Abstract
Glioblastomas are grade IV brain tumors characterized by high aggressiveness and invasiveness, giving patients a poor prognosis. We investigated the effects of the multi-kinase inhibitor sorafenib on six cultures isolated from human glioblastomas and maintained in tumor initiating cells-enriching conditions. These cell subpopulations are thought to be responsible for tumor recurrence and radio- and chemo-resistance, representing the perfect target for glioblastoma therapy. Sorafenib reduces proliferation of glioblastoma cultures, and this effect depends, at least in part, on the inhibition of PI3K/Akt and MAPK pathways, both involved in gliomagenesis. Sorafenib significantly induces apoptosis/cell death via downregulation of the survival factor Mcl-1. We provide evidence that sorafenib has a selective action on glioblastoma stem cells, causing enrichment of cultures in differentiated cells, downregulation of the expression of stemness markers required to maintain malignancy (nestin, Olig2 and Sox2) and reducing cell clonogenic ability in vitro and tumorigenic potential in vivo. The selectivity of sorafenib effects on glioblastoma stem cells is confirmed by the lower sensitivity of glioblastoma cultures after differentiation as compared with the undifferentiated counterpart. Since current GBM therapy enriches the tumor in cancer stem cells, the evidence of a selective action of sorafenib on these cells is therapeutically relevant, even if, so far, results from first phase II clinical trials did not demonstrate its efficacy.
Keywords: glioblastoma, sorafenib, therapy, tumor initiating cells, stemness, Mcl-1
Introduction
Glioblastomas (GBM) are the most common and aggressive astrocytic tumors. According to histological features and genetic alterations, the World Health Organization (WHO) classified GBM as grade IV tumors.1 As the old denomination “multiforme” reminds, these tumors present high cytopathological and genetic heterogeneity.2 The heterogeneous cellular phenotype of GBM is ascribed to the presence within the tumor of a subpopulation of cells known as tumor-initiating cells (TICs).3 TICs share some properties with normal stem cells, such as self-renewal and capacity to originate more differentiated cells4,5 and, importantly, represent the only cell subpopulation endowed with the ability to form tumor when transplanted in immunocompromised mice, recreating original tumor complexity and establishing a hierarchy of cells with different tumorigenic potentials.3,6 Cell heterogeneity and infiltrative nature concur to make GBM one of the most difficult tumor to treat.7 The high frequency of relapse occurring in GBM patients8 after surgical debulking and therapies could be sustained by TICs. In fact, these cells are more radio- and chemoresistant than more differentiated tumor cells,9 and for this reason, after conventional treatments, residual cancer cell foci could be enriched in TICs that will promote the relapse of the disease.10 Based on this evidence, targeting TICs represents an absolute requirement for the development of more efficacious therapies against GBM.11 Several drugs have been tested on GBM stem cells. Recently, the effectiveness of disulfiram, a widely used drug for alcoholism treatment, was reported on self-renewing GBM stem cells and temozolomide (TMZ)-resistant cells.12,13 The anti-diabetic drug metformin, which exerts antitumor activity against several solid tumors, demonstrates a greater efficacy on GBM TICs than on differentiated GBM cells.14 Several constitutively active or upregulated intracellular pathways were identified in GBM and gained interest for the development of novel therapeutic approaches, including NFkB,15 p53, Rb and pathways downstream receptor tyrosine kinases (RTKs).16 In the last few years a large number of studies have been undertaken to test the effects of targeted therapies against RTKs in several malignancies.17 Amplification/overexpression of RTKs genes (as epithelial growth factor receptor, EGFR; platelet derived growth factor receptor, PDGFR; vascular endothelial growth factor receptor, VEGFR; MET and KIT) is very frequent in GBM and drives the process of tumorigenesis,18 transducing signals through pathways such as those of MAPK and PI3K/Akt, which induce cell proliferation, survival, migration and drug resistance.19,20
We previously reported that inhibition of EGF-stimulated Akt by EGFR tyrosine kinase inhibitors (gefitinib and erlotinib) is required to affect human GBM TIC survival in vitro.21 However, likely because of the simultaneous co-activation of multiple RTKs,22 single RTK inhibition failed to demonstrate survival benefits in patients.23,24 More favorable effects could be achieved combining drugs against different RTKs or using multi-target drugs in order to completely abrogate the transduction pathways on which they converge and, thus, to bypass parallel signaling activation.16,24
Sorafenib is an oral multikinase inhibitor that targets several RTKs, including VEGFR2 and VEGFR3, PDGFRβ, fibroblast growth factor receptor 1 (FGFR1), Flt-3, RET and c-Kit.25 Sorafenib can also act directly inhibiting the downstream serine/threonine kinase Raf, a pivotal member of the MEK/ERK signal transduction pathway. Recent studies demonstrated that Raf1 activation provokes glioma formation in mice,26,27 underlining its importance in gliomagenesis. Sorafenib showed efficacy against different solid tumors25 and has already received FDA and EMA approval for the treatment of patients with advanced renal carcinoma28 and unresectable hepatocellular carcinoma (HCC).29 However, Raf/MEK/ERK pathway is not the only target of sorafenib in HCC cells, and a series of genes modulated by sorafenib that could be helpful to find new mechanisms of action was identified.30
Sorafenib inhibits the proliferation of human GBM cell lines, acting synergistically with bortezomib,31 and currently, several phase I/II trials are completed or ongoing32 in combination with TMZ, bevacizumab or radiotherapy.
In the present study we evaluated the anti-proliferative and pro-apoptotic effects of sorafenib on GBM TIC-enriched cultures showing a selective effect on the stem cells’ compartment, resulting in impairment of clonogenic ability in vitro and tumorigenic potential in vivo.
Results
Sorafenib exerts in vitro antiproliferative effects in GBM TICs
During preliminary experiments, cell concentration ensuring an exponential proliferation for the entire duration of treatment (up to 4 d) was determined for each GBM TIC culture, as we previously reported.21 From these experiments we identified 2,500, 3,000, 25,000, 12,000, 5,000 and 15,000 cells/well for GBM 3, 23, 24, 17, 19 and 6, respectively, as the optimal cell number for seeding each culture in a 96-wells plate (data not shown).
To evaluate whether sorafenib exerts antiproliferative effects on GBM TICs in vitro, cultures derived from all six GBMs were treated with increasing concentrations of sorafenib (from 5 to 20 µM) for different time intervals (48 and 72 h). Viability of cells was measured at each time point by MTT assay, and results were expressed as percentage of treated cells vs. control (vehicle treated) cells. As summarized in Figure 1A, sorafenib induced a dose- and time-dependent reduction of cell number in all GBM TIC cultures. Sorafenib half-maximal inhibitory concentration (IC50), measured at 48 h from dose-response curves (data not shown), was very similar in all the cultures: 15 µM for GBM 3, 23, 24 and 17; 12 µM for GBM 19 and 8 µM for GBM 6.
Figure 1. Inhibitory effect of sorafenib on proliferation and DNA synthesis of human GBM TICs. (A) TICs from six GBMs were plated in 96-well microplates and then treated with different concentrations of sorafenib 5 µM (left), 10 µM (center) and 20 µM (right) for 48 and 72 h. Results are expressed as means of percentage of cells vs. vehicle treated controls (measured by MTT assay) from four independent experiments, each performed in quadruplicate. All SE are lower than 5% of each mean value. (B) Sorafenib effects on DNA synthesis of GBM TIC cultures evaluated by BrdU incorporation in control (vehicle-treated) and in 24 h sorafenib-treated cells (IC50). Results are expressed as means, and error bars represent standard deviations from three different experiments. * p < 0.05; ** p < 0.01.
Sorafenib inhibition of GBM TIC DNA synthesis
To investigate whether sorafenib antiproliferative activity also involves a reduction of GBM TIC DNA synthesis, we measured the effects of this drug on BrdU incorporation after 24 h of treatment. As compared with vehicle-treated cells, the percentage of BrdU-positive cells was significantly decreased (Fig. 1B) from 20.1% to 0.7% in GBM 3 (p < 0.01), from 34.8% to 5.3% in GBM 23 (p < 0.01), from 15.5% to 0.8% in GBM 24 (p < 0.05), from 10.3% to 0.6% in GBM 17 (p < 0.05), from 20.2% to 2.7% in GBM 19 (p < 0.05) and from 13.2% to 0.8% in GBM 6 (p < 0.01).
These results clearly indicated that sorafenib induces growth arrest in all GBM TICs in the first 24 h of treatment, preventing the entering the S phase.
Sorafenib induces apoptosis in the vast majority of GBM TICs cultures
The contribution of cell death to the antiproliferative effects of sorafenib in GBM TICs was measured after 24 and 48 h of treatment with drug concentrations corresponding to the respective IC50. To accurately assess TIC viability, apoptosis was analyzed using annexin-V binding assay, in conjunction with propidium iodide (PI) staining to detect necrotic cells (Fig. 2). Data are reported as percentage of overall cell death, including PI-positive necrotic cells, early apoptotic cells (annexin-V-positive) and late apoptotic cells (labeled with both PI and annexin-V). The response to sorafenib treatment widely differed among cultures: TICs from GBM 3, 23, 24 and 17 showed a considerable increase of overall cell death compared with control, already significant at 24 h and reaching maximal values at 48 h of treatment (Fig. 2): from 8.8% to 44.7% in GBM 3, from 7.9% to 68.4% in GBM 23, from 10.1% to 74.2% in GBM 24 and from 20.1% to 72.2% in GBM 17. GBM 19 showed a lower cell death induction that became statistically significant only after 48 h (from 8.6% to 35.6%). Conversely, no evidence of cell death induction was detected in GBM 6, suggesting that, in this case, cell death and apoptosis do not contribute to the antiproliferative effect of sorafenib. The contribution of the apoptotic process to cell growth arrest exerted by sorafenib in GBM TICs is highlighted in Figure 2 by bars including the percentage of apoptotic fraction in each culture. Importantly, the apoptotic component in all TIC cultures (with exception of GBM 6 and partially GBM 19) represented a high percentage of the total cell death reaching values largely greater than 50%.

Figure 2. Sorafenib induces apoptosis in GBM TICs. Apoptosis/cell death induction in GBM TICs after 24 and 48 h of treatment with sorafenib IC50 (S) or control (C; vehicle-treated). Bars represent overall cell death and white boxes the apoptosis contribution (early and late apoptosis). Results are expressed as means, and error bars represent standard deviations from four different experiments. * p < 0.05; ** p < 0.01.
Intracellular mechanisms mediating sorafenib pro-apoptotic effects
To define the intracellular mechanisms by which sorafenib affects human GBM TIC survival in vitro, we analyzed the activation of intracellular pathways related to GBM development and, in particular, STAT-3, MEK/ERK1/2 and Akt pathways. The analysis was performed by western blot in control cells and in cells treated for 24 h with sorafenib, using concentrations corresponding to 0.5X, 1X and 2X-fold the calculated IC50 for each GBM TIC culture. Sorafenib-induced modulation of STAT-3 phosphorylation, one of the downstream molecules involved in GBM invasion and proliferation, was not strictly correlated with antiproliferative or pro-apoptotic effects observed in GBM TIC cultures, STAT-3 activation being variably modulated or unaffected (Fig. 3). Conversely, as expected according to the known mechanism of action of sorafenib, the treatment caused a significant reduction of the activation of MEK and ERK1/2 as well as of Akt in the responsive cultures (GBM 3, 23, 24 and 17) (Fig. 3; Fig. S1 for the densitometric quantification of the immunoreactive bands). This effect was obtained only for Akt at the highest concentration tested (2X IC50) in the partially responsive culture GBM 19, and completely absent in GBM 6, which we’ve demonstrated to be insensitive to apoptotic effects of the drug (Fig. 3).

Figure 3. Effects of sorafenib on STAT-3, MAPK pathway, Akt and Mcl-1. Summary of western blots that evaluate the levels of phosphorylated/active forms of STAT-3, MEK, ERK1/2, Akt and Mcl-1 content after 24 h treatment with three concentration of sorafenib (0.5X IC50, IC50 and 2x IC50) are showed. Results are representative blots from three independent experiments. Tubulin expression was evaluated to normalize immunoreactive band to total protein load. Densitometric evaluations of the data are reported in the Figure S1. Sorafenib treatment reduces the phosphorylation of MEK and ERK1/2 as well as of Akt in the more responsive cultures (GBM 3, 23, 24 and 17); in the less responsive culture (GBM 19), only a reduction of Akt activity at the higher dose is observed, and no changes are detected in GBM 6 culture. The STAT-3 phosphorylation levels differ among the six cultures tested without any correlation to the anti-proliferative or pro-apoptotic effects of sorafenib. The reduction of Mcl-1 correlates to the pro-apoptotic effects.
Since Mcl-1, an anti-apoptotic member of the Bcl-2 family, was reported to play a key role in pro-apoptotic effects of sorafenib,33 we investigated its expression in GBM TICs. The activity of sorafenib on MEK/ERK and Akt activation was paralleled by the dramatic downregulation of Mcl-1, in sorafenib-responsive TICs, starting from the lower concentration used (0.5X IC50) and proportionally increasing up to the 2X IC50. In the GBM 19 TICs that showed low induction of the apoptotic fraction after exposure to sorafenib, we observed a slight reduction of Mcl-1 only at the highest concentration tested, while Mcl-1 expression was unaffected in GBM 6, which did not activate the apoptotic process in response to sorafenib (Fig. 3; Fig. S1).
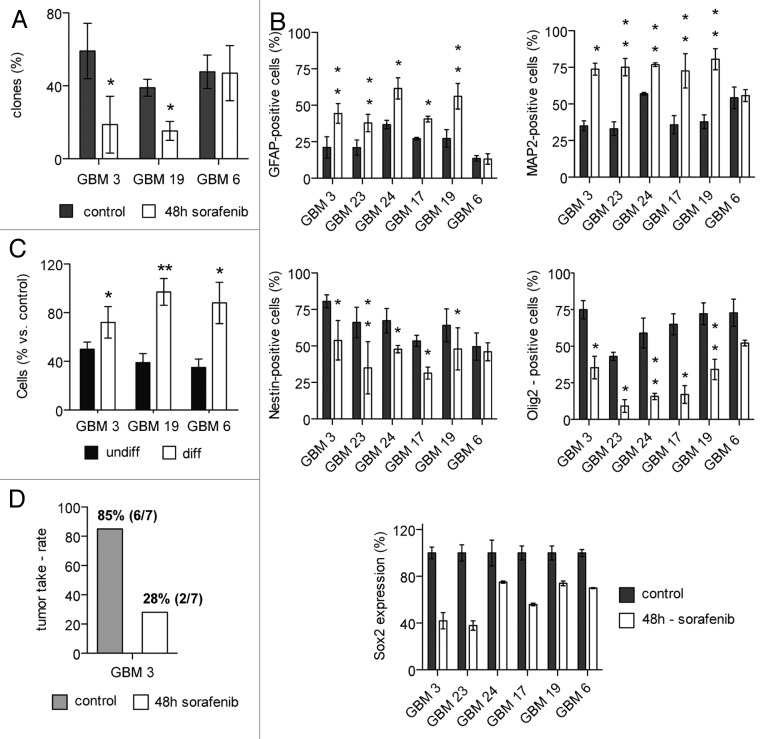
Sorafenib impairs the clonogenic potential in vitro and the tumorigenicity in vivo of sensitive GBM TICs
In order to understand whether sorafenib preferentially reduces the viability of the stem cells subset, or whether it also might affect the differentiated subpopulation present in GBM cultures, the clonogenic potential of cells surviving 48 h treatment with sorafenib was evaluated in three GBM TIC cultures showing different levels of sensitivity to the pro-apoptotic effect of sorafenib (namely, GBM 3, 19 and 6). The same number of viable cells was plated for control and sorafenib-treated conditions. Clonogenic assays identify single cell-derived colonies grown above an arbitrarily designated cut-off size in a given time: committed cells with a limited residual proliferative potential give rise to small colonies, while stem-like cells with self-renewal capacity are able to originate large colonies. The cut-off chosen was 100 cells after 2 weeks of culture (see “Materials and Methods”). As shown in Figure 4A, sorafenib treatment induced a significant reduction of the clonogenic potential of GBM 3 and GBM 19: from 67.9% to 26.8%, and from 38.9% to 11.9%, respectively. In contrast, sorafenib did not reduce clonogenicity of GBM 6 TICs (Fig. 4A). Correspondingly, a significant reduction of the clonogenic potential after treatment was confirmed in GBM 3 and GBM 19 by MTT colorimetric assays performed three weeks after cell seeding, using an absorbance value of 0.400 as a threshold (data not shown).
Figure 4. Sorafenib selectivity for cancer stem cells in GBM TIC cultures: Modulation of marker expression and impairment of clonogenic and tumorigenic potential. (A) Effects of sorafenib on in vitro clonogenic potential of GBM 3, 19 and 6. Cells were treated for 48 h with sorafenib (IC50) or vehicle (control), and clone formation was estimated after two weeks from clonal seeding. Results are reported as mean percentage of clones from three independent experiments, and error bars represent standard deviations. (B) Expression of differentiation (GFAP and MAP2, upper panels) and stemness (Nestin and Olig2, middle panels and Sox2, lower panel) markers in GBM TIC cultures after 48 h of treatment with sorafenib (IC50) or vehicle. Results are reported as mean percentage from three different experiments and error bars represent standard deviations. All determinations were performed by FACS, except that of Sox2 that was performed by western blot. (C) Reduced sensitivity to sorafenib of differentiated GBM cultures, as compared with undifferentiated TICs. Undifferentiated or differentiated cells were treated for 48 h with sorafenib (20 µM), and cell number was determined by MTT assay. Results are expressed as mean percentage of cells vs. control and error bars represent standard deviations of two experiments. (D) Reduction of GBM TIC tumorigenic potential after sorafenib treatment. Results are represented as tumor take rate in mice injected with untreated or in vitro sorafenib treated (48 h) cells from GBM 3. *p < 0.05; **p < 0.01 vs. control.
These results suggest a selective activity of sorafenib on the clonogenic potential, an in vitro test to evaluate cell stemness. To confirm the depletion of the stem cells pool by sorafenib, the expression of differentiation (glial fibrillary acidic protein, GFAP and microtubule-associated protein 2, MAP2) or stemness (nestin, Olig2 and Sox2) markers was evaluated in treated and untreated cells. The levels of basal expression of GFAP, astroglial differentiation marker and MAP2, neuronal differentiation marker, considerably differed among the GBM TICs used in this study (Fig. 4B, upper panels). With the only exception of GBM 6, whose GFAP- and MAP2-positive cell percentages were not affected by sorafenib (from 13.6% to 13.2% of GFAP-positive cells and from 54.2% to 55.7% of MAP2-positive cells in untreated and treated cells, respectively), all other cultures showed a significant increment of the fraction of GFAP-positive cells: from 21.1% to 44.4% in GBM 3, from 21.0% to 37.9% in GBM 23, from 36.8% to 61.6% in GBM 24, from 27.1% to 40.6% in GBM 17 and from 27.3% to 56.2% in GBM 19. The cell populations which survived the treatment also included a consistent fraction of MAP2-positive cells that generally was at least 2-fold greater in sorafenib-treated cells as compared with controls (Fig. 4B, upper panel).
The analysis of stem-specific markers evidenced that, with the exception of GBM 6, all cultures significantly reduced the percentage of nestin- and Olig2-expressing cells after 48-h exposure to sorafenib (Fig. 4B, middle panels). In particular, the amount of sorafenib-dependent decrease was about 35% (min -25% for GBM 19/max -47% for GBM 23) for nestin positivity, and more marked (average -67%; min -53% for GBM 19/max -79% for GBM 23) as far as Olig2 expression. GBM 6 showed no reduction in nestin-expressing cell content and a low decrease in Olig2-positive cells (from 72.9% to 52.2%) that did not reach statistical significance (Fig. 4B, middle panels). Furthermore, the expression levels of Sox2, a marker for normal neural cells also linked to self-renewal properties of GBM stem cells, were evaluated by western blot analysis. After 48 h of treatment, sorafenib decreased Sox2 protein levels in all GBM cultures. GBM 3, 17 and 23 cells showed the highest reduction of Sox2 expression after sorafenib treatment in comparison with vehicle-treated control cells (-70%, -74% and -75%, respectively). GBM 6, 19 and 24 displayed a lower, but still significant decrease in Sox2 expression (-42%, -56% and -38%, respectively) (Fig. 4B, lower panel).
Since GBM TICs that survived sorafenib treatment are enriched in cells expressing differentiation markers and depleted in markers associated with stemness, we evaluated whether this drug might have selective effects toward TIC subpopulation. Thus the effects of sorafenib were tested on GBM 3, 19 and 6 cultures after they were induced to differentiate for two weeks in a medium containing 10% of fetal bovine serum. After this period, cells were treated with sorafenib 20 µM (the highest concentration tested on GBM cultures) for 48 h. Cell proliferation was measured by MTT assay, and results are expressed as percentage of cells detected after treatment vs. control (vehicle-treated). Differentiated GBM cells showed a significantly lower sensitivity to sorafenib than the corresponding undifferentiated cultures (Fig. 4C). In particular, we observed 72% of cells in differentiated vs. 50% in undifferentiated GBM 3, 97% in differentiated vs. 39% in undifferentiated GBM 19, and 88% in differentiated vs. 35% in undifferentiated GBM 6 (Fig. 4C). The reduced in vitro sensitivity of GBM differentiated cells, together with both enrichment in differentiation markers and inhibition of the clonogenic potential of GBM TICs after treatment with sorafenib, strongly suggests a selective action of sorafenib on the stem (undifferentiated) compartment of GBM cultures.
To confirm the in vitro results, seven mice were orthotopically transplanted with 100,000 GBM 3 cells, survived to 48 h treatment with sorafenib or left untreated. Six out of seven (85%) NOD/SCID mice intracranially injected with untreated GBM 3 cells developed a tumor mass, while only two out of seven (28%) mice injected with in vitro sorafenib-treated GBM 3 cells formed a tumor (Fig. 4D).
Discussion
Despite the introduction of TMZ improved the survival of newly diagnosed GBM patients, their prognosis remains poor, with a median survival of about 14.6 mo.34 GBM is a very aggressive and infiltrative tumor, characterized by a complex heterogeneity that hampers the development of efficacious therapies. RTKs are often amplified in GBM,18,35 and over the years different attempts to target RTKs have been made.
The aim of this study was to test the efficacy of sorafenib on primary GBM TIC-enriched cultures. TIC subpopulations represent a tumor component refractory to chemo- and radio-therapy and are supposed to be responsible for the recurrence of the tumor. Sorafenib is a small-molecule inhibitor of different RTKs, such as PDGFRβ, RET, VEGFR2 and 3, often deregulated/amplified in GBM, and the intracellular serine/threonine kinase Raf (C-Raf and both wild-type and mutant B-Raf isoforms), which has been reported to have a role in GBM tumorigenesis.26 First, our data show inhibitory effect of sorafenib on cell proliferation in all the GBM TICs tested, as assessed by cell number evaluation in MTT assay and DNA synthesis activity in BrdU incorporation assay. Conversely, the cultures differ from each other as far as the ability of sorafenib to induce apoptotic effects. In particular, the group made up by GBM 3, 23, 24 and 17 is more sensitive, showing a consistent apoptotic response after both 24 and 48 h of treatment. GBM 19 showed a significant cell death response only after 48 h of treatment, whereas GBM 6 was insensitive to cell death induction and responded to sorafenib only with cell proliferation arrest.
Both MAPK and PI3K/Akt pathways are relevant for the biology of GBM. As reported by others, the cooperation of the MAPK pathway and Akt is important to induce glioma in vivo.36,37 Akt, in particular, seems to correlate with the malignancy of GBM. Akt activation is frequent in the more tumorigenic and invasive cells, and in the stem cells of the mass38 and it is able to promote the transformation of the less malignant anaplastic astrocytoma into GBM.39
Unexpectedly, from our data we observed that the anti-proliferative activity of sorafenib, which is relevant in each culture tested, could be not only dependent on ERK1/2 and Akt inhibition. In fact, GBM 6 culture has a consistent anti-proliferative response, even if none of these pathways is altered. Thus, other still undetermined pathways are regulated by sorafenib to induce its anti-proliferative activity.
In addition, according to the pro-apoptotic effects observed, sorafenib determines a downregulation of the anti-apoptotic member of Bcl-2 family, Mcl-1. Our study demonstrates that Mcl-1 inhibition, as previously shown in multiple tumor cell lines of varying histotypes,31 is independent from ERK1/2 downregulation, since in the presence of the MEK inhibitor PD98059 a limited reduction of Mcl-1 content was observed that did not reach the statistical significance (data not shown). However, we demonstrated that Mcl-1 inhibition plays a crucial role in sorafenib activity in GBM TICs, and our results showed that sustained Mcl-1 expression might represent a possible mechanism of resistance, as evidenced by the lack of Mcl-1 modulation in GBM 6, in which sorafenib was unable to induce cell death. Interestingly, sorafenib downregulates Mcl-1 only in the drug-sensitive TIC cultures, thus suggesting that in GBM this protein plays a key role in determining sensitivity or resistance to this drug.
On the basis of experimental evidence showing that high STAT-3 activity is frequently present in human tumors, including GBM, and that STAT-3 is involved in tumorigenic processes such as cell cycle progression, apoptosis and angiogenesis,40 we analyzed the effects of sorafenib on STAT-3 phosphorylation. However, modulation of STAT-3 activation, whose inhibition was reported to reduce proliferation and induce apoptosis in both cell lines and primary cultures of GBM after sorafenib treatment41 does not seem to be implicated in the effects of sorafenib on GBM TIC cultures, further highlighting the relevance of this cancer stem cells model to test in vitro antiproliferative activity of anticancer drugs.42
We demonstrated that sorafenib reduces the in vitro clonogenic potential of cell death-responsive cultures in GBM 3 and 19 (up to 1/3 of the control values), while in GBM 6, which did not undergo to cell death, clonogenic potential was unaffected. This evidence suggests a selective induction of cell death on the stem compartment of the cultures, sparing the cell subpopulation expressing high levels of differentiation markers, such as GFAP and MAP2. In fact, in sorafenib surviving cells, higher percentages of GFAP- and MAP2-expressing cells were observed, with a concomitant reduction of cells expressing stemness markers such as nestin, and Olig2, a glioma stem cells marker crucial for their proliferation43 and considered a negative prognostic indicator in GBM.44 Interestingly, we also observed a reduction in Sox2 expression. The decrease of Sox2, which contributes to the malignant phenotype of GBM,45 could be responsible for the reduced clonogenic capability observed after sorafenib treatment.
The hypothesis of the selective action on GBM stem cells is strengthened by the lower effectiveness of sorafenib on GBM differentiated cells compared with the same cultures maintained in “stem-permissive” conditions. To further confirm the reduced stemness of cells surviving the in vitro treatment, the tumorigenicity of GBM 3 was evaluated. Transplanting GBM cells, six out of seven mice injected with untreated cells developed a tumor mass, while only two out of seven mice injected with treated cells developed a tumor, demonstrating a reduced tumorigenic potential to 1/3 of the control. The reduction of the tumor engraftment frequency paralleled the reduction of clonogenicity, confirming that the in vitro evaluation of stemness by clonogenic limiting dilution is a reliable method to be used on GBM TIC cultures. This evidence is fundamental in the tumor-initiating cells model of GBM, by reason of which a therapy could not only hit the majority of cells composing the tumor mass, but should also target the stem compartment. In fact, the latter is characterized by unlimited proliferative capacity46 and increased resistance to radio- and chemotherapy as compared with more differentiated cells within the tumor mass,9,47 thus being responsible for recurrences. Bleau et al. demonstrated that high levels of Akt activation are associated with chemo-resistance, improving the ability of GBM stem subset to expel drugs outside the cell through the activation of specific ABC transporters.48 Considering the importance of Akt in the maintenance of the GBM stem cell pool, we can hypothesize that the downregulation of Akt seen in GBM 3 and 19 TIC cultures is involved in the reduction of their clonogenic potential after sorafenib treatment. Since it has been demonstrated that TMZ increases the percentage of GBM stem cells, raising the aggressiveness of the tumor,48 the reduction of GBM stem cell compartment induced by sorafenib gains more importance in GBM therapy. Sorafenib already showed its anti-glioma activity both in vitro and in vivo, although these experiments were limited to GBM cell lines or primary cultures.49,50 To our knowledge, this is the first time that it has been demonstrated that sorafenib acts against the stem component of cultures derived from GBM patients, although the establishment of its efficacy on this cell population in vivo will require further studies.
In conclusion, using a single drug, we obtained the inhibition of two important pathways for GBM biology, MAPK and PI3K/Akt, leading to the block of proliferation and, most of all, the induction of apoptosis via Mcl-1 inhibition in GBM TICs. Moreover, in vitro and in vivo results demonstrating that sorafenib preferentially affects TIC subpopulation, thus reducing the source of feeding of the tumor mass, suggest potential usefulness that may help in treatment of drug resistance and tumor relapse. Even if our and other in vitro results are encouraging, it cannot be ignored that sorafenib has been already used in clinics, and results from first phase II trials did not demonstrate sorafenib efficacy on GBM patients.51,52 However, further investigation will be necessary to verify the optimal dosage of sorafenib to obtain a therapeutic concentration at the tumor site.
Materials and Methods
Isolation of human glioblastoma tumor-initiating cells
Tumor specimens from GBM resections obtained after patients’ informed consent and Institutional Ethical Committee approval from the Neurosurgery Department (IRCCS-AOU San Martino-IST) were histologically classified according to the WHO classification as astrocytic glioma grade IV. Tumors from six patients, three males and three females, with an average age of 53.8 y (41–70) entered this study and were coded as GBM 3, 23, 24, 17, 19 and 6. Four out of six tumor samples (GBM 3, 23, 17 and 6) derived from primary GBMs, GBM 19 from a secondary GBM and one sample (GBM 24) was from a recurrence after initial radio- and chemotherapy. After surgery, tumor samples were immediately processed for isolation of TICs as previously described.21,45 Briefly, single cells were obtained by mechanical dissociation and plated in Dulbecco’s modified Eagle medium (DMEM)/F12 and Neurobasal (Gibco-Life Technologies, #31331 and #21103), supplemented with B27 (Gibco-Life Technologies, #12587–010), 2 mM L-glutamine (Euroclone, #3000D, penicillin-streptomycin (Euroclone, #ECB1001D), recombinant human basic FGF (10 ng/ml; Peprotech, #100–18B) and recombinant human EGF (20 ng/ml; Peprotech, #AF-100–15). All GBM cultures gave rise to neurospheres and were also able to grew as a monolayer on Matrigel (BD, #354234)-coated flask without losing their spherogenic and tumorigenic properties. To induce differentiation, GBM TICs were seeded and maintained for 2 wk in DMEM/F12 medium supplemented with 2 mM L-glutamine, penicillin-streptomycin and 10% of fetal bovin serum (FBS; Euroclone, #ECS0180L).
Intracranial tumor assays
Non-obese diabetic severe combined immunodeficient (NOD-SCID) mice were used to evaluate the tumorigenicity of cell cultures previously treated in vitro with sorafenib. Animals were housed in pathogenic-free conditions, and they were handled in agreement with guidelines conforming to current Italian regulations for the protection of animals used for scientific purposes (D.lvo 27/01/1992, no.116). Procedures were approved by the Ethical Committee for Animal Experimentation of the National Institute of Cancer Research and by the Italian Ministry of Health and were performed according to the National Regulation on Animal Research Resources. Intracranial inoculations were performed on adult NOD-SCID mice (6–8 weeks) anesthetized with i.m. ketamine and xylazine. Animals were positioned into a stereotactic apparatus (Model 900; David Kopf Instruments), and a hole was made using a 21-gauge needle, 2 mm lateral and 1 mm anterior from the intersection of the coronal and sagittal sutures (bregma). Up to 5 µl of cell suspension, containing 100,000 trypan blue-negative GBM TICs, were injected using a Hamilton syringe (series 7000; Sigma-Aldrich, #Z120359) at the depth of 3 mm. After six months, all mice were sacrificed by CO2 asphyxiation, and brains were cryopreserved. Analysis/identification of tumors was performed on 10 μm coronal sections stained with hematoxylin-eosin, as previously described.45
Cell proliferation assay
To test sorafenib effects on cell proliferation, MTT assays were performed.53 Sorafenib, purchased from US Biological (#S5343–15), was reconstituted in dimethyl sulfoxide (DMSO) at the concentration of 10 mM. GBM TICs were plated in Matrigel coated 96-well microplates. The number of cells of each culture was adjusted to a density that allowed an exponential growth for the duration of the assay (72 h) ranging from 2,500 to 25,000 cells/well. After exposure to sorafenib at increasing concentrations (from 5 to 20 μM) for different time intervals (0, 48, 72 h), cells were incubated for 4 h in MTT solution (2 mg/ml in PBS; Sigma-Aldrich, #1001089589). After removing MTT, 150 µl of DMSO were added in each well to dissolve formazan crystals, and the absorbance was determined at 540-nm wavelength using a plate reader. Dose-response curves were generated, and IC50 values were calculated by Graph Pad Prism 5 software.
BrdU incorporation assays
Effects of sorafenib on DNA synthesis were evaluated by 5-bromo2'-deoxy-uridine (BrdU, Sigma-Aldrich, #B5002) incorporation assay. Briefly, after a treatment of 24 h, cells were incubated with 10 µM BrdU for 30 min at 37°C and 5% CO2. Cells were harvested and fixed with ethanol for 30 min. Following fixation cells were treated with PureLink RNaseA (1 mg/ml; Invitrogen, #12091–021) and incubated in 0.1 N HCl for 10 min on ice. After a denaturation step (90°C for 20 min then 2 min on ice), cells were incubated with mouse anti-BrdU antibody (1:100; DAKO, #M0744) for 30 min and with FITC-conjugated goat anti-mouse IgG (1%; Jackson ImmunoResearch, #115–095–003) for 20 min at room temperature. Subsequently cells were stained with 10 µg/ml propidium iodide and analyzed by flow cytometry.
Apoptosis/cell death
To assess the effect of sorafenib on cell survival, treated and control cultures were double stained with Annexin V-FITC and propidium iodide (20 µg/ml) (Annexin V – FITC Apoptosis Detection kit; eBioscience, #BMS500FI), following the manifacture guidelines. Each sample was analyzed by flow cytometry with a FACScalibur (BD Bioscience) recording 10,000 events per sample.
Clonogenic assay
To verify the effect of sorafenib on stemness of GBM TIC cultures, the ability of single cells to form a colony was tested. Untreated or sorafenib-treated (48 h, using IC50 concentration for each culture) cells were seeded out in 96-well microplates with a density of 0.8 alive cell/well. The day after plating, cells were observed under light transmitted microscope, and wells containing no alive cells or more than one cell were excluded. Cells were maintained in complete medium for a period of time of 2 wk to allow the growth of colonies. Then the dimension of each colony was determined counting the cells in each well by microscope inspection. A cut off of 100 cells was chosen to discriminate between clusters derived from committed cells from clones originated from clonogenic cells. This value was chosen, because it showed the better correlation with the tumorigenic ability of GBM TICs. After three weeks, cell content of clones was measured by MTT assay, as previously described. An absorbance threshold value of 0.4 was used to discriminate true clones from clusters.
Immunofluorescence
To determine the expression of different markers of stemness and differentiation in GBM cultures, untreated or sorafenib-treated cells were plated onto matrigel-coated glass coverslips and then fixed with 4% paraformaldehyde for 15 min at r.t. Cells were incubated with primary antibodies as follows: mouse anti-human nestin (1:10.000; Abcam, #ab6329), rabbit anti-Olig2 (1:500; Millipore, #AB9610), rabbit anti-GFAP (1:10:000; DAKO, #Z0334) and mouse anti-MAP2 (1:1.000; Chemicon International, #MAB378). Immunocomplexes were detected with secondary fluorescent antibodies as DyLight 488 goat anti-mouse IgG and DyLight 459-goat anti-Rabbit IgG (Jackson ImmunoResearch, #111–485–003 and #111–505–003). Cells were counterstained with Hoechst 33342 dye (Sigma-Aldrich, #14533) to identify all nuclei. Images were acquired by automated Zeiss AxioImager M2 equipped with an Axiocam MRM (Zeiss). Results are showed as percentage of stained cells from randomly selected fields.
Western blot
Cells were cultured for 24 h in complete medium before being treated. Cells were lysed and total proteins were isolated and measured as standard methods.54 Primary antibodies (all from Cell Signaling Technology): against phospho-Akt (Ser-473, #9271), phospho-ERK1/2 (#9101), phospho-STAT-3 (#9134), phospho-MEK (#9121), Mcl-1 (#5453), total Akt (#9272), total ERK1/2 (#9102), total MEK (#9122) and α-tubulin (Sigma Aldrich, #T5168) and Sox2 (Millipore, #AB5603). Secondary ECL mouse (#GEHNA931) and rabbit (#GEHNA934) HRP-linked whole antibodies were from GE Healthcare. Immunocomplexes were detected using chemiluminescence system according to manufacturer's instructions (Immobilon, Millipore, #WBKLS0500). Densitometric analysis of resulting bands was performed on a Chemi-Doc system by the Image Lab Software (Bio-Rad Laboratories).
Statistical analysis
Experiments were repeated at least three times and statistical significance determined by two-tailed t test. P values lower than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
Funding from Italian association for Cancer Research (AIRC 13563), Fondazione San Paolo and University of Genova (PRA 2011) to T.F. and A.D. are gratefully acknowledged.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/23372
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/23372
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 3.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones DL, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 6.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 7.Nicholas MK, Lukas RV, Chmura S, Yamini B, Lesniak M, Pytel P. Molecular heterogeneity in glioblastoma: therapeutic opportunities and challenges. Semin Oncol. 2011;38:243–53. doi: 10.1053/j.seminoncol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 9.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 10.Ghiaur G, Gerber J, Jones RJ. Concise review: Cancer stem cells and minimal residual disease. Stem Cells. 2012;30:89–93. doi: 10.1002/stem.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florio T, Barbieri F. The status of the art of human malignant glioma management: the promising role of targeting tumor-initiating cells. Drug Discov Today. 2012;17:1103–10. doi: 10.1016/j.drudis.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Hothi P, Martins TJ, Chen L, Deleyrolle L, Yoon JG, Reynolds B, et al. High-throughput chemical screens identify disulfiram as an inhibitor of human glioblastoma stem cells. Oncotarget. 2012;3:1124–36. doi: 10.18632/oncotarget.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Triscott J, Lee C, Hu K, Fotovati A, Berns R, Pambid M, et al. Disulfiram, a drug widely used to control alcoholism, suppresses the self-renewal of glioblastoma and over-rides resistance to temozolomide. Oncotarget. 2012;3:1124–36. doi: 10.18632/oncotarget.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wurth R, Pattarozzi A, Gatti M, Bajetto A, Corsaro A, Parodi A, et al. Metformin selectively affects human glioblastoma blastoma tumor-initiating cell viability: A role for metformin-induced inhibition of Akt. Cell Cycle. 2013;12:145–56. doi: 10.4161/cc.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogueira L, Ruiz-Ontañon P, Vazquez-Barquero A, Moris F, Fernandez-Luna JL. The NFκB pathway: a therapeutic target in glioblastoma. Oncotarget. 2011;2:646–53. doi: 10.18632/oncotarget.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favoni RE, Daga A, Malatesta P, Florio T. Preclinical studies identify novel targeted pharmacological strategies for treatment of human malignant pleural mesothelioma. Br J Pharmacol. 2012;166:532–53. doi: 10.1111/j.1476-5381.2012.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puputti M, Tynninen O, Sihto H, Blom T, Mäenpää H, Isola J, et al. Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol Cancer Res. 2006;4:927–34. doi: 10.1158/1541-7786.MCR-06-0085. [DOI] [PubMed] [Google Scholar]
- 19.Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–64. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–79. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Griffero F, Daga A, Marubbi D, Capra MC, Melotti A, Pattarozzi A, et al. Different response of human glioma tumor-initiating cells to epidermal growth factor receptor kinase inhibitors. J Biol Chem. 2009;284:7138–48. doi: 10.1074/jbc.M807111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snuderl M, Fazlollahi L, Le LP, Nitta M, Zhelyazkova BH, Davidson CJ, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20:810–7. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A. 2007;104:12867–72. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–90. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 26.Lyustikman Y, Momota H, Pao W, Holland EC. Constitutive activation of Raf-1 induces glioma formation in mice. Neoplasia. 2008;10:501–10. doi: 10.1593/neo.08206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson JP, VanBrocklin MW, Guilbeault AR, Signorelli DL, Brandner S, Holmen SL. Activated BRAF induces gliomas in mice when combined with Ink4a/Arf loss or Akt activation. Oncogene. 2010;29:335–44. doi: 10.1038/onc.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. TARGET Study Group Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 29.Kane RC, Farrell AT, Madabushi R, Booth B, Chattopadhyay S, Sridhara R, et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist. 2009;14:95–100. doi: 10.1634/theoncologist.2008-0185. [DOI] [PubMed] [Google Scholar]
- 30.Cervello M, Bachvarov D, Lampiasi N, Cusimano A, Azzolina A, McCubrey JA, et al. Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle. 2012;11:2843–55. doi: 10.4161/cc.21193. [DOI] [PubMed] [Google Scholar]
- 31.Yu C, Friday BB, Lai J-P, Yang L, Sarkaria J, Kay NE, et al. Cytotoxic synergy between the multikinase inhibitor sorafenib and the proteasome inhibitor bortezomib in vitro: induction of apoptosis through Akt and c-Jun NH2-terminal kinase pathways. Mol Cancer Ther. 2006;5:2378–87. doi: 10.1158/1535-7163.MCT-06-0235. [DOI] [PubMed] [Google Scholar]
- 32.Wick W, Weller M, Weiler M, Batchelor T, Yung AWK, Platten M. Pathway inhibition: emerging molecular targets for treating glioblastoma. Neuro Oncol. 2011;13:566–79. doi: 10.1093/neuonc/nor039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Secchiero P, Melloni E, Voltan R, Norcio A, Celeghini C, Zauli G. MCL1 down-regulation plays a critical role in mediating the higher anti-leukaemic activity of the multi-kinase inhibitor Sorafenib with respect to Dasatinib. Br J Haematol. 2012;157:510–4. doi: 10.1111/j.1365-2141.2012.09042.x. [DOI] [PubMed] [Google Scholar]
- 34.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 35.Joensuu H, Puputti M, Sihto H, Tynninen O, Nupponen NN. Amplification of genes encoding KIT, PDGFRalpha and VEGFR2 receptor tyrosine kinases is frequent in glioblastoma multiforme. J Pathol. 2005;207:224–31. doi: 10.1002/path.1823. [DOI] [PubMed] [Google Scholar]
- 36.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–7. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 37.Robinson JP, Vanbrocklin MW, McKinney AJ, Gach HM, Holmen SL. Akt signaling is required for glioblastoma maintenance in vivo. Am J Cancer Res. 2011;1:155–67. [PMC free article] [PubMed] [Google Scholar]
- 38.Molina JR, Hayashi Y, Stephens C, Georgescu MM. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia. 2010;12:453–63. doi: 10.1593/neo.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonoda Y, Ozawa T, Aldape KD, Deen DF, Berger MS, Pieper RO. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001;61:6674–8. [PubMed] [Google Scholar]
- 40.de la Iglesia N, Puram SV, Bonni A. STAT3 regulation of glioblastoma pathogenesis. Curr Mol Med. 2009;9:580–90. doi: 10.2174/156652409788488739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang F, Brown C, Buettner R, Hedvat M, Starr R, Scuto A, et al. Sorafenib induces growth arrest and apoptosis of human glioblastoma cells through the dephosphorylation of signal transducers and activators of transcription 3. Mol Cancer Ther. 2010;9:953–62. doi: 10.1158/1535-7163.MCT-09-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 43.Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–17. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang Y, Diehn M, Watson N, Bollen AW, Aldape KD, Nicholas MK, et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci U S A. 2005;102:5814–9. doi: 10.1073/pnas.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gangemi RMR, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–8. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 46.Bonavia R, Inda MM, Cavenee WK, Furnari FB. Heterogeneity maintenance in glioblastoma: a social network. Cancer Res. 2011;71:4055–60. doi: 10.1158/0008-5472.CAN-11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bleau A-M, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–35. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegelin MD, Raskett CM, Gilbert CA, Ross AH, Altieri DC. Sorafenib exerts anti-glioma activity in vitro and in vivo. Neurosci Lett. 2010;478:165–70. doi: 10.1016/j.neulet.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang F, Brown C, Buettner R, Hedvat M, Starr R, Scuto A, et al. Sorafenib induces growth arrest and apoptosis of human glioblastoma cells through the dephosphorylation of signal transducers and activators of transcription 3. Mol Cancer Ther. 2010;9:953–62. doi: 10.1158/1535-7163.MCT-09-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reardon DA, Vredenburgh JJ, Desjardins A, Peters K, Gururangan S, Sampson JH, et al. Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol. 2011;101:57–66. doi: 10.1007/s11060-010-0217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Den RB, Kamrava M, Sheng Z, Werner-Wasik M, Dougherty E, Marinucchi M, et al. A Phase I Study of the Combination of Sorafenib With Temozolomide and Radiation Therapy for the Treatment of Primary and Recurrent High-Grade Gliomas. International Journal of Radiation Oncology Biology Physics. 2012 doi: 10.1016/j.ijrobp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Favoni RE, Pattarozzi A, Lo Casto M, Barbieri F, Gatti M, Paleari L, et al. Gefitinib targets EGFR dimerization and ERK1/2 phosphorylation to inhibit pleural mesothelioma cell proliferation. Curr Cancer Drug Targets. 2010;10:176–91. doi: 10.2174/156800910791054130. [DOI] [PubMed] [Google Scholar]
- 54.Barbieri F, Würth R, Favoni RE, Pattarozzi A, Gatti M, Ratto A, et al. Receptor tyrosine kinase inhibitors and cytotoxic drugs affect pleural mesothelioma cell proliferation: insight into EGFR and ERK1/2 as antitumor targets. Biochem Pharmacol. 2011;82:1467–77. doi: 10.1016/j.bcp.2011.07.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.