocytes are the key cellular mediators of this system in which the enormous diversity and exquisite specificity of the antigen receptors enables T and B cells to recognize virtually any foreign molecule. Antigen recognition alone, however, is insufficient to initiate effective immune responses. A key feature of the adaptive immune system is that lymphocytes depend on signals from neighboring cells to perform specific functions in the context of various immune conditions. For example, B cells cannot properly complete their primary task of making antibodies without signals from CD4 T cells. Similarly, CD4 T cells can differentiate into a variety of effector subsets depending on costimulatory signals and the cytokine milieu generated from other immune cells of the microenvironment. To prevent autoimmunity, lymphocyte self-tolerance must be enforced and certain conditions should favor activation of regulatory cells that suppress rather than promote immune responses. Integration of diverse extracellular cues to achieve the required cellular response involves a complex network of intracellular signaling events. This concept also applies to tumors of lymphoid origin, whose survival typically depends on both cell-intrinsic and cell-extrinsic signals from the microenvironment.
The phosphoinositide 3-kinase (PI3K) signaling network plays a fundamental role in signal transduction in mammalian cells [1, 2]. Hence, it is not surprising that PI3K is activated by diverse stimuli in lymphocytes and is required for the maintenance of proper adaptive immunity and self-tolerance [3, 4]. In this review we summarize recent advances in the understanding of PI3K signaling in B and T cells. These advances have been spurred largely by two technological breakthroughs: refinements in gene-targeted mouse models and discovery of PI3K isoform-selective inhibitors. We emphasize two key concepts. First, PI3K activation should not be viewed as a simple on/off switch but rather as a “rheostat” whose output must be properly balanced for effective cellular responses. Both effector and regulatory cell populations engage PI3K signaling pathways, as do cells of the innate immune system. Therefore, decreased PI3K signaling can lead to immunosuppression or, in some contexts, to increased inflammation and improved pathogen clearance, and even to autoimmunity. The second concept is that despite this complexity, the p110δ catalytic isoform of class I PI3K has emerged as a central driver of lymphocyte clonal selection, differentiation and trafficking. We conclude by describing progress in the development of PI3K inhibitors for therapeutic uses. Indeed, development of highly specific PI3K inhibitors for clinical use is one of the major goals in the pharmaceutical industry today. Of particular interest is the surprising success of a selective p110δ inhibitor CAL-101 in human B cell malignancies, where CAL-101 seems to act mainly by perturbing the signals received from the tumor microenvironment.
Overview of PI3K
PI3Ks are a family of lipid kinases that phosphorylate the 3′-hydroxyl group of phosphatidylinositol (PtdIns) and phosphoinositides (phosphorylated derivatives of PtdIns) [2]. Unlike yeast, whose genome encodes only one PI3K isoform (class III: Vps34) whose main role is in vesicle trafficking, the mammalian PI3Ks include eight enzymes with diverse roles in both vesicle trafficking and signal transduction. These enzymes are grouped into the categories known as class I, class II, and class III, based on substrate preference and structure. Only the class I PI3Ks have the ability to use PtdIns-4,5-bisphosphate (PtdIns-4,5-P2) as a substrate to generate the important second messenger PtdIns-3,4,5-trisphosphate (PIP3). Certain proteins containing a pleckstrin homology (PH) domain can specifically bind PIP3 and be recruited to membranes where PI3K is active [5]. Hence, class I PI3K acts as a signaling hub at the plasma membrane to change the lipid composition in a way that links transmembrane receptors to the organization of multiprotein complexes, also known as signalosomes [6]. The composition of these signalosomes and the specific PH domain-containing PI3K effector proteins recruited to these assemblies varies according to the receptor that is engaged. In most cells, the serine/threonine kinase AKT (also known as protein kinase B, PKB) is a key PI3K effector and AKT phosphorylation is a common readout of PI3K activation [7]. Two amino acid residues in AKT are phosphorylated in a PI3K-dependent manner: T308 by phosphoinositide-dependent kinase-1 (PDK-1), and S473 by TORC2 (a complex of the target of rapamycin (TOR), rictor and other proteins).
PI3K and AKT coordinate many aspects of the response to antigen receptor engagement. In B and T cells, the formation of signalosomes that drive antigen receptor-dependent Ca2+ mobilization is partially dependent on PI3K [6, 8]. PIP3 produced upon BCR or TCR clustering binds to PH domains in TEC family kinases to promote activation of phospholipase C-γ and subsequent hydrolysis of PtdIns-4,5-P2. This results in release of soluble IP3 to initiate Ca2+ mobilization, and accumulation of diacylglycerol in the membrane to activate protein kinase C isoforms and GTP exchange factors for RAS. A role for PI3K upstream of RAS in lymphocytes is supported by the observation of decreased ERK phosphorylation in PI3K-deficient T and B cells stimulated through antigen receptors [9]. Activated AKT has many substrates with key roles in lymphocyte activation and trafficking. The Forkhead Box Subgroup O (FOXO) transcription factors are one key group of AKT substrates in lymphocytes [3]. AKT-mediated phosphorylation inhibits DNA binding by FOXO factors and promotes their nuclear exit and cytoplasmic sequestration and degradation. AKT also can phosphorylate Tuberous Sclerosis 2 (TSC2) and Proline-Rich AKT substrate of 40kDa (PRAS40) to increase the activity of TORC1 (TOR complex-1), a multifunctional signaling protein that coordinates cell growth and metabolism [10]. However, the dependence of TORC1 activation on PI3K and AKT varies in lymphocytes according to the cell subset and stimulus [11, 12].
Elevation of cellular PIP3 is transient and is controlled by lipid phosphatases [13, 14]. Phosphatase and Tensin homolog (PTEN) is a phosphoinositide 3-phosphatase that converts PIP3 back to PtdIns-4,5-P2. In cells lacking PTEN, basal PIP3 amounts are increased and receptor stimulation causes exaggerated PI3K signaling. SH2 domain-containing inositol phosphatases (SHIP1 and SHIP2) are 5-phosphatases that convert PIP3 to PtdIns-3,4-bisphosphate (PtdIns-3,4-P2). The latter lipid species can still recruit and activate PDK-1 and AKT but has other signaling functions that do not overlap with PIP3. For example, the PH domains of TEC family kinases have high affinity for PIP3 but not PtdIns-3,4-P2, whereas the converse is true for the adaptor proteins Bam32, TAPP1 and TAPP2 [15].
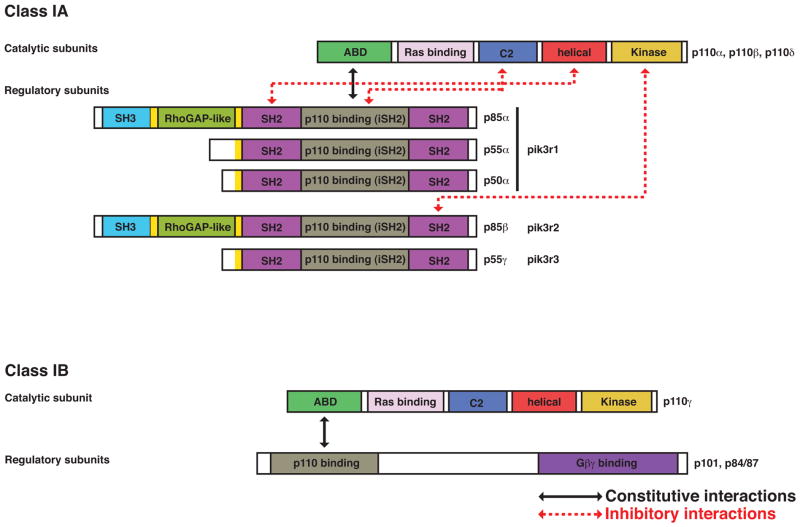
Class I PI3Ks are further divided into two subgroups: class IA and class IB [2]. Class IA PI3Ks contain one of three distinct 110 kDa catalytic isoforms (p110α, p110β, or p110δ) that forms a heterodimer with one of the five regulatory (adaptor) isoforms (p85α, p55α, p50α, p85β, or p55γ). The overall structure of p110 subunits is very similar with the following domains: adaptor-binding (ABD), Ras-binding, C2, helical and lipid kinase (Figure 1). The class IA regulatory isoforms share a similar C-terminal half that contains two Src-homology-2 (SH2) domains that flank a coiled-coil domain (also called iSH2) that binds tightly to the p110 ABD to form the heterodimer. The N-terminal portions of p85α and p85β contain an additional Src-homology-3 (SH3) domain and a RhoGAP-homology region flanked by proline-rich motifs. Class IB PI3K is composed of a single catalytic isoform (p110γ) bound to one of two regulatory isoforms (p101 or p84). In general, class IA PI3Ks are activated by tyrosine kinase (TK)-based signals whereas class IB PI3Ks are activated by G protein-coupled receptors (GPCRs). However, these distinctions have been challenged by numerous studies linking p110β and p110δ to GPCRs and p110γ to TKs [2, 16]. Whereas p110α and p110β are expressed ubiquitously, p110δ and p110γ are predominantly expressed in the cells of the immune system compared to other cell types. This suggests that p110δ and p110γ may have evolved to serve specific roles in immunity, a hypothesis supported by many published studies and emphasized herein. The possibility that p110α and p110β isoforms might also be important in lymphocytes will also be discussed.
Figure 1. Domain structure of class IA and class IB PI3K isoforms.
Black arrows indicate constitutive interactions of the heterodimers. Dashed red arrows indicate inhibitory interactions between the regulatory and catalytic isoforms of class IA PI3K that maintain low basal activity of the enzyme. Phosphorylation of tyrosine (Y) residues on the conserved pY-X-X-M motifs on various receptors or adaptor proteins (CD19/BCAP for B cells, CD28/ICOS for T cells) recruits the regulatory isoforms via the two SH2 domains and this binding releases the inhibitory interactions. Other protein:protein interactions also contribute to class IA and IB recruitment and activation.
The mechanism of class IA PI3K activation has been clarified through structural studies and has been reviewed in detail elsewhere [17, 18]. Briefly, the regulatory subunit has different functions in the absence or presence of stimulation. In the basal state, the regulatory subunit stabilizes the catalytic subunit while suppressing its activity. This suppression is mediated by several inter-subunit contacts including helical(p110)/N-SH2(p85) and C2(p110)/iSH2(p85) (Figure 1). The C-SH2 domain of p85β can also form an inhibitory contact with the kinase domain of p110β. In response to tyrosine kinase-based signals, the SH2 domains become bound to phosphotyrosine (pTyr) residues on receptors and adaptor proteins (consensus motif pTyr-X-X-Met), releasing the inhibitory contacts and bringing the catalytic subunit to the membrane where its substrates reside. Additional interactions involving the N-terminal domains of p85 proteins might also contribute to PI3K recruitment and activation in some contexts. Naturally occurring mutations in p85α and p85β have been identified in tumors of humans and mice [19–23]; in general these are gain-of-function mutations that disrupt the inhibitory interfaces and elevate PI3K enzyme activity while maintaining the stability of the catalytic subunit.
How do receptors on lymphocytes engage class I PI3K? Antigen receptors, costimulatory molecules, cytokine receptors, and chemokine receptors can all trigger an increase in PIP3 and phosphorylation of AKT. Crosslinking of the B cell receptor (BCR) leads to tyrosine phosphorylation of the co-receptor CD19 and B-cell adaptor protein (BCAP), and each of these proteins recruits class IA PI3K through SH2 domain interactions [4, 24, 25] (Figure 2). T cell receptor (TCR) engagement also activates class IA PI3K but the molecular details remain to be fully established. Possible mechanisms include: binding of p85 proline-rich domains to SH3 domains of Src family kinases [26, 27]; interaction of p85 Rho-GAP domains with Rac-GTP downstream of the Vav exchange factors [28–31]; and association of SH2 domains in p85 or p50 with pTyr residues in proteins associated with TCR signalosomes [32, 33]. Two co-stimulatory molecules on T cells, CD28 and ICOS, recruit PI3K through pTyr-X-X-Met motifs [34]. In the case of CD28, recruitment of PI3K appears dispensable for most of the initial costimulatory signals but is essential for CD28 function in effector T cells [4]. The role of PI3K in ICOS signaling will be discussed below. Other costimulatory molecules and cytokine receptors on B and T cells recruit and activate class IA PI3K through diverse mechanisms [35, 36]. The class IB p110γ isoform is mainly activated by GPCR signals (i.e. chemokine receptors) in cells of the innate immune system such as neutrophils [37]. The βγ subunits of heterotrimeric G proteins directly bind to p110γ and mediate enzyme activation through p101 or p84 adaptor subunits (Figure 1). However, the situation is more complicated in lymphocytes in that chemokines activate p110γ in T cells but p110δ in B cells [38, 39]. The mechanism linking GPCRs to p110δ in B cells is not known. Furthermore, there is some evidence that TCR signaling activates p110γ, possibly via G-alpha proteins or the small GTPase RAS [40, 41]. All of the class I catalytic subunits including p110γ contain RAS-binding domains (Figure 1), and the interaction of p110γ with RAS is required for T cell development at the β-selection stage [41]. An interesting and largely unexplored question is whether different receptors generate distinct pools of PIP3 during lymphocyte activation, with selective localization and downstream effectors. This concept was validated in a study of p110γ activation in mast cells, where p101-bound and p84-bound p110γ produced distinct pools of PIP3 with separable function [42].
Figure 2. PI3K engagement in B lymphocytes and the rheostat concept.
BCR engagement triggers tyrosine (Y) phosphorylation on CD19 and BCAP to recruit PI3K dimers mainly consisting of p85α and p110δ. PI3K activation promotes signalosome assembly for Ca2+ mobilization and diacylglycerol (DAG) production, and increases activity of AKT. BCR dependent Ca2+ flux, AKT activation and proliferation is mainly dependent on PIP3 pools generated by dimers of the p110δ catalytic isoform with the p85α regulatory isoform. Cytokine (BAFF and IL-4) dependent survival signals require p110α as well, which might generate distinct pools of PIP3 as shown. Two parallel membranes drawn in light brown represent a 3-dimensional cell surface rather than distinct membranes. In vivo, overall PI3K activity serves as a ‘rheostat’ (gray circle) whose signal output strength determines the nature of the response. In B cells, high PI3K activity opposes class switch recombination and promotes plasma cell differentiation. Low PI3K activity promotes class switch recombination, with p110δ inhibition selectively augmenting IgE production in mice. A similar rheostat concept applies in CD4 T cells to generate the variety of different subsets required depending on the immune context.
Tools to study PI3K signaling in lymphocytes
Following the molecular cloning of class I PI3K isoforms in the early 1990s, PI3K signaling in lymphocytes was studied mainly using established cell lines derived from lymphoid tumors. This approach was convenient for biochemical studies because cell lines have more cytoplasm and protein content than primary T and B cells. Cell line studies also avoided the problem that primary lymphocytes die rapidly in culture and cannot easily be metabolically labeled or transfected. However, tumor cells have severe drawbacks for signal transduction research as they usually have dysregulated PI3K signaling, and cell proliferation is typically uncoupled from physiological extracellular controls. Furthermore, the PI3K inhibitors used in early experiments (wortmannin and LY294002) are non-selective compounds that inhibit all PI3K isoforms as well as mTOR and other lipid and protein kinases in cells [43–45]. A more precise understanding of PI3K signaling required better tools.
The first major technical advance was the creation of genetically modified mouse strains lacking individual class I PI3K isoforms. Germline and/or tissue-specific knockouts of the genes encoding each class I catalytic subunit, and of the genes encoding p85α/p55α/p50α and p85β, have been generated and characterized (Supplementary Table 1). Insights gained from these mouse strains have been summarized in many reviews (for example, [4, 35, 46]) and new findings are discussed herein. However, PI3K gene knockouts have significant limitations even when deletion is tissue-specific. One problem is that loss of one isoform of PI3K often leads to altered expression of non-targeted isoforms [47]. For example, deletion of the Pik3r1 gene encoding p85α/p55α/p50α causes reduced expression of p110 proteins and increased expression of p85β. Deletion of individual p110 isoforms results in functional compensation of other catalytic isoforms. Hence, an important step forward was the generation of knock-in mice that express intact proteins with point mutations causing loss-of-function. This strategy was first used by Okkenhaug, Vanhaesebroeck and colleagues to generate p110δD910A/D910A mice with a point mutation inactivating p110δ kinase function [9], and has been used subsequently to generate kinase-inactive (KI) alleles of each of the p110 isoforms (Supplementary Table 1). This strategy prevents compensatory changes in expression or function and provides a more precise model of chemical kinase inhibition. However, the knock-in strategy is still limited to some degree by the fact that the mutation can affect lymphocyte development, resulting in an altered pool of mature T or B cells. In addition, p110αKI mice display embryonic lethality at a stage before lymphoid precursors can be isolated. Therefore, data from knockout and knockin mice are most conclusive when combined with studies of wild-type cells treated with selective PI3K inhibitors.
The discovery and validation of selective, ATP-competitive PI3K inhibitors has been driven by efforts from both industry and academia. Although the kinase domains of class I PI3K isoforms are highly conserved, X-ray crystal structures have shown that the ATP-binding pockets have distinct topologies and flexibilities that can allow selective binding of distinct chemical structures [18]. These properties have allowed the development of compounds with good selectivity for single class I PI3K isoforms. There are also several compounds targeting all class I isoforms with minimal off-target effects on other kinases (termed “pan-class I PI3K inhibitors”). Examples of isoform-selective and pan-class I PI3K inhibitors are shown in Table 1. Many of these can be purchased from commercial vendors, and most have optimized pharmacological properties to allow dosing of animals in vivo. Despite these powerful additions to the PI3K inhibitor toolkit, many investigators continue to test PI3K function using wortmannin or LY294002. The importance of choosing the right PI3K inhibitor at the optimal concentration (or in vivo dose) cannot be overstated.
Table 1.
Selected PI3K inhibitors currently available for basic research or in clinical trials1
| Targeted Isoform(s) | Compound Name | Status |
|---|---|---|
| Pan-class I | GDC-0941 | Basic and clinical |
| ZSTK474 | Basic and clinical | |
| XL147 | Basic and clinical | |
| p110α high selectivity | A66 | Basic |
| INK1117 | Clinical | |
| BYL-719 | Clinical | |
| p110α moderate selectivity | PIK-75 | Basic |
| p110α/γ/δ | PIK-90 | Basic |
| p110β | TGX-115, TGX-221 | Basic |
| AZD6482 | Basic and clinical | |
| p110γ | AS252424, AS604850 | Basic |
| p110δ | IC87114 | Basic |
| CAL-101/GS1101 | Clinical | |
| CAL-263 | Clinical | |
| AMG-319 | Clinical | |
| Dual p110γ/p110δ | IPI-145 | Clinical |
Dual PI3K/TOR inhibitors not included in the table
In addition to PI3K gene knockouts and knock-ins, other genetically engineered mouse models have been produced to study PI3K signaling in lymphocytes. These include knockouts and knock-in mutations in PI3K effectors such as PDK-1 and AKT, and knockouts of the genes encoding PIP3 phosphatases PTEN and SHIP1. These models have proven very useful for dissecting PI3K signaling pathways in lymphocytes, and some recent advances are described in this review. Mouse strains with mutations affecting TOR signaling, FOXO transcription factors and other PI3K-regulated signaling components have also yielded important advances that we will discuss. Another common approach has been to generate transgenic mice expressing membrane-targeted forms of AKT in lymphocytes. The rationale for this strategy is that it will reveal the functions of AKT, independent of other PI3K effectors. These mice exhibit dramatic phenotypes with altered lymphocyte development, lymphoproliferation and loss of immune homeostasis [48–51]. However, the phenotypes of AKT gain-of-function mutations should be interpreted with caution. In normal immune cells, AKT is recruited transiently to cell membranes and once activated it travels to different subcellular compartments to phosphorylate diverse substrates. The activation state of AKT is also controlled by a large array of phosphatases and other regulatory proteins. Therefore, constitutive anchoring in the membrane does not faithfully recapitulate the functions of active AKT. Nevertheless, T cell-specific deletion of PTEN or expression of a constitutively active PI3K also causes lymphoproliferation and autoimmunity in mice [52, 53]. Together these approaches concur that artificially elevating PI3K signaling output in T cells causes a loss of immune homeostasis.
Fluorescent bioprobes have provided valuable insights into the kinetics and subcellular localization of PI3K lipid production in lymphocytes, and will continue to be useful tools [54]. However, these probes do not provide a comprehensive and quantitative view of different PI3K lipid species. Further advances in this direction are likely to emerge from the application of novel mass spectrometry technologies. The group of Stephens and Hawkins has reported a method for sensitive quantitation of PIP3 without radioactive or heavy isotope labeling [55]. The method also allows the discrimination of PIP3 species based on distinct fatty acyl chain composition. Combining this technique with genetic or pharmacological inhibition of specific PI3K isoforms might uncover important aspects of PI3K signaling specificity in lymphocytes.
B lymphocytes
B cell development and tolerance
B cells develop in the bone marrow from lymphoid progenitors that undergo an ordered series of gene rearrangement steps punctuated by stringent checkpoints. The immunoglobulin (Ig) heavy chain rearrangement occurs at the pro-B cell stage and when successful, the heavy chain pairs with surrogate light chains and Igα-Igβ heterodimers to form the pre-BCR at the cell surface. Assembly of the pre-BCR triggers tonic (basal) signaling that is critical for repression of RAG (recombination-activating gene) expression to prevent further heavy chain rearrangement. At this stage cells proliferate and are termed large pre-B (or pre-B-I) cells. Ig light chain rearrangement proceeds at the small pre-B (pre-B-II) cell stage and this leads to surface expression of surface IgM containing μ heavy chains and either κ or λ light chains. Immature B cells expressing intact surface IgM are further screened for self-reactivity in the bone marrow and spleen before entering peripheral B cell pools.
The first evidence suggesting a role for PI3K in B cell development came from the original studies of p85α and p110δ knockout, and p110δKI models [9, 56–59]. The numbers of pre-B cells are reduced in mice lacking p85α or p110δ indicating a clear role for class IA PI3K in the pro-B/pre-B transition, though residual development suggested that other isoforms might also function in early B cell development. To address this possibility, Okkenhaug and colleagues analyzed mice with lymphocyte-specific (CD2-Cre) deletion of p110α or p110β [60]. No significant B cell deficits were observed. Crossing each conditional knockout strains with the p110δKI strain revealed that combined inactivation of p110α and p110δ results in a near complete absence of pre-B-II cells, whereas deletion of p110β does not alter the p110δKI phenotype. Using a fluorescence-based microscopy technique, it was demonstrated that sorted pro-B/pre-B-I cells lacking p110α/p110δ have elevated Rag gene expression. This defect in Rag repression correlates with enhanced frequency of rearranged heavy chain alleles in sorted pro/pre-B cells. Thus, both p110α and p110δ both seem to be important for allelic exclusion and developmental progression at the pre-BCR checkpoint whereas p110β is not involved. In addition, p110α and p110δ were found to have overlapping, required functions in IL-7-driven proliferation of pro-B cells in vitro. The class IB isoform p110γ is expressed in the B lineage but is not essential for B cell development or activation. However, combined inactivation of p110γ and p110δ does impair B cell development and reduce peripheral B cell numbers to a greater extent than p110δ inactivation alone [61]. Overlapping functions of p110γ and p110δ are also apparent in thymocyte development, as discussed below.
FOXO factors bind to the promoters of Rag-1 and Rag-2 genes and promote transcription [62]. Deletion of Foxo1 in the B lineage using Mb1-Cre reduces expression of Rag genes, causing impaired heavy and light chain rearrangement and a reduction in pre-B cell numbers [63]. Together with the results of the p110α/δ deletion study this suggests a model in which tonic pre-BCR signaling activates PI3K and AKT, suppressing FOXO factors to extinguish Rag expression. In accord, Su and colleagues reported that deletion of Sin1, an essential component of TORC2, prevents phosphorylation of AKT on S473 and enhances Foxo1-dependent Rag expression in developing B cells [64]. This study also identified a selective role for the AKT2 isoform in Foxo1 phosphorylation and suppression of Rag expression in cultured pro-B cells. However, a required role for AKT2 in this process in vivo is challenged by a study of fetal liver chimeric mice reconstituted with AKT1/AKT2 double knockout cells [65]. In this system, combined deletion of both AKT1 and AKT2 actually resulted to an increase in bone marrow pre-B and immature B cells. One possibility is that the AKT3 isoform can compensate for loss of AKT1 and AKT2 in early B cell development in vivo; mRNA measurements suggest that AKT1 and AKT3 are expressed at higher levels than AKT2 at the pro-B and pre-B cell stages [65].
Kurosaki and colleagues reported that development of B cells from CD19/BCAP double knockout mice is blocked at the pre-BCR checkpoint stage [24]. Both CD19 and BCAP have multiple tyrosine residues that, when phosphorylated, represent a consensus motif for binding to p85 adaptor subunits. Reconstitution of wild-type but not tyrosine-mutated CD19 or BCAP into double knockout cells restores PI3K/AKT signaling and B cell development. These findings suggest that ligand-independent pre-BCR signaling triggers tyrosine phosphorylation of CD19 and BCAP, leading to recruitment of class IA heterodimers containing p85α and either p110α or p110δ. In order for light chain rearrangement to commence, PI3K/AKT signaling needs to be extinguished to allow reactivation of FOXO-dependent Rag expression. Another component of the pre-BCR signaling complex, the adaptor protein SLP-65 (also known as BLNK), seems to be required for attenuation of PI3K/AKT signaling [66].
There are several tolerance mechanisms that prevent the emergence of self-reactive B cells and the production of autoantibodies. In the bone marrow, immature B cells expressing surface IgM recognizing multivalent self-antigen undergo receptor editing, and eventually clonal deletion if self-reactivity persists. In the absence of self-antigen recognition, tonic signaling by the mature surface IgM on immature B cells activates PI3K to extinguish Rag expression, similar to the pre-BCR checkpoint [67]. Immature B cells recognizing monovalent self-antigens in the bone marrow or periphery enter a state known as anergy, in which they fail to respond even to strong stimulation. Anergic B cells have a short lifespan and are rapidly removed from the repertoire. Together, negative selection by clonal deletion and anergy induction produce a self-tolerant peripheral B cell repertoire. Rickert and colleagues have shown that both negative selection and anergy are regulated at the level of PIP3 production [68]. Using a transgenic mouse model, it was observed that anergic B cells have significantly lower production of PIP3 and activation of AKT upon BCR stimulation. Reduced PI3K activation correlates with diminished phosphorylation of CD19, and elevated expression of PTEN. Co-deletion of PTEN reverses the PI3K/AKT activation defects and prevents the induction of anergy. In a negative selection model using immature B cells derived from non-transgenic mice, BCR crosslinking causes a block in proliferation and differentiation that can be overcome by PTEN deletion [68]. An unanswered question is whether a specific PI3K isoform is involved in generating the PIP3 pools responsible for preventing tolerance in PTEN-deficient B cells. Whether PI3K inhibition can induce tolerance in autoreactive B cells also remains to be determined, but is suggested by experiments described in the next section.
Peripheral B cells
At an early stage in B cell development, a lineage split occurs resulting in two subsets of peripheral B cells: the innate-like B-1 B cells that reside in body cavities, and B-2 B cells in blood and secondary lymphoid organs. The B-2 B cells are further divided into marginal zone (MZ) B cells that reside in the MZ of the spleen and do not circulate; and follicular (FO) B cells that recirculate through blood, lymph, and lymphoid tissues. In mouse strains lacking p85α or p110δ, or in chimeric mice with AKT1/AKT2-deficient B cells, B-1 and MZ B cells are nearly absent [9, 56, 58, 65, 69, 70]. This phenotype is similar to mice lacking CD19, a component of BCR signalosomes [71]. This suggests that a major of function of CD19 is to activate PI3K and AKT to allow commitment to the B-1 or MZ lineages at key stages of B cell development. The CD19/PI3K/AKT signal appears to act through inactivation of Foxo1, since Foxo1 deletion in peripheral B cells expands the MZ B cell population and reverses the MZ deficiency in CD19 knockouts [72].
The absence of B-1 and MZ B cells in PI3K knockout strains made it difficult to interpret whether PI3K activity is important for the function of fully developed B-1 and MZ B cells. Gold and colleagues addressed this question by utilizing a p110δ-selective inhibitor IC87114 (Table 1) in immune assays using normal B-1 and MZ B cells [73]. Selective inhibition of p110δ in these cells completely inhibits AKT activation by Toll-like receptor (TLR) ligands and chemoattractants, suggesting that p110δ is the main isoform linking these extracellular signals to AKT. p110δ inhibition also suppresses chemotaxis, proliferation and antibody production by B-1 and MZ cells. Notably, treatment of mice with IC87114 disrupts the localization of B cells with a MZ surface phenotype (IgMhiIgDlo), suggesting that p110δ is required for chemotactic and adhesive signals that position MZ cells in the marginal zone. B-1 and MZ cells are thought to be the major source of natural antibodies to common microbial antigens and some self-antigens. Consistent with the reduced B-1 and MZ compartments in p110δKI mice, natural antibody production is reduced. Autoantibody production is also reduced in p110δKI mice and in mice treated with p110δ inhibitor. These findings suggest that selective p110δ inhibitors have potential for the treatment of autoimmune diseases driven by autoantibodies. Whether p110δ inhibition enforces B cell tolerance through anergy induction, or acts mainly by blocking proliferation and differentiation of self-reactive B cells remains to be established. Interestingly, p110δ inactivation suppresses the expansion of B-1 cells and MZ B cells in the absence of PTEN [74].
The main function of FO B cells is to produce antibodies in response to T cell-dependent (protein) antigens. It is well established that FO B cell function is highly dependent on class IA PI3K. B cells lacking p85α or p110δ, or expressing kinase-inactive p110δ show severely impaired signaling downstream of the BCR and a complete inability to proliferate following BCR crosslinking [9, 56–59, 70, 75]. These defects are not simply due to altered development because wild-type B cells treated with IC87114 display equivalent defects [76]. A primary role of PI3K following BCR crosslinking is to promote signalosome assembly leading to diacylglycerol production, PKCβ activation, and NFκB nuclear translocation to activate gene expression (Figure 2) [3, 8].
Class IA PI3K signals also contribute to antigen presentation by B cells [77], adhesion of T:B conjugates [78], and to differentiation of follicular helper T cells (Tfh cells, see below). This predicts that PI3K inhibition would prevent the differentiation and function of T cells capable of delivering helper signals to B cells. It is surprising, therefore, that T cell-dependent antibody responses, while reduced, are not abolished by genetic disruption of p110δ or p85α [9, 56, 59, 73] nor by IC87114 treatment of mice [73, 79]. It is important to note that PI3K is not required for that FO B cell proliferation in vitro driven by CD40 ligand (CD40L) and interleukin-4 (IL-4), a model for B cell response to T cell help [57, 80]. Proliferation of FO B cells driven by TLR ligands is also partially preserved when PI3K is inhibited [9, 56–59, 80, 81]. Therefore, the defects in BCR signaling might be overcome by PI3K-independent signals from T cells or TLR ligands. Further, PI3K inhibitors promote immunoglobulin (Ig) class switching under conditions where B cell proliferation is maintained (e.g. CD40L + IL-4, or LPS), whereas PTEN deletion suppresses class switching [79, 82, 83]. Ig class switching requires activation-induced cytidine deaminase (AID), whose gene transcription is controlled by Foxo1 [63]. All of these observations emphasize that PI3K is not a universal “on” switch for B cell responses or for T cell help (Figure 2). Ultimately, the balance of positive and negative effects of PI3K inhibition probably explains the retention of a reasonably robust T-dependent antibody response in vivo. Also of interest is the finding that p110δ inhibition or p85α deletion causes a selective elevation in basal and antigen-specific IgE production [79, 84]. Whether this phenomenon will affect the clinical success of p110δ inhibitors, particularly in inflammatory diseases, is a topic of interest.
FO B cell survival mainly depends on the cytokines B-cell activating factor (BAFF) and IL-4 as well as tonic (basal) signaling through the BCR. Cytokine-dependent survival is highly dependent on p110δ activity in vitro [76, 85]. However, FO B cell numbers are only modestly reduced in p110δKI mice suggesting that survival signals from cytokines and/or BCR tonic signaling in vivo are not absolutely p110δ-dependent. A study from Rajewsky’s group showed that in the absence of the BCR, a constitutively active form of p110α is sufficient to maintain FO B cell survival in the periphery [86]. The converse experiment using a conditional p110α knockout approach showed that only in the absence of both p110α and p110δ were FO B cells completely depleted [60] (Figure 2). These results again show the importance of dissecting PI3K signaling through both gain-of-function and loss-of-function approaches. It is likely that expression of a constitutively active p110α isoform can elevate PIP3 beyond the physiological level, bypassing the need for p110δ engagement by tonic signals from an intact BCR. Several interesting questions arise from this work. First, do p110α and p110δ produce distinct PIP3 pools with different spatial location and effector molecules? Alternatively, each isoform might contribute quantitatively to shared signaling outputs. Is the absence of FO B cells in p110α/p110δ-double knockouts due only to impaired BCR tonic signaling or also to a block in survival signals from cytokines?
An intriguing issue raised by the p110α/p110δ-double knockout study is whether acute treatment with pan-class I PI3K inhibitors would cause a significant reduction in the peripheral B cell population, relative to selective inhibitors of individual PI3K isoforms. Given that pan-PI3K inhibitors are entering clinical trials for cancer therapy, it will be important to evaluate their impact on immune homeostasis compared to more selective compounds. One might predict that selective p110δ inhibitors would mainly affect the B-1 and MZ compartments without eliminating FO B cell numbers or function. Of particular interest is targeting p110α because activating mutations in the PIK3CA gene are very common in many solid tumors and are considered oncogenic drivers [87]. Development of a highly selective p110α inhibitor would perhaps have the potential to selectively act on PIK3CA mutant tumor cells while sparing B-1 and MZ B cells and maintaining normal FO B cell function in the periphery. Of course, the impact of different PI3K inhibitors on all immune subsets that regulate the tumor microenvironment is a subject of great significance. Later in this review we will address the issue of targeting PI3K isoforms in tumors of B cell origin.
T cells
Thymocytes
T cells develop in the thymus where CD4-CD8- double negative (DN) precursors arriving from the bone marrow undergo a differentiation program characterized by sequential VDJ recombination of the TCRβ and TCRα chains. The DN precursor cells can further be divided into DN1-4 stages based on the differential expression of CD44 and CD25. Proper rearrangement of TCRβ is required at the DN3 stage to proceed to DN4 stage (β-selection) and subsequently to CD4+CD8+ double positive (DP) cells that now rearrange and express the TCRα chain. TCRαβ+ DP cells are subjected to positive and negative selection and CD4 or CD8 single positive (SP) cells migrate out of the thymus to populate the lymph nodes and spleen. It is now clear that PI3K activity plays a crucial role in thymocyte development especially during β-selection [88].
Genetic models have suggested roles for both class IA and IB PI3K in thymocyte development. Loss of p110δ, p85α or both p85α/β does not significantly reduce overall numbers of thymocytes. However, pre-TCR signaling is attenuated in the absence of p85α or p110δ [89, 90]. The role of class IB PI3K is more prominent. p110γ knockout mice have increased apoptosis of DP thymocytes and strikingly, p110δ/γ double knockout markedly reduces the number of thymocytes due to a defect at the DN3-DN4 transition stage [91, 92]. Turner and colleagues have addressed the molecular basis for this defect using genetic models and an in vitro DN3 differentiation system [89]. To a similar extent as the p110δ/γ double knockout mice, DP thymocyte numbers are reduced in mice lacking p110δ and the p101 regulatory subunit of class IB PI3K. Both p110δ and p101/p110γ are required at the DN3-DN4 stage but act downstream of different receptors. AKT activation through the pre-TCR is entirely dependent on p110δ whereas CXCR4-stimulated AKT activation is mainly dependent on p101/p110γ with a smaller role for p110δ. A functional role of CXCR4 in thymocyte development was confirmed using both genetic and pharmacological approaches. Using a knock-in approach in which p110γ is mutated to prevent its binding to RAS, the same group has shown that CXCR4 activates p110γ in a RAS-dependent manner during thymocyte β-selection [41]. These studies strongly support the concept that antigen receptors can cooperate with chemokine receptors to activate both class IA and IB PI3K isoforms to promote cellular responses. An important question is whether this concept also applies to activation of effector T cells, which express chemokine receptors with costimulatory function [93]. If so, it would suggest that dual p110δ/γ inhibitors would be more effective than isoform-selective compounds with respect to blocking effector T cell activation. An interesting detail to address is whether p110δ and p110γ create distinct pools of PIP3 with different downstream effectors.
One process driven by preTCR-dependent PI3K activation is increased cellular metabolism driven by PDK-1 and AKT. A striking feature of thymocytes lacking PDK-1 is a severe defect in the ability to upregulate nutrient receptors CD71 (transferrin receptor) and CD98 (amino acid transporter subunit) at the DN3 stage. Conversely, PTEN-deficient thymocytes upregulate these receptors and progress to the DN4 stage in the absence of a pre-TCR signal [94]. Increased expression of nutrient transporters might be required for the rapid proliferative expansion at this stage. AKT is likely the key mediator downstream of PI3K and PDK-1, since nutrient receptor upregulation is maintained by a PDK1 mutant (L155E) that can activate AKT but not other PDK1 substrates [95]. AKT1/AKT2 double knockout DN3 cells show a defect in development and reduced glucose uptake [96].
CD4 T cells
Resting CD4 T cells recirculate among lymphoid tissues, searching for foreign antigenic peptides presented by antigen-presenting cells (APCs). Antigen recognition with costimulation from dendritic cells leads to T cell clonal expansion and differentiation into effector CD4 T cells. These are usually termed T helper (Th) cells as they provide help to other immune cells such as macrophages, B cells and CD8 T cells to orchestrate the overall immune response depending on the immune context. The functional variety of T helper cells is achieved by the differentiation of naïve uncommitted CD4 T cells to distinct subsets including Th1, Th2, Th17, and T follicular helper (Tfh) cells (Figure 3). Distinct types of CD4 T cells with suppressive potential, termed regulatory T cells (Tregs), are also produced either during thymocyte selection (natural Tregs; nTreg) or upon stimulation under tolerogenic conditions (induced Tregs; iTreg). The differentiation of CD4 T cells to each subset is dictated by sensing of extracellular cues in the form of cytokines and cell contact-dependent signals from the microenvironment, with distinct outcomes depending on the immune context.
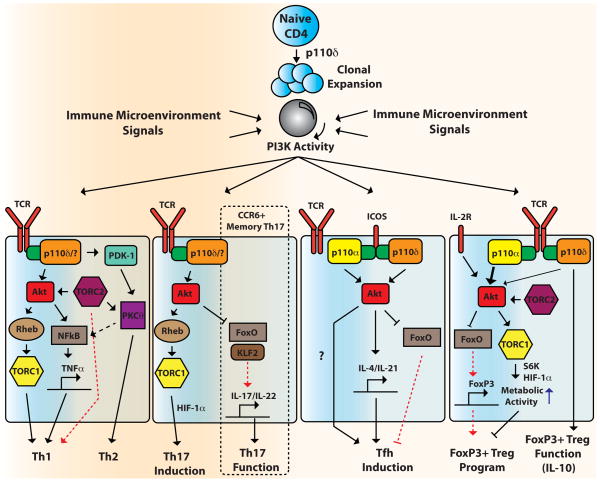
Figure 3. Role of PI3K and downstream effectors in the differentiation of activated CD4 T lymphocytes.
Antigen encounter triggers clonal expansion of naïve CD4 T cells that is mainly dependent on p110δ. Depending on the immune microenvironment (cytokines, etc.), primed CD4 T cells differentiate into different CD4 T cell subsets (Th1, Th2, Th17, Tfh, and iTregs). TORC2-dependent AKT activation promotes Th1 differentiation through TORC1 and NFκB, and TORC2-dependent PKCθ activation promotes Th2 differentiation. PDK-1 also triggers NFκB activation through a complex with PKCθ. AKT increases Th17 differentiation through TORC1 and HIF-1α activation. Already established CCR6+ human memory Th17 cell function (IL-17/IL-22 secretion) also depends on PI3K/AKT activity via modulation of FOXO and its target genes including KLF2. ICOS stimulation triggers Tfh differentiation through a p110δ-dependent pathway, with a speculative role for p110α binding to ICOS. The total PI3K output acts as a rheostat (gray circle) with decreased PI3K/AKT activity favoring iTreg induction. Isoforms other than p110δ including p110α probably suppress iTreg induction in this context. PI3K/AKT blockade results in the induction of FOXO-mediated FoxP3 expression and low TORC1 activity leads to decreased metabolic activity via S6K and HIF-1α. Established FoxP3+ Tregs also depend on p110δ activity for proper suppressive function such as IL-10 secretion. Due to space constraints, the nucleus is not depicted where all of the genes are regulated.
The proliferation of CD4 T cells in vitro driven by antibody-mediated clustering of TCR with CD28 is largely PI3K-independent [9, 57, 97–99]. It is likely that artificial TCR/CD28 clustering overrides certain physiological signaling requirements, as clonal expansion of CD4 T cells driven by antigen (or superantigen) and APCs is markedly suppressed by PI3K blockade, with a major role for p110δ [97–99] (Figure 3). Furthermore, numerous studies indicate that CD4 T cell differentiation is highly regulated by PI3K signaling and its downstream effectors, particularly the AKT/FOXO axis and TOR.
Th1 and Th2 cells
Th1 and Th2 cells were the first subsets of CD4 T cells to be discovered and categorized by the distinctive cytokines they secrete. The signature cytokine produced by Th1 cells is IFNγ, which provides essential help to macrophages for the destruction of vesicular pathogens. IFNγ and IL-2 produced by Th1 cells also help CD8 T cells differentiate into cytotoxic T lymphocytes (CTL) that are essential for cell-mediated immunity to viruses. Th2 cells secrete IL-4 and other cytokines to provide help to B cells for optimal antibody production to combat extracellular pathogens. These general distinctions are not absolute as Th1 cytokines can drive class switching to certain antibody isotypes, and Th2 cytokines can contribute to alternative macrophage polarization. T cells from p110δKI mice have a defect in both Th1 and Th2 differentiation and cytokine production, suggesting a main role of p110δ in this process [98]. p110δKI mice also are protected from Th2-mediated inflammation in a model of ovalbumin-dependent airway hypersensitivity [100]. In a more recent study the Okkenhaug group used IC87114 to revisit the role of p110δ in proliferation and differentiation of both murine and human CD4 T cells [101]. A noteworthy finding was that concentrations of IC87114 above 1 μM inhibit proliferation of p110δKI T cells lacking p110δ kinase activity. Therefore, off-target effects likely contribute to the cellular actions of IC87114 when used at > 1 μM. Nevertheless, lower concentrations (0.01–1 μM) of IC87114 do partially suppress proliferation and strongly inhibit Th1 and Th2 cytokine production from naïve and effector/memory T cells from both murine and human sources (Figure 3). In vivo treatment with IC87114 also suppresses Th1 cytokine production and reduces ear swelling in a contact hypersensitivity model.
Genetic or pharmacological inhibition of p110δ does not fully block proliferation of naïve CD4 T cells or differentiation of Th1 or Th2 subsets [98, 101]. Therefore, other class IA isoforms might contribute to PI3K signaling that drives T helper expansion and differentiation (Figure 3). Supporting this idea, total class IA PI3K activity measured by an in vitro kinase assay using immunoprecipitated p85 is reduced only 50% in p110δKI T cells suggesting that p110α and/or p110β activity might also be linked to signaling by the TCR and other receptors on T cells [98]. Immune assays using isoform selective inhibitors along with generation of T cell-specific p110α or p110β conditional knockout mice can help resolve the contribution of these isoforms in T cell activation.
PI3K influences Th1 and Th2 differentiation through complex mechanisms. One key signaling protein linking PI3K activation to Th differentiation is TOR (often referred to as mTOR, for mammalian (or mechanistic) target of rapamycin). Originally identified in searches for rapamycin targets in yeast and mammalian cells, this serine/threonine kinase is now known to have multiple functions and only some of these are rapamycin-sensitive [102, 103]. TOR is encoded by a single gene in mammals (MTOR) but is the catalytic subunit of two distinct multi-protein assemblies known as TOR complex-1 (TORC1) and TOR complex-2 (TORC2). TORC1 contains the protein raptor and is nutrient-sensitive; TORC2 contains a distinct protein rictor and is nutrient-insensitive. The activation of both TORC1 and TORC2 is at least partially PI3K-dependent [103–105]. TORC1 phosphorylates many substrates, but the best studied are two protein families that control mRNA translation: the ribosomal S6 kinases (S6Ks) and the eIF4E-binding proteins (4EBPs). TORC2 phosphorylates the hydrophobic motif of AKT and other AGC family kinases including serum and glucocorticoid-inducible kinase (SGK) and certain protein kinase C (PKC) isoforms. In mouse T cells, conditional knockout of Mtor (the gene was previously known as Frap1) blunts clonal expansion and completely blocks differentiation into Th1, Th2 or Th17 subsets [106]. In contrast, the induction of FoxP3 expression, the hallmark transcription factor driving a Treg program, is increased in TOR-deficient T cells [106]. This observation fits with abundant literature that rapamycin promotes Treg formation [107, 108].
Both TORC1 and TORC2 contribute to Th differentiation (Figure 3). Deletion of the small GTPase Rheb1 to block TORC1 activation prevents Th1 and Th17 differentiation, but not Th2 differentiation [109]. Deletion of rictor, an essential component of TORC2, impairs Th2 but not Th17 differentiation [109, 110]. Boothby and colleagues reported that rictor deletion also impairs Th1 differentiation, but this was not observed in the study of Powell and colleagues [109, 110]. Inhibition of both TORC1 and TORC2 is required for efficient induction of Tregs. Rheb1-deficient or rictor-deficient T cells show only a small increase in Treg generation [109]. Low concentrations of rapamycin, which inhibit TORC1 but not TORC2, achieve weak Treg induction unless TORC2 is genetically inactivated by rictor knockout [109].
The mechanisms that modulate Th differentiation downstream of TORC1 and TORC2 are beginning to be defined. Powell’s group provided evidence that TORC1 and TORC2 control differentiation programs through distinct effects on signal transducer and activator of transcription (STAT) phosphorylation and suppressor of cytokine signaling (SOCS) protein expression [109]. The signaling components linking TOR complexes to the STAT pathway are not yet assigned. Boothby and colleagues showed that a constitutively active PKCθ restored Th2 differentiation in rictor-deficient T cells [110]. This suggests that TORC2-dependent phosphorylation of PKCθ is required for signals leading to the Th2 program.
There is also evidence that AKT activity promotes Th1 differentiation. An early study indicated that constitutively active AKT could restore Th1 cytokine production in CD28-deficient T cells [111], and a rictor knockout study reported that active AKT could restore Th1 differentiation in TORC2-deficient T cells [110]. Activated AKT can associate with the CARMA-1/MALT1/BCL10 complex in T cells and promote NFκB activation [112–114]. A selective inhibitor of AKT1 and AKT2 (AKTi-1/2) suppresses a subset of NFκB-dependent genes, particularly the Th1 cytokine TNFα [115]. PI3K might also promote NFκB activation through PDK-1, which associates with PKCθ following TCR stimulation to promote the interaction of the CARMA-1/MALT1/BCL10 and IKK complexes [116, 117] (Figure 3).
The ability of AKT to phosphorylate and inactivate the FOXO family of transcription factors might also regulate Th1 and Th2 differentiation. Mice with FOXO-deficient T cells develop inflammatory disorders with elevated Th1 and Th2 cytokines [118–120]. However, FOXO factors activate the FoxP3 promoter and FOXO inactivation causes a dramatic impairment in Treg numbers and function [118, 120, 121]. Conversely, AKT inhibition potentiates Treg induction [122]. Thus, modulation of AKT activity in T cells can indirectly restrain the differentiation of T helper subsets through increased Treg-mediated suppression.
A related concept is that during immune responses in vivo, PI3K inhibition can affect T cells indirectly through altered function of accessory cells. PI3K/TOR signaling in dendritic cells and macrophages suppresses production of inflammatory cytokines [107, 123, 124]. Hence, PI3K inhibition augments the production of inflammatory mediators such as IL-12 that can promote Th1 differentiation. This likely explains an observed Th1 bias in p85α-deficient mice [125] and could contribute to elevated Th1 cytokine production in an allergy model using p110δKI mice [100].
Th17 cells
The Th17 subset of CD4 T cells, discovered in 2003, is developmentally and functionally distinct from Th1 or Th2 cells. These cells produce a different set of cytokines (IL-17A, IL-17F, IL-21 and IL-22) and are specialized to protect against microbial pathogens at epithelial surfaces. Th17 cells gained major interest based on their primary role in autoimmune diseases such as colitis and experimental autoimmune encephalomyelitis (EAE), a mouse model for multiple sclerosis. The role of PI3K in Th17 differentiation has been explored recently. However, data from three different groups show apparently conflicting results. SHIP KO T cells, which presumably have elevated PIP3 levels, fail to differentiate into Th17 cells both in vitro and in vivo. [126]. T cells expressing a myristoylated AKT transgene (myr-AKT) likewise cannot differentiate into Th17 cells in vitro [127]. The simplest interpretation of these results is that PI3K signaling suppresses Th17 differentiation. However, PI3K inhibition also blocks Th17 differentiation. p110δKI T cells cannot differentiate into Th17 cells in vitro and p110δKI mice are less susceptible to EAE due to a Th17 defect in vivo [128] (Figure 3). It is possible to reconcile these observations by considering that PI3K lipid production and hydrolysis is not a simple on/off switch; each of these genetic models has qualitatively distinct effects on the PI3K signaling network. SHIP dephosphorylates the 5-position of PIP3 to produce PtdIns(3,4)P2. Since both PIP3 and PtdIns(3,4)P2 can promote PDK-1- mediated AKT phosphorylation, loss of SHIP should not necessarily affect AKT activation. Indeed, activation of AKT through TCR engagement in SHIP KO T cells is unchanged [129]. It is possible that loss of SHIP prevents the recruitment of effector proteins that specifically bind to PtdIns(3,4)P2, and that these proteins transmit signals to induce Th17 differentiation. This model is consistent with the data from p110δKI T cells, which should also have reduced PtdIns(3,4)P2 levels. Both p110δKI and SHIP knockout T cells also have reduced Th2 differentiation [98, 129]. The observation that myristoylated AKT blocks Th17 differentiation could result from the atypical localization or activation kinetics of this AKT variant. The increased Treg differentiation observed in this Myr-AKT strain [127] is also at odds with other approaches to modulate PI3K/AKT activity (see below). An important future question to address is whether PI3K isoforms other than p110δ have a distinct role in Th17 differentiation. It will also be interesting to identify PtdIns(3,4)P2 effectors that function in T cells; PtdIns(3,4)P2-selective binding proteins Bam32, TAPP1 and TAPP2 have important roles in B cells [78, 130]. In addition, it is worth considering whether SHIP and potential PtdIns(3,4)P2 effectors function mainly in signaling from the TCR or from cytokine receptors that influence Th17 differentiation.
A positive role for PI3K/AKT signaling in Th17 is supported by studies of T cells lacking TORC1 function. Mice with T cell-specific deletion of Mtor or Rheb1 have a drastic reduction in Th17 differentiation and are protected from EAE [106, 109] (Figure 3). Further supporting a role for TORC1, rapamycin treatment also suppresses Th17 differentiation and ameliorates EAE [131, 132]. Other work suggests that TORC1 regulates the balance of Th17/Treg differentiation through a central regulator of cellular metabolism, hypoxia-inducible factor-1α (HIF-1α) [133, 134]. HIF-1α expression is increased by TORC1 in a manner independent of hypoxia, and contributes to increased glycolysis during Th17 differentiation. The idea that TORC1 controls metabolic programs that determine Th differentiation is supported by other recent work [135–137].
Human memory Th17 cells can be defined by surface expression of the chemokine receptor CCR6. Culturing these cells in the presence of cytokines that signal through the γC (gamma-common) chain (e.g. IL-2, IL-7, IL-15) induces production of IL-17, IL-22 and other cytokines. Under these conditions, pharmacological inhibition of PI3K or AKT suppresses IL17/IL-22 production by human CCR6+ cells [138]. This indicates that PI3K activation is important not only for establishment of Th17 effector cells but also for their function (Figure 3). Further mechanistic experiments suggest that PI3K/AKT signaling promotes Th17 cytokine production through suppression of FOXO and KLF transcription factors [138]. The ability of PI3K/AKT signaling to suppress FOXO and KLF-dependent gene expression is also responsible for changes in homing and trafficking that accompany activation of both CD4 and CD8 T cells in mice [139].
Tfh cells
The T follicular helper (Tfh) cell subset is specialized to provide help to B cells during germinal center (GC) reactions. Tfh cells are defined by the expression of the chemokine receptor CXCR5 and their specific requirement for the transcription factor B cell lymphoma-6 (BCL6) and the inducible costimulatory (ICOS) transmembrane receptor. Following antigen encounter, B cells that initiate the GC reaction undergo somatic hypermutation and isotype switching to increase antibody affinity and diversify effector functions. By expressing CXCR5, primed T cells can migrate into the B cell follicle along the CXCL13 gradient and first receive signals from B cells in the form of antigen-mediated TCR signaling and costimulatory signals to initiate the Tfh program. Many costimulatory signals provided by activated B cells are crucial for Tfh signaling. Of these, PI3K signaling downstream of ICOS appears to play a nonredundant role in regulating the abundance and effector functions of Tfh cells [140].
Interruption of ICOS-ICOS ligand (ICOSL) interaction or ICOS deficiency (ICOS−/− mice) leads to impaired GC responses [140]. To address the question of whether PI3K signaling downstream of ICOS is the main mediator for this defect, an ICOS knock-in transgenic mouse (ICOS-YF) was generated that harbors a mutation in the crucial tyrosine Y181 that is required for p85 recruitment [141]. The ability of ICOS to strongly enhance TCR-mediated AKT phosphorylation is blocked in ICOS-YF T cells. Class switched Ig levels as well as the magnitude of the GC response are severely impaired in these mice. This phenotype is mainly due to a defect in the generation of Tfh cells and the effects are very similar in ICOS−/− mice. This suggests that class IA PI3K is a central player in ICOS signaling leading to Tfh differentiation (Figure 3). FOXO inhibition might play an important role in this pathway as Foxo1/Foxo3-deletion in T cells promotes Tfh generation [118].
A subsequent study from Turner and colleagues provided evidence that p110δ is the primary catalytic isoform that couples ICOS engagement to Tfh generation [142] (Figure 3). Conditional deletion of Pik3cd (encoding p110δ) in mature T cells (using CD4-Cre) or in primed T cells (using OX40-Cre) prevents the expression of Tfh-selective cytokines IL-4 and IL-21. T cell-specific deletion of p110δ reduces Tfh numbers with an accompanying decrease in GC B cells and high affinity antibody production. Importantly, amplifying PIP3 through PTEN deletion in activated T cells has the converse effect of increasing Tfh cells, GC B cells and antibody affinity. A surprising finding from this study was that B cell-specific ablation of p110δ using CD19-Cre does not impair the GC response or antibody production, other than an increase in IgE production. Hence, the decreased T-dependent antibody response in the original “whole body” p110δKI mice can be attributed to defects in T cells rather than in B cells. Although p110δ controls many facets of B cell development and T cell-independent antibody production, this experiment suggests that the B cell intrinsic role of p110δ in T-dependent responses is mainly restricted to suppression of IgE isotype switching during the germinal center response.
A number of observations add complexity to the simple model that p110δ in T cells controls Tfh differentiation and antibody responses. Acute inhibition of p110δ using IC87114 in mice reduces GC B cell numbers, but does not reduce antigen-specific IgG1 and markedly increases the IgE response [73, 79]. This indicates that extrafollicular B cell differentiation and class switching can still proceed when the germinal center response is suppressed. It is also possible that PI3K isoforms other than p110δ might contribute to ICOS signaling. Using synthetic ICOS peptides and ICOS immunoprecipitations, a significant amount of p110α bound to ICOS can be detected after stimulation of the CD4 T cell line D10 and primary CD4 T cell blasts [143]. siRNA-mediated knockdown or pharmacological inhibition of p110α partially reduces ICOS-dependent AKT phosphorylation in D10 cells. The application of newer inhibitors with greater selectivity and improved pharmacological properties should further resolve the roles of PI3K isoforms in ICOS function in vitro and in vivo. With the entry of isoform-selective inhibitors and pan-class I PI3K inhibitors in clinical trials, we will also begin to learn how different PI3K target profiles affect antibody isotypes in humans.
Tregs
The discussion thus far makes it clear that PI3K activation plays a generally positive role in signaling to generate different effector CD4 T cell subsets (Figure 3). The major catalytic isoform responsible in most contexts seems to be p110δ although other isoforms contribute. This function for PI3K in CD4 T cell expansion and differentiation is not surprising given the central role of PI3K in the growth factor responses of many other cell types. In this respect, it was initially perplexing that reduced PI3K function in immune cells could lead to autoimmunity in mice. p110δKI mice develop colitis [9] and r1δT/r2n mice (a strain with T cell-specific deletion of Pik3r1 encoding p85α, and germline deletion of Pik3r2 encoding p85β) develop an exocrinopathy that resembles primary Sjögren’s Syndrome [144]. It now seems likely that both of these phenotypes ban be attributed to reduced numbers and/or function of Tregs ([144, 145] and unpublished data). Accumulating data suggest that PI3K activity is intimately linked to both development and function of Tregs.
Tregs are essential for peripheral tolerance induction. Both nTregs and iTregs can suppress proliferation of potentially autoreactive T cells, through direct contact with effector T cells and dendritic cells as well as through secreted factors. Thymic development of nTregs seems to be constrained by PI3K activity as p110δKI mice have increased thymic Treg numbers [145]. Recent studies suggest a critical role for FOXO transcription factors in linking PI3K/AKT signaling to the thymic development of nTregs. Although a single allele of Foxo1 or Foxo3 is enough to maintain nTreg development, combined deletion of both genes leads to a severe defect in FoxP3+ nTregs in the thymus [118, 120]. FOXO factors directly activate FoxP3 expression through direct binding to FOXO consensus sequences in the Foxp3 promoter [120, 121]. Thus, when PI3K activity is reduced FOXO factors can relocate the nucleus to turn on a Treg transcriptional program that includes FoxP3 expression (Figure 3). On the other hand, elevated PI3K/AKT signaling does not necessarily suppress nTreg development as thymic nTreg development is unaltered in a mouse model where PTEN is deleted specifically in T cells (CD4-Cre) [146]. Conflicting results have been obtained using activated AKT transgenes in two different models of nTreg development in thymocytes [127, 147].
PI3K/AKT signaling has complex roles in mature Tregs. Despite the marked decrease in thymic nTregs in the T cell-specific Foxo1/Foxo3 double knockouts, peripheral (spleen and lymph node) Tregs are only modestly reduced [118, 120]. This suggests that PI3K/AKT signaling promotes, and FOXO factors suppress, homeostatic maintenance of peripheral Tregs. In accord, peripheral Treg numbers are decreased in both p110δKI and r1δT/r2n mice [144, 145]. Adding further complexity, PI3K/AKT signaling is attenuated in mature Tregs. Compared to conventional CD4 T cells, isolated Tregs show blunted activation of AKT in response to TCR engagement or IL-2 [126, 146, 148]. This phenotype correlates with elevated PTEN expression in Tregs, and PTEN-deletion promotes IL-2-dependent proliferation of Tregs [120]. It is conceivable that a low level of PI3K/AKT signaling is required to prevent FOXO-dependent cell cycle arrest and apoptosis, but that higher levels could suppress the Treg phenotype.
PI3K activity is also required for the function of peripheral Tregs. Purified Tregs from p110δKI mice have reduced suppressive activity and severely impaired secretion of IL-10, an anti-inflammatory cytokine [145] (Figure 3). Interestingly, this defect in Treg function provides protection of mice from Leishmania major infection [149]. Despite having impaired Th1 responses, p110δKI mice are able to contain the parasite and this phenotype is reversed by adoptive transfer of wild-type Tregs. This opens up the possibility of developing p110δ-selective inhibitors for Leishmania treatment.
An extensive focus in Treg biology has been the study of ex vivo expansion of naïve conventional CD4 T cells to iTregs. This process is relevant to peripheral tolerance induction and has therapeutic implications with respect to adoptive transfer of Tregs. There is strong evidence that limiting PI3K/AKT/TOR activity during T cell priming promotes the conversion of conventional CD4 T cells to iTregs. Merkenschlager and colleagues showed that withdrawal of TCR stimulus after 18 hours triggers iTreg conversion, and used small molecule inhibitors to probe the signaling pathways responsible for this effect [122]. Inhibition of PI3K and/or TOR, or AKT inhibition, led to greatly increased FoxP3+ iTregs in this system. Although the p110δ inhibitor IC87114 modestly increased iTregs, the effect of a p110α inhibitor with some activity against p110γ and p110δ (PIK-90; see Table 1) was more profound. This result further supports involvement of diverse p110 isoforms in different immune contexts. Other work supports a role for PI3K/AKT signaling to oppose iTreg conversion through suppression of FOXO activity (Figure 3). In CD4 T cells lacking the ubiquitin ligase Cbl-b, phosphorylation of AKT and FOXO factors are elevated and TGFβ-induced iTreg conversion is greatly reduced [121]. This phenotype can be rescued by overexpressing Foxo1 or Foxo3. Conversely, iTreg induction is abolished in Foxo1/Foxo3 double knockout T cells or in cells expressing constitutively active AKT [79, 120, 147]. Together these data indicate that similar to thymic nTregs, FOXO-driven FoxP3 expression is also important for iTreg generation in peripheral T cells.
As discussed above, TOR activity also opposes iTreg induction. It is interesting that inhibition of both TORC1 and TORC2 are required for conversion of conventional CD4 T cells to Tregs [132, 138]. It makes sense that TORC2 inhibition should augment FOXO-dependent Treg programming through AKT suppression (Figure 3). What is the role of TORC1? We have obtained evidence that S6K activity downstream of TORC1 opposes iTreg differentiation. Genetic or pharmacological suppression of S6K increases iTreg induction in vitro and increases Treg numbers in vivo [135]. A search of the patent literature reveals S6K inhibition as a strategy for ex vivo expansion of Tregs (US 2009/0142318). TORC1 also opposes the iTreg fate through HIF-1α, which binds to FoxP3 and promotes its degradation [133, 134]. Whether PI3K inhibitors promote iTreg conversion primarily through the AKT/FOXO axis or also through TORC1 inhibition remains to be determined.
CD8 T cells
Upon antigen encounter, resting CD8 T cells differentiate into effector CTLs with a fundamental role in anti-viral and anti-tumor responses. Cantrell and colleagues have reported key roles for PI3K/ATK/TOR signaling in CD8 T cells, with interesting distinctions from PI3K function in CD4 T cells [150]. Whereas PI3K inhibition suppresses antigen-driven clonal expansion of CD4 T cells, PI3K is not required for CD8 T cell proliferation or associated metabolic changes such as increased glucose uptake. Clonal expansion of antigen-primed CD8 T cells cultured in the presence of IL-2 are not affected by the p110δ inhibitor IC87114, a specific AKT inhibitor, nor in CD8 T cells from p110δKI mice. Proliferation and glucose uptake are impaired in PDK-1 null cells, implying that AGC kinases regulated by PDK1 can compensate for AKT in this context. However, PI3K/AKT signaling is required in activated CD8 T cells to initiate the transcriptional program for CTL effector function. Expression of cytotoxic effector molecules including granzymes and perforin, and production of the cytokine IFNγ correlate with AKT activity and FOXO inactivation. AKT activation also coordinates changes in expression of homing and trafficking receptors to allow effector CTLs to leave lymph nodes and traffic to sites of infection. Specifically, AKT inhibition or genetic interference with PDK-1-mediated AKT activation prevents the downregulation of FOXO target genes encoding proteins important for naïve CD8 T cell trafficking: L-selectin (CD62L), KLF2, CC motif chemokine receptor 7 (CCR7), and sphingosine-1 phosphate receptor 1 (S1P1). An earlier study from this group reported that rapamycin treatment of antigen-primed CD8 T cells also prevents the downregulation of L-selectin (CD62L), KLF2, CCR7 and S1P1 [151]. Whether rapamycin acts in this system through inhibition of TORC2/AKT or TORC1 signaling remains to be resolved [139].
The role of PI3K signaling in cytotoxicity of mature CTLs has not been studied in detail. However, there is evidence that the p110γ isoform is required for CTL chemotaxis and trafficking to sites of infection [152]. Similarly, the p110γ isoform has a significant role in trafficking and survival of effector CD4 cells [153]. This raises the possibility that drugs in development that target p110γ, either alone or with other class I isoforms, will suppress effector T cell trafficking and compromise host defense.
Although natural killer (NK) cells are traditionally classified as part of the innate immune system, they are lymphocytes and display some features of adaptive immune cells [154]. Both p110δ and p110γ appear to play important roles in NK cell maturation and function, including cytokine production and cytotoxicity. The functions of p110δ and p110γ in NK cells and the discrepancies among experimental systems were reviewed recently by Kerr and Collucci [155].
CAL-101 and the potential of PI3K inhibitors in lymphoid malignancies
Starting from the initial description of wortmannin as a PI3K inhibitor that suppresses neutrophil responses [156, 157], there has been interest in developing PI3K inhibitors for inflammatory and autoimmune diseases. Most efforts have focused on inhibitors of p110δ and/or p110γ based on the leukocyte-restricted expression of these isoforms and the immune phenotypes of gene-targeted mice [158]. The p110γ isoform has received particular attention for inflammatory diseases, as p110γ plays an integral role in chemotaxis and respiratory burst of neutrophils during inflammation [159], and amplifies mast cell degranulation and allergic responses [160]. p110γ has a limited role in T cells and is largely dispensable in B cells. p110δ has been considered a more promising target for suppression of adaptive immune responses, based on the fundamental role of this isoform in both B and T cells [46]. The discovery of compounds with high selectivity for p110δ has proceeded more rapidly than for selective p110γ inhibitors, and many pharmaceutical companies have selective p110δ inhibitors in their development pipelines [161] (current clinical candidates are listed in Table 1). These have potential application for allergy and autoimmune diseases, as discussed in the Future Directions section.
In parallel to studies of PI3K inhibitors in inflammation, there has been a tremendous effort to develop pan-class I PI3K inhibitors, isoform-selective PI3K inhibitors, dual PI3K/TOR inhibitors and selective TOR inhibitors for cancer [161–167]. Notably, the most exciting success in the clinic so far has been the efficacy of the p110δ inhibitor CAL-101 in a subset of cancers of B cell origin. Although the results of CAL-101 clinical trials have not been published in peer-reviewed journals, some of the data have been presented at professional meetings and conferences [168, 169]. Remarkably, treatment with CAL-101 as a single agent provided durable remissions to a significant percentage of patients with chronic lymphocytic leukemia (CLL), indolent non-Hodgkin’s lymphoma (iNHL) or mantle cell lymphoma (MCL). Most of these patients had relapsed from multiple other treatment regimens, yet responded to the p110δ inhibitor. CAL-101 also showed impressive efficacy when combined with standard-of-care agents in CLL and iNHL (rituximab and bendamustine).
CAL-101 was discovered by Calistoga and is a chemical derivative of IC87114. CAL-101 inhibits p110δ in vitro at a concentration 40- to 300-fold lower than other class I PI3K isoforms [170, 171]. The compound also shows great selectivity when profiled against other protein and lipid kinases. In cell-based assays, CAL-101 inhibits p110δ-dependent responses a low nanomolar concentration. At concentrations above 1 μM, CAL-101 significantly inhibits cellular responses mediated by p110β and p110γ. These concentration ranges are important to remember when considering the mechanism of action of CAL-101 in cellular model systems as well as in patients.
The actions of CAL-101 have been studied in cell lines and primary patient samples representing diverse B cell malignancies including CLL, diffuse large B cell lymphoma (DLBCL) and multiple myeloma (MM) [170–173]. In general, CAL-101 has weak cytotoxic activity on isolated cancer cells at submicromolar concentrations, and variable cytotoxicity in the range of 1–10 μM. This suggests that p110δ is not required for intrinsic or serum-dependent survival signaling in culture. However, lower concentrations of CAL-101 (0.1–0.5 μM) do effectively suppress the survival signals provided by stromal cells and extracellular factors including TNFα, BAFF, fibronectin and soluble CD40L. Importantly, submicromolar concentrations of CAL-101 effectively suppress chemotaxis of CLL cells to CXCL12 and CXCL13 [173], chemokines that are commonly present in B cell follicles of lymph nodes. This suggests that in malignant B cells, similar to normal B cells, chemokine receptors signal via the class IA isoform p110δ. In addition, p110δ may serve important functions in stromal cells as CAL-101 suppresses the secretion of cytokines by monocyte-derived “nurse-like” cells in co-culture with CLL cells [173]. Together these findings indicate that CAL-101 disrupts the microenvironment and might block access of tumor cells to niches that provide extrinsic survival signals (Figure 4).
Figure 4. A model for the efficacy of a p110δ inhibitor in CLL.
The two illustrations show a lymph node containing CLL tumor cells before treatment (Left panel) or after treatment with the p110δ inhibitor, CAL-101 (Right panel). Chemokines drive the entry of CLL cells and other lymphocytes into the lymph node from blood vessels via the high endothelial venules (HEV). Cells exit the lymph node via the efferent lymph. The legend at the lower right explains the arrows and symbols. The scale at the upper right depicts the differential p110δ enzyme activity in cells shown in the figure. NLC = nurse-like cells. Th = T helper. Other stromal cells also interact with CLL cells in the tumor cell niche.
This model is strongly supported by clinical data. In a phase I trial of CAL-101 in CLL (Clinical Trials identifier NCT00710528), a large majority of patients (> 80%) across all dose levels showed significant shrinkage of lymph nodes but this was accompanied by an elevation of circulating lymphocytes. This is consistent with release of CLL cells from lymphoid tissue and/or failure of circulating cells to home to lymph nodes. Patients treated with CAL-101 showed markedly reduced amounts of chemokines CCL2, CCL3 and CXCL13 [173], in accord with the in vitro experiments mentioned above. Importantly, the peripheral lymphocytosis resolved over time suggesting that tumor cells died out when prevented from accessing lymph nodes (Figure 4). It remains unclear whether direct cytotoxic effects of CAL-101 contribute to efficacy in CLL patients. The peak plasma levels of CAL-101 are greater than 2 μM suggesting that partial blockade of p110β and/or p110γ together with complete p110δ inhibition might cause direct tumor cell killing. Nevertheless, the lymphocytosis indicates that many tumor cells do not die immediately. In patients treated with combination of CAL-101 and either Rituximab or Bendamustine, lymphocytosis was not observed. This suggests that release from the protective niche by CAL-101 sensitizes leukemia/lymphoma cells to the companion agent.
Calistoga is now part of the company Gilead, which has plans to develop CAL-101 (now GS-1101) rapidly for oncology applications. When representatives of these companies have presented clinical data on CAL-101, the excitement among audience members has been palpable. The emerging success of CAL-101 validates more than a decade of intense PI3K drug discovery efforts across academia and industry. The CAL-101 results also raise many questions, some of which are discussed here (also see [161]).
One question is whether inhibition of microenvironmental signals is also the basis for CAL-101 efficacy in iNHL and MCL. When clinical data become available it will be interesting to learn whether CAL-101 causes transient lymphocytosis and reduced plasma chemokine levels in these patient populations. A related issue is whether CAL-101 fails to block microenvironmental signals in malignancies where the drug has not shown efficacy, such as DLBCL, AML and MM. A more general point is that CAL-101 studies have underscored the importance of modeling cancer cell interactions with the microenvironment even at early stages of development of PI3K-targeted therapies. It is likely that the efficacy of PI3K inhibitors in cancer patients does not correlate linearly with the IC50 for cytotoxic effects in vitro. In other words, the potential of a certain inhibitor can be either underestimated or overestimated by studies of isolated tumor cell lines.
Another question is whether greater efficacy can be achieved using less selective PI3K inhibitors. Some tumors arising from mature B cells depend on chronic BCR signaling [174]; if these signals require both p110α and p110δ then a dual inhibitor of these two isoforms might provide a potent cytotoxic effect. Perhaps pan-PI3K inhibitors would have even broader activity in leukemia and lymphoma subtypes that have been resistant to CAL-101 in early clinical studies. The potentially greater efficacy might be counterbalanced by reduced tolerability and general immunosuppression. It is even possible that broader PI3K inhibition will promote tumor survival through increased production of Tregs.
Conclusions and Future Directions
Thus far, a p110δ inhibitor in B cell malignancies is the biggest success story in the world of PI3K drug development. Where does this leave the community of inflammation and autoimmunity investigators? In some ways the barriers are greater for development of drugs in this arena. Most of the target diseases are not terminal, so there is a higher standard for safety. There are usually other treatment options with reasonable efficacy. Furthermore, animal models of inflammation and autoimmunity are generally of limited predictive value for efficacy in humans. Nevertheless, there remains a medical need and large potential market for improved anti-inflammatory and immunosuppressive drugs. Also, PI3K inhibitors seem to cause more manageable side effects in humans than initially feared. Considering these issues, it makes sense to move PI3K inhibitors forward quickly, establishing safe doses first in healthy volunteers and then testing in patient populations. This is where the promise of PI3K inhibitors will truly be tested: in human beings.
If one accepts this premise, then the question is which isoform(s) to target. Both p110δ and p110γ are preferentially expressed in leukocytes and are central mediators of immune responses. Mice lacking either protein are healthy, suggesting that compounds selective for one of these isoforms will be well tolerated. Yet given the overlap in function between these isoforms, and possible roles of p110α and/or p110β, it might not be possible to shut down pathological immune responses or cancer cells by inhibiting just one isoform. Compounds that inhibit both p110δ and p110γ (e.g. IPI-145, Table 1) might offer better control of autoimmunity and inflammation, but what will be the effect on host defense? Again, the best choice might be to test different target profiles in various disease states to establish which profile provides the best therapeutic window. It is important to keep in mind that compounds with “partial” selectivity might provide the best balance between efficacy and tolerability. For example, a p110δ inhibitor that partially blocks p110γ at the effective dose might suppress effector T cell function without interfering with the myriad functions of p110γ in innate immunity. An equally important consideration is that each compound has its own pharmacological profile. Two experimental agents with similar isoform selectivity might have distinct efficacy in humans based on their pharmacokinetics. Relating such findings back to the biology of PI3K in different immune cells will be a challenge.
One research question that deserves more detailed investigation is the role of different PI3K isoforms in memory lymphocytes. Unlike laboratory mice maintained in pathogen-free conditions, which have mostly naïve T and B cells, human beings are constantly exposed to microbes and the majority of peripheral lymphocytes are memory or effector cells. A typical autoimmune disease patient will have an even greater fraction of such cells. There has been some progress in studying PI3K signaling in human memory lymphocytes. As noted above, memory Th17 cells stimulated with cytokines produce IL-17 through a PI3K-dependent pathway [138], and p110δ inhibition reduces proliferation and cytokine production by effector/memory T cells from humans with inflammatory diseases [101]. Similarly, human lupus patients have increased PI3K activity in peripheral lymphocytes, with a direct correlation between p110δ activity and the fraction of activated/memory T cells [175]. We need to learn more about how different PI3K inhibitors affect the function and trafficking of pathogenic lymphocytes in different immune disease states. At the same time it will be important to consider whether PI3K inhibitors that effectively suppress autoimmunity also interfere with immunological memory to pathogens. Although the road ahead is certain to be filled with challenges, the fundamental premise that PI3K inhibition will be useful for human disease therapy seems more and more solid.
Supplementary Material
Acknowledgments
We thank Lawrence Kane and Sung Su Yea for helpful comments on the manuscript. PI3K and TOR research in our laboratory has been supported by NIH grant R03-AI85462, a Discovery Grant from the University of California Industry-University Cooperative Research Program, and a Sponsored Research Agreement from Intellikine, Inc.
Abbreviations
- 4EBP
eIF4E-binding protein
- ABD
Adaptor-binding domain
- AID
Activation-induced cytidine deaminase
- AKT/PKB
Protein kinase B
- APC
Antigen-presenting cell
- BAFF
B-cell activating factor
- BCAP
B-cell adaptor protein
- BCL6
B cell lymphoma-6
- BCR
B cell receptor
- CCR7
CC motif chemokine receptor 7
- CD40L
CD40 ligand
- CD62L
L-selectin
- CD71
Transferrin receptor
- CD98
Amino acid transporter subunit
- CLL
Chronic lymphocytic leukemia
- CTL
Cytotoxic T lymphocyte
- DLBCL
Diffuse large B cell lymphoma
- EAE
Experimental autoimmune encephalomyelitis
- eIF4E
Eukaryotic translation initiation factor 4E
- FO
Follicular
- FOXO
Forkhead Box Subgroup O
- GC
Germinal center
- GPCR
G protein-coupled receptor
- HIF-1α
Hypoxia-inducible factor-1 alpha
- ICOS
Inducible costimulatory
- IFNγ
Interferon-gamma
- IKK
IκB kinase
- IL-4
Interleukin-4
- iNHL
Indolent non-Hodgkin’s lymphoma
- iTreg
Induced Treg
- KLF
Kruppel-like factor
- LPS
Lipopolysaccharide
- MCL
Mantle cell lymphoma
- MM
Multiple myeloma
- mTOR
Mammalian/mechanistic target of rapamycin
- Myr-AKT
Myristoylated AKT
- MZ
Marginal zone
- NFκB
Nuclear factor kappa B
- NK cell
Natural killer cell
- nTreg
Natural Treg
- PDK-1
Phosphoinositide-dependent kinase-1
- PI3K
Phosphoinositide 3-kinase
- PIP3
PtdIns-3,4,5-trisphosphate
- PKC
Protein kinase C
- PRAS40
Proline-Rich AKT substrate of 40kDa
- PtdIns
Phosphatidylinositol
- PTEN
Phosphatase and Tensin homolog
- pTyr
Phosphotyrosine
- RAG
Recombination-activating gene
- Raptor
Regulatory-associated protein of mTOR
- Rictor
Rapamycin-insensitive companion of mTOR
- S1P1
Sphingosine-1 phosphate receptor 1
- S6K
Ribosomal S6 kinase
- SGK
Serum and glucocorticoid-inducible kinase
- SH2
Src-homology-2 domain
- SH3
Src-homology-3 domain
- SHIP
SH2 domain-containing inositol phosphatase
- siRNA
Small interfering RNA
- SOCS
Suppressor of cytokine signaling
- STAT
Signal transducer and activator of transcription
- TCR
T cell receptor
- Tfh
Follicular helper T cell
- Th
T helper cell
- TK
Tyrosine kinase
- TNFa
Tumor necrosis factor alpha
- TOR
Target of rapamycin
- TORC1
TOR complex 1
- TORC2
TOR complex 2
- Treg
Regulatory T cell
- TSC2
Tuberous Sclerosis 2
Footnotes
Competing Interests Statement
D.A.F. is a scientific advisor to Intellikine, a company developing PI3K and TOR inhibitors.
References
- 1.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 3.Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228:253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 4.Okkenhaug K, Fruman DA. PI3Ks in Lymphocyte Signaling and Development. Curr Top Microbiol Immunol. 2011;346:57–85. doi: 10.1007/82_2010_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 6.Fruman DA, Satterthwaite AB, Witte ON. Xid-like phenotypes: a B cell signalosome takes shape. Immunity. 2000;13:1–3. doi: 10.1016/s1074-7613(00)00002-9. [DOI] [PubMed] [Google Scholar]
- 7.Fayard E, Xue G, Parcellier A, Bozulic L, Hemmings BA. Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Current topics in microbiology and immunology. 2010;346:31–56. doi: 10.1007/82_2010_58. [DOI] [PubMed] [Google Scholar]
- 8.Fruman DA. Phosphoinositide 3-kinase and its targets in B-cell and T-cell signaling. Curr Opin Immunol. 2004;16:314–320. doi: 10.1016/j.coi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJ, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 10.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donahue AC, Fruman DA. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur J Immunol. 2007;37:2923–2936. doi: 10.1002/eji.200737281. [DOI] [PubMed] [Google Scholar]
- 12.Salmond RJ, Emery J, Okkenhaug K, Zamoyska R. MAPK, phosphatidylinositol 3-kinase, and mammalian target of rapamycin pathways converge at the level of ribosomal protein S6 phosphorylation to control metabolic signaling in CD8 T cells. J Immunol. 2009;183:7388–7397. doi: 10.4049/jimmunol.0902294. [DOI] [PubMed] [Google Scholar]
- 13.Huang YH, Sauer K. Lipid signaling in T-cell development and function. Cold Spring Harb Perspect Biol. 2010;2:a002428. doi: 10.1101/cshperspect.a002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baracho GV, Miletic AV, Omori SA, Cato MH, Rickert RC. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr Opin Immunol. 2011;23:178–183. doi: 10.1016/j.coi.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang TT, Li H, Cheung SM, Costantini JL, Hou S, Al-Alwan M, Marshall AJ. Phosphoinositide 3-kinase-regulated adapters in lymphocyte activation. Immunol Rev. 2009;232:255–272. doi: 10.1111/j.1600-065X.2009.00838.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, Acevedo LM, Manglicmot JR, Song X, Wrasidlo W, Blair SL, Ginsberg MH, Cheresh DA, Hirsch E, Field SJ, Varner JA. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3Kg, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19:715–727. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backer JM. The regulation of class IA PI 3-kinases by inter-subunit interactions. Curr Top Microbiol Immunol. 2010;346:87–114. doi: 10.1007/82_2010_52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vadas O, Burke JE, Zhang X, Berndt A, Williams RL. Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci Signal. 2011;4:re2. doi: 10.1126/scisignal.2002165. [DOI] [PubMed] [Google Scholar]
- 19.Cheung LW, Hennessy BT, Li J, Yu S, Myers AP, Djordjevic B, Lu Y, Stemke-Hale K, Zhang F, Ju Z, Cantley LC, Scherer SE, Liang H, Lu KH, Broaddus RR, Mills GB. High Frequency of PIK3R1 and PIK3R2 Mutations in Endometrial Cancer Elucidates a Novel Mechanism for Regulation of PTEN Protein Stability. Cancer Discov. 2011;1:170–185. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaiswal BS, Janakiraman V, Kljavin NM, Chaudhuri S, Stern HM, Wang W, Kan Z, Dbouk HA, Peters BA, Waring P, Dela Vega T, Kenski DM, Bowman KK, Lorenzo M, Li H, Wu J, Modrusan Z, Stinson J, Eby M, Yue P, Kaminker JS, de Sauvage FJ, Backer JM, Seshagiri S. Somatic Mutations in p85alpha Promote Tumorigenesis through Class IA PI3K Activation. Cancer Cell. 2009;16:463–474. doi: 10.1016/j.ccr.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen JW, Schleithoff L, Bartram CR, Schulz AS. An oncogenic fusion product of the phosphatidylinositol 3-kinase p85beta subunit and HUMORF8, a putative deubiquitinating enzyme. Oncogene. 1998;16:1767–1772. doi: 10.1038/sj.onc.1201695. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez C, Jones DR, Rodriguez-Viciana P, Gonzalez-Garcia A, Leonardo E, Wennstrom S, von Kobbe C, Toran JL, LRB, Calvo V, Copin SG, Albar JP, Gaspar ML, Diez E, Marcos MA, Downward J, Martinez AC, Merida I, Carrera AC. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. Embo J. 1998;17:743–753. doi: 10.1093/emboj/17.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ, Phillips WA. The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–7429. [PubMed] [Google Scholar]
- 24.Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497–1503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Brooks SR, Li X, Anzelon AN, Rickert RC, Carter RH. The physiologic role of CD19 cytoplasmic tyrosines. Immunity. 2002;17:501–514. doi: 10.1016/s1074-7613(02)00426-0. [DOI] [PubMed] [Google Scholar]
- 26.Kapeller R, Prasad KV, Janssen O, Hou W, Schaffhausen BS, Rudd CE, Cantley LC. Identification of two SH3-binding motifs in the regulatory subunit of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:1927–1933. [PubMed] [Google Scholar]
- 27.Prasad KV, Janssen O, Kapeller R, Raab M, Cantley LC, Rudd CE. Src-homology 3 domain of protein kinase p59fyn mediates binding to phosphatidylinositol 3-kinase in T cells. Proc Natl Acad Sci USA. 1993;90:7366–7370. doi: 10.1073/pnas.90.15.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds LF, Smyth LA, Norton T, Freshney N, Downward J, Kioussis D, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways. J Exp Med. 2002;195:1103–1114. doi: 10.1084/jem.20011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saveliev A, Vanes L, Ksionda O, Rapley J, Smerdon SJ, Rittinger K, Tybulewicz VL. Function of the nucleotide exchange activity of vav1 in T cell development and activation. Sci signal. 2009;2:ra83. doi: 10.1126/scisignal.2000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigorito E, Bardi G, Glassford J, Lam EW, Clayton E, Turner M. Vav-dependent and vav-independent phosphatidylinositol 3-kinase activation in murine B cells determined by the nature of the stimulus. J Immunol. 2004;173:3209–3214. doi: 10.4049/jimmunol.173.5.3209. [DOI] [PubMed] [Google Scholar]
- 31.Walmsley MJ, Ooi SK, Reynolds LF, Smith SH, Ruf S, Mathiot A, Vanes L, Williams DA, Cancro MP, Tybulewicz VL. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 32.Bruyns E, Marie-Cardine A, Kirchgessner H, Sagolla K, Shevchenko A, Mann M, Autschbach F, Bensussan A, Meuer S, Schraven B. T cell receptor (TCR) interacting molecule (TRIM), a novel disulfide-linked dimer associated with the TCR-CD3-zeta complex, recruits intracellular signaling proteins to the plasma membrane. J Exp Med. 1998;188:561–575. doi: 10.1084/jem.188.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osman N, Turner H, Lucas S, Reif K, Cantrell DA. The protein interactions of the immunoglobulin receptor family tyrosine-based activation motifs present in the T cell receptor zeta subunits and the CD3 gamma, delta and epsilon chains. Eur J Immunol. 1996;26:1063–1068. doi: 10.1002/eji.1830260516. [DOI] [PubMed] [Google Scholar]
- 34.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 36.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011;244:115–133. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawkins PT, Stephens LR, Suire S, Wilson M. PI3K signaling in neutrophils. Curr Top Microbiol Immunol. 2010;346:183–202. doi: 10.1007/82_2010_40. [DOI] [PubMed] [Google Scholar]
- 38.Nombela-Arrieta C, Lacalle RA, Montoya MC, Kunisaki Y, Megias D, Marques M, Carrera AC, Manes S, Fukui Y, Martinez AC, Stein JV. Differential requirements for DOCK2 and phosphoinositide-3-kinase gamma during T and B lymphocyte homing. Immunity. 2004;21:429–441. doi: 10.1016/j.immuni.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Reif K, Okkenhaug K, Sasaki T, Penninger JM, Vanhaesebroeck B, Cyster JG. Cutting edge: differential roles for phosphoinositide 3-kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J Immunol. 2004;173:2236–2240. doi: 10.4049/jimmunol.173.4.2236. [DOI] [PubMed] [Google Scholar]
- 40.Alcazar I, Marques M, Kumar A, Hirsch E, Wymann M, Carrera AC, Barber DF. Phosphoinositide 3-kinase gamma participates in T cell receptor-induced T cell activation. J Exp Med. 2007;204:2977–2987. doi: 10.1084/jem.20070366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janas ML, Turner M. Interaction of Ras with P110{gamma} Is Required for Thymic {beta}-Selection in the Mouse. J Immunol. 2011;187:4667–4675. doi: 10.4049/jimmunol.1101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bohnacker T, Marone R, Collmann E, Calvez R, Hirsch E, Wymann MP. PI3Kgamma adaptor subunits define coupling to degranulation and cell motility by distinct PtdIns(3,4,5)P3 pools in mast cells. Sci signal. 2009;2:ra27. doi: 10.1126/scisignal.2000259. [DOI] [PubMed] [Google Scholar]
- 43.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. Embo J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobs MD, Black J, Futer O, Swenson L, Hare B, Fleming M, Saxena K. Pim-1 ligand-bound structures reveal the mechanism of serine/threonine kinase inhibition by LY294002. J Biol Chem. 2005;280:13728–13734. doi: 10.1074/jbc.M413155200. [DOI] [PubMed] [Google Scholar]
- 45.Knight ZA. A pharmacological map of the PI3-K family defines a role for p110[alpha] in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okkenhaug K, Ali K, Vanhaesebroeck B. Antigen receptor signalling: a distinctive role for the p110delta isoform of PI3K. Trends Immunol. 2007;28:80–87. doi: 10.1016/j.it.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parsons MJ, Jones RG, Tsao MS, Odermatt B, Ohashi PS, Woodgett JR. Expression of active protein kinase B in T cells perturbs both T and B cell homeostasis and promotes inflammation. J Immunol. 2001;167:42–48. doi: 10.4049/jimmunol.167.1.42. [DOI] [PubMed] [Google Scholar]
- 50.Patra AK, Na SY, Bommhardt U. Active protein kinase B regulates TCR responsiveness by modulating cytoplasmic-nuclear localization of NFAT and NF-kappa B proteins. J Immunol. 2004;172:4812–4820. doi: 10.4049/jimmunol.172.8.4812. [DOI] [PubMed] [Google Scholar]
- 51.Rathmell JC, Elstrom RL, Cinalli RM, Thompson CB. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur J Immunol. 2003;33:2223–2232. doi: 10.1002/eji.200324048. [DOI] [PubMed] [Google Scholar]
- 52.Borlado LR, Redondo C, Alvarez B, Jimenez C, Criado LM, Flores J, Marcos MA, Martinez AC, Balomenos D, Carrera AC. Increased phosphoinositide 3-kinase activity induces a lymphoproliferative disorder and contributes to tumor generation in vivo. Faseb J. 2000;14:895–903. doi: 10.1096/fasebj.14.7.895. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, Tsubata T, Ohashi PS, Koyasu S, Penninger JM, Nakano T, Mak TW. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 54.Varnai P, Balla T. Live cell imaging of phosphoinositides with expressed inositide binding protein domains. Methods. 2008;46:167–176. doi: 10.1016/j.ymeth.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark J, Anderson KE, Juvin V, Smith TS, Karpe F, Wakelam MJ, Stephens LR, Hawkins PT. Quantification of PtdInsP3 molecular species in cells and tissues by mass spectrometry. Nat Methods. 2011;8:267–272. doi: 10.1038/nmeth.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, Humphries LA, Rawlings D, Reynolds H, Vigorito E, Turner M. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196:753–763. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, Cantley LC. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 58.Jou ST, Carpino N, Takahashi Y, Piekorz R, Chao JR, Carpino N, Wang D, Ihle JN. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol. 2002;22:8580–8591. doi: 10.1128/MCB.22.24.8580-8591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, Koyasu S. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science. 1999;283:390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 60.Ramadani F, Bolland DJ, Garcon F, Emery JL, Vanhaesebroeck B, Corcoran AE, Okkenhaug K. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Sci signal. 2010;3:ra60. doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beer-Hammer S, Zebedin E, von Holleben M, Alferink J, Reis B, Dresing P, Degrandi D, Scheu S, Hirsch E, Sexl V, Pfeffer K, Nurnberg B, Piekorz RP. The catalytic PI3K isoforms p110gamma and p110delta contribute to B cell development and maintenance, transformation, and proliferation. J Leuk Biol. 2010;87:1083–1095. doi: 10.1189/jlb.0809585. [DOI] [PubMed] [Google Scholar]
- 62.Amin RH, Schlissel MS. Foxo1 directly regulates the transcription of recombination-activating genes during B cell development. Nat Immunol. 2008;9:613–622. doi: 10.1038/ni.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dengler HS, Baracho GV, Omori SA, Bruckner S, Arden KC, Castrillon DH, DePinho RA, Rickert RC. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat Immunol. 2008;9:1388–1398. doi: 10.1038/ni.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazorchak AS, Liu D, Facchinetti V, Di Lorenzo A, Sessa WC, Schatz DG, Su B. Sin1-mTORC2 suppresses rag and il7r gene expression through Akt2 in B cells. Mol Cell. 2010;39:433–443. doi: 10.1016/j.molcel.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calamito M, Juntilla MM, Thomas M, Northrup DL, Rathmell J, Birnbaum MJ, Koretzky G, Allman D. Akt1 and Akt2 promote peripheral B-cell maturation and survival. Blood. 2010;115:4043–4050. doi: 10.1182/blood-2009-09-241638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herzog S, Hug E, Meixlsperger S, Paik JH, DePinho RA, Reth M, Jumaa H. SLP-65 regulates immunoglobulin light chain gene recombination through the PI(3)K-PKB-Foxo pathway. Nat Immunol. 2008;9:623–631. doi: 10.1038/ni.1616. [DOI] [PubMed] [Google Scholar]
- 67.Verkoczy L, Duong B, Skog P, Ait-Azzouzene D, Puri K, Vela JL, Nemazee D. Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection. J Immunol. 2007;178:6332–6341. doi: 10.4049/jimmunol.178.10.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Browne CD, Del Nagro CJ, Cato MH, Dengler HS, Rickert RC. Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity. 2009;31:749–760. doi: 10.1016/j.immuni.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donahue AC, Hess KL, Ng KL, Fruman DA. Altered splenic B cell subset development in mice lacking phosphoinositide 3-kinase p85alpha. Int Immunol. 2004;16:1789–1798. doi: 10.1093/intimm/dxh180. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki H, Matsuda S, Terauchi Y, Fujiwara M, Ohteki T, Asano T, Behrens TW, Kouro T, Takatsu K, Kadowaki T, Koyasu S. PI3K and Btk differentially regulate B cell antigen receptor-mediated signal transduction. Nat Immunol. 2003;4:280–286. doi: 10.1038/ni890. [DOI] [PubMed] [Google Scholar]
- 71.Depoil D, Fleire S, Treanor BL, Weber M, Harwood NE, Marchbank KL, Tybulewicz VL, Batista FD. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol. 2008;9:63–72. doi: 10.1038/ni1547. [DOI] [PubMed] [Google Scholar]
- 72.Chen J, Limon JJ, Blanc C, Peng SL, Fruman DA. Foxo1 regulates marginal zone B-cell development. Eur J Immunol. 2010;40:1890–1896. doi: 10.1002/eji.200939817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Durand CA, Hartvigsen K, Fogelstrand L, Kim S, Iritani S, Vanhaesebroeck B, Witztum JL, Puri KD, Gold MR. Phosphoinositide 3-kinase p110delta regulates natural antibody production, marginal zone and B-1 B cell function, and autoantibody responses. J Immunol. 2009;183:5673–5684. doi: 10.4049/jimmunol.0900432. [DOI] [PubMed] [Google Scholar]
- 74.Janas ML, Hodson D, Stamataki Z, Hill S, Welch K, Gambardella L, Trotman LC, Pandolfi PP, Vigorito E, Turner M. The effect of deleting p110delta on the phenotype and function of PTEN-deficient B cells. J Immunol. 2008;180:739–746. doi: 10.4049/jimmunol.180.2.739. [DOI] [PubMed] [Google Scholar]
- 75.Hess KL, Donahue AC, Ng KL, Moore TI, Oak J, Fruman DA. Frontline: The p85alpha isoform of phosphoinositide 3-kinase is essential for a subset of B cell receptor-initiated signaling responses. Eur J Immunol. 2004;34:2968–2976. doi: 10.1002/eji.200425326. [DOI] [PubMed] [Google Scholar]
- 76.Bilancio A, Okkenhaug K, Camps M, Emery JL, Ruckle T, Rommel C, Vanhaesebroeck B. Key role of the p110delta isoform of PI3K in B-cell antigen and IL-4 receptor signaling: comparative analysis of genetic and pharmacologic interference with p110delta function in B cells. Blood. 2006;107:642–650. doi: 10.1182/blood-2005-07-3041. [DOI] [PubMed] [Google Scholar]
- 77.Al-Alwan MM, Okkenhaug K, Vanhaesebroeck B, Hayflick JS, Marshall AJ. Requirement for phosphoinositide 3-kinase p110delta signaling in B cell antigen receptor-mediated antigen presentation. J Immunol. 2007;178:2328–2335. doi: 10.4049/jimmunol.178.4.2328. [DOI] [PubMed] [Google Scholar]
- 78.Al-Alwan M, Hou S, Zhang TT, Makondo K, Marshall AJ. Bam32/DAPP1 promotes B cell adhesion and formation of polarized conjugates with T cells. J Immunol. 2010;184:6961–6969. doi: 10.4049/jimmunol.0904176. [DOI] [PubMed] [Google Scholar]
- 79.Zhang TT, Okkenhaug K, Nashed BF, Puri KD, Knight ZA, Shokat KM, Vanhaesebroeck B, Marshall AJ. Genetic or pharmaceutical blockade of p110delta phosphoinositide 3-kinase enhances IgE production. J Allergy Clin Immunol. 2008;122:811–819. e812. doi: 10.1016/j.jaci.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 80.Donahue AC, Fruman DA. Proliferation and Survival of Activated B Cells Requires Sustained Antigen Receptor Engagement and Phosphoinositide 3-Kinase Activation. J Immunol. 2003;170:5851–5860. doi: 10.4049/jimmunol.170.12.5851. [DOI] [PubMed] [Google Scholar]
- 81.Dil N, Marshall AJ. Role of phosphoinositide 3-kinase p110 delta in TLR4- and TLR9-mediated B cell cytokine production and differentiation. Mol Immunol. 2009;46:1970–1978. doi: 10.1016/j.molimm.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 82.Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doi T, Obayashi K, Kadowaki T, Fujii H, Koyasu S. PI3K is a negative regulator of IgE production. Int Immunol. 2008;20:499–508. doi: 10.1093/intimm/dxn009. [DOI] [PubMed] [Google Scholar]
- 85.Henley T, Kovesdi D, Turner M. B-cell responses to B-cell activation factor of the TNF family (BAFF) are impaired in the absence of PI3K delta. Eur J Immunol. 2008;38:3543–3548. doi: 10.1002/eji.200838618. [DOI] [PubMed] [Google Scholar]
- 86.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 88.Fayard E, Moncayo G, Hemmings BA, Hollander GA. Phosphatidylinositol 3-kinase signaling in thymocytes: the need for stringent control. Sci Signal. 2010;3:re5. doi: 10.1126/scisignal.3135re5. [DOI] [PubMed] [Google Scholar]
- 89.Janas ML, Varano G, Gudmundsson K, Noda M, Nagasawa T, Turner M. Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J Exp Med. 2010;207:247–261. S241–242. doi: 10.1084/jem.20091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shiroki F, Matsuda S, Doi T, Fujiwara M, Mochizuki Y, Kadowaki T, Suzuki H, Koyasu S. The p85alpha regulatory subunit of class IA phosphoinositide 3-kinase regulates beta-selection in thymocyte development. J Immunol. 2007;178:1349–1356. doi: 10.4049/jimmunol.178.3.1349. [DOI] [PubMed] [Google Scholar]
- 91.Swat W, Montgrain V, Doggett TA, Douangpanya J, Puri K, Vermi W, Diacovo TG. Essential role of PI3Kdelta and PI3Kgamma in thymocyte survival. Blood. 2006;107:2415–2422. doi: 10.1182/blood-2005-08-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005;175:2783–2787. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- 93.Molon B, Gri G, Bettella M, Gomez-Mouton C, Lanzavecchia A, Martinez AC, Manes S, Viola A. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6:465–471. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 94.Hagenbeek TJ, Naspetti M, Malergue F, Garcon F, Nunes JA, Cleutjens KB, Trapman J, Krimpenfort P, Spits H. The loss of PTEN allows TCR alphabeta lineage thymocytes to bypass IL-7 and Pre-TCR-mediated signaling. J Exp Med. 2004;200:883–894. doi: 10.1084/jem.20040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kelly AP, Finlay DK, Hinton HJ, Clarke RG, Fiorini E, Radtke F, Cantrell DA. Notch-induced T cell development requires phosphoinositide-dependent kinase 1. Embo J. 2007;26:3441–3450. doi: 10.1038/sj.emboj.7601761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proc Natl Acad Sci U S A. 2007;104:12105–12110. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deane JA, Kharas MG, Oak JS, Stiles LN, Luo J, Moore TI, Ji H, Rommel C, Cantley LC, Lane TE, Fruman DA. T cell function is partially maintained in the absence of class IA phosphoinositide 3-kinase signaling. Blood. 2007;109:2894–2902. doi: 10.1182/blood-2006-07-038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 99.Shi J, Cinek T, Truitt KE, Imboden JB. Wortmannin, a phosphatidylinositol 3-kinase inhibitor, blocks antigen-mediated, but not CD3 monoclonal antibody-induced, activation of murine CD4+ T cells. J Immunol. 1997;158:4688–4695. [PubMed] [Google Scholar]
- Nashed BF, Zhang T, Al-Alwan M, Srinivasan G, Halayko AJ, Okkenhaug K, Vanhaesebroeck B, Hayglass KT, Marshall AJ. Role of the phosphoinositide 3-kinase p110delta in generation of type 2 cytokine responses and allergic airway inflammation. Eur J Immunol. 2007;37:416–424. doi: 10.1002/eji.200636401. [DOI] [PubMed] [Google Scholar]
- 101.Soond DR, Bjorgo E, Moltu K, Dale VQ, Patton DT, Torgersen KM, Galleway F, Twomey B, Clark J, Gaston JS, Tasken K, Bunyard P, Okkenhaug K. PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010;115:2203–2213. doi: 10.1182/blood-2009-07-232330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thoreen CC, Sabatini DM. Rapamycin inhibits mTORC1, but not completely. Autophagy. 2009;5:725–726. doi: 10.4161/auto.5.5.8504. [DOI] [PubMed] [Google Scholar]
- 103.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gan X, Wang J, Su B, Wu D. Evidence for Direct Activation of mTORC2 Kinase Activity by Phosphatidylinositol 3,4,5-Trisphosphate. J Biol Chem. 2011;286:10998–11002. doi: 10.1074/jbc.M110.195016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 106.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Janes MR, Fruman DA. Immune regulation by rapamycin: moving beyond T cells. Sci Signal. 2009;2:pe25. doi: 10.1126/scisignal.267pe25. [DOI] [PubMed] [Google Scholar]
- 108.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- 112.Kane LP, Mollenauer MN, Xu Z, Turck CW, Weiss A. Akt-dependent phosphorylation specifically regulates Cot induction of NF-kappa B-dependent transcription. Mol Cell Biol. 2002;22:5962–5974. doi: 10.1128/MCB.22.16.5962-5974.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Narayan P, Holt B, Tosti R, Kane LP. CARMA1 is required for Akt-mediated NF-kappaB activation in T cells. Mol Cell Biol. 2006;26:2327–2336. doi: 10.1128/MCB.26.6.2327-2336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qiao G, Li Z, Molinero L, Alegre ML, Ying H, Sun Z, Penninger JM, Zhang J. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol Cell Biol. 2008;28:2470–2480. doi: 10.1128/MCB.01505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng J, Phong B, Wilson DC, Hirsch R, Kane LP. Akt Fine-tunes NF-{kappa}B-dependent Gene Expression during T Cell Activation. J Biol Chem. 2011;286:36076–36085. doi: 10.1074/jbc.M111.259549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee KY, D’Acquisto F, Hayden MS, Shim JH, Ghosh S. PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation. Science. 2005;308:114–118. doi: 10.1126/science.1107107. [DOI] [PubMed] [Google Scholar]
- 117.Park SG, Schulze-Luehrman J, Hayden MS, Hashimoto N, Ogawa W, Kasuga M, Ghosh S. The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF-kappaB and activate T cells. Nat Immunol. 2009;10:158–166. doi: 10.1038/ni.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch’en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 120.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 121.Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. 2010;207:1381–1391. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 124.Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30:218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 125.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 126.Locke NR, Patterson SJ, Hamilton MJ, Sly LM, Krystal G, Levings MK. SHIP regulates the reciprocal development of T regulatory and Th17 cells. J Immunol. 2009;183:975–983. doi: 10.4049/jimmunol.0803749. [DOI] [PubMed] [Google Scholar]
- 127.Pierau M, Engelmann S, Reinhold D, Lapp T, Schraven B, Bommhardt UH. Protein kinase B/Akt signals impair Th17 differentiation and support natural regulatory T cell function and induced regulatory T cell formation. J Immunol. 2009;183:6124–6134. doi: 10.4049/jimmunol.0900246. [DOI] [PubMed] [Google Scholar]
- 128.Haylock-Jacobs S, Comerford I, Bunting M, Kara E, Townley S, Klingler-Hoffmann M, Vanhaesebroeck B, Puri KD, McColl SR. PI3Kdelta drives the pathogenesis of experimental autoimmune encephalomyelitis by inhibiting effector T cell apoptosis and promoting Th17 differentiation. J Autoimmun. 2011;36:278–287. doi: 10.1016/j.jaut.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 129.Tarasenko T, Kole HK, Chi AW, Mentink-Kane MM, Wynn TA, Bolland S. T cell-specific deletion of the inositol phosphatase SHIP reveals its role in regulating Th1/Th2 and cytotoxic responses. Proc Natl Acad Sci U S A. 2007;104:11382–11387. doi: 10.1073/pnas.0704853104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Allam A, Marshall AJ. Role of the adaptor proteins Bam32, TAPP1 and TAPP2 in lymphocyte activation. Immunol Lett. 2005;97:7–17. doi: 10.1016/j.imlet.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 131.Donia M, Mangano K, Amoroso A, Mazzarino MC, Imbesi R, Castrogiovanni P, Coco M, Meroni P, Nicoletti F. Treatment with rapamycin ameliorates clinical and histological signs of protracted relapsing experimental allergic encephalomyelitis in Dark Agouti rats and induces expansion of peripheral CD4+CD25+Foxp3+ regulatory T cells. J Autoimmun. 2009;33:135–140. doi: 10.1016/j.jaut.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 132.Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7:1819–1824. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hernandez JB, Michalek RD, Weist BM, Newton RH, Limon JJ, Carrillo MA, Patterson EK, Nguyen LV, Fruman DA, Rathmell JC, Walsh CM. Control of T cell metabolism and regulatory T cell generation by a DRAK2/p70S6K1 signaling axis. 2011. submitted. [Google Scholar]
- 136.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wan Q, Kozhaya L, ElHed A, Ramesh R, Carlson TJ, Djuretic IM, Sundrud MS, Unutmaz D. Cytokine signals through PI-3 kinase pathway modulate Th17 cytokine production by CCR6+ human memory T cells. J Exp Med. 2011;208:1875–1887. doi: 10.1084/jem.20102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol. 2011;11:109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 141.Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, Suh WK. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2009;106:20371–20376. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rolf J, Bell SE, Kovesdi D, Janas ML, Soond DR, Webb LM, Santinelli S, Saunders T, Hebeis B, Killeen N, Okkenhaug K, Turner M. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J Immunol. 2010;185:4042–4052. doi: 10.4049/jimmunol.1001730. [DOI] [PubMed] [Google Scholar]
- 143.Acosta YY, Zafra MP, Ojeda G, Bernardone IS, Dianzani U, Portoles P, Rojo JM. Biased binding of class IA phosphatidyl inositol 3-kinase subunits to inducible costimulator (CD278) Cell Mol Life Sci. 2011;68:3065–3079. doi: 10.1007/s00018-010-0606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oak JS, Deane JA, Kharas MG, Luo J, Lane TE, Cantley LC, Fruman DA. Sjogren’s syndrome-like disease in mice with T cells lacking class 1A phosphoinositide-3-kinase. Proc Natl Acad Sci U S A. 2006;103:16882–16887. doi: 10.1073/pnas.0607984103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E, Rowan WC, Sancho S, Walker LS, Vanhaesebroeck B, Okkenhaug K. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 146.Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, Bensinger SJ, Hancock WW, Turka LA. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest. 2006;116:2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 149.Liu D, Zhang T, Marshall AJ, Okkenhaug K, Vanhaesebroeck B, Uzonna JE. The p110delta isoform of phosphatidylinositol 3-kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. J Immunol. 2009;183:1921–1933. doi: 10.4049/jimmunol.0901099. [DOI] [PubMed] [Google Scholar]
- 150.Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, Feijoo C, Okkenhaug K, Cantrell DA. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martin AL, Schwartz MD, Jameson SC, Shimizu Y. Selective regulation of CD8 effector T cell migration by the p110 gamma isoform of phosphatidylinositol 3-kinase. J Immunol. 2008;180:2081–2088. doi: 10.4049/jimmunol.180.4.2081. [DOI] [PubMed] [Google Scholar]
- 153.Thomas MS, Mitchell JS, DeNucci CC, Martin AL, Shimizu Y. The p110gamma isoform of phosphatidylinositol 3-kinase regulates migration of effector CD4 T lymphocytes into peripheral inflammatory sites. J Leuk Biol. 2008;84:814–823. doi: 10.1189/jlb.0807561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kerr WG, Colucci F. Inositol phospholipid signaling and the biology of natural killer cells. J Innate Immun. 2011;3:249–257. doi: 10.1159/000323920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-triphosphate in neutrophil responses. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baggiolini M, Dewald B, Schnyder J, Ruch W, Cooper PH, Payne TG. Inhibition of the phagocytosis-induced respiratory burst by the fungal metabolite wortmannin and some analogues. Exp Cell Res. 1987;169:408–418. doi: 10.1016/0014-4827(87)90201-1. [DOI] [PubMed] [Google Scholar]
- 158.Rommel C. Taking PI3Kdelta and PI3Kgamma one step ahead: dual active PI3Kdelta/gamma inhibitors for the treatment of immune-mediated inflammatory diseases. Curr Top Microbiol Immunol. 2010;346:279–299. doi: 10.1007/82_2010_79. [DOI] [PubMed] [Google Scholar]
- 159.Ruckle T, Schwarz MK, Rommel C. PI3Kgamma inhibition: towards an ‘aspirin of the 21st century’? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 160.Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–451. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 161.Fruman DA, Rommel C. PI3Kd inhibitors in cancer: rationale and serendipity merge in the clinic. Cancer Discov. 2011 doi: 10.1158/2159-8290.CD-11-0249. Epub Nov. 29. [DOI] [PubMed] [Google Scholar]
- 162.Abraham RT, Eng CH. Mammalian target of rapamycin as a therapeutic target in oncology. Expert opinion on therapeutic targets. 2008;12:209–222. doi: 10.1517/14728222.12.2.209. [DOI] [PubMed] [Google Scholar]
- 163.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27:5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 164.Janes MR, Fruman DA. Targeting TOR dependence in cancer. Oncotarget. 2010;1:69–76. doi: 10.18632/oncotarget.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 166.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Workman P, Clarke PA, Raynaud FI, van Montfort RL. Drugging the PI3 Kinome: From Chemical Tools to Drugs in the Clinic. Cancer Res. 2010;70:2146–2157. doi: 10.1158/0008-5472.CAN-09-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM, Wagner-Johnston ND, Flinn IW, Kahl BS, Spurgeon SE, Lannutti B, Giese NA, Webb HK, Ulrich RG, Peterman S, Holes LM, Yu AS. CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110{delta}, Demonstrates Clinical Activity and Pharmacodynamic Effects In Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. ASH Annual Meeting Abstracts. 2010;116:55. [Google Scholar]
- 169.Furman RR, Byrd JC, Flinn IW, Coutre SE, MBD, Brown JR, Kahl BS, Wagner-Johnston ND, Giese NA, Yu AS. Interim results from a phase I study of CAL-101, a selective oral inhibitor of phosphatidylinositol 3-kinase p110d isoform, in patients with relapsed or refractory hematologic malignancies. J Clin Oncol. 2010;28:15s, abstr 3032. [Google Scholar]
- 170.Ikeda H, Hideshima T, Fulciniti M, Perrone G, Miura N, Yasui H, Okawa Y, Kiziltepe T, Santo L, Vallet S, Cristea D, Calabrese E, Gorgun G, Raje NS, Richardson P, Munshi NC, Lannutti BJ, Puri KD, Giese NA, Anderson KC. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood. 2010;116:1460–1468. doi: 10.1182/blood-2009-06-222943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M, Druker BJ, Puri KD, Ulrich RG, Giese NA. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, Jones J, Andritsos L, Puri KD, Lannutti BJ, Giese NA, Zhang X, Wei L, Byrd JC, Johnson AJ. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, Giese N, O’Brien S, Yu A, Miller LL, Lannutti BJ, Burger JA. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, Xu W, Shaffer AL, Wright G, Xiao W, Powell J, Jiang JK, Thomas CJ, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Connors JM, Johnson NA, Rimsza LM, Campo E, Jaffe ES, Wilson WH, Delabie J, Smeland EB, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Pierce SK, Staudt LM. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Suarez-Fueyo A, Barber DF, Martinez-Ara J, Zea-Mendoza AC, Carrera AC. Enhanced phosphoinositide 3-kinase delta activity is a frequent event in systemic lupus erythematosus that confers resistance to activation-induced T cell death. J Immunol. 2011;187:2376–2385. doi: 10.4049/jimmunol.1101602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.