Abstract
Glucose-6-phosphate dehydrogenase (G6PD) catalyzes the rate-determining step in the pentose phosphate pathway and produces NADPH to fuel glutathione recycling. G6PD deficiency is the most common enzyme deficiency in humans and affects over 400 million people worldwide; however, its impact on cardiovascular disease is poorly understood. The glutathione pathway is paramount to antioxidant defense, and G6PD-deficient cells do not cope well with oxidative damage. Limited clinical evidence indicates that G6PD deficiency may be associated with hypertension. However, there are also data to support a protective role of G6PD deficiency in decreasing the risk of heart disease and cardiovascular-associated deaths, perhaps through a decrease in cholesterol synthesis. Studies in G6PD-deficient (G6PDX) mice are mixed and provide evidence for both protective and deleterious effects. G6PD deficiency may provide a protective effect through decreasing cholesterol synthesis, superoxide production, and reductive stress. However, recent studies indicate that G6PDX mice are moderately more susceptible to ventricular dilation in response to myocardial infarction or pressure overload-induced heart failure. Furthermore, G6PDX hearts do not recover as well as nondeficient mice when faced with ischemia-reperfusion injury, and G6PDX mice are susceptible to the development of age-associated cardiac hypertrophy. Overall, the limited available data indicate a complex interplay in which adverse effects of G6PD deficiency may outweigh potential protective effects in the face of cardiac stress. Definitive clinical studies in large populations are needed to determine the effects of G6PD deficiency on the development of cardiovascular disease and subsequent outcomes.
Keywords: G6PD, heart failure, NADPH, ROS, oxidative stress
this article is part of a collection on Nutrients and Cardiovascular Health and Disease: Glucose, Fatty Acids, and Beyond. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
Glucose-6-phosphate dehydrogenase (G6PD) catalyzes the conversion of G6P to 6-phosphogluconolactone and the formation of NADPH from NADP+. G6PD activity is a key determinant of the NADPH-to-NADP+ ratio in the cytoplasm and thus contributes to replenishment of the antioxidant glutathione system. In addition, by influencing the concentration of NADPH, G6PD activity can influence NADPH-dependent superoxide production (3, 83, 107, 110). G6PD deficiency is the most common enzyme deficiency in the world, with an estimated >400 million people with G6PD deficiency (12, 59, 109a). G6PD deficiency is common throughout sub-Saharan Africa, regions in the Mediterranean, and parts of Southeast Asia and is thought to be preserved in these populations because it results in few gross complications and it confers a selective advantage against malaria (59). Here we will review the biochemical and physiological effects of G6PD deficiency on the heart and discuss the potential impact on the development and progression of heart disease. Furthermore, we will address recent evidence suggesting adverse effects of G6PD deficiency on heart failure.
G6PD Deficiency
G6PD deficiency is caused by a diverse array of mutations in the g6pdx gene (82, 103, 109a), which is X-linked, and thus G6PD deficiency is most common in males. Common mutant gene variants produce a defective enzyme that is rapidly degraded, resulting in a decrease in the amount of G6PD and in its overall enzymatic activity (69, 72). The World Health Organization has divided the deficiency by varying degrees: class I is very severe deficiency (<1% of normal G6PD activity), class II is severe deficiency (1–10% of normal activity), class III is moderate deficiency (10–60% of normal activity), class IV is normal activity (60–150% activity), and class V is increased activity (>150% of normal activity) (12, 109a). The most common deficient alleles (A− and Med) result in moderate deficiency (class III). G6PD A− is largely found in African populations and results in a residual G6PD activity of ∼20% (6). Between 0.5 and 2.9% of Americans are G6PD deficient (12). Most Americans that have G6PD deficiency are of African descent, and ∼10% of black Americans are G6PD deficient (12, 57). Most deficient individuals are moderately deficient (class II and III), and have few problems (6, 7, 58), though very severe deficiency (class I) results in chronic hemolytic anemia (6, 7). The most common complications are neonatal jaundice and acute hemolytic anemia in response to oxidizing stimuli such as fava bean consumption, microbial infection, or specific medications (6, 12). The avoidance of these stimuli and vaccination against common pathogens generally prevent anemia in most deficient patients. If severe hemolysis occurs, a blood transfusion is generally sufficient to alleviate the response. In the case of neonatal jaundice, most hospitals are equipped to administer phototherapy to alleviate this condition (96). Thus, for most patients, G6PD deficiency manifests only as a minor episodic treatable condition, and the majority of G6PD-deficient patients live normal lives without any major complications.
G6PD Biochemistry and Cell Biology
G6PD is a cytoplasmic enzyme that controls the entry of G6P into the pentose phosphate pathway (Fig. 1). The pentose phosphate pathway is characterized by the following reactions: 1) G6PD oxidizes G6P to 6-phosphogluconolactone; 2) lactonase hydrolyzes circular 6-phosphogluconolactone to form a linear product, 6-phosphogluconate; and 3) 6-phosphogluconate dehydrogenase (6PGD) converts 6-phosphogluconate to ribulose-5-phosphate. The reaction catalyzed by 6PGD cleaves the 1° carbon from 6-phosphogluconate to release CO2. These three reactions are known as the oxidative phase of the pathway. G6PD and 6PGD both reduce NADP+ to NADPH, but the reaction catalyzed by G6PD is a rate-determining step of the pentose phosphate pathway, and decreasing the activity of G6PD lowers NADPH levels (27, 31, 32, 35, 44). In the nonoxidative phase, ribulose-5-phosphate may be used for nucleotide synthesis or aromatic amino acid synthesis or may be converted to fructose-6-phosphate and glyceraldehydes-3-phosphate through a series of aldolases and transketolases that reenter the pathway or are oxidized as fuel. It is important to note that there is also NADPH in the mitochondria which is maintained by isocitrate dehydrogenase, glutamate dehydrogenase, and malic enzyme and that isocitrate dehydrogenase has a cytoplasmic isoform that produces NADPH; however, G6PD is the major producer of cytoplasmic NADPH (27, 44).
Fig. 1.
The pentose phosphate pathway. The oxidative phase of the pentose phosphate pathway produces NADPH from NADP+ and ribulose-5-phosphate. Ribulose-5-phosphate may be converted to glyceraldehydes-3-phosphate and fructose-6-phosphate through a series of nonoxidative reactions. 6PGD, 6-phosphogluconate dehydrogenase; G6PD, glucose-6-phosphate dehydrogenase.
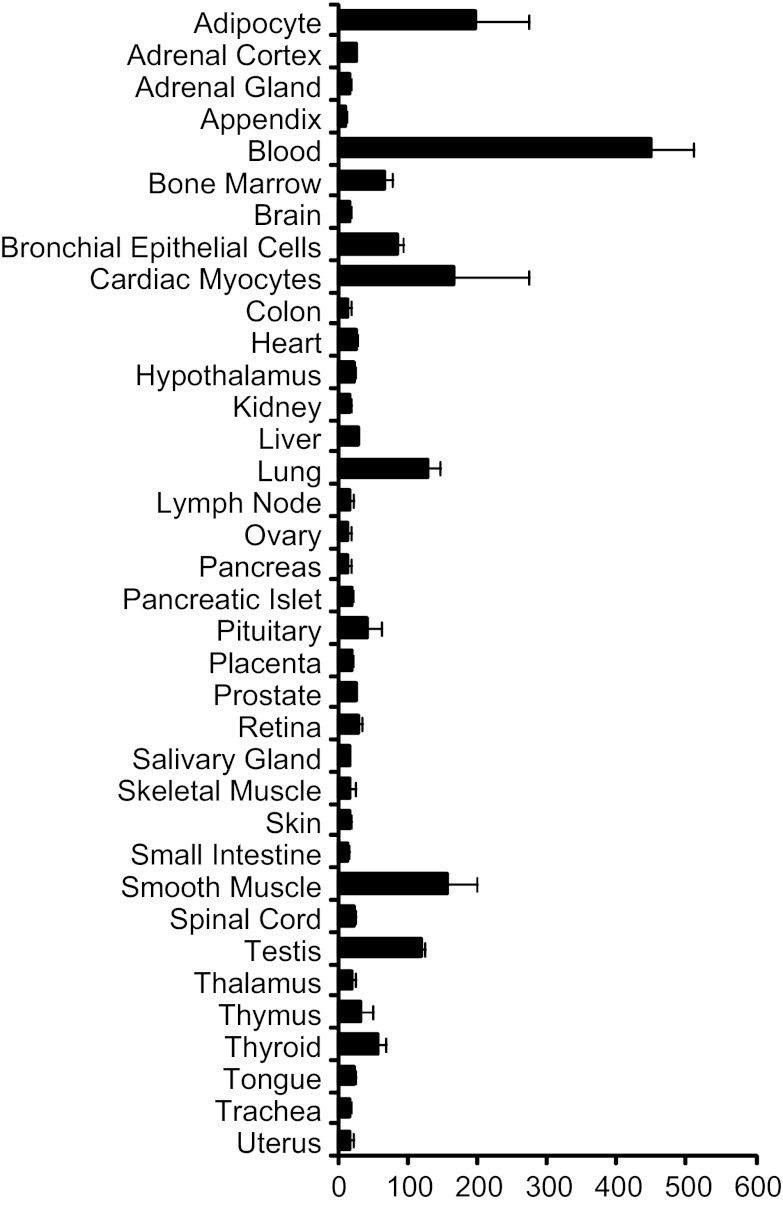
NADPH from G6PD is required by cellular antioxidant systems to reduce reactive oxygen species (ROS) (19, 21, 23, 24, 44, 45, 101). The glutathione system requires NADPH to remove excess hydrogen peroxide (H2O2) (Fig. 2). In this system, glutathione reductase uses NADPH to convert oxidized glutathione (GSSG) to its reduced form (GSH). GSH is then converted back to GSSG by glutathione peroxidase to reduce H2O2 to water. Thus NADPH fuels the removal of H2O2 by the glutathione system (65). This system is generally considered to be beneficial because excessive ROS would likely adversely affect cell function through damaging reactions with proteins, nucleotides, and lipids (99, 107). Because of the requirement for NADPH in the reduction of ROS, G6PD-deficient cells are sensitive to oxidizing stimuli and more easily succumb to oxidative stress than nondeficient cells (23, 24, 104), whereas the overexpression of G6PD protects cells against oxidative damage (91). This increased susceptibility for cell death in G6PD-deficient cells can lead to adverse physiological effects under conditions of increased oxidative stress in tissues where the specific cell type is indispensable (44, 45, 48, 75, 101, 105, 111, 115). In particular, cell types that normally have low G6PD expression in nondeficient individuals may be likely to succumb when faced with oxidant stress in deficient individuals where the G6PD expression is even lower than normal (39, 115) (Fig. 3).
Fig. 2.
Role of G6PD in both antioxidant and oxidant formation pathways. The pentose phosphate pathway produces NADPH through G6PD and 6PGD. NADPH supports the antioxidant glutathione pathway in which glutathione reductase (GR) uses NADPH to reduce oxidized glutathione (GSSG) to reduced glutathione (GSH) for use by glutathione peroxidase (GPx) to reduce H2O2 to H2O. On the other hand, NADPH is also used to produce superoxide (O2·−) via NADPH oxidase, uncoupled nitric oxide synthase, and xanthine oxidase. O2·− is converted to H2O2 by O2·− dismutase (SOD), and the overproduction of these reactive oxygen species may adversely affect cell function.
Fig. 3.
G6PD expression in various human tissues. Graphical microarray mRNA expression data were obtained from biogps.org using the GeneAtlas U133A, gcrma data set from Su et al. (102a).
The short-term effects of decreased G6PD activity have been examined in cardiomyocytes (44). In nondeficient cells, H2O2 stimulates G6PD activity and short-term pharmacological inhibition of G6PD in the absence of H2O2 decreased GSH/GSSG and increased ROS. The increase in ROS resulted in contractile dysfunction, as indicated by decreased cell shortening and prolonged relengthening. These functional impairments corresponded with impaired Ca2+ transport and were rescued by antioxidant treatment, but not by ribose supplementation, indicating that they were likely due to a decrease in the capacity of the antioxidant glutathione system.
It is important to note that the nonoxidative phase is critical for ribose synthesis and the subsequent production of nucleotides, and this could potentially impact the G6PD-deficient cell. This is particularly important under conditions of rapid growth, such as cancerous cells where extra ribose may be needed to produce DNA for replication (10); however, this topic is outside the focus of this review. The effects of ribose supplementation in the G6PD-deficient state have been investigated (44, 45, 105) and found that it did not impact the physiology or pathophysiology of G6PD deficiency in the heart (44, 45). On the other hand, the NADPH produced by G6PD is critical both for protecting the cell against oxidative damage and for cell growth (104, 105).
G6PD Deficiency in Human Cardiovascular Disease
Despite its known role in oxidant defense, there is a surprisingly limited amount of clinical information about G6PD deficiency in the cardiovascular system. G6PD deficiency may increase diabetes and renal failure. This is indicated by increased serum creatinine and an increased risk for diabetes in G6PD-deficient subjects (28, 74, 90, 108, 109) and albuminuria and metabolic dysfunction in deficient animals (40, 111, 115), which could have adverse cardiac effects. G6PD is upregulated in response to a number of stress-induced stimuli, including heart failure, (9, 32, 35, 38, 40, 44, 45, 101, 102), and deficient individuals may be unable to upregulate G6PD in response to stress (19–21, 68). An increase in the activity of G6PD is generally thought of as being protective because it should increase the capacity of antioxidant defense systems. G6PD activity was increased in postinfarct surviving myocardium and in failing myocardium in both human and animal models of disease (9, 32, 35, 38, 39, 45). This could be a compensatory mechanism necessary to oppose the increased generation of ROS in failing myocardium (9).
In 1967, cardiovascular parameters in association with G6PD deficiency were examined among 1,473 black American men (57). This study found a higher incidence of hypertension and idiopathic cardiomyopathy among those with G6PD deficiency. However, this study also found evidence of decreased coronary artery disease among G6PD-deficient patients compared with a normal population. Similar evidence has been observed more recently in two small studies that suggest G6PD deficiency may decrease the risk of coronary heart disease and cardiovascular associated death (15, 67). The first study reported on the mortality in 1,756 G6PD-deficient men in Sardinia (15) and found that during a 5-yr follow-up period, 29 men with G6PD deficiency died of cardiovascular-associated death, whereas 62.6 cardiovascular deaths were expected based on population data. This suggests that G6PD deficiency may decrease cardiovascular-associated death. The second study was a case-control study that reported that among 314 cases of Sardinian men with coronary artery disease, 11.8% were G6PD deficient, whereas among 424 controls, 18.6% were G6PD deficient (67). These findings suggest that G6PD deficiency protects against coronary heart disease. Thus, despite the role of G6PD in protecting against oxidative damage in cell and tissue based studies, limited population studies do not support an adverse role for G6PD deficiency in human cardiovascular disease, although findings from these studies are not definitive as they cannot exclude a survivor benefit.
Effects of Deficient G6PD Activity in Experimental Systems
Animal model of G6PD deficiency.
Complete genetic deletion of G6PD produces embryonic lethality (58). However, the generation of G6PD-deficient (G6PDX) mice that recapitulate key aspects of clinical deficiency was reported in 1988 (84). This was done by treating male mice with a DNA ethylating agent 1-ethyl-1-nitrosourea to induce DNA mutations, and G6PD deficiency was identified in subsequent offspring. G6PDX mice have an A:T mutation in the 5′-untranslated region at the splice site of the 3′-end of exon 1 (93). This mutation results in decreased translation of G6PD and leads to ∼20–40% residual G6PD activity (class III deficiency) in G6PDX mice compared with wild-type (WT) littermate control mice. G6PDX mice mature normally but have been reported to develop modest cardiac hypertrophy at 9 mo of age (44). Overall, G6PDX mice provide a clinically relevant model of G6PD deficiency that reflects the extent of limited G6PD activity commonly found in human patients with G6PD deficiency (6, 12).
Statin-like effect.
A protective cardiovascular role for G6PD deficiency is conceivable in light of the effects of G6PD deficiency on cholesterol synthesis (71). The enzyme 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase is rate determining in the cholesterol synthesis pathway and is dependent on NADPH to catalyze its reaction. Decreased cholesterol synthesis was found in peripheral lymphomononuclear cells obtained from G6PD-deficient subjects (5), and evidence suggests that G6PD deficiency may decrease the overall cholesterol content in the blood of deficient subjects (71). We also found evidence for decreased cholesterol synthesis in G6PDX mice (88). Furthermore, in an apolipoprotein E-deficient mouse model of atherosclerosis, G6PD deficiency decreased cholesterol and the development of vascular lesions (63). Thus G6PD deficiency may provide a statin-like cholesterol-lowering effect and thereby protect against atherosclerosis (57, 70, 71, 94).
Reductive stress.
In addition to decreasing cholesterol synthesis, G6PD deficiency may be protective under conditions of reductive stress (85). Reductive stress refers to an increase in NADPH and GSH and may result in adverse cellular events such as protein misfolding and aggregation, mitochondrial dysfunction, pathological gene expression, and increased susceptibility to apoptosis (11, 14, 60, 98, 106, 112, 114). Evidence for a role of reductive stress in heart failure was found in a mouse model of desmin-related cardiomyopathy induced by overexpression of mutant human αB-crystallin (hR120GCryAB) in mice (CryAB mice) (85–87). This cardiomyopathy model results in protein aggregation within the cell, hypertrophy, and failure of the ventricle (29, 64, 85). Support for the role of reductive stress in the cardiomyopathy observed in CryAB mice was provided by the observation that G6PD and glutathione reductase activities were increased with a concomitant increase in GSH and the GSH-to-GSSG ratio (GSH/GSSG) (85). An increase in GSH contrasts with the typically reported decrease in GSH/GSSG that is associated with oxidative stress (86). When CryAB mice were crossed with G6PDX mice, there was a decrease in protein aggregation and cardiac hypertrophy, thus supporting a protective role for G6PD deficiency in reducing reductive stress in protein aggregation-associated cardiomyopathy (85). Overall, the data suggest that decreasing G6PD activity may alleviate reductive stress (17).
G6PD fuels superoxide production.
Although G6PD supplies the antioxidant glutathione system with NADPH, the NADPH produced by G6PD is also used to produce superoxide via NADPH oxidase (Nox), uncoupled nitric oxide synthase, and xanthine oxidase (Fig. 2) (3, 83, 107, 110). These reactions are all implicated in heart failure, and decreasing the production of ROS by these enzymes may exert beneficial effects on cardiac hypertrophy and dysfunction (18, 37, 41, 42, 49, 110). An upregulation of G6PD expression may fuel these superoxide-producing enzymes in failing myocardium (32, 35). Specifically, G6PD expression and activity were increased in failing myocardium in dogs and humans (Fig. 4) (32, 35). The increase in G6PD corresponded with an increase in NADPH levels and superoxide production, and pharmacological inhibition of G6PD in vitro decreases NADPH levels and ROS production.
Fig. 4.
G6PD inhibition decreases NADPH levels and O2·− production in failing myocardium. Myocardium was obtained from failing and nonfailing patients undergoing cardiac surgery. NADPH levels and O2·− production were assessed in myocardial homogenates in the presence or absence of the G6PD inhibitor 6-aminonicotinamide. *P < 0.05 vs. normal; #P < 0.04 vs. heart failure. Reprinted with permission from Gupte et al. (32) with modifications.
Similar evidence for a role of G6PD in deriving NADPH-dependent ROS was observed in a number of other experimental models with different cell and tissue types (2, 36, 51, 78–80). These include a decrease in aortic superoxide production in apolipoprotein E-deficient atherosclerotic mice that are crossed with G6PDX mice (63). G6PD deficiency also decreased aortic dihydroethidium and nitrotyrosine and lowered the hypertensive response to angiotensin-II infusion (62). Superoxide production is increased in the aorta, heart, and liver in genetically obese Zucker rats, and in vitro pharmacological inhibition of G6PD decreased the generation of superoxide in these tissues (31, 97). Recently, G6PD was reported to decrease superoxide production by Nox4 in isolated liver nuclei (100). Overall, the production of superoxide may be increased when G6PD activity is increased or decreased when there is limited G6PD activity.
Suppression of myocardial ROS generation in response to pharmacological G6PD inhibition suggests that inhibiting G6PD may also decrease the generation of ROS in vivo (32, 35) and thus suggests a potential therapeutic approach for treating heart failure patients (33, 34). Agents that inhibit G6PD include 6-aminonicotinamide and dehydroepiandrosterone (DHEA). 6-Aminonicotinamide is not amenable to in vivo use due to toxic side effects. DHEA is a naturally occurring hormone with pleiotropic effects such as an inhibition of growth that may be unrelated to the inhibition of G6PD (8, 33, 73, 81). Although DHEA inhibits G6PD activity, it may also increase the transcription of G6PD that confounds its inhibitory effect (35, 43, 73) and makes it impossible to attribute and observed effects to G6PD inhibition. Taken together, current pharmacological inhibitors of G6PD are inadequate for evaluating the long-term cardiovascular effects of G6PD inhibition.
It is important to note that most of the studies that showed decreased superoxide production in response to G6PD inhibition did not examine the balance between decreased superoxide production and GSH capacity. This is important because although G6PD deficiency could be beneficial if it decreases excessive superoxide production (18, 37, 41, 42, 49, 110), G6PD deficiency could still have adverse cardiac effects if it lowers antioxidant defenses (39, 44, 45). Furthermore, it should be kept in mind that an increase in superoxide production does not always adversely affect cardiac function (38, 66). In fact, superoxide is a signaling molecule that is necessary for vascular function, and Nox4 deficiency, which decreases superoxide levels, decreased angiogenesis and accelerated the development of heart failure (1, 26, 77, 95, 113). In light of the recent finding that Nox4-derived superoxide is necessary for vascular growth (89, 95, 113), others found that G6PD may fuel Nox4-derived superoxide production (100). Furthermore, we found decreased superoxide and capillary density in failing G6PD-deficient myocardium (39). Overall, the finding that limiting G6PD activity also decreases superoxide production does not necessarily mean that this is a beneficial effect of G6PD deficiency.
G6PD deficiency and cardiac ischemia-reperfusion.
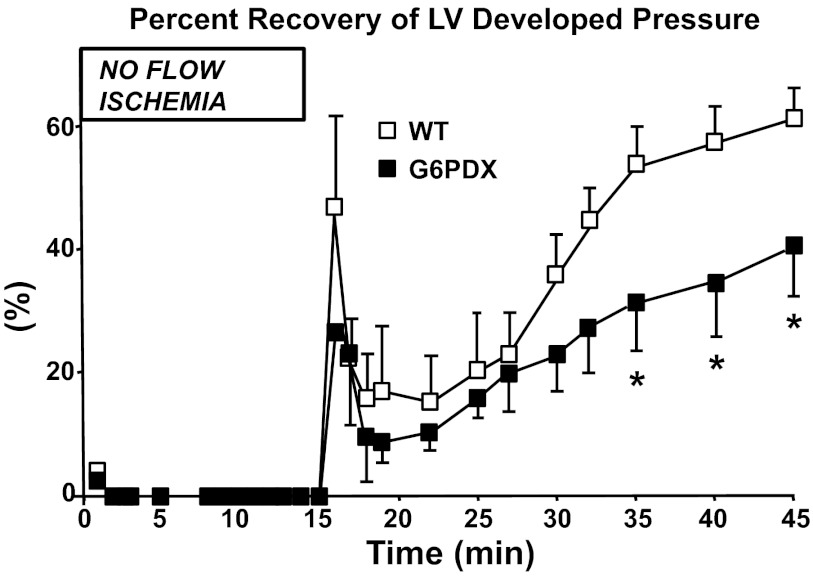
Because G6PD deficiency decreases myocardial antioxidant capacity, it may exacerbate the adverse cardiac effects of acute oxidative stress such as with ischemia-reperfusion injury (92). Isolated perfused hearts from G6PDX mice displayed a greater impairment in relaxation and pressure development after injury compared with WT mice (Fig. 5) (45). In these experiments, acute ischemia-reperfusion injury increased G6PD activity in WT mice, whereas G6PD-deficient myocardium was unable to compensate for the increased oxidative stress as indicated by a decreased GSH/GSSG ratio in these hearts (45). The exacerbated impairments in relaxation and pressure development in G6PDX mice were rescued by antioxidant treatment, but not by ribose supplementation, demonstrating that the deficit was due to a deficiency in antioxidant defenses rather than a ribose deficiency resultant from the nonoxidative phase of the pentose phosphate pathway. Thus G6PD deficiency increases the sensitivity of the myocardium for ROS and exacerbates contractile dysfunction in the face of oxidative injury.
Fig. 5.
G6PD deficiency adversely affects the outcome of ischemia-reperfusion injury. G6PD-deficient (G6PDX) mouse hearts have a decreased capacity to recover from acute ischemia-reperfusion injury. Isolated mouse hearts were perfused retrograde using Langendorff system, where isovolumic left ventricular (LV) pressure was measured at baseline and with 15 min of no-flow global ischemia followed by 30 min of reperfusion. WT, wild-type. *P < 0.05 vs. WT. Reprinted with permission from Jain et al. (45).
Heart failure and G6PD deficiency.
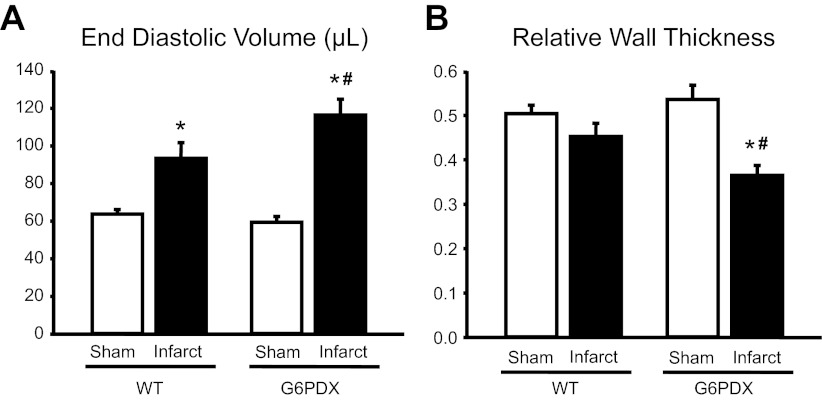
We recently tested the effects of G6PD deficiency on the development of heart failure and found that G6PD deficiency exacerbated cardiac remodeling after chronic stress (39). G6PDX mice were subjected to 6 wk of pressure overload or to myocardial infarction with 12 wk of follow-up. In these experiments, G6PD deficiency worsened left ventricular dilation (Fig. 6) without affecting the overall heart mass or diastolic or systolic function. We further stressed pressure-overloaded mice with a high fructose diet and found that in this case, G6PD deficiency increased systolic dysfunction, fetal gene expression, and hypertrophy among high fructose-fed mice. Thus G6PD deficiency adversely affected the development of left ventricular chamber expansion and heart failure.
Fig. 6.
Adverse effects of G6PD deficiency on heart failure. Sixteen week-old male mice underwent sham surgery or permanent coronary occlusion to induce myocardial infarction. Cardiac function was assessed at 11 wk by echocardiography. G6PDX mice developed greater LV chamber expansion (A) and dilation (B) in response to myocardial infarction. *P < 0.05 vs. respective sham; #P < 0.05 vs. WT infarct. Reprinted from Hecker et al. (39).
Interestingly, in these studies NADPH levels were increased in G6PD-deficient myocardium in response to either infarction or pressure overload (39), suggesting that there is a compensatory mechanism that increases NADPH levels in G6PD deficiency. This compensatory increase in NADPH levels could be due to an increase in mitochondrial NADPH-producing enzymes and may not affect the cytoplasmic pool of NADPH levels. In this regard, superoxide production was decreased in G6PDX mice, possibly due to decreased cytoplasmic availability of NADPH for use by Nox. However, despite the compensatory increase in NADPH levels and the decrease in superoxide production, G6PD-deficient myocardium had increased oxidative stress in response to pressure overload as indicated by a decrease in GSH, indicating an adverse effect of G6PD deficiency.
In the myocardial infarction model of heart failure described above, there was no effect of G6PD deficiency on superoxide production, but there was an increase in lipid peroxidation products in G6PDX mice, indicating increased oxidative stress in this heart failure model as well (39). A possible explanation for the increased oxidative stress despite there being an increase in NADPH levels and no overall change in superoxide generation in this case is that G6PD-deficient myocardium are unable to fully alleviate oxidative stress. Here it should be considered that the measured level of NADPH does not necessarily reflect the flux through G6PD or the rate of production of NADPH but simply reflects the concentration of NADPH at equilibrium. Also, measures of G6PD activity are generally reflective only of the amount of enzyme, rather than the activity within the cell, because at physiological concentrations of NADPH, G6PD is almost completely inhibited (22). Thus activation of G6PD is a matter of de-repression of the enzyme. In the 1970s, Eggleston and Krebs found that GSSG de-represses G6PD through the activation of some cofactor (22, 76). Although their data have been challenged, the activation of G6PD by GSSG remains an intriguing possibility (56). If GSSG activates G6PD, then it may take high levels of GSSG to sufficiently activate G6PD to counterbalance oxidative stress in G6PD-deficient myocardium. This would result in greater oxidative stress as observed by a decreased GSH/GSSG ratio, but not necessarily an increase in the production of ROS. This change in the levels of GSH/GSSG (and NADPH) may result in aberrant signaling and the adverse changes that we observed with G6PD deficiency with heart failure (11, 14, 16, 60, 85, 106).
Beneficial effects of pharmacologic G6PD activation in heart failure.
Benfotiamine is a vitamin B1 analog that activates the pentose phosphate pathway by increasing the activity of G6PD and transketolase (46). A recent investigation in a mouse model of myocardial infarction found administration of benfotiamine for 4 wk before coronary ligation increased G6PD activity; decreased infarct size and oxidative stress; and improved survival, capillary density, blood flow, and functional parameters in the heart (46). Other studies found benfotiamine reduced diastolic dysfunction associated with diabetes (13, 46, 47). In cardiomyocytes exposed to hypoxia, silencing of G6PD prevented antiapoptotic signaling in response to benfotiamine, indicating that the beneficial effects of benfotiamine on cardiomyocytes may be due to increased activation of G6PD. Overall, these studies show that the activation of G6PD may be beneficial in heart failure.
G6PD deficiency and vascular dysfunction.
The pro- and antioxidant effects of G6PD also play an important role in vascular redox homeostasis and vascular reactivity. In the vascular endothelium, G6PD deficiency increases oxidant stress and decreases bioavailable nitric oxide (52). In vitro studies in human coronary artery endothelial cells and in vivo studies in G6PDX mice have shown that this occurs as a result of uncoupling of endothelial nitric oxide synthase (eNOS) to increase ROS formation and is associated with a decrease in GSH levels and the GSH/GSSG ratio (52, 53). G6PD deficiency is also associated with a decrease in nitric oxide levels owing to increased consumption by ROS as well as a decrease in eNOS activity owing to limited NADPH stores (52). In contrast, overexpression of G6PD limits endothelial oxidant stress and increases nitric oxide levels (55). With the use of intravital videomicroscopy, G6PDX mice were shown to have an impaired vasodilator response to the endothelium-dependent vasodilator acetylcholine (53). These findings were supported in a small study of G6PD-deficient individuals that examined endothelial function using brachial artery vascular reactivity. When compared with age-matched controls, G6PD-deficient subjects demonstrated impaired forearm blood flow responses, and this was associated with increased levels of 8-isoprostanes, a marker of systemic oxidant stress (25).
Elevated levels of aldosterone, which is a steroid hormone that is structurally similar to DHEA, decrease endothelial G6PD activity leading to endothelial dysfunction (53). This acquired G6PD-deficient state is associated with eNOS uncoupling and eNOS-derived ROS formation, decreased GSH levels and GSH/GSSG ratio, and an increase in peroxynitrite formation. Aldosterone decreased endothelium-dependent vasodilation in WT mice but had no further effect on G6PDX mice and G6PD activity, and vasodilator responses were restored with vascular gene transfer of G6PD (53). The increase in ROS associated with this acquired G6PD-deficient state was also found to inhibit cGMP formation through a mechanism that involved oxidative posttranslational modification of guanylyl cyclase (61). Taken together, these findings indicate that G6PD deficiency has important consequences for nitric oxide-mediated vasodilator responses.
Vascular contraction with potassium chloride or amphotericin B leads to an increase in G6PD activity that occurs in a protein kinase Cδ-dependent manner (30). Under these conditions, inhibition of G6PD decreased ROS and contractility. Aortas from G6PDX mice were also found to have a decrease in potassium chloride-evoked force compared with WT mice (30). Further work revealed that G6PD inhibition also modulates calcium stores to promote precontracted vascular smooth muscle relaxation by decreasing calcium influx and increasing sequestration as well as inhibiting Rho kinase (2). Thus, in the contractile state, G6PD deficiency decreases oxidant stress and promotes vascular relaxation.
Clinical Implications and Future Directions
The clinical effects of G6PD deficiency on the heart remain largely unexplored despite it being the most common known enzyme deficiency in the world (12). It is important to gain further insight of G6PD deficiency in the context of human cardiovascular health and disease. Although studies suggested that G6PD deficiency may decrease superoxide production in failing myocardium and that G6PD deficiency may decrease the risk of developing coronary heart disease (67), our recent studies in mice indicate increased oxidative stress in G6PD-deficient failing myocardium and that G6PD deficiency adversely affects the development of heart failure (39).
Because G6PD deficiency increases oxidative stress and adversely affects the development of heart failure in mice (39, 45), mechanistic studies should be performed in humans to assess the effects of deficiency on indexes of ROS in heart failure patients. Failing myocardium from tissue banks and screened for G6PD deficiency may be easily identified by common alleles such as the G6PD A− allele or the Med allele. A relatively minor amount of myocardial tissue is required to assess the production of ROS and oxidative stress (32). G6PD activity and NADPH levels can also be assessed in a relatively small amount of myocardial tissue. Thus one could determine the effect of G6PD deficiency on NADPH levels, ROS production, and oxidative stress in failing human myocardium.
The effects of G6PD deficiency on the development and progression of heart failure in human patients could be explored by screening hypertensive patients for G6PD deficiency and then following these patients over an extended period to see whether G6PD deficiency affects the development of heart failure in these patients. Another study could examine G6PD-deficient patients who have already developed heart failure to determine whether G6PD deficiency positively or negatively affects prognosis. Thus the development of heart failure should be examined in G6PD-deficient patients.
Summary and Conclusions
Overall, G6PD deficiency may decrease the rate of cardiovascular disease development among humans through its effect on atherogenesis (15, 63, 67, 70). However, these conclusions come from limited data. Furthermore, it appears that in response to stress, G6PD deficiency sensitizes the myocardium to an allowance for increased levels of oxidative damage and may thus lead to worsened disease outcomes (Fig. 7) (39, 44–46, 54). More population studies in humans are needed to better elucidate the effects of G6PD deficiency on the pathophysiology of cardiovascular disease.
Fig. 7.
Effects of changes in NADPH levels. Increasing NADPH fuels superoxide production by NADPH oxidase or may contribute to reductive stress. Decreasing NADPH may limit cholesterol synthesis but also decreases antioxidant capacity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants P01-HL-074237, HL-105301, and T32-HL-072751.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.A.H. prepared figures; P.A.H., S.A.G., and W.C.S. drafted manuscript; P.A.H., S.A.G., and W.C.S. edited and revised manuscript; P.A.H., S.A.G., and W.C.S. approved final version of manuscript.
REFERENCES
- 1.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69: 2327–2343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ata H, Rawat DK, Lincoln T, Gupte SA. Mechanism of glucose-6-phosphate dehydrogenase-mediated regulation of coronary artery contractility. Am J Physiol Heart Circ Physiol 300: H2054–H2063, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babior BM. NADPH oxidase: an update. Blood 93: 1464–1476, 1999 [PubMed] [Google Scholar]
- 5.Batetta B, Bonatesta RR, Sanna F, Putzolu M, Mulas MF, Collu M, Dessi S. Cell growth and cholesterol metabolism in human glucose-6-phosphate dehydrogenase deficient lymphomononuclear cells. Cell Prolif 35: 143–154, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutler E. G6PD deficiency. Blood 84: 3613–3636, 1994 [PubMed] [Google Scholar]
- 7.Beutler E. Glucose-6-phosphate dehydrogenase deficiency: a historical perspective. Blood 111: 16–24, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Biaglow JE, Ayene IS, Koch CJ, Donahue J, Stamato TD, Tuttle SW. G6PD deficient cells and the bioreduction of disulfides: effects of DHEA, GSH depletion and phenylarsine oxide. Biochem Biophys Res Commun 273: 846–852, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Bing RJ, Gudbjarnason S, Tschopp H, Braasch W. Molecular changes in myocardial infarction in heart muscle. Ann NY Acad Sci 156: 583–593, 1969 [DOI] [PubMed] [Google Scholar]
- 10.Boros LG, Puigjaner J, Cascante M, Lee WN, Brandes JL, Bassilian S, Yusuf FI, Williams RD, Muscarella P, Melvin WS, Schirmer WJ. Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Res 57: 4242–4248, 1997 [PubMed] [Google Scholar]
- 11.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell 125: 443–451, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 371: 64–74, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Ceylan-Isik AF, Wu S, Li Q, Li SY, Ren J. High-dose benfotiamine rescues cardiomyocyte contractile dysfunction in streptozotocin-induced diabetes mellitus. J Appl Physiol 100: 150–156, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Christians ES, Benjamin IJ. Proteostasis and REDOX state in the heart. Am J Physiol Heart Circ Physiol 302: H24–H37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocco P, Todde P, Fornera S, Manca MB, Manca P, Sias AR. Mortality in a cohort of men expressing the glucose-6-phosphate dehydrogenase deficiency. Blood 91: 706–709, 1998 [PubMed] [Google Scholar]
- 16.Das DK. Redox regulation of cardiomyocyte survival and death. Antioxid Redox Signal 3: 23–37, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Dimmeler S, Zeiher AM. A “reductionist” view of cardiomyopathy. Cell 130: 401–402, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, Sorrentino S, Manes C, Schieffer B, Drexler H, Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res 100: 894–903, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Efferth T, Fabry U, Glatte P, Osieka R. Increased induction of apoptosis in mononuclear cells of a glucose-6-phosphate dehydrogenase deficient patient. J Mol Med (Berl) 73: 47–49, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Efferth T, Fabry U, Osieka R. DNA damage and apoptosis in mononuclear cells from glucose-6-phosphate dehydrogenase-deficient patients (G6PD Aachen variant) after UV irradiation. J Leukoc Biol 69: 340–342, 2001 [PubMed] [Google Scholar]
- 21.Efferth T, Schwarzl SM, Smith J, Osieka R. Role of glucose-6-phosphate dehydrogenase for oxidative stress and apoptosis. Cell Death Differ 13: 527–528, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Eggleston LV, Krebs HA. Regulation of the pentose phosphate cycle. Biochem J 138: 425–435, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fico A, Paglialunga F, Cigliano L, Abrescia P, Verde P, Martini G, Iaccarino I, Filosa S. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ 11: 823–831, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Filosa S, Fico A, Paglialunga F, Balestrieri M, Crooke A, Verde P, Abrescia P, Bautista JM, Martini G. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem J 370: 935–943, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forgione MA, Loscalzo J, Holbrook M, Scribner AW, Palmisano J, Maxwell C, Baldwin C, Vita J. The A326G (A+) variant of the glucose-6-phosphate dehydrogenase gene is associated with endothelial dysfunction in African Americans (Abstract). J Am Coll Cardiol. 41, 249A 2003. 12535818 [Google Scholar]
- 26.Frazziano G, Champion HC, Pagano PJ. NADPH oxidase-derived ROS and the regulation of pulmonary vessel tone. Am J Physiol Heart Circ Physiol 302: H2166–H2177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frederiks WM, Kummerlin IP, Bosch KS, Vreeling-Sindelarova H, Jonker A, Van Noorden CJ. NADPH production by the pentose phosphate pathway in the zona fasciculata of rat adrenal gland. J Histochem Cytochem 55: 975–980, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109: 433–438, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Guan J, Mishra S, Falk RH, Liao R. Current perspectives on cardiac amyloidosis. Am J Physiol Heart Circ Physiol 302: H544–H552, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupte RS, Ata H, Rawat D, Abe M, Taylor MS, Ochi R, Gupte SA. Glucose-6-phosphate dehydrogenase is a regulator of vascular smooth muscle contraction. Antioxid Redox Signal 14: 543–558, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupte RS, Floyd BC, Kozicky M, George S, Ungvari ZI, Neito V, Wolin MS, Gupte SA. Synergistic activation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase by Src kinase elevates superoxide in type 2 diabetic, Zucker fa/fa, rat liver. Free Radic Biol Med 47: 219–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupte RS, Vijay V, Marks B, Levine RJ, Sabbah HN, Wolin MS, Recchia FA, Gupte SA. Upregulation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase activity increases oxidative stress in failing human heart. J Card Fail 13: 497–506, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Gupte SA. Glucose-6-phosphate dehydrogenase: a novel therapeutic target in cardiovascular diseases. Curr Opin Investig Drugs 9: 993–1000, 2008 [PubMed] [Google Scholar]
- 34.Gupte SA. Targeting the pentose phosphate pathway in syndrome X-related cardiovascular complications. Drug Dev Res 71: 161–167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, Floyd BC, Ojaimi C, Bellomo M, Wolin MS, Recchia FA. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol 41: 340–349, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Han CY, Umemoto T, Omer M, Den Hartigh LJ, Chiba T, Leboeuf R, Buller CL, Sweet IR, Pennathur S, Abel ED, Chait A. NADPH oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes. J Biol Chem 287: 10379–10393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi K, Kimata H, Obata K, Matsushita A, Fukata A, Hashimoto K, Noda A, Iwase M, Koike Y, Yokota M, Nagata K. Xanthine oxidase inhibition improves left ventricular dysfunction in dilated cardiomyopathic hamsters. J Card Fail 14: 238–244, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Hecker PA, Galvao TF, O'Shea KM, Brown BH, Henderson R, Jr, Riggle H, Gupte SA, Stanley WC. High-sugar intake does not exacerbate metabolic abnormalities or cardiac dysfunction in genetic cardiomyopathy. Nutrition 28: 520–526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hecker PA, Lionetti V, Ribeiro RF, Jr, Rastogi S, Brown BH, O'Connell KA, Cox JW, Shekar KC, Gamble D, Sabbah HN, Leopold JA, Gupte SA, Recchia FA, Stanley WC. Glucose 6-phosphate dehydrogenase deficiency increases redox stress and moderately accelerates the development of heart failure. Circ Heart Fail. First published on November 20, 2012; doi:10.1161/CIRCHEARTFAILURE.112.969576. [DOI] [PMC free article] [PubMed]
- 40.Hecker PA, Mapanga RF, Kimar CP, Ribeiro RF, Jr, Brown BH, O'Connell KA, Cox JW, Shekar KC, Asemu G, Essop MF, Stanley WC. Effects of glucose 6-phosphate-dehydrogenase deficiency on the metabolic and cardiac responses to obesogenic or high-fructose diets. Am J Physiol Endocrinol Metab 303: E959–E972, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41: 2164–2171, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, Sharma RV, Davisson RL. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res 106: 1763–1774, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izumo K, Horiuchi M, Komatsu M, Aoyama K, Bandow K, Matsuguchi T, Takeuchi M, Takeuchi T. Dehydroepiandrosterone increased oxidative stress in a human cell line during differentiation. Free Radic Res 43: 922–931, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Jain M, Brenner DA, Cui L, Lim CC, Wang B, Pimentel DR, Koh S, Sawyer DB, Leopold JA, Handy DE, Loscalzo J, Apstein CS, Liao R. Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ Res 93: e9–e16, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Jain M, Cui L, Brenner DA, Wang B, Handy DE, Leopold JA, Loscalzo J, Apstein CS, Liao R. Increased myocardial dysfunction after ischemia-reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation 109: 898–903, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Katare R, Caporali A, Emanueli C, Madeddu P. Benfotiamine improves functional recovery of the infarcted heart via activation of pro-survival G6PD/Akt signaling pathway and modulation of neurohormonal response. J Mol Cell Cardiol 49: 625–638, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katare RG, Caporali A, Oikawa A, Meloni M, Emanueli C, Madeddu P. Vitamin B1 analog benfotiamine prevents diabetes-induced diastolic dysfunction and heart failure through Akt/Pim-1-mediated survival pathway. Circ Heart Fail 3: 294–305, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ko CH, Li K, Li CL, Ng PC, Fung KP, James AE, Wong RP, Gu GJ, Fok TF. Development of a novel mouse model of severe glucose-6-phosphate dehydrogenase (G6PD)-deficiency for in vitro and in vivo assessment of hemolytic toxicity to red blood cells. Blood Cells Mol Dis 47: 176–181, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA 107: 15565–15570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JW, Choi AH, Ham M, Kim JW, Choe SS, Park J, Lee GY, Yoon KH, Kim JB. G6PD up-regulation promotes pancreatic beta-cell dysfunction. Endocrinology 152: 793–803, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Leopold JA, Cap A, Scribner AW, Stanton RC, Loscalzo J. Glucose-6-phosphate dehydrogenase deficiency promotes endothelial oxidant stress and decreases endothelial nitric oxide bioavailability. FASEB J 15: 1771–1773, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Leopold JA, Dam A, Maron BA, Scribner AW, Liao R, Handy DE, Stanton RC, Pitt B, Loscalzo J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med 13: 189–197, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leopold JA, Loscalzo J. Oxidative enzymopathies and vascular disease. Arterioscler Thromb Vasc Biol 25: 1332–1340, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Leopold JA, Zhang YY, Scribner AW, Stanton RC, Loscalzo J. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arterioscler Thromb Vasc Biol 23: 411–417, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Levy HR, Christoff M. A critical appraisal of the effect of oxidized glutathione on hepatic glucose 6-phosphate dehydrogenase activity. Biochem J 214: 959–965, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long WK, Wilson SW, Frenkel EP. Associations between red cell glucose-6-phosphate dehydrogenase variants and vascular diseases. Am J Hum Genet 19: 35–53, 1967 [PMC free article] [PubMed] [Google Scholar]
- 58.Longo L, Vanegas OC, Patel M, Rosti V, Li H, Waka J, Merghoub T, Pandolfi PP, Notaro R, Manova K, Luzzatto L. Maternally transmitted severe glucose 6-phosphate dehydrogenase deficiency is an embryonic lethal. EMBO J 21: 4229–4239, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luzzatto L, Notaro Malaria R. Protecting against bad air. Science 293: 442–443, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Maloyan A, Sanbe A, Osinska H, Westfall M, Robinson D, Imahashi K, Murphy E, Robbins J. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-related cardiomyopathy. Circulation 112: 3451–3461, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maron BA, Zhang YY, Handy DE, Beuve A, Tang SS, Loscalzo J, Leopold JA. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem 284: 7665–7672, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsui R, Xu S, Maitland KA, Hayes A, Leopold JA, Handy DE, Loscalzo J, Cohen RA. Glucose-6 phosphate dehydrogenase deficiency decreases the vascular response to angiotensin II. Circulation 112: 257–263, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Matsui R, Xu S, Maitland KA, Mastroianni R, Leopold JA, Handy DE, Loscalzo J, Cohen RA. Glucose-6-phosphate dehydrogenase deficiency decreases vascular superoxide and atherosclerotic lesions in apolipoprotein E−/− mice. Arterioscler Thromb Vasc Biol 26: 910–916, 2006 [DOI] [PubMed] [Google Scholar]
- 64.McLendon PM, Robbins J. Desmin-related cardiomyopathy: an unfolding story. Am J Physiol Heart Circ Physiol 301: H1220–H1228, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meldrum NU, Tarr HL. The reduction of glutathione by the Warburg-Christian system. Biochem J 29: 108–115, 1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mellor K, Ritchie RH, Meredith G, Woodman OL, Morris MJ, Delbridge LM. High-fructose diet elevates myocardial superoxide generation in mice in the absence of cardiac hypertrophy. Nutrition 26: 842–848, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Meloni L, Manca MR, Loddo I, Cioglia G, Cocco P, Schwartz A, Muntoni S, Muntoni S. Glucose-6-phosphate dehydrogenase deficiency protects against coronary heart disease. J Inherit Metab Dis 31: 412–417, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Mesbah-Namin SA, Nemati A, Tiraihi T. Evaluation of DNA damage in leukocytes of G6PD-deficient Iranian newborns (Mediterranean variant) using comet assay. Mutat Res 568: 179–185, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Morelli A, Benatti U, Gaetani GF, De Flora A. Biochemical mechanisms of glucose-6-phosphate dehydrogenase deficiency. Proc Natl Acad Sci USA 75: 1979–1983, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muntoni S, Batetta B, Dessi S, Muntoni S, Pani P. Serum lipoprotein profile in the Mediterranean variant of glucose-6-phosphate dehydrogenase deficiency. Eur J Epidemiol 8, Suppl 1: 48–53, 1992 [DOI] [PubMed] [Google Scholar]
- 71.Muntoni S, Muntoni S. Gene-nutrient interactions in G6PD-deficient subjects–implications for cardiovascular disease susceptibility. J Nutrigenet Nutrigenomics 1: 49–54, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Naylor CE, Rowland P, Basak AK, Gover S, Mason PJ, Bautista JM, Vulliamy TJ, Luzzatto L, Adams MJ. Glucose 6-phosphate dehydrogenase mutations causing enzyme deficiency in a model of the tertiary structure of the human enzyme. Blood 87: 2974–2982, 1996 [PubMed] [Google Scholar]
- 73.Ng HP, Wang YF, Lee CY, Hu ML. Toxicological and antioxidant effects of short-term dehydroepiandrosterone injection in young rats fed diets deficient or adequate in vitamin E. Food Chem Toxicol 37: 503–508, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Niazi GA. Glucose-6-phosphate dehydrogenase deficiency and diabetes mellitus. Int J Hematol 54: 295–298, 1991 [PubMed] [Google Scholar]
- 75.Nichols KD, Kirby GM. Expression of cytochrome P450 2A5 in a glucose-6-phosphate dehydrogenase-deficient mouse model of oxidative stress. Biochem Pharmacol 75: 1230–1239, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Nogueira M, Garcia G, Mejuto C, Freire M. Regulation of the pentose phosphate cycle. Cofactor that controls the inhibition of glucose-6-phosphate dehydrogenase by NADPH in rat liver. Biochem J 239: 553–558, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oshikawa J, Kim SJ, Furuta E, Caliceti C, Chen GF, McKinney RD, Kuhr F, Levitan I, Fukai T, Ushio-Fukai M. Novel role of p66Shc in ROS-dependent VEGF signaling and angiogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 302: H724–H732, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park J, Choe SS, Choi AH, Kim KH, Yoon MJ, Suganami T, Ogawa Y, Kim JB. Increase in glucose-6-phosphate dehydrogenase in adipocytes stimulates oxidative stress and inflammatory signals. Diabetes 55: 2939–2949, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Park J, Chung JJ, Kim JB. New evaluations of redox regulating system in adipose tissue of obesity. Diabetes Res Clin Pract 77, Suppl 1: S11–S16, 2007 [DOI] [PubMed] [Google Scholar]
- 80.Park J, Rho HK, Kim KH, Choe SS, Lee YS, Kim JB. Overexpression of glucose-6-phosphate dehydrogenase is associated with lipid dysregulation and insulin resistance in obesity. Mol Cell Biol 25: 5146–5157, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 301: H1798–H1809, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Persico MG, Viglietto G, Martini G, Toniolo D, Paonessa G, Moscatelli C, Dono R, Vulliamy T, Luzzatto L, D'Urso M. Isolation of human glucose-6-phosphate dehydrogenase (G6PD) cDNA clones: primary structure of the protein and unusual 5′ non-coding region. Nucleic Acids Res 14: 2511–2522, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Porras AG, Olson JS, Palmer G. The reaction of reduced xanthine oxidase with oxygen. Kinetics of peroxide and superoxide formation. J Biol Chem 256: 9006–9103, 1981 [PubMed] [Google Scholar]
- 84.Pretsch W, Charles DJ, Merkle S. X-linked glucose-6-phosphate dehydrogenase deficiency in Mus musculus. Biochem Genet 26: 89–103, 1988 [DOI] [PubMed] [Google Scholar]
- 85.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell 130: 427–439, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rajasekaran NS, Firpo MA, Milash BA, Weiss RB, Benjamin IJ. Global expression profiling identifies a novel biosignature for protein aggregation R120GCryAB cardiomyopathy in mice. Physiol Genomics 35: 165–172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rajasekaran NS, Varadharaj S, Khanderao GD, Davidson CJ, Kannan S, Firpo MA, Zweier JL, Benjamin IJ. Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxid Redox Signal 14: 957–971, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rawat DK, Hecker P, Watanabe M, Chettimada S, Levy RJ, Okada T, Edwards JG, Gupte SA. Glucose-6-phosphate dehydrogenase and NADPH redox regulates cardiac myocyte L-type calcium channel activity and myocardial contractile function. PLoS One 7: e45365, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, om-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011 [DOI] [PubMed] [Google Scholar]
- 90.Saeed TK, Hamamy HA, Alwan AA. Association of glucose-6-phosphate dehydrogenase deficiency with diabetes mellitus. Diabet Med 2: 110–112, 1985 [DOI] [PubMed] [Google Scholar]
- 91.Salvemini F, Franze A, Iervolino A, Filosa S, Salzano S, Ursini MV. Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression. J Biol Chem 274: 2750–2757, 1999 [DOI] [PubMed] [Google Scholar]
- 92.Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol 301: H1723–H1741, 2011 [DOI] [PubMed] [Google Scholar]
- 93.Sanders S, Smith DP, Thomas GA, Williams ED. A glucose-6-phosphate dehydrogenase (G6PD) splice site consensus sequence mutation associated with G6PD enzyme deficiency. Mutat Res 374: 79–87, 1997 [DOI] [PubMed] [Google Scholar]
- 94.Sanna F, Bonatesta RR, Frongia B, Uda S, Banni S, Melis MP, Collu M, Madeddu C, Serpe R, Puddu S, Porcu G, Dessi S, Batetta B. Production of inflammatory molecules in peripheral blood mononuclear cells from severely glucose-6-phosphate dehydrogenase-deficient subjects. J Vasc Res 44: 253–263, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care 27: 884–889, 2011 [DOI] [PubMed] [Google Scholar]
- 97.Serpillon S, Floyd BC, Gupte RS, George S, Kozicky M, Neito V, Recchia F, Stanley W, Wolin MS, Gupte SA. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol 297: H153–H162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shao D, Oka S, Brady CD, Haendeler J, Eaton P, Sadoshima J. Redox modification of cell signaling in the cardiovascular system. J Mol Cell Cardiol 52: 550–558, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soliman H, Gador A, Lu YH, Lin G, Bankar G, Macleod KM. Diabetes-induced increased oxidative stress in cardiomyocytes is sustained by a positive feedback loop involving Rho kinase and PKCbeta2. Am J Physiol Heart Circ Physiol 303: H989–H1000, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spencer NY, Yan Z, Boudreau RL, Zhang Y, Luo M, Li Q, Tian X, Shah AM, Davisson RL, Davidson B, Banfi B, Engelhardt JF. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. J Biol Chem 286: 8977–8987, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stanton RC. Glucose-6-phosphate dehydrogenase, nadph, and cell survival. IUBMB Life 64: 362–369, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stanton RC, Seifter JL, Boxer DC, Zimmerman E, Cantley LC. Rapid release of bound glucose-6-phosphate dehydrogenase by growth factors. Correlation with increased enzymatic activity. J Biol Chem 266: 12442–12448, 1991 [PubMed] [Google Scholar]
- 102a.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 101: 6062–6067, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takizawa T, Huang IY, Ikuta T, Yoshida A. Human glucose-6-phosphate dehydrogenase: primary structure and cDNA cloning. Proc Natl Acad Sci USA 83: 4157–4161, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tian WN, Braunstein LD, Apse K, Pang J, Rose M, Tian X, Stanton RC. Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am J Physiol Cell Physiol 276: C1121–C1131, 1999 [DOI] [PubMed] [Google Scholar]
- 105.Tian WN, Braunstein LD, Pang J, Stuhlmeier KM, Xi QC, Tian X, Stanton RC. Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J Biol Chem 273: 10609–10617, 1998 [DOI] [PubMed] [Google Scholar]
- 106.Tome ME, Johnson DB, Samulitis BK, Dorr RT, Briehl MM. Glucose 6-phosphate dehydrogenase overexpression models glucose deprivation and sensitizes lymphoma cells to apoptosis. Antioxid Redox Signal 8: 1315–1327, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301: H2181–H2190, 2011 [DOI] [PubMed] [Google Scholar]
- 108.Wan GH, Tsai SC, Chiu DT. Decreased blood activity of glucose-6-phosphate dehydrogenase associates with increased risk for diabetes mellitus. Endocrine 19: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 109.Wiesenfeld SL, Petrakis NL, Sams BJ, Collen MF, Cutler JL. Elevated blood pressure, pulse rate and serum creatinine in Negro males deficient in glucose-6-phosphate dehydrogenase. N Engl J Med 282: 1001–1002, 1970 [DOI] [PubMed] [Google Scholar]
- 109a.World Health Organization Working Group. Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ 67: 601–611, 1989 [PMC free article] [PubMed] [Google Scholar]
- 110.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA 93: 6770–6774, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu Y, Zhang Z, Hu J, Stillman IE, Leopold JA, Handy DE, Loscalzo J, Stanton RC. Glucose-6-phosphate dehydrogenase-deficient mice have increased renal oxidative stress and increased albuminuria. FASEB J 24: 609–616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang H, Limphong P, Pieper J, Liu Q, Rodesch CK, Christians E, Benjamin IJ. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J 26: 1442–1451, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang X, Min X, Li C, Benjamin IJ, Qian B, Zhang X, Ding Z, Gao X, Yao Y, Ma Y, Cheng Y, Liu L. Involvement of reductive stress in the cardiomyopathy in transgenic mice with cardiac-specific overexpression of heat shock protein 27. Hypertension 55: 1412–1417, 2010 [DOI] [PubMed] [Google Scholar]
- 115.Zhang Z, Liew CW, Handy DE, Zhang Y, Leopold JA, Hu J, Guo L, Kulkarni RN, Loscalzo J, Stanton RC. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and beta-cell apoptosis. FASEB J 24: 1497–1505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]