Abstract
Increased NADP reduced (NADPH) oxidase 4 (Nox4) and reduced expression of the nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ) contribute to hypoxia-induced pulmonary hypertension (PH). To examine the role of Nox4 activity in pulmonary vascular cell proliferation and PH, the current study used a novel Nox4 inhibitor, GKT137831, in hypoxia-exposed human pulmonary artery endothelial or smooth muscle cells (HPAECs or HPASMCs) in vitro and in hypoxia-treated mice in vivo. HPAECs or HPASMCs were exposed to normoxia or hypoxia (1% O2) for 72 hours with or without GKT137831. Cell proliferation and Nox4, PPARγ, and transforming growth factor (TGF)β1 expression were measured. C57Bl/6 mice were exposed to normoxia or hypoxia (10% O2) for 3 weeks with or without GKT137831 treatment during the final 10 days of exposure. Lung PPARγ and TGF-β1 expression, right ventricular hypertrophy (RVH), right ventricular systolic pressure (RVSP), and pulmonary vascular remodeling were measured. GKT137831 attenuated hypoxia-induced H2O2 release, proliferation, and TGF-β1 expression and blunted reductions in PPARγ in HPAECs and HPASMCs in vitro. In vivo GKT137831 inhibited hypoxia-induced increases in TGF-β1 and reductions in PPARγ expression and attenuated RVH and pulmonary artery wall thickness but not increases in RVSP or muscularization of small arterioles. This study shows that Nox4 plays a critical role in modulating proliferative responses of pulmonary vascular wall cells. Targeting Nox4 with GKT137831 provides a novel strategy to attenuate hypoxia-induced alterations in pulmonary vascular wall cells that contribute to vascular remodeling and RVH, key features involved in PH pathogenesis.
Keywords: rosiglitazone, PPARγ, TGF-β, pulmonary hypertension
Clinical Relevance
Our findings add to an existing body of literature that identifies NADPH oxidases as critical mediators involved in the pathogenesis of pulmonary hypertension (PH). We provide evidence that Nox4 can be therapeutically targeted with a novel bioavailable Nox4 inhibitor to attenuate the development of hypoxia-induced pulmonary vascular remodeling and right ventricular dysfunction. Our findings support the continued investigation of therapies targeting Nox4 as novel therapeutic approaches in PH.
Pulmonary hypertension (PH) is a progressive disorder associated with significant morbidity and mortality. Although recent therapeutic advances have improved survival for patients with PH, the prognosis remains poor (1). The pathobiology of PH is complex, and factors that contribute to endothelial dysfunction have been implicated in pathogenesis (2, 3). Among these factors, NADP reduced (NADPH) oxidase enzymes that produce reactive oxygen species (ROS) contribute to the development of a variety of vascular diseases, such as atherosclerosis (4) and systemic (5) and pulmonary hypertension (6). NADPH oxidases catalyze the reduction of molecular oxygen to generate superoxide (O2.−), hydrogen peroxide (H2O2), or secondary oxidants (7). Seven isoforms of the catalytic moiety of the nonphagocytic NADPH oxidase enzyme have been described (Nox1–5, Duox1–2). These subunits are homologous to the catalytic moiety of the prototype phagocytic NADPH oxidase Nox2 (or gp91phox) but differ from each other regarding cellular localization, tissue distribution, regulation, activation, and expression (7, 8). For example, although both Nox1 and Nox4 are expressed in vascular smooth muscle cells (VSMCs), they are targeted to discreet intracellular locations, are differentially regulated in response to growth factors and vascular injury, and are activated by distinct mechanisms (9).
Experimental evidence suggests that Nox4 is involved in PH pathogenesis. Nox4 was the most abundant gp91phox homolog identified in endothelial cells (ECs) and VSMCs (4, 7), and its expression was associated with pulmonary vascular remodeling (10), vasoconstriction (11), and enhanced proliferation of vascular wall cells (8). Nox4 was selectively up-regulated in the pulmonary arteries of chronic hypoxia-exposed mice and in the vascular lesions of patients with idiopathic pulmonary arterial hypertension (IPAH) (10). In addition, chronic hypoxia exposure selectively increased Nox4 expression in the lungs of mice in vivo (12), and Nox4 RNA interference attenuated hypoxia-induced human pulmonary artery smooth muscle cell (HPASMC) proliferation in vitro (13). Chronic hypoxia–induced increases in Nox4 were also associated with reductions in the expression of the nuclear transcription factor, peroxisome proliferator-activated receptor γ (PPARγ), in HPASMCs and human pulmonary artery endothelial cells (HPAECs) (12). PPARγ, an important regulator of lipid and glucose metabolism (14), modulates growth and proliferation of ECs (15) and SMCs (16). Activation of PPARγ with the synthetic ligand rosiglitazone attenuates hypoxia-induced increases in mouse lung Nox4 expression in vivo (12) and in HPAECs (12) and HPASMCs (13) in vitro. Rosiglitazone also attenuates right ventricular hypertrophy (RVH), PH, or pulmonary vascular remodeling in chronic hypoxia–exposed mice (12) or rats (17, 18). Collectively, these studies suggest that hypoxic increases in Nox4 expression and activity contribute to PH pathogenesis and that treatment strategies that inhibit Nox4 expression or activity may have therapeutic potential in PH.
Until recently, specific pharmacological NADPH oxidase inhibitors with high selectivity, potency, and bioavailability have not been available (19, 20). The current study used GKT137831, a recently described pyrazolopyridinedione derivative that inhibits Nox4 and Nox1 (21), to further examine the role of Nox4 in hypoxia-induced alterations in pulmonary vascular cell proliferation and PH. GKT137831 [2-(2-chlorophenyl)-4-[3-(dimethylamino)phenyl]-5-methyl-1H-pyrazolo[4,3-c]pyridine-3,6(2H,5H)-dione] is a small molecule that inhibits Nox4 and Nox1 isoforms (Ki 100–150 nM) in cell-free assays of ROS production using membranes prepared from cells heterologously overexpressing specific Nox isoforms. GKT137831 is a weak inhibitor of Nox2 in cell-free assays and did not significantly inhibit neutrophil oxidative burst. GKT137831 had no radical scavenging or antioxidant activity and did not show significant in vitro off-target inhibition of 170 different proteins, indicating high Nox specificity (21, 22).
Our findings demonstrate that GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation, TGF-β1 expression, and reductions in PPARγ in vitro and in vivo and blunts hypoxia-induced RVH and pulmonary vascular remodeling. These findings provide novel evidence that Nox4 plays a critical role in hypoxia-induced alterations in pulmonary vascular wall cells and indicate that directly targeting Nox4 represents a viable therapeutic strategy in PH.
Materials and Methods
Cell Culture and In Vitro Hypoxia Exposure
Monolayers of HPAECs and HPASMCs were propagated in culture and placed in normoxic (21% O2, 5% CO2) or hypoxic (1% O2, 5% CO2) conditions for 72 hours as previously reported (13). GKT137831 (0.1–20 μM), provided through a material transfer agreement from GenKyoTex (Geneva, Switzerland), or vehicle (1% DMSO) were added to the culture medium at the onset (prevention regimen) or during the last 24 hours (intervention regimen) of a 72-hour hypoxia exposure regimen. More detailed information about GKT137831 dosing and specificity is provided in the online supplement. Pulmonary artery endothelial cells were isolated from the lungs of control subjects or patients with idiopathic pulmonary arterial hypertension (IPAH) as described (23), and lysates of these cells were generously provided by Dr. Suzy Comhair (Cleveland Clinic, Cleveland, OH).
Measurements of Cellular Proliferation and H2O2 Generation
Hypoxic HPASMC and HPAEC proliferation was determined using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT; ATCC, Manassas, VA) as recently reported (13), by Western blotting to detect proliferating cell nuclear antigen (PCNA) expression, or by manual cell counting after Trypan blue staining. Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen, Molecular Probes, Eugene, OR) was used to measure H2O2 released into the culture media from HPAECs or HPASMCs as previously described (13). After exposure to control or hypoxic environments for 72 hours, Amplex Red reagent was added, and the cells were returned to control or hypoxic environments for an additional hour before fluorescence measurements.
Measurement of Nox4, PPARγ, and TGF-β1 mRNA
Quantitative RT-PCR was performed to measure the mRNA levels of selected gene targets. In selected studies, small interfering RNA to Nox4 was used to inhibit Nox4 activity. Detailed methods are provided in the online supplement.
Mouse Model of Chronic Hypoxia Exposure
Mice were exposed to normoxia or hypoxia (10% O2) for 3 weeks as we reported (12). During the final 10 days of exposure to hypoxic or normoxic conditions, each animal was given rosiglitazone (10 mg/kg/d) or GKT137831 (30 or 60 mg/kg/d) daily by oral gavage. At the conclusion of these exposures, right ventricular systolic pressure (RVSP), right ventricular hypertrophy (RVH), and pulmonary vascular remodeling were determined, and the expression of selected targets was examined in lung tissue as described in the online supplement.
Western Blot Analysis of Lung Tissue and Cell Lysates
Levels of Nox4, PPARγ, and TGF-β1 proteins were determined by Western blotting as described in the online supplement.
Statistical Analysis
Statistical analysis was performed by one-way ANOVA, followed by a Tukey’s post hoc analysis. The level of statistical significance was set at an α value of P ≤ 0.05.
Results
GKT137831 Attenuates Hypoxia-Induced HPAEC and HPASMC Proliferation In Vitro
Hypoxia is a potent proliferative stimulus that can enhance PASMC proliferation in a Nox4-mediated manner (13, 24, 25). As expected, exposure to hypoxia increased the proliferation of HPAECs and HPASMCs in vitro (Figures 1A and 1B and E1A and E1B). Treatment with graded concentrations of GKT137831 during the last 24 hours of exposure (“intervention paradigm,” Figure 1A) attenuated cell proliferation in normoxic HPASMCs but had no significant effect on proliferation in normoxic HPAECs. Hypoxia increased proliferation in HPASMCs and HPAECs, which was attenuated by intervention with 20 μM GKT137831. Similarly, administration of GKT137831 throughout the 72-hour period of normoxia or hypoxia exposure (“prevention paradigm,” Figure 1B) attenuated HPASMC proliferation under normoxic conditions at the 20 μM concentration but had no effect on proliferation in normoxic HPAECs. In the prevention paradigm, GKT137831 attenuated hypoxia-induced HPASMC and HPAEC proliferation at 5 and 20 μM. Complementary assays of cell proliferation measuring the expression of PCNA or manual cell counting confirmed that GKT137831 attenuated hypoxia-induced pulmonary vascular cell proliferation (Figures E1A and E1B).
Figure 1.
Hypoxia-induced human pulmonary artery smooth muscle cell (HPASMC) and human pulmonary artery endothelial cell (HPAEC) proliferation was attenuated by GKT137831. HPAECs and HPASMCs were exposed to control (21% O2) or hypoxic (1% O2) environments for 72 hours. Cells were treated with vehicle or with GKT137831 (0.1–20 μM) for the final 24 hours (A) or for the entire 72-hour exposure (B). Proliferation was then determined by MTT assay. Each bar represents mean ± SEM cell proliferation expressed as fold-change relative to control samples (n = 3 HPAECs; n = 3 HPASMCs). $P < 0.05 versus 0-Control, +P < 0.01 versus 0-Control, *P < 0.001 versus 0-Control, #P < 0.05 versus 0-Hypoxia, ##P < 0.01 versus 0-Hypoxia, and ∧P < 0.001 versus 0-Hypoxia.
Nox4 siRNA Attenuates Hypoxia-Induced Proliferation of Pulmonary Vascular Wall Cells
To more specifically confirm the role of Nox4 in hypoxic pulmonary vascular cell proliferation, HPASMCs and HPAECs were treated with Nox4 siRNA. Graded doses of siNox4 decreased Nox4 protein levels by 50% and attenuated hypoxia-induced HPASMC and HPAEC proliferation in a fashion similar to that observed after treatment with GKT137831 (Figures 2A and 2B).
Figure 2.
Nox4 RNA interference inhibits hypoxia-induced HPAEC and HPASMC proliferation. Selected HPAECs and HPASMCs were transfected with control (−) or Nox4 (+) siRNA and propogated for 96 hours. (A) The siNox4 concentration was titrated to achieve a 50% reduction in target protein expression. Each bar represents mean ± SEM Nox4 protein expressed relative to control samples (n = 3). *P < 0.001 versus control. (B) After transfection, HPASMCs and HPAECs were exposed to normoxic or hypoxic conditions for 72 hours, and proliferation was determined by MTT assay. Each bar represents mean ± SEM cell proliferation expressed as fold-change relative to normoxic control samples (n = 7 HPAECs; n = 3 HPASMCs). *P < 0.001 versus (−) normoxia; #P < 0.001 versus (−) hypoxia.
GKT137831 Attenuates Hypoxia-Induced H2O2 Generation in HPAECs and HPASMCs
H2O2 is the major product of Nox4 activation (26, 27). Hypoxia exposure increases H2O2 generation by pulmonary vascular wall cells (3, 13), and hypoxia-induced increases in HPASMC H2O2 release and proliferation are abrogated by Nox4 siRNA (13). To assess the effect of Nox4 inhibition on hypoxia-induced H2O2 generation, graded concentrations of GKT137831 were added to HPAECs or HPASMCs as described in Figure 1. As shown in Figures 3A and 3B, hypoxia-induced HPAEC and HPASMC H2O2 generation was attenuated in a concentration-dependent fashion by administration of GKT137831. GKT137831-induced reductions in H2O2 production were not associated with reductions in Nox4 protein levels (data not shown). To confirm that H2O2 generation was the critical target of GKT137831, polyethylene glycol catalase (PEG-CAT) was used as an alternative approach to reduce hypoxic increases in H2O2 levels. Consistent with Nox4 siRNA- or GKT137831-mediated inhibition of hypoxia-induced HPASMC and HPAEC proliferation, PEG-CAT attenuated cell proliferation (Figure 3C). Taken together, these studies confirm the importance of Nox4-mediated H2O2 generation in the proliferative response of pulmonary vascular wall cells to hypoxia.
Figure 3.
Hypoxia-induced increases in HPASMC and HPAEC H2O2 production were attenuated by GKT137831, and catalase attenuated cell proliferation. HPAECs (A) and HPASMCs (B) were exposed to control (21% O2) or hypoxic (1% O2) environments for 72 hours. Cells were treated with vehicle or GKT137831 (0.1, 5, or 20 μM) for the final 24 hours of exposure. H2O2 production was measured by Amplex Red assay. Each bar represents mean ± SEM concentration of H2O2 expressed as fold-change relative to control samples (n = 3). *P < 0.001 versus control; #P < 0.05 versus hypoxia. (C) HPASMCs and HPAECs were treated with polyethylene glycol-catalase (1,000 U/ml) during the final 24 hours of exposure to hypoxic or normoxic environments, and proliferation was measured by MTT assay. Each bar represents mean ± SEM proliferation expressed as fold-change relative to normoxic control samples (n = 3 HPAECs; n = 5 HPASMCs). *P < 0.01 versus normoxia; #P < 0.05 versus hypoxia.
Hypoxia-Induced Reductions in HPAEC and HPASMC PPARγ Expression Are Attenuated by Treatment with GKT137831
PPARγ expression was significantly decreased in HPAECs and HPASMCs after exposure to hypoxia for 72 hours (Figures 4A and 4B). GKT137831, whether administered during the final 24 hours of hypoxia exposure (Figure 4A) or during the entire 72-hour hypoxia exposure (Figure E2A), attenuated hypoxic reductions in HPAEC PPARγ expression. Similarly, administration of GKT137831 during the last 24 hours (Figure 4B) or at the onset of exposure to hypoxia (Figure E2B) attenuated hypoxic reductions in HPASMC PPARγ expression in a dose-dependent manner. To further confirm the relevance of hypoxia-induced increases in Nox4 expression and decreases in PPARγ expression, the expression of these targets was examined in lysates of pulmonary artery endothelial cells isolated from control subjects or patients with IPAH as previously described (23). In pulmonary artery endothelial cells from patients with IPAH compared with control patients, Nox4 mRNA levels were increased nearly 2.5-fold, whereas PPARγ mRNA levels were significantly diminished (Figures 4C and 4D).
Figure 4.
Treatment with GKT137831 attenuated hypoxic reductions of peroxisome proliferator-activated receptor (PPAR)γ expression in HPASMCs and HPAECs. HPAECs and HPASMCs were exposed to control (21% O2) or hypoxic (1% O2) environments for 72 hours. Selected HPAECs (A) and HPASMCs (B) were then treated with vehicle or with GKT137831 (5–20 μM) during the final 24 hours of exposure. Cells were then collected, and proteins were isolated for Western blot analysis of PPARγ and CDK4. Each bar represents mean ± SEM density of PPARγ bands relative to CDK4 expressed as fold-change relative to control values (n = 4–6). *P < 0.05 versus control. (C and D) Pulmonary artery endothelial cells were isolated from the lungs of control subjects or patients with idiopathic pulmonary arterial hypertension (IPAH). Quantitative real-time PCR was performed on deidentified lysates for Nox4 (C) and PPARγ (D). Each bar represents mean ± SEM of Nox4 and PPARγ mRNA expressed relative to 9S (n = 3). *P < 0.05 versus control.
Treatment with GKT137831 In Vivo Attenuates Chronic Hypoxia-Induced Right Ventricular Hypertrophy, Pulmonary Vascular Remodeling, and Proliferation but Not Increased RVSP
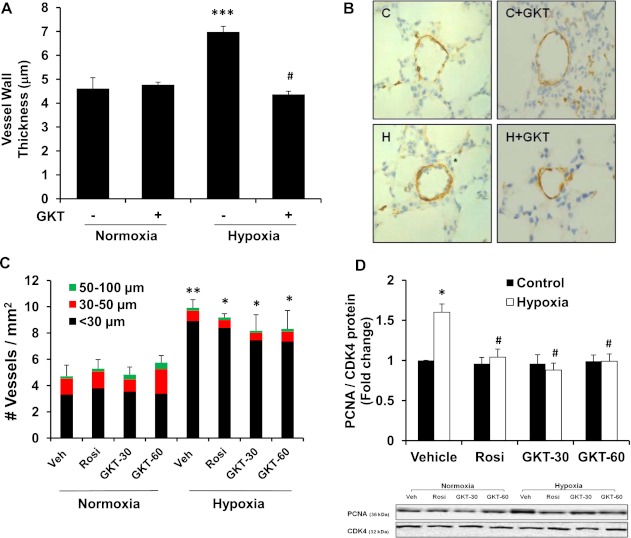
To determine the contribution of enhanced Nox4 expression to the development of hypoxia-induced PH in vivo, C57Bl/6 mice were exposed to normoxic or hypoxic conditions for 3 weeks, and GKT137831 was administered during the final 10 days. Because we have previously shown that treatment with the PPARγ ligand rosiglitazone reduced Nox4 expression in the lungs of chronic hypoxia-exposed mice and attenuated increases in RVSP and RVH (12), rosiglitazone was included as a therapeutic arm for comparative purposes. Consistent with our previous studies, chronic hypoxia–exposed mice developed significant increases in RVSP and RVH (Figures 5A and 5B). Administration of GKT137831 (30 or 60 mg/kg/d) failed to attenuate hypoxia-induced increases in RVSP but significantly attenuated RVH. In contrast, rosiglitazone treatment attenuated hypoxic increases in lung Nox4 expression, RVSP, and RVH as previously reported (12). As illustrated in Figures 6A and 6B, the administration of GKT137831 (60 mg/kg/d) attenuated hypoxia-induced increases in vessel wall thickness. Hypoxia increased α-SMA positive vessels in the lung yet neither GKT137831 nor rosiglitazone had a significant effect on this increase (Figure 6C). In contrast, GKT137831 (30 or 60 mg) and rosiglitazone attenuated chronic hypoxia–induced proliferation in vivo in the mouse lung (Figure 6D). These findings suggest that the GKT137831 regimens used were sufficient to reduce proliferation and vascular wall thickness in this model but insufficient to reduce the number of small vessels staining with α-SMA.
Figure 5.
GKT137831 attenuated hypoxia-induced increases in right ventricular hypertrophy but not elevations in right ventricular systolic pressure (RVSP). Mice were exposed to hypoxia (10% O2) or normoxia (control, 21% O2) for 3 weeks. Vehicle, rosiglitazone (Rosi, 10 mg/kg/d), or GKT137831 (GKT, 30 or 60 mg/kg/d) was given daily by oral gavage for the final 10 days of exposure. (A) RVSP was measured by advancing a pressure transducer into the right ventricle. Each bar represents the mean RVSP ± SEM in mm Hg (n = 4–8). *P < 0.05 versus normoxic control mice; #P < 0.01 versus normoxic control mice. (B) Hearts were dissected into the right ventricle and left ventricle plus septum. Each bar represents the mean ± SEM ratio of the weight of the right ventricle to left ventricle plus septum (n = 8). *P < 0.05 versus normoxic control mice; #P < 0.05 versus hypoxic control mice.
Figure 6.
GKT137831 or rosiglitazone attenuated hypoxia-induced vascular remodeling and proliferating cell nuclear antigen expression in vivo. Mice were exposed to hypoxia (10% O2) or normoxia (21% O2) for 3 weeks. Vehicle control (Veh), rosiglitazone (Rosi, 10 mg/kg/d), or GKT137831 (GKT, 30 or 60 mg/kg/d) were given daily by oral gavage for the final 10 days of exposure. Tissue sections generated from mouse lungs were stained with antibodies to α-SMA, and the vessel wall thickness and vessel density were measured for vessels with diameter < 100 μm. (A) Bars represent the mean ± SEM vessel wall thickness relative to normoxic control samples (n = 3–4). ***P < 0.001 versus normoxia; #P < 0.001 versus hypoxia. (B) Representative photomicrographs of α-SMA–stained vessels exposed to normoxia or hypoxia with or without GKT137831 are demonstrated. Labeling is shown (C, control; C+GKT, control + GKT137831; H, hypoxia; H + GKT, hypoxia + GKT137831), and the scale bar in each image = 50 μm. (C) Bars represent the mean ± SEM number of α-SMA staining vessels per mm2 relative to normoxic control samples (n = 3–4). **P < 0.01 versus normoxia-veh; *P < 0.05 versus normoxia-veh. (GKT-30 = GKT-30 mg/kg; GKT-60 = GKT-60 mg/kg; Rosi = rosiglitazone; Veh = vehicle). (D) Whole lung lysates were isolated for Western blot analysis of PCNA and CDK4 to determine cell proliferation. Each bar represents mean ± SEM density of PCNA bands relative to CDK4 expressed as fold-change relative to control values (n = 8). *P < 0.05 versus control-vehicle; #P < 0.05 versus hypoxia-vehicle.
Administration of GKT137831 or Rosiglitazone Attenuates Hypoxia-Induced Reductions in PPARγ and Increases in TGF-β1 Expression In Vivo
Because hypoxia reduced PPARγ expression in HPAECs and HPASMCs in vitro, the lungs of animals exposed to hypoxia for 3 weeks were examined for PPARγ expression. Chronic hypoxia exposure significantly reduced mouse lung PPARγ expression (Figure 7A). Administration of GKT137831 or rosiglitazone attenuated hypoxic reductions in lung PPARγ expression (Figure 7A). Transforming growth factor (TGF)-β1 contributes to hypoxia-mediated Nox4 expression and activity and HPASMC proliferation (24, 28–30), and PPARγ ligands have been shown to modulate TGF-β1 signaling in the lung (31–33). We therefore examined the impact of Nox4 inhibition with GKT137831 on hypoxia-induced TGF-β1 expression in the lung. As expected, hypoxia increased TGF-β1 expression in the mouse lung, and treatment with GKT137831 or rosiglitazone attenuated hypoxia-induced TGF-β1 expression (Figure 7B). To better define signaling interactions between Nox4, H2O2, and TGF-β1, we examined the impact of GKT137831 or PEG-CAT on hypoxic increases in TGF-β1 in vitro. Hypoxia stimulated TGF-β1 expression as expected (Figure 7C). Administration of GKT137831 or PEG-CAT during the last 24 hours of hypoxia exposure attenuated increases in TGF-β1 expression. These results suggest that Nox4-derived H2O2 stimulates increases in TGF-β1 and reductions in PPARγ expression.
Figure 7.
GKT137831 or rosiglitazone attenuated hypoxic alterations in PPARγ in vivo and TGF-β1 in vivo and in vitro. Animals were exposed to hypoxia (10% O2) or normoxia (21% O2) for 3 weeks. Control (Vehicle), rosiglitazone (Rosi, 10 mg/kg/d), or GKT137831 (GKT, 30 or 60 mg/kg/d) were given daily by oral gavage for the final 10 days of exposure. Protein was prepared from whole lung homogenate for Western blot analysis. Each bar represents the mean ± SEM density of PPARγ (A) or TGF-β1 (B) bands relative to CDK4 expressed as fold-change relative to control samples (n = 8). *P < 0.05 versus normoxic control lung; #P < 0.05 versus hypoxic vehicle-treated lung. (C) HPAECs were exposed to hypoxia for 72 hours with or without GKT137831 (20 μM) or polyethylene glycol (PEG) catalase (1,000 U/ml) administered during the last 24 hours. Each bar represents the mean ± SEM TGF-β1 mRNA expression relative to control samples (n = 3). *P < 0.01 versus (–) normoxia; **P < 0.001 versus (–) normoxia; #P < 0.01 versus (–) hypoxia.
Discussion
Nox4 is an important mediator of hypoxia-induced derangements in the pulmonary circulation that contribute to the development of PH (10, 12). The goals of the current study were to further define the role of hypoxia-induced alterations in Nox4 on HPAEC and HPASMC proliferation and to explore the benefit of Nox4-specific inhibition in modulating proliferative pathways implicated in PH pathogenesis. The primary conclusions from our in vitro studies demonstrate that the novel Nox4 inhibitor GKT137831 attenuates hypoxia-induced 1) HPASMC and HPAEC proliferation, 2) H2O2 generation, 3) TGF-β1 expression, and 4) reductions in PPARγ levels in HPAECs and HPASMCs. Our in vivo studies demonstrate that GKT137831 also attenuates chronic hypoxia–induced RVH, vascular remodeling, lung cell proliferation, and hypoxic alterations in lung PPARγ and TGF-β1 expression.
As the predominant Nox isoform expressed in VSMCs and ECs, Nox4 constitutes an attractive target for PH therapy. However, until recently, specific pharmacological inhibitors of NADPH oxidase were not available. For example, diphenyleneiodonium is commonly used as a synthetic NADPH oxidase inhibitor (34). However, diphenyleneiodonium nonspecifically inhibits the action of multiple flavoprotein dehydrogenases (35). Apocynin, another commonly used NADPH oxidase inhibitor, inhibits NADPH oxidases by interfering with p47 and assembly of the activated enzyme complex (36). Apocynin also functions as an antioxidant in vascular ECs and SMCs, cells that lack the myeloperoxidase activity necessary for apocynin activation (37). Recently, pyrazolopyridine derivatives have been reported to inhibit NADPH oxidases with a high potency and specific affinity for Nox1 and Nox4 isoforms (38, 39). The pyrazolopyridine derivative GKT137831 is a first-in-class, potent, and orally bioavailable Nox4 and Nox1 inhibitor (21). GKT137831 was used in the current study for its ability to specifically inhibit the Nox4 isoform, which has been shown to be selectively up-regulated in hypoxia (10) and associated with hypoxia-induced HPASMC proliferation (13, 24), vascular remodeling, and PH (10, 12).
The current study indicates that Nox4 can be targeted to modulate hypoxic proliferation of pulmonary vascular wall cells. The findings illustrated in Figures 1, 2, and 3 demonstrate that attenuating hypoxia-induced H2O2 with Nox4 siRNA, GKT137831, or PEG-CAT consistently attenuates proliferation, emphasizing the important role of Nox4-derived H2O2 in pulmonary vascular wall cells in response to hypoxia. Nox4-derived ROS contribute to cellular proliferation through well established pathways involving activation of upstream kinases (e.g., c-SRC, ERK 1/2, MAPK, Akt), increased expression and activity of growth promoting transcription factors (e.g., c-fos, c-jun, c-myc), and inactivation of protein tyrosine phosphatases (40, 41). As indicated in the online supplement, studies to detect apoptosis or necrosis in hypoxia-exposed HPAECs or HPASMCs showed that neither hypoxia nor treatment with GKT137831 significantly altered the fate of pulmonary vascular wall cells under these conditions.
The current findings and previous reports indicate that PPARγ is an important target of Nox4-derived H2O2 and also a critical upstream regulator of Nox4 expression. For example, the current study and others have demonstrated that hypoxia significantly reduces PPARγ expression in the lung (17) and in vascular wall cells (12). Coupled with evidence that hypoxia selectively up-regulates Nox4 in the lung (10), that Nox4 generates H2O2 (26, 27), and that H2O2 reduces PPARγ expression in endothelial cells (42), these findings indicate that Nox4-derived ROS mediate reductions in PPARγ during hypoxia. Consistent with this postulate, GKT137831 attenuates hypoxia-induced reductions in PPARγ expression and HPAEC and HPASMC proliferation in vitro in the current study. Loss of PPARγ is associated with SMC proliferation, migration, and vascular remodeling (43). The mechanisms by which Nox4 reduces PPARγ may relate to H2O2–mediated activation of the transcription factor AP1, which negatively regulates PPARγ (42). Furthermore, pulmonary vascular tissue from patients with IPAH demonstrates reduced PPARγ (15) and increased Nox4 expression (10). The current study extends these findings by demonstrating that PPARγ mRNA levels were decreased and Nox4 mRNA levels were increased in pulmonary artery endothelial cells isolated from patients with IPAH when compared with cells from control patients (Figure 4). To our knowledge, these findings are the first to demonstrate simultaneous derangements in the expression of Nox4 and PPARγ in the same pulmonary vascular cell compartment in IPAH. These results provide additional evidence for the relevance of hypoxia-induced alterations in PPARγ and Nox4 to the pathobiology of IPAH.
Several lines of evidence suggest that loss of PPARγ expression causes significant alterations in pulmonary vascular function. Targeted deletion of PPARγ in endothelial (44) or smooth muscle cells (45) is associated with the spontaneous development of PH. In the current study, chronic hypoxia exposure reduced PPARγ expression in the mouse lung in vivo, and these reductions in PPARγ levels were inhibited by GKT137831 administration (Figure 7A). Furthermore, GKT137831, rosiglitazone, or PEG-CAT attenuated hypoxic increases in TGF-β1 levels (Figures 7B and 7C). By attenuating alterations in the expression of targets downstream of Nox4-derived ROS, such as PPARγ or TGF-β1, Nox4 inhibition with GKT137831 provides a novel strategy attenuating proliferative responses in pulmonary vascular wall cells in PH.
On the other hand, mounting evidence indicates that PPARγ plays an important upstream role in the regulation of Nox4 expression. We demonstrated that treatment with the synthetic PPARγ ligand rosiglitazone attenuates hypoxic PH, RVH, pulmonary vascular remodeling, and increases in Nox4 expression in the hypoxic mouse model of PH (12). Others have reported similar therapeutic effects of PPARγ ligands in other models of PH (12, 17, 18, 46, 47). The molecular mechanisms underlying PPARγ-induced suppression of Nox4 derive in part from the ability of PPARγ to inhibit NF-κB binding to and activation of the Nox4 promoter (13). Collectively, these findings demonstrate that PPARγ not only regulates the upstream transcriptional expression of Nox4 but that Nox4-derived H2O2 also regulates PPARγ levels and function. These relationships between PPARγ and Nox4 are illustrated schematically in the online supplement (Figure E3).
Because the PPARγ ligand rosiglitazone was previously shown to attenuate hypoxic increases in lung Nox4 expression and activity and to reduce RVH, PH, and vascular remodeling (12), we used a rosiglitazone arm in the current study for comparative purposes. Inhibiting Nox4 with GKT137831 attenuated chronic hypoxia–induced RVH but not increases in RVSP (Figures 5A and 5B). These results were surprising because we had anticipated that the attenuation of RVH would be mediated by reductions in pulmonary vascular resistance and RVSP. We considered two potential mechanisms for these seemingly discrepant observations.
First, previous studies in a rat model of hypoxia-induced PH described a similar scenario wherein a therapeutic intervention attenuated hypoxia-induced increases in RVH without lowering RVSP due to persistent rho kinase-mediated pulmonary arterial vasoconstriction (18). Our findings demonstrate that hypoxia caused pulmonary vascular remodeling with thickening of the vascular wall in vessels less than 100 μm and increased numbers of α-SMA–positive vessels. Although the regimens of GKT137831 used in our studies were sufficient to fully attenuate the observed vascular wall thickening and markers of cell proliferation (PCNA), they did not significantly attenuate the enhanced number of α-SMA–positive vessels. Taken together, our findings suggest that inhibitory effects of GKT137831 on Nox4-mediated ROS may be sufficient to overcome proliferative signals that contribute to vascular remodeling and RVH but insufficient to counteract integrated redox sensitive signaling pathways that promote sustained hypoxic vasoconstriction.
A second potential mechanism for the observed GKT137831-mediated reductions in hypoxic increases in RVH but not RVSP could be attributable to direct effects of GKT137831 on the right ventricle. ROS contribute to the development of cardiac hypertrophy, pathogenic ventricular fibrosis, and remodeling (48). For example, Nox4 was an important mediator of cardiac hypertrophy induced by aortic constriction in an experimental pressure overload model (49). However, little is known about the role of Nox4 in RV dysfunction. Our findings suggest that GKT137831 may inhibit potential hypertrophy-inducing effects of Nox-mediated ROS in the right ventricle. The potential role of Nox4 in right ventricular dysfunction in PH remains an area of active investigation in our lab.
Like PPARγ, our results indicate that TGF-β1 is both upstream and downstream of Nox4 signaling. GKT137831 or PEG-CAT inhibited hypoxic increases in TGF-β1 expression, indicating a role for Nox4-derived ROS in hypoxic TGF-β1 induction. On the other hand, TGF-β1 is an upstream mediator that stimulates Nox4 expression and promotes HPASMC proliferation in normoxic (29) and hypoxic (24) environments. By preventing loss of PPARγ function, GKT137831 may preserve the ability of the PPARγ pathway to attenuate Nox4 expression and TGF-β1 signaling. Collectively, our results indicate that inhibiting Nox4 activity with GKT137831 can attenuate pulmonary vascular cell proliferation, vascular remodeling, and RVH in response to hypoxia by reducing alterations in PPARγ, TGF-β1, and other pathways that mediate hypoxic proliferation.
Because ROS can modulate physiologic and pathologic processes, future translational studies should evaluate for potential adverse effects of systemic Nox4 inhibition on cellular signaling, differentiation, and gene expression. GKT137831 continues to be studied experimentally in diabetic complications including nephropathy and retinopathy and in cardiovascular disorders and was granted orphan drug status by the EMA and FDA in 2010 for treatment of idiopathic pulmonary fibrosis (50). The findings in our study highlight the potential benefits of GKT137831 for attenuating proliferative pathways in PH and support the continued investigation of its use as an orally bioavailable drug for the treatment of PH. Our findings also demonstrate similar patterns of altered Nox4 and PPARγ expression in experimental models and in endothelial cells isolated from patients with IPAH. These findings highlight the translational relevance of our in vitro findings and support the continued investigation of therapies targeting Nox4 as novel therapeutic approaches in PH. Future studies should define if GKT137831 treatment dosage and duration can be optimized to enhance its safety and efficacy profile in PH management.
Supplementary Material
Acknowledgments
GKT137831 was provided through a material transfer agreement from GenKyoTex (Geneva, Switzerland). Pulmonary artery endothelial cells isolated from control subjects or patients with IPAH were kindly provided by S.A.A. Comhair and S.C. Erzurum, who are supported by National Institutes of Health grants R01 HL60917 and R01 HL081064.
Footnotes
This work was supported by NHLBI T32 training grant HL076118–06 (D.E.G.) and by the Research Service of the Atlanta Veterans Affairs Medical Center and the National Institutes of Health grants R01 DK 074518 and R01 HL 102167.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0418OC on August 16, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–163 [DOI] [PubMed] [Google Scholar]
- 2.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 2010;121:2045–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fresquet F, Pourageaud F, Leblais V, Brandes RP, Savineau JP, Marthan R, Muller B. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. Br J Pharmacol 2006;148:714–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 2002;105:1429–1435 [DOI] [PubMed] [Google Scholar]
- 5.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 2006;8:691–728 [DOI] [PubMed] [Google Scholar]
- 6.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 2006;290:L2–L10 [DOI] [PubMed] [Google Scholar]
- 7.Bedard K, Krause KH. The Nox family of Ros-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007;87:245–313 [DOI] [PubMed] [Google Scholar]
- 8.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 2010;30:653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2004;24:677–683 [DOI] [PubMed] [Google Scholar]
- 10.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit Nox4 in the pulmonary vasculature. Circ Res 2007;101:258–267 [DOI] [PubMed] [Google Scholar]
- 11.Ahmad M, Kelly MR, Zhao X, Kandhi S, Wolin MS. Roles for Nox4 in the contractile response of bovine pulmonary arteries to hypoxia. Am J Physiol Heart Circ Physiol 2010;298:H1879–H1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 2010;42:482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X, Murphy TC, Nanes MS, Hart CM. PPAR gamma regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-kappa B. Am J Physiol Lung Cell Mol Physiol 2010;299:L559–L566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukunaga Y, Itoh H, Doi K, Tanaka T, Yamashita J, Chun TH, Inoue M, Masatsugu K, Sawada N, Saito T, et al. Thiazolidinediones, peroxisome proliferator-activated receptor gamma agonists, regulate endothelial cell growth and secretion of vasoactive peptides. Atherosclerosis 2001;158:113–119 [DOI] [PubMed] [Google Scholar]
- 15.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res 2003;92:1162–1169 [DOI] [PubMed] [Google Scholar]
- 16.de Dios ST, Bruemmer D, Dilley RJ, Ivey ME, Jennings GL, Law RE, Little PJ. Inhibitory activity of clinical thiazolidinedione peroxisome proliferator activating receptor-gamma ligands toward internal mammary artery, radial artery, and saphenous vein smooth muscle cell proliferation. Circulation 2003;107:2548–2550 [DOI] [PubMed] [Google Scholar]
- 17.Kim EK, Lee JH, Oh YM, Lee YS, Lee SD. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. Respirology 2010;15:659–668 [DOI] [PubMed] [Google Scholar]
- 18.Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 2007;292:L885–L897 [DOI] [PubMed] [Google Scholar]
- 19.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 2006;71:331–341 [DOI] [PubMed] [Google Scholar]
- 20.Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. Small-molecule Nox inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal 2009;11:2535–2552 [DOI] [PubMed] [Google Scholar]
- 21.Aoyama TPY-H, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, et al. Nicotinamide adenine dinucleotide phosphate oxidase (Nox) in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schroder K, Brandes RP, Devaraj S, Torok NJ. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel Nox4/Nox1 inhibitor in vivo. Free Radic Biol Med 2012;2:289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2007;293:L548–L554 [DOI] [PubMed] [Google Scholar]
- 24.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. Nox4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 2009;296:L489–L499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diebold I, Petry A, Hess J, Gorlach A. The NADPH oxidase subunit Nox4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 2010;21:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med 2009;47:1239–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 2006;18:69–82 [DOI] [PubMed] [Google Scholar]
- 28.Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P, Wilson K, Hoidal JR, Kennedy TP. Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2007;292:L1543–L1555 [DOI] [PubMed] [Google Scholar]
- 29.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, et al. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2006;290:L661–L673 [DOI] [PubMed] [Google Scholar]
- 30.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 2005;97:900–907 [DOI] [PubMed] [Google Scholar]
- 31.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPAR gamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2005;288:L1146–L1153 [DOI] [PubMed] [Google Scholar]
- 32.Nicola T, Ambalavanan N, Zhang W, James ML, Rehan V, Halloran B, Olave N, Bulger A, Oparil S, Chen YF. Hypoxia-induced inhibition of lung development is attenuated by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Am J Physiol Lung Cell Mol Physiol 2011;301:L125–L134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong K, Xing D, Li P, Aksut B, Ambalavanan N, Yang Q, Nozell SE, Oparil S, Chen YF. Hypoxia induces downregulation of PPAR{gamma} in isolated pulmonary arterial smooth muscle cells and in rat lung via transforming growth factor-beta signaling. Am J Physiol Lung Cell Mol Physiol 2011;301:L899–L907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borbely G, Szabadkai I, Horvath Z, Marko P, Varga Z, Breza N, Baska F, Vantus T, Huszar M, Geiszt M, et al. Small-molecule inhibitors of NADPH oxidase 4. J Med Chem 2010;53:6758–6762 [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 1993;290:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 1994;11:95–102 [DOI] [PubMed] [Google Scholar]
- 37.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008;51:211–217 [DOI] [PubMed] [Google Scholar]
- 38.Kim JA, Neupane GP, Lee ES, Jeong BS, Park BC, Thapa P. NADPH oxidase inhibitors: a patent review. Expert Opin Ther Pat 2011 [DOI] [PubMed] [Google Scholar]
- 39.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, et al. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 2010;53:7715–7730 [DOI] [PubMed] [Google Scholar]
- 40.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 2000;20:2175–2183 [DOI] [PubMed] [Google Scholar]
- 41.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 2003;285:R277–R297 [DOI] [PubMed] [Google Scholar]
- 42.Blanquicett C, Kang BY, Ritzenthaler JD, Jones DP, Hart CM. Oxidative stress modulates PPAR gamma in vascular endothelial cells. Free Radic Biol Med 2010;48:1618–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meredith D, Panchatcharam M, Miriyala S, Tsai YS, Morris AJ, Maeda N, Stouffer GA, Smyth SS. Dominant-negative loss of PPAR gamma function enhances smooth muscle cell proliferation, migration, and vascular remodeling. Arterioscler Thromb Vasc Biol 2009;29:465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-gamma in mice causes PDGF receptor-beta-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol 2009;297:L1082–L1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, et al. An antiproliferative BMP-2/PPARgamma/ApoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 2008;118:1846–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuda Y, Hoshikawa Y, Ameshima S, Suzuki S, Okada Y, Tabata T, Sugawara T, Matsumura Y, Kondo T. Effects of peroxisome proliferator-activated receptor gamma ligands on monocrotaline-induced pulmonary hypertension in rats (in Japanese). Nihon Kokyuki Gakkai Zasshi 2005;43:283–288 [PubMed] [Google Scholar]
- 47.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation 2007;115:1275–1284 [DOI] [PubMed] [Google Scholar]
- 48.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 2007;93:903–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II–induced cardiac hypertrophy. Circ Res 2003;93:802–805 [DOI] [PubMed] [Google Scholar]
- 50.GenKyoTex N. GKT137831 granted orphan drug status for idiopathic pulmonary fibrosis by the EC (EMEA). 2010. [accessed July 17, 2011]. Available from: http://www.genkyotex.com/index.php?rubID=7.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.