Summary
Chromatin modifications have been implicated in the self-renewal and differentiation of embryonic stem cells (ESCs). However, the function of histone variant H2A.Z in ESCs remains unclear. We show that H2A.Z is highly enriched at promoters and enhancers and is required for both efficient self-renewal and differentiation of murine ESCs. H2A.Z deposition leads to an abnormal nucleosome structure, decreased nucleosome occupancy and increased chromatin accessibility. In self-renewing ESCs, knockdown of H2A.Z compromises OCT4 binding to its target genes and leads to decreased binding of MLL complexes to active genes and of PRC2 complex to repressed genes. During differentiation of ESCs, inhibition of H2A.Z also compromises RA-induced RARα binding, activation of differentiation markers and the repression of pluripotency genes. We propose that H2A.Z mediates such contrasting activities by acting as a ‘general facilitator’ that generates access for a variety of complexes both activating and repressive.
INTRODUCTION
Recent studies have revealed that eukaryotic genomes are characterized by a large number of histone modification patterns and chromatin states, which are dependent on the differentiation status of cells (Ernst et al., 2011). In all cell types, inactive genes are mainly associated with the repressive H3K27me3 mark, while nucleosomes at active promoters and enhancers are often associated with multiple different modifications including a variety of histone methylations, histone acetylation and deposition of histone variants (Barski et al., 2007; Bruce et al., 2005; Goldberg et al., 2007; Guenther et al., 2010; Heintzmen et al., 2007; Jin et al., 2009; Kim et al., 2005; Mikkelsen et al., 2007; Wang et al., 2008).
The histone variant H2A.Z is conserved from yeast to humans and is implicated in multiple nuclear processes including genome integrity, X-chromosome inactivation, DNA repair and transcriptional regulation (Zlatanova and Thakar, 2008) by impacting chromatin structure (Goldman et al., 2010; Jin and Felsenfeld, 2007; Kim et al., 2009; Thambirajah et al., 2006; Tolstorukov et al., 2009; Tremethick et al., 2002; Tremethick et al., 2004). H2A.Z is localized to promoters of active genes in various systems, suggesting a positive role in gene regulation (Barski et al., 2007; Bruce et al., 2005; Conerly et al., 2010; Cui et al., 2009; Dryhurst et al., 2009; Hardy et al., 2009; Leach et al., 2000; Ren and Gorovsky, 2001; Valdes-Mora et al., 2011; Zilberman et al., 2008). In contrast, H2A.Z was first described as a component of pericentric heterochromatin (Rangasamy et al., 2003). Particularly in ES cells, H2A.Z was reported to localize exclusively at polycomb complex target genes and mediate the targeting of PRC2 and repression of these genes but not be required for the self-renewal of ES cells (Creyghton et al., 2008).
To clarify the function of H2A.Z in ES cells and to understand how H2A.Z functions together with other mechanisms of chromatin modification such as histone methylation by the MLL family of histone H3K4 methyltransferases, we investigated the genomic distribution profiles of H2A.Z, acetylated H2A.Z, histone modifications H3K4me3, and H3K27me3, and RbBP5 (a core subunit of the MLL complexes) in mouse ES cells using ChIP-Seq and tested the activities of H2A.Z in ESC self-renewal and differentiation. Our data indicate that H2A.Z is enriched at active enhancers and promoters and facilitates chromatin accessiblity to allow binding of a variety of active and repressive complexes required for ESC self-renewal and differentiation.
RESULTS
H2A.Z is highly enriched at active promoters and enhancers in ES cells
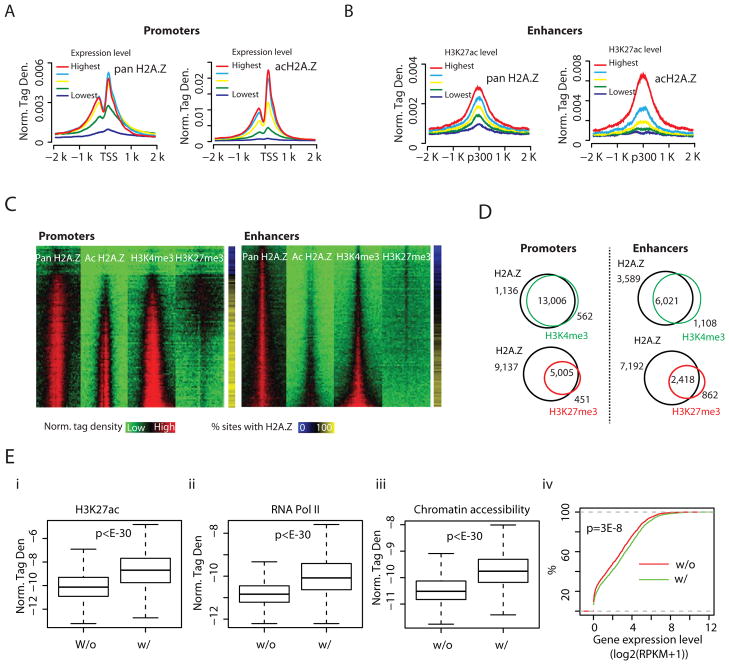
We profiled the genome-wide distribution of H2A.Z in mouse ES cells with ChIP-Seq using antibodies that recognize acetylated H2A.Z (hereafter acH2A.Z) (Bruce et al., 2005) and antibodies that recognize H2A.Z irrespective of its state of acetylation (hereafter pan H2A.Z). This led to the identification of 51,536 islands of pan H2A.Z and 32,248 islands of acH2A.Z using the SICER program (Zang et al., 2009). The islands are highly enriched at promoter and enhancer regions (Figure S1A–C). A positive correlation between gene expression levels and the enrichment of pan H2A.Z and acH2A.Z surrounding TSSs was found (Figure 1A). The percentage of H2A.Z acetylation increased with gene expression level (Figure S1D). Interestingly, even at the promoters of genes without detectable levels of expression, two-thirds of the associated H2A.Z islands were acetylated (Figure S1D). We next examined the pan H2A.Z and acH2A.Z levels at enhancers, defined by intergenic p300 sites, and grouped by the levels of H3K27ac, a marker of active enhancers (Hawkins et al., 2011; Jaenisch et al., 2010; Rada-Iglesias et al., 2011). The average profiles of pan H2A.Z and acH2A.Z tag density were positively correlated with the level of H3K27ac (Figures 1B). Again, the percentage of H2A.Z acetylation reached close to 100% for enhancers having the highest levels of H3K27ac (Figure S1E).
Figure 1. H2A.Z marks open chromatin and is co-localized with H3K4me3 at promoters and enhancers (see also Figure S1).
A. Left panel: relationship of normalized, averaged tag density of pan H2A.Z across TSSs to expression levels. All non-locally redundant genes were sorted into 5 equal size groups according to their mRNA expression values (RPKM). Right Panel: a similar plot for acH2A.Z across TSSs.
B. Left panel: normalized tag density of pan H2A.Z across intergenic p300 binding sites, sorted into 5 equal-size groups by their H3K27ac level. Right Panel: a similar plot for acH2A.Z across intergenic p300 binding sites.
C. Heatmaps of pan H2A.Z, acH2A.Z and histone modifications H3K4me3 and H3K27me3 in promoter regions (left panel) and enhancer regions (right panel), ±3 Kb around TSS and intergenic p300 binding sites, respectively (see Supplemental Experimental Procedures for details). The rightmost bars indicate the percentage of promoters and enhancers that showed enrichment of pan H2A.Z for each bin.
D. Venn diagrams for promoters (left panel) and enhancers (right panel) occupied by pan H2A.Z and either H3K4me3 (green) or H3K27me3 (red).
E. Enhancers associated with both H2A.Z and H3K4me3 are more likely to be active. Boxplots of (i) H3K27ac ChIP-seq tag densities, (ii) RNA Pol II ChIP-seq tag densities, and (iii) BNase-Seq tag densities, which measure chromatin accessibility around intergenic p300 binding sites that are bound by H2A.Z (+/− 2.5K bps; H2A.Z bound enhancers). The binding sites are separated into two groups based on presence (w/) or absence (w/o) of H3K4me3 islands. The top and bottom of the box represent 25th and 75th percentile, respectively. The upper whisker represents the smaller of the maximum and upper quartile + 1.5 IQR (interquartile range), while the lower whisker represents the larger of the minimun and lower quartile − 1.5 IQR. P-values were calculated by the t-test. (iv) % of H2A.Z-bound enhancers (Y-axis) whose target genes exhibit lower expression than the value specified by the X-axis. The potential target of an H2A.Z-bound enhancer is defined by its nearest gene within 100Kbp. A line shifted to the left side indicates a globally lower level of expression. The P-value was calculated by the Kolmogorov-Smirnov Test.
H2A.Z and H3K4me3 are co-localized at both promoters and enhancers
To test whether H2A.Z is positively correlated with active or repressive histone modifications in ES cells, we compared our H2A.Z profiles with H3K4me3 and H3K27me3 profiles determined by others (Meissner et al., 2008; Mikkelsen et al., 2007) using a clustering analysis of all promoters ranked from lowest to highest gene expression levels in ES cells (Figure 1C, left panel). Both pan H2A.Z and acH2A.Z were highly correlated with H3K4me3 at active promoters. Globally, 92% of the H2A.Z-bound promoters were associated with H3K4me3 (Figure 1D, left panel). Examination of the distribution of H2A.Z, H3K4me3 and H3K27me3 at the Hoxa10 promoter indicated a better spatial correlation between H2A.Z and H3K4me3 than with H3K27me3 (Figure S1F). Global analysis revealed that the spatial distribution of H2A.Z was highly correlated with H3K4me3 but less so with H3K27me3 (Figure S1G).
While enhancers are generally associated with H3K4me1 (Heintzman et al., 2007), active enhancers are also marked by H3K4me2 (He et al., 2010; Pekowska et al., 2010; Wang et al., 2008), H3K4me3 (Pekowska et al., 2011; Wang et al., 2008), H3K27ac (Jaenisch et al., 2010; Wang et al., 2008), and H3K9acK14ac (Roh et al., 2005; Roh et al., 2007) and poised enhancers are associated with H3K27me3 (Rada-Iglesias et al., 2011). The positive correlation of H2A.Z with H3K27ac (Figure 1B) suggested that it may also be co-localized with H3K4me3 at enhancers. We therefore compared the binding levels of H2A.Z and H3K4me3 modification using a clustering analysis of intergenic p300 binding sites ranked by their H3K27ac levels. We found that the pattern of pan H2A.Z distribution paralleled that of H3K4me3 at enhancers as well as promoters (Figure 1C, right panel). Only a minor fraction of the enhancers were associated with both H2A.Z and H3K27me3: these were bivalent enhancers as indicated by the co-existence of H3K4me3 and H3K27me3. The acH2A.Z distribution showed an inverse pattern from H3K27me3, suggesting that it is mainly located in active enhancers. In total, among the 9,610 H2A.Z-bound enhancers, 63% were also associated with H3K4me3 (Figure 1D, right panel). The Pearson coefficient analysis indicated that H2A.Z was much better correlated with H3K4me3 than with H3K27me3 at enhancers (Figure S1H). Using H3K4me1 as an enhancer marker revealed a similar correlation between H2A.Z and H3K4me3 at enhancers (Figures S1I,J). These data indicate that H2A.Z is co-localized with H3K4me3 at active regulatory regions of transcription in ES cells. We further anlyzed how H2A.Z-containing nucleosomes are associated with histone acetylation and methylation at a resolution of individual nucleosomes and found that H2A.Z-containing nucleosomes are more frequently modified by H3K4me3 than H3K27me3 and prefer H3K27ac to H3K9ac in the genome (Figure S1K).
Enhancers associated with both H2A.Z and H3K4me3 are more likely to be active
Among the H2A.Z-bound enhancers, 63% are also associated with H3K4me3. We found that the H2A.Z-bound enhancers having H3K4me3 are associated with higher levels of H3K27ac and RNA Pol II and have higher chromatin accessibility than enhancers lacking H3K4me3 (Figure 1E, panels i–iii). The expression levels of putative target genes (the nearest gene within +/− 100K bps) for H2A.Z-bound enhancers with H3K4me3 are significantly higher than those without H3K4me3 (Figure 1E, panel iv), suggesting that enhancers with both H2A.Z and H3K4me3 are more likely to be active. The top GO-terms of target genes of H3K4me3-positive H2A.Z-containing enhancers are mainly associated with house-keeping functions such as the regulation of cellular, metabolic and biosynthetic processes (Figure S1L, panel i), while those for H3K4me3-negative enhancers show preferences towards system development and cell differentiation (Figure S1L, panel ii).
H2A.Z promotes H3K4me3 and H3K27me3 by facilitating the binding of the MLL and PRC2 complexes
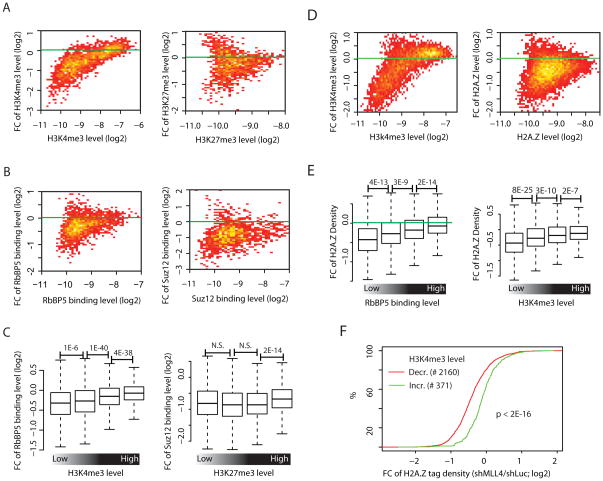
To test if H2A.Z facilitates histone methylation, we knocked down H2A.Z using shRNAs (Figure S2A), and measured global changes in H3K4me3 and H3K27me3 by ChIP-Seq. We first quantified the histone modifications in defined regions (promoters, enhancers) and plotted the fold change (FC) of each histone modification between control and H2A.Z knockdown cells versus the average level of the modification (termed MA analyses hereafter). At promoters we found that H3K4me3 modification was only modestly affected by knocking down H2A.Z, whereas a stronger effect was detected with the H3K27me3 modification (Figure S2B). At enhancers, H2A.Z knockdown resulted in a substantial decrease in both the H3K4me3 and H3K27me3 signals (Figure 2A). It is interesting to note that greater decreases in H3K4me3 were detected at weaker sites than at stronger sites (Figure 2A, left panel).
Figure 2. Inter-regulation of H2A.Z and H3K4me3 and H3K27me3 at enhancers (see also Figure S2, Table S1).
A. MA analysis (see Supplemental Experimental Procedures for details) for H3K4me3 (left panel) and H3K27me3 (right panel) in enhancer regions. The analysis was made from H2A.Z knockdown cells and shLuc control..
B. MA analysis for RbBP5 (left panel) and Suz12 (right panel) in enhancer regions, as in Panel A.
C. Left panel: RbBP5 islands from enhancer regions were sorted into quartiles by the levels of H3K4me3. Shown for each group is a boxplot (see Figure 1E legend for explanation) for the fold change of RbBP5 tag density (shH2A.Z/shLuc). Right panel: as left panel, except that the calculation was made for SUZ12 and H3K27me3. P values were calculated by the t-test.
D. MA analysis for H3K4me3 (left panel) and H2A.Z (right panel) in enhancer regions. The analysis was made from MLL4 knockdown cells and shLuc control.
E. Left panel: H2A.Z islands from enhancer regions were sorted into quartiles by the levels of RbBP5 binding. Shown for each group is a boxplot (see Figure 1E legend for explanation) for the fold change of RbBP5 tag density (shMLL4/shLuc). Right panel: as left panel, except that the calculation was made for H2A.Z and H3K4me3. P values were calculated by the t-test.
F. Empirical cumulative distribution for the fold change of H2A.Z ChIP-Seq tag density (shMLL4/shLuc) for enhancers that show H3K4me3 decrease by more than 1.5 fold upon MLL4 knockdown (red line). Y-axis shows the % of enhancers that exhibit a lower FC of H2A.Z level than the value specified by the X-axis. Enhancers that show H3K4me3 increase by more than 1.5 fold are chosen as control (green line). A line shifted to the left means a systematically more decrease in the H2A.Z levels. P-value was calculated by Kolmogorov-Smirnov Test.
H3K4me3 and H3K27me3 modifications are generated by the MLL and PRC2 complexes, respectively (Schuettengruber et al., 2007). Our data showed that RbBP5, a core subunit of MLL complexes (Cho et al., 2007; Dou et al., 2006), co-localizes with the H3K4me3 and pan/acH2A.Z islands (Figure S2C, upper panels), while SUZ12, a core subunit of the PRC2 complex (Cao and Zhang, 2004), predominantly localizes to the H3K27me3 islands (Figure S2C, lower panels). Consistent with the histone modification changes, both RbBP5 and SUZ12 exhibited substantial decreases in binding to enhancers (Figure 2B) and to promoters in the H2A.Z knockdown cells (Figure S2D). Interestingly, H2A.Z knockdown led to greater decreases in RbBP5 binding at enhancers having lower levels of H3K4me3 in contrast to sites having higher levels of H3K4me3 (Figure 2C, left panel), which is consistent with the greater loss of H3K4me3 at enhancers having lower H3K4me3 levels (Figure 2A, left panel). In contrast, Suz12 decreased at all H3K27me3 sites (Figure 2C, right panel).
These results indicate that H2A.Z regulates H3K4me3 and H3K27me3 methylations by facilitating the targeting of the MLL and PRC2 complexes to promoters and particularly to enhancers.
H3K4me3 promotes H2A.Z deposition at enhancers
To test whether H3K4me3 modification influences H2A.Z deposition, we knocked down MLL4, an H3K4 methyltransferase highly expressed in ES cells (Cho et al., 2009) (Figure S2E). Although the expression of MLL4 was decreased by 80%, we did not find detectible changes in overall levels of H3K4me3 by Western blotting (data not shown). To determine whether MLL4 nevertheless regulates H3K4 methylation at specific enhancers, we mapped the genome-wide distribution of H3K4me3 in the control and MLL4 knockdown cells, and quantified the changes at enhancers by MA analyses. Interestingly, H3K4me3 decreased at a large number of enhancers following MLL4 knockdown, with more severe loss of methylation signals being at enhancers having lower levels of this modification (Figure 2D, left panel). Profiling the distribution of H2A.Z in these cells revealed substantial decreases of H2A.Z at enhancers in the MLL4 knockdown cells, particularly at enhancers with lower levels of H2A.Z (Figure 2D, right panel). To test directly how the H2A.Z change is related to the binding of the MLL complex at enhancers, we compared the fold change of H2A.Z with RbBP5 binding levels. Interestingly, the analysis revealed greater reductions in H2A.Z deposition in the regions having lower levels of RbBP5 binding after MLL4 knockdown (Figure 2E, left panel), which is consistent with the observation that greater losses of H2A.Z were detected at sites of weaker H3K4 methylations (Figure 2E, right panel). In addition, enhancers that showed decreased levels of H3K4me3 exhibited significantly more loss of H2A.Z deposition than those that showed an increase (Figure 2F), suggesting a causal link between the H3K4me3 change and changes in H2A.Z deposition in the MLL4 knockdown cells.
These results indicate that H3K4me3 modification facilitates the deposition of H2A.Z at enhancers. Furthermore, the results also suggest that higher levels of active histone modifications help to maintain the robustness of chromatin configuration and gene expression. In support of this hypothesis, we found that knockdown of H2A.Z resulted in significantly more genes having changed expression in the poorly methylated group than in the highly methylated group (Figure S2F).
H2A.Z regulates chromatin accessibility
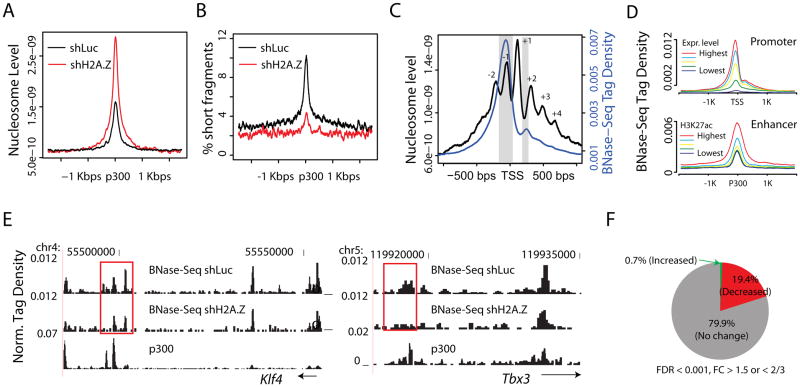
We previously proposed that deposition of histone variants H2A.Z and H3.3 in transcriptional regulatory regions destabilizes nucleosome structure and provides opportunities for the binding of transcription factors (Jin et al., 2009). Another recent report suggested that in colon cancer cells H2A.Z is an essential factor for gene reactivation by facilitating nucleosome removal (Yang et al., 2012). To test whether deposition of H2A.Z in ES cells regulates nucleosome occupancy and chromatin accessibility at transcriptional regulatory regions, we determined the global nucleosome distribution in control and H2A.Z knockdown cells using MNase-Seq (Schones et al., 2008). Consistent with the previous reports (Hu et al., 2011; Tillo et al., 2010), in control ES cells enhancers are associated with increased nucleosome occupancy relative to neighboring regions (Figure 3A, black line) and H2A.Z knockdown leads to increased nucleosome levels at enhancer sites (Figure 3A, red vs black lines). These data indicate that replacement of H2A.Z with canonical H2A leads to enhanced nucleosome deposition. Thus H2A.Z binding at enhancers destabilizes the local nucleosome structure and facilitates nucleosome removal.
Figure 3. H2A.Z regulates nucleosome organization and chromatin accessibility at enhancers (see also Figure S3).
A. The nucleosome level at p300-bound enhancers is significantly increased after knockdown of H2A.Z.
B. Percentage of short fragments (<120 bps) as a function of position relative to the p300 binding site.
C. Normalized BNase-Seq tag density (blue) and nucleosome tag density (black) in regions ±1 Kbp around TSS. Inferred nucleosome positions are numbered as indicated. The regions immediately upstream of the TSS and the linker region between nucleosomes +1 and +2 that are preferentially digested by Benzonase are highlighted in gray.
D. Normalized BNase-Seq tag densities across TSSs (upper panel) and across intergenic p300 binding sites (lower panel). The TSSs and p300 binding sites were sorted into five equal-sized groups based on gene expression level and H3K27ac level, respectively.
E. UCSC Genome Browser images for the BNase-Seq tag distribution in H2A.Z knockdown and control cells. Potential enhancer regions associated with Klf4 (left) and Tbx3 (right) are chosen as examples. p300 genomic regions that show significantly decreased levels of chromatin accessibility following knockdown of H2A.Z are highlighted with red rectangles.
F. The percentages of hypersensitive sites that show a significant increase in accessibility (green), decrease in accessibility (red), or no change in accessibility (gray) following knockdown of H2A.Z.
In human T cells, nucleosomes with H2A.Z protect only ~120bp of DNA from MNase digestion, a fragment shorter than that given by canonical nucleosomes (Tolstorukov et al., 2009). In ESCs, short DNA fragments (90–120bp) resulting from MNase digestion were enriched in regions surrounding intergenic p300 binding sites and positively correlated with enhancer activities as measured by the level of H3K27ac (Figure S3A). This is consistent with the hypothesis that nucleosomes are unstable at these regulatory regions. Importantly, knockdown of H2A.Z resulted in a 2–3 fold reduction in the density of short fragments surrounding intergenic p300 binding sites (Figure 3B), indicating that H2A.Z deposition contributes to the generation of short DNA fragments, i.e. to an unstable nucleosome structure and decreased nucleosome occupancy at enhancers.
To test whether H2A.Z deposition affects chromatin accessibility, we treated the control and H2A.Z knockdown cells with Benzonase (the nuclease from Serratia marcescens), followed by deep sequencing of chromatin cleavage sites (BNase-Seq). This approach is similar to DNase-Seq but has the advantage of being less sensitive to enzyme concentration (Grontved et al., 2012). The BNase cleavage sites were highly enriched immediately upstream of TSS and in linker regions (Figure 3C). The BNase-Seq tag density was positively correlated with gene expression levels at promoters and with H3K27ac levels at enhancers (Figure 3D), indicating that cleavage by Benzonase is a good indicator of chromatin accessibility. Moreover, intergenic regions more accessible to Benzonase were associated with higher levels of H2A.Z acetylation and H3K4me3 and with lower levels of H3K27me3 (Figure S3B). Interestingly, knockdown of H2A.Z resulted in significantly decreased chromatin accessibility at a subset of genomic regions, as exemplified by several enhancer regions near to Klf4 and Tbx3 (Figure 3E, highlighted in red boxes). Globally, we found that 19.4% of Benzonase hypersensitive sites showed a significant reduction of BNase cleavage following H2A.Z knockdown, while only 0.7% exhibited increases (Figure 3F). Intriguingly, the regions with lower chromatin accessibility, as measured by the average Benzonase tag density, were subject to greater decreases in chromatin accessibility after knockdown (Figure S3C, D). To determine if the reduced chromatin accessibility is correlated with decreased binding of MLL complexes, we monitored RbBP5 binding and found that hypersensitive sites with reduced accessibility were subject to greater decreases in RbBP5 binding after the H2A.Z knockdown (Figure S3E). To test whether the decrease in chromatin accessibility is related to changes in gene expression, we identified 2986 genes with decreased accessibility at hypersensitive sites located +/− 100Kbp around the TSS and compared these with the 1204 genes having changed expression. The analysis revealed that 205 were shared between the two sets, which is highly significant (binominal test, p<0.0001) (Figure S3F). These 205 genes are enriched in GO terms of system development and embryonic development (Figure S3G).
Overall these results indicate that deposition of H2A.Z results in an abnormal and unstable nucleosome structure, leading to decreased nucleosome occupancy and thereby increasing chromatin accessibility, particularly at enhancers.
H2A.Z is required for efficient self-renewal and pluripotency of ES cells
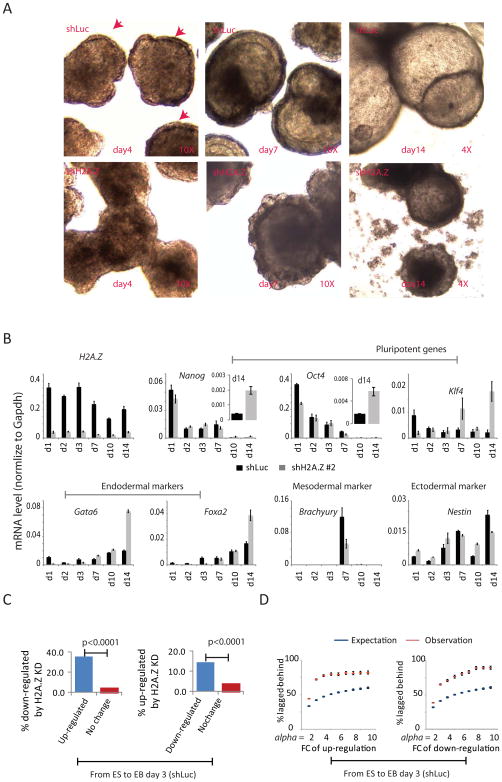
The observation that H2A.Z is critically involved in modulating nucleosome occupancy and chromatin accessibility at enhancers implies that it plays important roles in ESC function. We therefore investigated whether it contributes to the self-renewal of ESCs using several different assays. (1) By examining ESC colony morphology, we found that only 56% of colonies that grew for 7 days after H2A.Z knockdown (shH2A.Z) exhibited ES morphology, while 81% of the colonies that grew from the control ES cells (shLuc) maintained ES morphology (Figures 4A, B); (2) H2A.Z knockdown ES colonies tended to become flat and had lower alkaline phosphatase activity (Figure 4C); (3) Flow cytometry analysis revealed that a greater fraction of cells had lower SSEA1 expression after 2 days of H2A.Z knockdown (13.9% in control and 24.5% in knockdown cells) and increased SSEA4 expression (1.3% in control and 14.5% in knockdown cells), although OCT4 expression in the majority of cells did not change much (Figure 4D); (4) Knockdown of H2A.Z resulted in substantial decreases in expression of pluripotency genes such as Sox2, Esrrb, Tbx3 and Klf4 and increases in expression of differentiation genes such as Brachyury, HAND1 and WNT7B (Figure 4E & Figure S4A). In addition, genes responsive to H2A.Z knockdown in ES cells are enriched in GO terms of system development and embryonic development (Figure S4B). (5) Comparing genes responsive to H2A.Z knockdown in ESCs and those whose expression was altered at EB day 3 during EB formation revealed a highly significant overlap between the two groups (Figure 4F). Overall these results indicate a loss of pluripotency and self-renewal capacity of ESCs after knockdown of H2A.Z, accompanied by premature differentiation. Thus H2A.Z critically contributes to the self-renewal and pluripotency of ES cells as well as to their differentiation.
Figure 4. H2A.Z is required for ES cell self-renewal (see also Figure S4).
A. H2A.Z RNAi ES cell colonies are scored by morphology: compact and round (ES-like), flattened and intermediate. Scale bar, 100μm.
B. GFP+ ES cells were sorted into a 96-well plate having a MEF feeder layer. One week later, colonies were scored by morphology. The experiments were carried out for H2A.Z knockdown ES cells and control ES cells side-by-side and were repeated four times. The percentages of colonies that maintain ES-like morphology (compact and round) are represented as mean +/− SEM. The P-value was calculated by the t-test.
C. H2A.Z knockdown (shH2A.Z) and shLuc control ES cells stained for alkaline phosphatase activity.
D. FACS analysis showing the distribution of expression of SSEA-1 (a pluripotency marker for murine ESCs) and SSEA-4 (a differentiation marker for murine ESCs) relative to OCT4 (a pluripotency TF) for ES cells with H2A.Z knockdown (shH2A.Z) and shLuc control cells. The analysis was done after two days of culture following re-plating from individual ES colonies.
E. RT-PCR results (averages of three replicates) for mRNAs of four pluripotency genes and two early differentiation markers in the H2A.Z knockdown (shH2A.Z) and shLuc control ES cells. Data are represented as mean +/− standard deviation.
F. Venn diagrams for genes responsive to H2A.Z knockdown in ES cells (up-regulated or down-regulated by more than 1.5 fold and FDR < 0.001; left circles) and genes that are up-or down-regulated during EB formation from ESCs to EB day 3 (EB-associated genes; right circles).
H2A.Z facilitates targeting of OCT4 to genes critical to ESC pluripotency
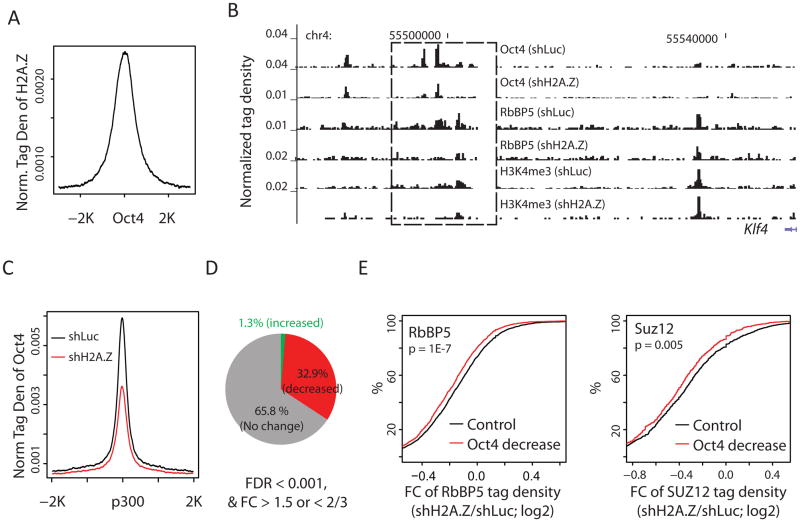
To understand the mechanisms whereby H2A.Z contributes to the self-renewal of ESCs, we tested whether H2A.Z regulates targeting of the transcription factor, OCT4. Using ChIP-Seq, we identified genome-wide OCT4 binding sites in the H2A.Z knockdown and control ESCs. Analysis indicated that H2A.Z binding is highly elevated at OCT4 binding sites in control ESCs (Figure 5A). However, knockdown of H2A.Z leads to substantially decreased OCT4 binding at several putative enhancers downstream of the Klf4 gene (Figure 5B), accompanied by decreased expression of the Klf4 gene (Figure 4E). Furthermore, H2A.Z knockdown resulted in a global decrease of OCT4 binding at p300-bound enhancers (Figure 5C), though the overall expression level of OCT4 itself was not significantly affected (Figure 4E). Quantitative analysis indicated that 33% of OCT4 binding sites showed decreased binding after H2A.Z knockdown, while only 1.3% showed increased binding (Figure 5D). Since OCT4 may directly interact with MLL complexes (Ang et al., 2011), reduced OCT4 binding after H2A.Z knockdown may compromise the recruitment of MLL complexes and thus H3K4 methylation. Indeed, upon knockdown of H2A.Z and loss of OCT4 binding, RbBP5 binding and H3K4me3 levels at the Klf4 enhancer regions markedly decreased (Figure 5B). Globally, significantly more RbBP5 loss was detected at enhancer sites that lost OCT4 binding than at sites that did not lose OCT4 (Figure 5E, left panel).
Figure 5. H2A.Z is required for efficient bindings of OCT4.
A. Average profile of H2A.Z tag density around OCT4 binding sites in mouse ESCs.
B. UCSC Genome Browser image of tag distribution of OCT4, RbBP5 and H3K4me3 in the putative enhancer regions downstream of the Klf4 gene in control and H2A.Z knockdown ESCs. The genomic region that shows significant decreases in the levels of Oct4, RbBP5 and H3K4me3 is highlighted by the rectangle.
C. Average profile of OCT4 tag density around intergenic p300 binding sites in the H2A.Z knockdown (shH2A.Z) and control (shLuc) ES cells.
D. The percentages of OCT4 binding sites that showed a significant increase (green), decrease (red), or no change (gray) after knockdown of H2A.Z.
E. Left: intergenic OCT4 binding sites that co-localize with RbBP5 are separated into two groups based on whether or not OCT4 binding is significantly decreased upon H2A.Z knockdown (FC > 1.5, FDR < 0.001). The empirical cumulative distribution is plotted for the fold change of RbBP5 ChIP-Seq tag density for each group (knockdown/control). Right: as the left panel, except that the calculation was made for SUZ12 (X-axis). A line shifting to the left means a greater decrease in RbBP5 (or SUZ12) binding levels. P-values were calculated by Kolmogorov-Smirnov Tests.
H2A.Z also plays an important role in the establishment of the H3K27me3 modification and Suz12 binding in the repression of differentiation genes, as seen from the results of H2A.Z knockdown in Figures 2A, 2B and 4E. Interestingly, the loss of SUZ12 at enhancers after H2A.Z knockdown was also significantly related to a decreased binding of OCT4 (Figure 5E, right panel).
These results indicate that H2A.Z is needed for efficient targeting of OCT4, which is likely involved in the recruitment of the MLL and PRC2 complexes to active and silent genes, respectively.
H2A.Z is required for efficient differentiation of ES cells
The results in Figure 4 indicate that ESCs with reduced H2A.Z expression tend to differentiate. To further test whether knockdown of H2A.Z promotes ESC differentiation, we monitored the formation of embryoid bodies (EB) from control and H2A.Z knockdown ESCs. The EBs from the H2A.Z knockdown cells were morphologically different from the control ESCs: although a primitive endoderm layer from control cells formed at day 4, this layer was not detectable in the knockdown cells at day 4, and only became apparent at day 7 (Figure 6A, red arrow heads). RT-PCR analyses showed that H2A.Z remained suppressed during the period of 14-day differentiation in the knockdown cells (Figure 6B). In the knockdown cells (shH2A.Z) the expression of pluripotency genes Nanog, Oct4 and Klf4 is somewhat compromised at day 1 of differentiation but they remain active at 14 days in contrast to the control cells (shLuc) (Figure 6B, inserts). Consistent with delayed endodermal differentiation phenotypes, knockdown of H2A.Z resulted in decreased induction of a number of endodermal transcription factors (Gata6, Hnf1b and Hnf4a), markers of visceral endoderm (Afp, Ttr and Bmp2) and of parietal endoderm (Dab2) at day 3 and to a lesser extent at day 7 (Figure 6B; Figure S5A,B). In addition, mesodermal markers Brachyury, Hand1, Nkx2-5, Bmp4 and Runx1, and ectodermal markers Sox4 and Nestin were aberrantly expressed, but showed no generalizable pattern of consistent up- or down-regulation (Figure 6B; Figure S5A,B). These results indicate that knockdown of H2A.Z compromised both gene activation and repression in EB formation.
Figure 6. H2A.Z is required for efficient differentiation of ESCs (see also Figure S5).
A. Comparative morphology of H2A.Z knockdown (shH2A.Z) and control (shLuc) EBs at day 4, day 7 and day 14. Primitive endoderm layers are indicated by red arrows.
B. RT-PCR results for mRNAs from the H2A.Z knockdown (shH2A.Z) and shLuc (control) EBs. Data are represented as mean +/− standard deviation.
C. Left: the percentage of genes down-regulated by H2A.Z knockdown at EB day 3 (Y-axis) plotted for genes that are significantly up-regulated (blue) and unchanged (red) in transition from wt ES cells to EB day 3. Right: the percentage of genes up-regulated by H2A.Z knockdown at EB day 3 (Y-axis) plotted for genes that are significantly down-regulated (blue) and unchanged (red) from wt ES cells to EB day 3. P-values were calculated by the t-test.
D. Left panel: each point represents genes up-regulated by at least alpha-fold from ESC to EB day 3 in the control cells. The percentage of genes with expression values that follow the order: EB day 3 (shLuc) > EB day 3 (shH2A.Z) > ES is calculated for each point (Y-axis). The expression values of all genes are randomly shuffled independently for EB day 3 (shLuc), EB day 3 (shH2A.Z) and ESCs and are repeated many times to estimate the expectated percentage. Right panel: similar to the left panel but for genes that are down-regulated by at least alpha-fold from ESCs to EB day 3 in the control cells. The Y-axis shows the percentage of genes with expression levels following the order EB day 3 (shLuc) < EB day 3 (shH2A.Z) < ES. Data are represented as mean +/− standard deviation
To test this notion systematically, we identified genes that are activated or repressed in day 3 EBs as compared to ESCs and then noted the proportion of these two groups of genes that failed to be efficiently activated or repressed in the H2A.Z knockdown EBs: this was 35.5% of the activated and 14.4% of the repressed genes, which is significantly higher than the number of genes that remained unchanged during EB formation (Figure 6C). This shows that during EB formation changes in gene expression are generally less in the H2A.Z knockdown cells than in the control cells, i.e. H2A.Z knockdown compromises both the activation of genes required for EB development and the repression of genes required for pluripotency. To test this rigorously, we first identified genes that are up-regulated during EB formation from control ESCs to day 3 and calculated the fraction of these genes that exhibited a lower induction level in the H2A.Z knockdown EBs as compared to the control EBs. We found that this fraction is significantly higher than expected (Figure 6D, left), indicating that knockdown of H2A.Z compromised the induction of these genes. Similarly, knockdown of H2A.Z also compromised the repression of genes during EB formation (Figure 6D, right. The defect in gene up-regulation was particularly significant for endodermal markers at day 3 (Figure S5A), which is consistent with the defect in endodermal phenotype shown in Figure 6A.
In conclusion, although knockdown of H2A.Z leads to decreased expression of many pluripotency genes and up-regulation of differentiation genes, it is also required for the silencing of ESC-specific genes and the optimal activation of differentiation genes during differentiation of ESCs.
H2A.Z is required for efficient binding of RARα following retinoic acid (RA) treatment
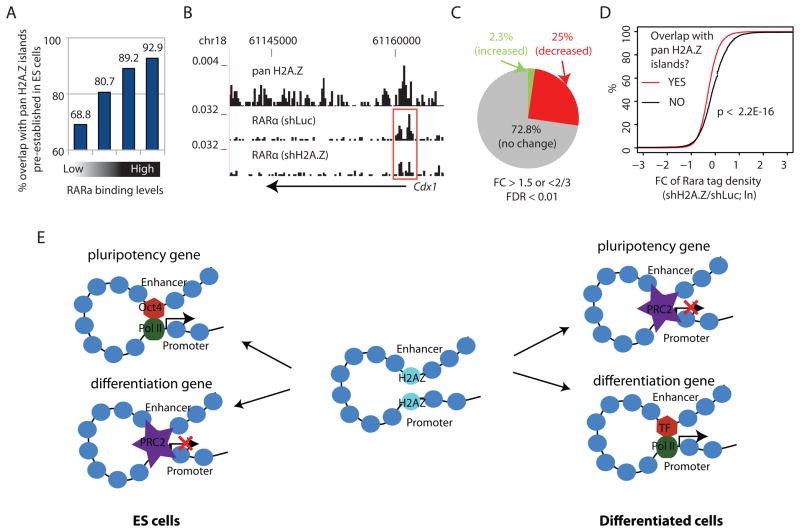
To test whether H2A.Z facilitates transcription factor binding during ESC differentiation, we analyzed the binding profiles of RARα following withdrawal of LIF and addition of RA to ESCs, which initiates ESC differentiation to the neuronal lineage (Diez del Corral and Storey, 2004). Interestingly, a vast majority of the genomic regions bound by RARα after RA exposure were enriched with H2A.Z in ESCs (Figures 7A, B), indicating that the chromatin of many developmental enhancers is pre-configured by H2A.Z at earlier stages of development (Amat and Gudas, 2011). Although RARα expression was not affected by H2A.Z knockdown, the binding of RARα was significantly compromised genome-wide: 25% of its bindings showed a decrease while only 2% showed an increase on knockdown of H2A.Z (Figures 7C). Remarkably, the RARα binding sites pre-configured with H2A.Z in ESCs exhibited more decrease in RARα binding than the sites without H2A.Z (Figure 7D), suggesting that setting a chromatin configuration by H2A.Z in ESCs facilitates transcription factor binding during later development.
Figure 7.
H2A.Z facilitates RARα binding during RA-induced ESC differentiation.
A. RARα bound genomic regions were sorted into quartiles based on RARα enrichment levels after 3 hours of RA exposure. The Y-axis indicates the percentage of RARα bound regions that overlap with pan H2A.Z islands identified in ESCs.
B. UCSC Genome Browser image of tag distributions of pan H2A.Z in ES cells and RARα in H2A.Z knockdown (shH2A.Z) and control (shLuc) cells 3 hrs after RA exposure for a known RAR target gene cdx1. The promoter region that shows a significant decrease in RARα binding is highlighted by the red rectangle.
C. The percentages of RARα binding sites that showed a significant increase (green), decrease (red), or no change (gray) in RARα binding after knockdown of H2A.Z.
D. RARα binding sites are separated into two groups based on whether or not overlapping with a pan H2A.Z island identified in the ES cells. The empirical cumulative distribution is plotted for the fold change of RARα ChIP-Seq tag density for each group (shH2A.Z/shLuc). A line shifting to the left means systematically more decrease in the RARα levels. The P-value was calculated by Kolmogorov-Smirnov Test.
E. Differential roles of H2A.Z in active genes and repressed genes in the self-renewal and differentiation of ESCs (see DISCUSSION for details).
DISCUSSION
How does H2A.Z contribute to the regulation of transcription?
Similar to differentiated cells (Barski et al., 2007; Bruce et al., 2005; Cui et al., 2009; Jin et al., 2009), we found that H2A.Z is linked to gene activation in murine ES cells and its acetylation state correlates with the level of gene expression. H2A.Z co-localizes with H3K4me3 in transcriptional regulatory regions, including promoters and enhancers. While this manuscript was being revised, the co-localization of H2A.Z with H3K4me3 in mouse ESCs was also reported by (Ku et al., 2012), who also noted the relationship between its state of acetylation and the level of gene expression. The H2A.Z level at enhancers is positively correlated with H3K27ac, a mark of enhancer activity (Hawkins et al., 2011; Jaenisch et al., 2010; Rada-Iglesias et al., 2011), suggesting that it also influences the activity of enhancers. Incorporation of H2A.Z into nucleosomes leads to an unstable structure in vitro (Jin et al., 2009). Furthermore, it was recently shown that H2A.Z deposition at promoter regions facilitates gene activation following 5-Aza-CdR-induced demethylation in cancer cells and this is achieved by a reduction of nucleosome occupancy and generating a ‘permissive’ environment (Yang et al., 2012). Consistent with these observations, inclusion of H2A.Z generates an abnormal nucleosome and protects only 120bp of DNA from MNase digestion in T cells (Tolstorukov et al., 2009). Thus H2A.Z appears to regulate chromatin accessibility by modulating nucleosome occupancy at regulatory regions. Indeed, our data indicate that knocking down H2A.Z in murine ES cells leads to a decrease in the number of smaller H2A.Z nucleosomes and an increase in general nucleosome occupancy at critical cis-regulatory regions. Consistent with these results, knockdown of H2A.Z results in decreased chromatin accessibility when probed by Benzonase digestion. These results support the hypothesis that H2A.Z as an essential component of an unstable nucleosome, from which the octamer can readily be evicted or displaced, as described earlier (Jin et al., 2009). The loss or displacement of nucleosomes from DNA allows the binding of factors/complexes at various cis elements where H2A.Z is found including 1) transcription complexes at active TSS, 2) PRC2 at bivalent TSS and 3) sequence specific transcription factors at enhancers. H2A.Z therefore appears as a ‘general facilitator’ for the activity of a wide variety of other functional factors/complexes.
How does H2A.Z contribute to the self-renewal and differentiation of ESCs?
We demonstrate that H2A.Z is required for efficient self-renewal of ES cells. In the H2A.Z knockdown cells, expression of many transcription factors such as Klf4, Tbx3 and Sox2 critical for ESC funtion were decreased. It has been shown previously that the pluripotency transcription factor, OCT4, binds to enhancers and mediates activation of characteristic ESC genes. OCT4 binding could directly promote the assembly of the transcription machinery or promote H3K4 methylation by recruiting the MLL complexes (Ang et al., 2011). Our data indicate that H2A.Z-mediated chromatin accessibility is critical for OCT4 targeting to enhancers: knockdown of H2A.Z compromises OCT4 binding to the downstream enhancers of the Klf4 gene. At a global level, inhibition of H2A.Z decreased OCT4 binding over a large fraction of its target sites, which leads to less efficient recruitment of MLL complexes, decreased H3K4 methylation and down-regulation of key ESC genes.
Inhibition of H2A.Z also led to increased expression of differentiation genes in ESCs. Others have shown (Creyghton et al., 2008) and we confirmed here that reduction of H2A.Z compromises targeting of the PRC2 complex and decreases H3K27 trimethylation, resulting in activation of differentiation genes normally repressed in ESCs. Our data suggest that H2A.Z-mediated OCT4 targeting might also be involved in recruitment of SUZ12 to a subset of the repressed enhancers.
Although inhibition of H2A.Z triggers premature differentiation of ESCs, H2A.Z is required for efficient ESC differentiation. Similar to the self-renewal of ESCs, the chromatin accessiblity mediated by deposition of H2A.Z may be critical for full activation of differentiation genes and complete repression of ESC-specific genes during differentiaiton of ESCs. Indeed, we observe that both gene activation and repression during EB formation were compromised on H2A.Z knockdown. Furthermore, we find that pre-configuration of chromatin by H2A.Z is required for optimal targeting of RARα during RA-induced differentiation of ESCs.
In summary, we provide data showing differential roles of H2A.Z at active genes and repressed genes in the self-renewal and differentiation of ESCs (Figures 7E). H2A.Z facilitates expression of many pluripotency genes and also the repression of differentiation genes by generating chromatin accessibility and thereby facilitating the efficient targeting of activating and repressive complexes, respectively. During differentiation of ESCs, optimal induction of differentiation genes and the complete silencing of pluripotency genes also requires H2A.Z to facilitate access of the appropriate complexes. For these reasons we propose that H2A.Z is a ‘general facilitator’ that generates access for a wide variety of activating and repressive complexes.
EXPERIMENTAL PROCEDURES
Cells & cell culture
CMTi, and R1 murine ES cell lines were routinely cultured on feeder coated dishes in ES-qualified DMEM supplemented with various growth factors. More details and analysis of ES cell morphology, embryoid body formation and retinoic acid treatment are described in Supplemental Experimental Procedures.
Knockdown of H2A.Z and MLL4 using shRNA
The RNA interference constructs targeting mouse H2A.Z and MLL4 were generated by inserting target sequences (Supplemental Experimental Procedures) into pGreenPuro Lentivector (System Biosciences). The lentiviral particles were packaged in 293T cells with the psPax2 packaging plasmid and lentiviral supernatants were then used to infect mouse ES cells for knockdown experiments.
Chromatin preparation, antibodies and ChIP-Seq
ChIP-Seq experiments were performed as described previously (Barski et al., 2007) with antibodies against pan H2A.Z and against acH2A.Z (Bruce et al., 2005). Other antibodies used in ChIP-Seq experiments of this study are listed in Table S1.
BNase-Seq for chromatin accessibility assay
The method of Benzonase digestion for a genome wide profiling of chromatin accessibility followed that described by Grontved et al (2012) with some modifications (Supplemental Experimental Procedures).
Public ChIP-Seq data
Other ChIP-Seq data obtained from the GEO database for mouse ES cells include H3K27ac, H3K4me3, H3K27me3 and H3K36me3 and p300 (Jaenisch et al., 2010; Mikkelsen et al., 2007).
Data analysis
Sequence alignment & peak calling, definition of genomic regions, heatmaps for the distribution of histone modifications at promoters and enhancers, Pearson coefficients for similarity of spatial distributions between two epigenetic markers, MA analysis and definition of differentially expressed genes are described in Supplemental Experimental Procedures.
Supplementary Material
Highlights.
H2A.Z is required for both self-renewal and differentiation of ES cells
H2A.Z destabilizes nucleosomes and increases chromatin accessibility at enhancers
H2A.Z promotes OCT4, MLLs and PRC2 binding for activation and repression in ESCs
H2A.Z promotes RARα binding, and gene activation and repression in differentiation
Acknowledgments
We thank Zhibin Wang, Dustin E. Schones, Iouri Chepelev, Brian J. Abraham, Gang Wei, Daniel Kraushaar, and Benjamin Kidder for helpful discussions. The DNA Sequencing Core and the Flow Cytometry Core of NHLBI assisted with this work. Supported was provided by the Division of Intramural Research Program of the National Institute of Heart, Lung and Blood Institute, NIH.
Footnotes
Accession number
The GEO accession number for data reported in this paper is GSE34483.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat R, Gudas LJ. RARgamma is required for correct deposition and removal of Suz12 and H2A.Z in embryonic stem cells. J Cell Physiol. 2011;226:293–298. doi: 10.1002/jcp.22420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, Ding J, Ge Y, Darr H, Chang B, et al. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145:183–197. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bruce K, Myers FA, Mantouvalou E, Lefevre P, Greaves I, Bonifer C, Tremethick DJ, Thorne AW, Crane-Robinson C. The replacement histone H2A.Z in a hyperacetylated form is a feature of active genes in the chicken. Nucleic acids research. 2005;33:5633–5639. doi: 10.1093/nar/gki874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Zhang Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol Cell. 2004;15:57–67. doi: 10.1016/j.molcel.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Cho YW, Hong S, Jin Q, Wang L, Lee JE, Gavrilova O, Ge K. Histone methylation regulator PTIP is required for PPARgamma and C/EBPalpha expression and adipogenesis. Cell Metab. 2009;10:27–39. doi: 10.1016/j.cmet.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YW, Hong T, Hong S, Guo H, Yu H, Kim D, Guszczynski T, Dressler GR, Copeland TD, Kalkum M, et al. PTIP associates with MLL3- and MLL4-containing histone H3 lysine 4 methyltransferase complex. J Biol Chem. 2007;282:20395–20406. doi: 10.1074/jbc.M701574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conerly ML, Teves SS, Diolaiti D, Ulrich M, Eisenman RN, Henikoff S. Changes in H2A.Z occupancy and DNA methylation during B-cell lymphomagenesis. Genome Research. 2010;20:1383–1390. doi: 10.1101/gr.106542.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton MP, Markoulaki S, Levine SS, Hanna J, Lodato MA, Sha K, Young RA, Jaenisch R, Boyer LA. H2AZ Is Enriched at Polycomb Complex Target Genes in ES Cells and Is Necessary for Lineage Commitment. Cell. 2008;135:649–661. doi: 10.1016/j.cell.2008.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui K, Zang C, Roh TY, Schones DE, Childs RW, Peng W, Zhao K. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4:80–93. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez del Corral R, Storey KG. Opposing FGF and retinoid pathways: a signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis. Bioessays. 2004;26:857–869. doi: 10.1002/bies.20080. [DOI] [PubMed] [Google Scholar]
- Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD, Roeder RG. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nature structural & molecular biology. 2006;13:713–719. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- Dryhurst D, Ishibashi T, Rose KL, Eirin-Lopez JM, McDonald D, Silva-Moreno B, Veldhoen N, Helbing CC, Hendzel MJ, Shabanowitz J, et al. Characterization of the histone H2A.Z-1 and H2A.Z-2 isoforms in vertebrates. Bmc Biology. 2009;7:86. doi: 10.1186/1741-7007-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473:43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JA, Garlick JD, Kingston RE. Chromatin remodeling by imitation switch (ISWI) class ATP-dependent remodelers is stimulated by histone variant H2A.Z. J Biol Chem. 2010;285:4645–4651. doi: 10.1074/jbc.M109.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grontved L, Bandle R, John S, Baek S, Chung HJ, Liu Y, Aguilera G, Oberholtzer C, Hager GL, Levens D. Rapid Genome-Scale Mapping of Chromatin Accessibility in Tissue. Epigenetics Chromatin. 2012;5:10. doi: 10.1186/1756-8935-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S, Jacques PE, Gevry N, Forest A, Fortin ME, Laflamme L, Gaudreau L, Robert F. The Euchromatic and Heterochromatic Landscapes Are Shaped by Antagonizing Effects of Transcription on H2A.Z Deposition. Plos Genetics. 2009;5:e1000687. doi: 10.1371/journal.pgen.1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Hon GC, Yang C, Antosiewicz-Bourget JE, Lee LK, Ngo QM, Klugman S, Ching KA, Edsall LE, Ye Z, et al. Dynamic chromatin states in human ES cells reveal potential regulatory sequences and genes involved in pluripotency. Cell Res. 2011;21:1393–1409. doi: 10.1038/cr.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HH, Meyer CA, Shin H, Bailey ST, Wei G, Wang Q, Zhang Y, Xu K, Ni M, Lupien M, et al. Nucleosome dynamics define transcriptional enhancers. Nature genetics. 2010;42:343–347. doi: 10.1038/ng.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nature genetics. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- Hu G, Schones DE, Cui K, Ybarra R, Northrup D, Tang Q, Gattinoni L, Restifo NP, Huang S, Zhao K. Regulation of nucleosome landscape and transcription factor targeting at tissue-specific enhancers by BRG1. Genome research. 2011;21:1650–1658. doi: 10.1101/gr.121145.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CY, Felsenfeld G. Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes & Development. 2007;21:1519–1529. doi: 10.1101/gad.1547707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CY, Zang CZ, Wei G, Cui KR, Peng WQ, Zhao KJ, Felsenfeld G. H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nature Genetics. 2009;41:941–945. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Vanoosthuyse V, Fillingham J, Roguev A, Watt S, Kislinger T, Treyer A, Carpenter LR, Bennett CS, Emili A, et al. An acetylated form of histone H2A.Z regulates chromosome architecture in Schizosaccharomyces pombe. Nature Structural & Molecular Biology. 2009;16:1286–U1107. doi: 10.1038/nsmb.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M, Jaffe JD, Koche RP, Rheinbay E, Endoh M, Koseki H, Carr SA, Bernstein BE. H2A.Z landscapes and dual modifications in pluripotent and multipotent stem cells underlie complex genome regulatory functions. Genome Biol. 2012;13:R85. doi: 10.1186/gb-2012-13-10-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach TJ, Mazzeo M, Chotkowski HL, Madigan JP, Wotring MG, Glaser RL. Histone H2A.Z is widely but nonrandomly distributed in chromosomes of Drosophila melanogaster. Journal of Biological Chemistry. 2000;275:23267–23272. doi: 10.1074/jbc.M910206199. [DOI] [PubMed] [Google Scholar]
- Meissner A, Mikkelsen TS, Gu HC, Wernig M, Hanna J, Sivachenko A, Zhang XL, Bernstein BE, Nusbaum C, Jaffe DB, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–U791. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku MC, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–U552. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekowska A, Benoukraf T, Ferrier P, Spicuglia S. A unique H3K4me2 profile marks tissue-specific gene regulation. Genome Res. 2010;20:1493–1502. doi: 10.1101/gr.109389.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekowska A, Benoukraf T, Zacarias-Cabeza J, Belhocine M, Koch F, Holota H, Imbert J, Andrau JC, Ferrier P, Spicuglia S. H3K4 tri-methylation provides an epigenetic signature of active enhancers. EMBO J. 2011;30:4198–4210. doi: 10.1038/emboj.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy D, Berven L, Ridgway P, Tremethick DJ. Pericentric heterochromatin becomes enriched with H2A.Z during early mammalian development. Embo Journal. 2003;22:1599–1607. doi: 10.1093/emboj/cdg160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren QH, Gorovsky MA. Histone H2A.Z acetylation modulates an essential charge patch. Molecular Cell. 2001;7:1329–1335. doi: 10.1016/s1097-2765(01)00269-6. [DOI] [PubMed] [Google Scholar]
- Roh TY, Cuddapah S, Zhao K. Active chromatin domains are defined by acetylation islands revealed by genome-wide mapping. Genes Dev. 2005;19:542–552. doi: 10.1101/gad.1272505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh TY, Wei G, Farrell CM, Zhao K. Genome-wide prediction of conserved and nonconserved enhancers by histone acetylation patterns. Genome Research. 2007;17:74–81. doi: 10.1101/gr.5767907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schones DE, Cui KR, Cuddapah S, Roh TY, Barski A, Wang ZB, Wei G, Zhao KJ. Dynamic regulation of nucleosome positioning in the human genome. Cell. 2008;132:887–898. doi: 10.1016/j.cell.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Thambirajah AA, Dryhurst D, Ishibashi T, Li A, Maffey AH, Ausio J. H2A.Z stabilizes chromatin in a way that is dependent on core histone acetylation. Journal of Biological Chemistry. 2006;281:20036–20044. doi: 10.1074/jbc.M601975200. [DOI] [PubMed] [Google Scholar]
- Tillo D, Kaplan N, Moore IK, Fondufe-Mittendorf Y, Gossett AJ, Field Y, Lieb JD, Widom J, Segal E, Hughes TR. High nucleosome occupancy is encoded at human regulatory sequences. PLoS One. 2010;5:e9129. doi: 10.1371/journal.pone.0009129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstorukov MY, Kharchenko PV, Goldman JA, Kingston RE, Park PJ. Comparative analysis of H2A.Z nucleosome organization in the human and yeast genomes. Genome Research. 2009;19:967–977. doi: 10.1101/gr.084830.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremethick DJ, Fan JY, Gordon F, Luger K, Hansen JC. The essential histone variant H2A.Z regulates the equilibrium between different chromatin conformational states. Nat Struct Biol. 2002;9:172–176. doi: 10.1038/nsb767. [DOI] [PubMed] [Google Scholar]
- Tremethick DJ, Fan JY, Rangasamy D, Luger K. H2A.Z alters the nucleosome surface to promote HP1 alpha-mediated chromatin fiber folding. Molecular Cell. 2004;16:655–661. doi: 10.1016/j.molcel.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Valdes-Mora F, Song JZ, Statham AL, Strbenac D, Robinson MD, Nair SS, Patterson KI, Tremethick DJ, Stirzaker C, Clark SJ. Acetylation of H2A.Z is a key epigenetic modification associated with gene deregulation and epigenetic remodeling in cancer. Genome Res. 2011 doi: 10.1101/gr.118919.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZB, Zang CZ, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui KR, Roh TY, Peng WQ, Zhang MQ, et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nature Genetics. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Noushmehr H, Han H, Andreu-Vieyra C, Liang G, Jones PA. Gene reactivation by 5-aza-2′-deoxycytidine-induced demethylation requires SRCAP-mediated H2A.Z insertion to establish nucleosome depleted regions. PLoS Genet. 2012;8:e1002604. doi: 10.1371/journal.pgen.1002604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang CZ, Schones DE, Zeng C, Cui KR, Zhao KJ, Peng WQ. A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics. 2009;25:1952–1958. doi: 10.1093/bioinformatics/btp340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman D, Coleman-Derr D, Ballinger T, Henikoff S. Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature. 2008;456:125–U114. doi: 10.1038/nature07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatanova J, Thakar A. H2A.Z: View from the top. Structure. 2008;16:166–179. doi: 10.1016/j.str.2007.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.