Abstract
We have recently demonstrated the remarkable efficiency of self-complementary (sc) AAV9 vectors for central nervous system (CNS) gene transfer following intravenous delivery in mice and larger animals. Here, we investigated whether gene delivery to motor neurons (MNs) could also be achieved via intramuscular (i.m.) scAAV9 injection and subsequent retrograde transport along the MNs axons. Unexpectedly, we found that a single injection of scAAV9 into the adult mouse gastrocnemius (GA) mediated widespread MN transduction along the whole spinal cord, without limitation to the MNs connected to the injected muscle. Spinal cord astrocytes and peripheral organs were also transduced, indicating vector spread from the injected muscle to both the CNS and the periphery through release into the blood circulation. Moreover, we showed that i.m. injection of scAAV9 vectors expressing “survival of motor neuron” (Smn) in spinal muscular atrophy (SMA) mice mediated high survival motor neuron (SMN) expression levels at both the CNS and the periphery, and increased the median lifespan from 12 days to 163 days. These findings represent to date the longest extent in survival obtained in SMA mice following i.m. viral vector gene delivery, and might generate a renewed interest in the use of i.m. adeno-associated viruses (AAV) delivery for the development of gene therapy strategies for MN diseases.
Introduction
Novel molecular approaches to the treatment of motor neuron (MN) diseases, based on gene transfer using recombinant viral gene vectors, have great potential for the delivery of therapeutic proteins to MNs. Intramuscular (i.m.) injection of viral vectors such as those derived from adenoviruses, adeno-associated viruses (AAVs), or lentiviruses has been used with varying degrees of success for MN gene transfer in animal models of MN diseases.1,2,3,4,5,6 This approach involves axonal retrograde transport of the vectors to the MN cell bodies connected to the injected muscles, probably though dynein-dependent microtubule binding.7,8,9 This method is particularly attractive for human gene therapy because it is easily accessible and not invasive, and has been recently translated to nonhuman primates.10 We recently demonstrated the remarkable efficiency of scAAV9 for widespread gene transfer to the central nervous system (CNS) after intravenous (i.v.) delivery in mice and translated this approach to a large animal model, the lix-1 cat model of spinal muscular atrophy (SMA).11,12 A large percentage of MNs were found to be transduced in both neonatal and adult animals of both species, demonstrating the efficiency of scAAV9 to reach the nervous parenchyma through the mature blood–brain barrier. The outstanding potential of i.v. scAAV9 for MN gene transfer was also reported by another group in neonatal mice, but unexpectedly, transduction was found restricted to astrocytes in the adult mice.13 Nevertheless, recent studies confirmed the efficiency of scAAV9 delivered via the i.v. route to transduce both glial and neuronal cells in adult mice and nonhuman primates, strengthening the interest of this approach in clinics.14,15 The considerable therapeutic potential of i.v. scAAV9 delivery was further demonstrated in a mouse model of severe SMA, an autosomal recessive MN disease mostly caused by mutation or deletion of the survival of motor neuron gene.16 Indeed, we and others showed that i.v. delivery of an scAAV9 expressing SMN1 in neonatal SMNdelta7 mice (SMN2+/+, SMNdelta7+/+, and Smn−/−)17 increased median survival from ~70 to 250 days, depending on the study.18,19,20 Of note, we found that mice treated with moderate amounts of scAAV9 containing an optimized human SMN1 expression cassette survived for up to 355 days (median life span of nearly 200 days), displaying a normal number of MNs at 14 days (disease end point), a complete restoration of motor activity, and a body weight close to normal values.18 It is noteworthy that only a modest increase of life span (3–5 days) had been previously demonstrated when severe SMA mice were injected into multiple muscle groups with survival motor neuron (SMN)-expressing pseudotyped lentiviral vectors (EIAV), although these vectors were reported to undergo efficient axonal retrograde transport to the MN cell bodies.1 Despite the poor mouse rescue reported in this study, this was the first proof of principle for a therapeutic effect of SMN delivery to MNs following i.m. injection of a viral vector. More recently, AAVs of serotype one to eight have been reported to undergo significant axonal retrograde transport and spread from the injected muscles to distant organs, excluding the CNS.21,22,23 Moreover, investigation of the retrograde transport properties of scAAV serotypes one to six following injection into the rat muscles revealed a relatively low level of reporter gene expression in the spinal cord MNs (~1–4.1% transduction).24 Because scAAV9 is among the most effective vector for CNS gene delivery, we examined its ability for axonal retrograde transport to MNs after i.m. delivery in both neonatal and adult mice. Unexpectedly, we found that a single injection of scAAV9 into the gastrocnemius (GA) muscle of adult mice was highly efficient to target a number of cells throughout the entire spinal cord and the peripheral organs. Finally, we demonstrated the high efficacy of the i.m. route for AAV-SMN gene therapy in a mouse model of SMA, showing for the first time that a limited number of i.m. injections of a scAAV9-SMN significantly prolonged the life expectancy of neonatal SMNdelta7 mice. These findings provide the basis for the utilization of i.m. scAAV9 delivery as a simple and practical method for therapeutic gene delivery to the spinal cord in both neonatal and adult animals.
Results
A single i.m. scAAV9 injection mediates efficient global gene delivery in adult mice
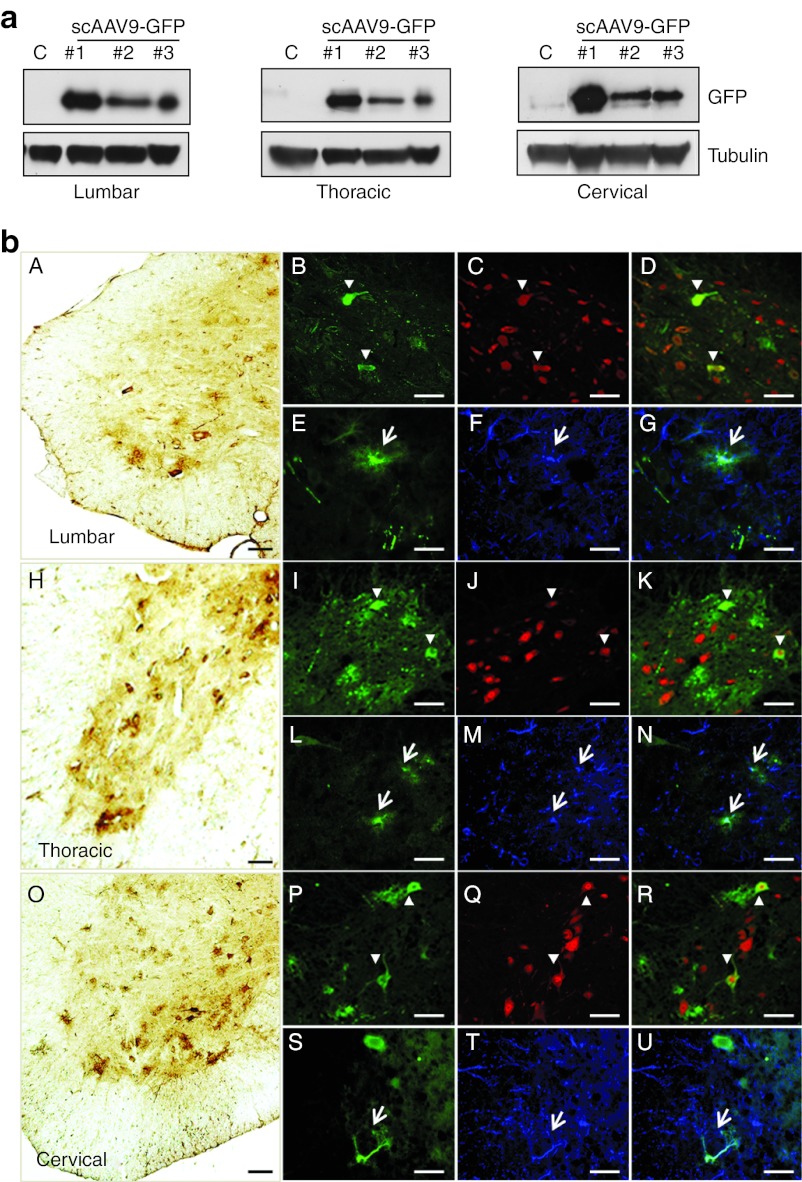
We first investigated whether recombinant scAAV9 was capable to mediate transduction of the spinal cord cells after i.m. delivery in adult mice. In this aim, scAAV9 expressing green fluorescent protein (GFP) under the control of the cytomegalovirus promoter (4.5 × 1012 vg/kg; 9 × 1010 vg per mouse in 100 µl) was unilaterally injected into the right GA of 2-months-old mice (n = 6). Four weeks later, the western blot analysis revealed the presence of GFP in protein extracts from the lumbar spinal cord (n = 3) (Figure 1a). This finding is consistent with retrograde axonal transport of the viral particles from the nerve terminals in the injected GA to the MN cell bodies in the lumbar spinal cord. Unexpectedly, GFP expression was also observed in the thoracic and cervical spinal cord segments which were not connected to the right GA, consistent with a vascular spread of the vector from the injection site (Figure 1a). For identification of the type of cell transduced in the spinal cord of the injected mice, transverse sections of the entire spinal cord were colabeled with antibodies directed against GFP and either the “neuronal nuclei,” (NeuN) a neuron-specific nuclear protein) or glial fibrillary acidic protein (GFAP), a marker of astrocytes). Both neuronal and glial cells were transduced throughout the spinal cord after the unilateral injection of AAV9-GFP into the GA (Figure 1b). Among all transduced cells in the cervical spinal cord, 48 ± 4% were neurons and 52 ± 4% were astrocytes (P = 0.5) (n = 5) (Figure 1b). Counting of GFP-positive neurons which displayed a typical MN morphology (large polygonal cell profiles (>20 µm)) revealed that 43 ± 3 % of total MNs were efficiently transduced in the cervical spinal cord following AAV9 injection into the mouse GA. It is noteworthy that injection of the same dose of scAAV9-GFP in a smaller volume (50 µl) induced similar MN transduction. No significant difference was found in the percentage of GFP-positive MNs in the cervical spinal cord after injection of 50 µl or 100 µl of viral suspension (40 ± 1 % versus 43 ± 3 %, respectively, P = 0.53).
Figure 1.
A single i.m. scAAV9 injection mediates widespread spinal cord transduction in adult mice. (a) Western blot analysis of GFP expression in spinal cord extracts from three adult wild-type (WT) mice injected into the right GA with 4.5 × 1012 vg/kg of scAAV9-GFP at 8 weeks of age (#1, #2, and #3), and from one age-matched control that received no injections (C). GFP is expressed in the lumbar, thoracic, and cervical spinal cord segments. (b) Representative transverse sections of the (A–G) lumbar, (H–N) thoracic, and (O–U) cervical segments of the spinal cord treated for (A,H,O) GFP immunohistochemistry, (B–D,I–K,P–R) GFP/NeuN double-immunofluorescence (green: GFP-positive cells; red: NeuN-positive neurons; yellow: merge) or (E–G,L–N,S–U) GFP/GFAP double-immunofluorescence (green: GFP-positive cells, blue: GFAP-positive astrocytes, turquoise blue: merge). Scale bars = 50 µm. AAV, adeno-associated viruses; GA, gastrocnemius; GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; NeuN, neuronal nuclei.
Widespread transduction of both MNs and astrocytes along the whole spinal cord strongly suggested that gene transfer did not result exclusively from retrograde axonal transport from the injected muscle to the connected MN cell bodies, but also involved vector spread into the bloodstream.
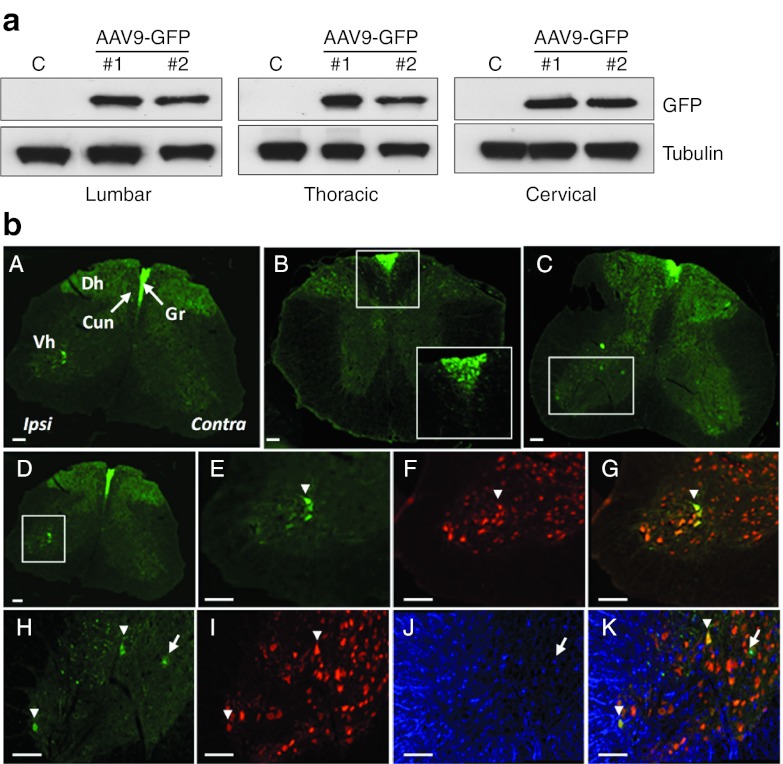
We thus investigated whether i.m. injected scAAV9 spread to the whole body through the bloodstream by analyzing GFP expression into the brain and in peripheral organs such as the contralateral GA or the liver using both western blotting and GFP immunofluorescence analysis. In accordance to the vascular spread hypothesis, substantial amounts of GFP were found in the contralateral GA, although the intensity of GFP staining was, as expected, lower than that in the injected GA (Figure 2b). GFP was also expressed in the liver and the brain (at variable levels) (Figure 2a) in which transduced cells were mainly found in the choroid plexus, the cerebellum, and the pons (Figure 2b). Together, these results showed that a single unilateral i.m. administration of scAAV9-GFP in adult mice resulted in the extensive spreading of viral particles through the bloodstream to remote nervous and peripheral tissues.
Figure 2.
A single scAAV9-GFP injection induces transduction of the brain and peripheral organs in adult mice. (a) Western blot analysis of GFP expression in protein extracts from the contralateral (noninjected) GA, the liver, and the brain of two adult wild-type mice injected with 4.5 × 1012 vg/kg of scAAV9-GFP at the age of 8 weeks (#1, #2), and from one age-matched control mice that received no injection (the weak signal detected in both the control and AAV9-GFP lanes corresponds to nonspecific signal) (C). (b) GFP-immunofluorescence analysis of representative transverse sections from (A) the injected GA (B) the contralateral GA, and (C,D) the liver of a (C) control and (D) scAAV9-GFP injected mouse (E–G) cerebellum and choroid plexus of a (E) control and (F,G) scAAV9-GFP injected mouse, showing an intense GFP expression in choroid epithelial cells and Purkinje cells. (H–J) Reticular formation in the pons showing GFP-positive neurons and astrocytes. Arrowheads: neuronal cells, arrows: astrocytes; Scale bars = 50 µm. AAV, adeno-associated viruses; GA, gastrocnemius; GFP, green fluorescent protein.
A single scAAV9-GFP injection into the GA leads to moderate levels of spinal cord transduction in neonatal mice
To determine whether i.m. scAAV9 injection could be potentially used for gene therapy of neonatal SMA mice, we checked the efficacy of this delivery route by injecting scAAV9-GFP in neonatal wild-type (WT) mice and analyzing GFP expression in different tissues 4 weeks later. As in the procedure used in adult mice, scAAV9-GFP (4.5 × 1012 vg/kg; 9 × 109 vg per mouse) was injected unilaterally into the GA of P0 mice (n = 6), and GFP expression was analyzed in the spinal cord by western blotting and immunofluorescence on tissue sections. Consistent with the findings in adults, GFP was detected in protein extracts from the cervical, thoracic, and lumbar segments of the spinal cord (n = 2) (Figure 3a). Immunofluorescence analysis of spinal cord sections (n = 4) confirmed the presence of GFP in the whole spinal cord. By contrast to the findings for adults, transduced MNs were mostly confined to the ipsilateral side of the ventral horn in the lumbar segment, consistent with a primary mechanism of axonal retrograde transport of the vectors from the injected muscle (Figure 3b). However, we also detected labeled MNs and astrocytes throughout the spinal cord, particularly in the cervical segment (Figure 3b). Intense GFP labeling was also observed in nerve fibers located within the dorsal spinal cord, with particularly strong GFP expression observed in the dorsal horn and the fasciculus gracilis (an ascending tract carrying sensory information from the middle thoracic and lower limbs). The fasciculus cuneatus (an ascending tract involved principally in the transmission of sensory information from the arms) also displayed GFP expression, but to a lesser extent.
Figure 3.
A single scAAV9-GFP injection resulted in moderate levels of spinal cord transduction in neonatal mice. (a) Western blot analysis of GFP expression in spinal cord extracts from two newborn wild-type mice injected into the GA with 4.5 × 1012 vg/kg of scAAV9-GFP at P0 (#1, #2) and from one age-matched control mouse that received no injection (C). (b) Representative transverse sections of the (A,D–G) lumbar, (B) thoracic and (C,H–K) cervical segments of the spinal cord treated for (A,B,D–G) GFP/NeuN double-immunofluorescence (green: GFP-positive cells; red: NeuN-positive neurons, yellow: merge) or (C, H–K) GFP/NeuN/GFAP triple-immunofluorescence (green: GFP-positive cells; red: NeuN-positive neurons, blue: GFAP-positive astrocytes, yellow: merge, turquoise blue: merge). The bilateral injection of scAAV9 into the GA of neonatal mice resulted in moderate levels of spinal cord transduction. Arrowheads: neuronal cells; arrows: astrocytes. Scale bars = 50 µm. AAV, adeno-associated viruses; Contra, contralateral side; Cun, fasciculus cuneatus; Dh, dorsal horn; GA, gastrocnemius; GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; Gr, fasciculus gracilis; Ipsi, ipsilateral side; Vh, ventral horn.
These results validate the use of i.m. scAAV9 for gene transfer to spinal cord cells in neonatal mice and suggest age-related differences in the efficiency with which the i.m. injected scAAV9 spreads to the spinal cord through the bloodstream.
Bilateral scAAV9-SMNopti injection into the hind limb muscles significantly increases SMA mouse survival
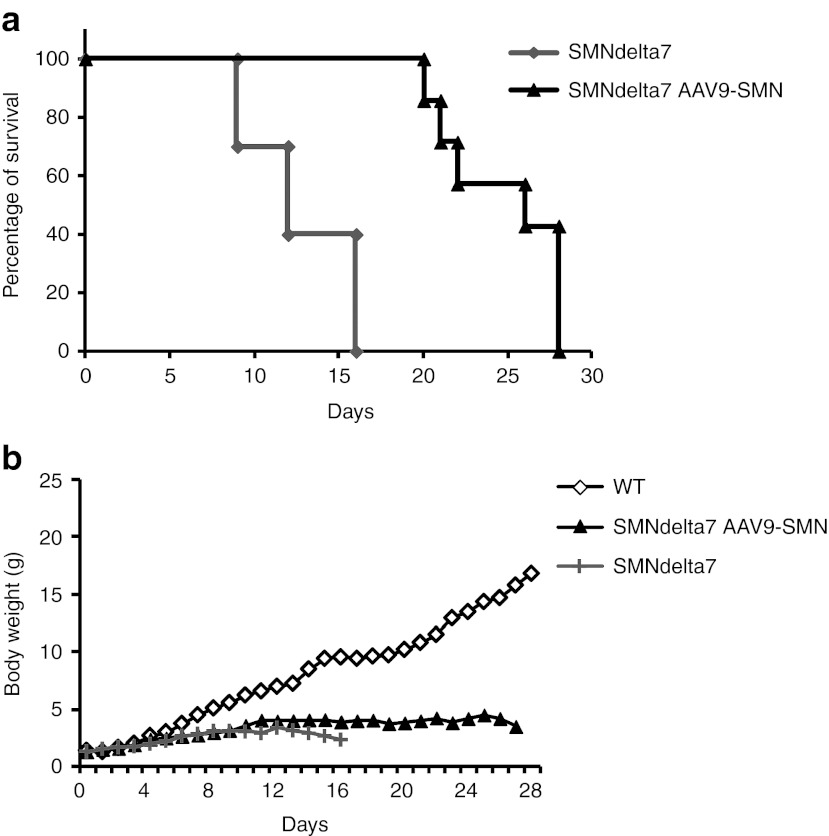
To maximize the potential therapeutic effect of i.m. scAAV9 SMN gene delivery, a high dose of scAAV9 carrying an improved human SMN expression cassette (scAAV9-SMNopti)18 was injected into the right and left GA of neonatal SMNdelta7 mice (5 × 1013 vg/kg, 7 × 1010 vg per mouse, n = 7) and the course of the disease was compared with that of noninjected SMNdelta7 (n = 10) and WT littermates (n = 9). SMNdelta7 mice had a particularly severe phenotype, with a significant weight loss by the age of 8 days (3 ± 0.15 g for SMNdelta7 mice versus 5.6 ± 0.3 g for WT controls, P < 0.05) and a mean life expectancy of 13 ± 1 days (median survival of 12 days) (Figure 4a). Remarkably, injection of scAAV9-SMNopti into both GAs in neonatal mice more than doubled the life expectancy of these animals (median survival of 26 days, mean survival of 25 ± 1 days, and maximal life span of 28 days) (P = 0.0001, χ² test) (Figure 4a).
Figure 4.
The bilateral i.m. injection of scAAV9-SMNopti into the hind limbs significantly increased the survival of SMNdelta7 mice. (a) Kaplan–Meier survival curve of neonatal SMNdelta7 mice injected into both GA with 5 × 1013 vg/kg of scAAV9-SMNopti (SMNdelta7 AAV9-SMN, n = 7, black) or noninjected (SMNdelta7, n = 10, gray). Treated SMNdelta7 mice survived for up to 28 days, with a median survival of 26 days versus 12 days for untreated mice (P = 0.0001, χ² test). (b) Weight curve of SMNdelta7 mice injected with scAAV9-SMNopti-injected (SMNdelta7 AAV9-SMN, black) or not injected (SMNdelta7, dark gray), and of wild-type littermates (WT, n = 9, white). AAV, adeno-associated viruses; GA, gastrocnemius; SMN, survival motor neuron.
The body weight of the treated mice slightly increased with age, reaching a steady state by 10 days of age. However, the treated mice remained smaller than the WT mice, the difference between the two groups appearing significant by the age of 8 days (3 ± 0.2 g for treated mice versus 5.6 ± 0.3 g for WT controls, P < 0.05). At 11 days of age, no significant difference was found in the body weight of the treated versus nontreated SMNdelta7 mice (4 ± 0.8 g versus 2.9 ± 0.3 g, respectively, P > 0.05; two-way analysis of variance, post-hoc Bonferroni test) (Figure 4b).
scAAV9-SMNopti injection into all four limbs greatly rescued SMNdelta7 mice
To further increase the therapeutic effect of scAAV9-SMN gene transfer, additional SMNdelta7 mice (n = 5) were injected at birth with scAAV9-SMNopti (5 × 1013 to 1 × 1014 vg/kg) into both the GA and triceps (TRI) muscles. Remarkably, all treated mice were still alive at 100 days of age (20% of the treated mice survived for up to 260 days), whereas all the untreated mice died within 16 days. Mean life expectancy was increased to 166 ± 27 days by the treatment (median of 163 days) (P = 0.0042, χ² test) (Figure 5a). The treated SMNdelta7 mice were significantly heavier than untreated mice from 14 days of age (6 ± 1.5 g versus 3 ± 0.07 g, P < 0.05; two-way analysis of variance, post-hoc Bonferroni test), their body weight increasing with age to reach a steady state by about 110 days (Figure 5b). However, they remained smaller than WT littermates (at 14 days of age, their mean weight body was 64% that of the wild-type (6 ± 1.5 g versus 9.4 ± 0.5 g, respectively; P < 0.01)) (Figure 5b).
Figure 5.
The injection of scAAV9-SMNopti into the GA and triceps (TRI) muscles greatly increases survival and improves the weight loss phenotype in SMNdelta7 mice. (a) Kaplan–Meier survival curve of neonatal SMNdelta7 mice injected bilaterally into both GA and TRI with 5 × 1013 vg/kg to 1 × 1014 vg/kg of scAAV9-SMNopti (SMNdelta7 AAV9-SMN, n = 5, black) and of SMNdelta7 mice that received no injection (SMNdelta7, gray). The treatment greatly increased life expectancy (up to 260 days, median survival of 163 days versus 12 days for untreated mice (P = 0.0042, χ² test). (b) Weight curves for treated (SMNdelta7 AAV9-SMN, black) and untreated SMNdelta7 mice (SMNdelta7, gray), and their wild-type littermates (WT, n = 9, white). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). AAV, adeno-associated viruses; GA, gastrocnemius; SMN, survival motor neuron.
Unlike untreated SMNdelta7 mice, which displayed severe muscular atrophy at P16, mice treated i.m. with scAAV9-SMNopti had a normal phenotype (Figure 6a) and moved normally in their cages. Surprisingly, the monitoring of spontaneous activity in an actimeter showed a deficit in rearing behavior (209 ± 94 rearing events versus 752 ± 28 in WT controls, P = 0.0015) but not in other behavioral parameters, such as mean speed (10.4 ± 3.3 cm/s versus 6.6 ± 0.2 cm/s, P = 0.29) and distance (22,985 ± 14,652 cm versus 19,872 ± 462 cm, P = 0.84) (Figure 6b). The animals in the treated group displayed clear interindividual variability in spontaneous activity that was not observed in the control group. This variability probably resulted from differences in SMN levels between mice, likely due to technical problems with the injection (see the differences in SMN protein levels between mice in Figure 6c). It is noteworthy that only few mice displayed the signs of hyperactivity previously reported after the i.v. injection of similar vectors.18,25 Like mice receiving i.v. injections, the i.m. treated mice displayed degenerative necrotic changes to the ears but, surprisingly, these pathological features appeared later in mice treated i.m. than in mice treated i.v. (~70 days versus ~25 days after i.v. injection). The treated mice also had shorter tails than the WT mice, as previously reported for SMA type III mice26 and treated SMNdelta7 mice.27,28
Figure 6.

The injection of scAAV9-SMNopti into the GA and triceps (TRI) muscles rescues the SMNdelta7 phenotype. (a) Photographs of heterozygous mice (control, Ctl), SMNdelta7 mice and SMNdelta7 mice injected into the GA and the TRI with 8 × 1010 vg of scAAV9-SMNopti, (A) highlighting the severity of the SMNdelta7 phenotype and (B,C) the rescue of treated SMNdelta7 mice at (B) 7 days and (C) 219 days of age. (D) The scAAV9-SMN-treated SMNdelta7 mice display ear necrosis and have small tails. (b) Monitoring of spontaneous activity (speed, distance and rearing behavior) in heterozygous (control) and scAAV9-SMNopti-treated SMNdelta7 mice using an actimeter. NS: no significant (Student t test) (c) Western blot analysis of SMN expression in spinal cord extracts from SMNdelta7 mice (n = 2), wild-type mice (WT, n = 2) and scAAV9-SMNopti-injected SMNdelta7 mice (n = 2) 13 days after injection. The levels of SMN in scAAV9-SMNopti-injected SMNdelta7 mice are similar to or greater than those in age-matched WT mice. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). AAV, adeno-associated viruses; GA, gastrocnemius; SMN, survival motor neuron.
scAAV9 injection into all four limbs of neonatal mice leads to substantial increase of transgene expression throughout the spinal cord
We then investigated SMN levels in spinal cord extracts from treated SMNdelta7 mice, untreated SMNdelta7 mice, and WT mice (n = 2 in each group). Fourteen days after injection, large amounts of SMN protein were found in the whole spinal cord, with the amount of protein found in one treated mouse exceeding that in both the WT mice (Figure 6c).
We also analyzed the spinal cord transduction profile by determining which type of cells expressed GFP after i.m. injection of scAAV9-GFP (5 × 1013 vg/kg, bilateral GA, and TRI injections) into neonatal WT mice (n = 3). Four weeks after injection, GFP was found to be expressed in the whole spinal cord (Figure 7) and double-immunostaining for GFP/NeuN and GFP/GFAP revealed the presence of GFP-positive MNs and astrocytes throughout the cervical to lumbar ventral horns. Strong GFP immunostaining was also observed in nerve fibers of the ventral and dorsal spinal cord, particularly in the dorsal horn (and the ventral horn to a lesser extent), in the fasciculus gracilis and fasciculus cuneatus), in the dorsal and ventral roots, and in the dorsal root ganglia (Figure 7).
Figure 7.
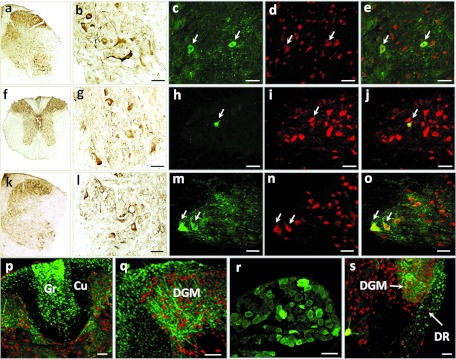
The injection of scAAV9 into the GA and triceps muscles leads to widespread spinal cord transduction in neonatal mice. Representative transverse sections of the (a–e) lumbar spinal cord, (f–j, p) thoracic spinal cord, (k–o,q,s) cervical spinal cord and (r) dorsal root ganglia of neonatal mice injected with 5 × 1013 vg/kg of scAAV9-GFP. Four weeks after injection of the vector, the sections were treated for (a,b,f,g,k,l) GFP immunohistochemistry, (c–e,h–j,m–q,s) GFP/NeuN double-immunofluorescence (the arrows indicate transduced motor neurons in the ventral spinal cord) or (r) GFP immunofluorescence (green: GFP-positive cells; red: NeuN-positive cells, yellow: merge). Scale bar = 50 µm. AAV, adeno-associated viruses; Cu, fasciculus cuneatus; DGM, dorsal gray matter; DR, dorsal root; GA, gastrocnemius; GFP, green fluorescent protein; Gr, fasciculus gracilis; NeuN, neuronal nuclei.
Discussion
The sustained and noninvasive SMN gene delivery to the spinal cord MNs has long been a challenge for SMA gene therapy. We recently demonstrated the remarkable capacity of scAAV9 vectors to mediate widespread gene transfer to the spinal cord MNs following i.v. injection in both neonatal and adult animals.12,13 Another team also reported the potential of i.v. scAAV9 injection for CNS gene transfer, but in contrast to what we described, this vector was reported to target solely the astrocytes in adult mice.13 We and others subsequently showed the dramatic efficiency of this approach for rescuing SMA mice using SMN-encoding vectors.18,19,25
As AAV vectors were reported to undergo retrograde axonal transport after i.m. injection, this study was designed to analyze the potential of scAAV9 for gene transfer to MNs after i.m. injection and to investigate the feasibility of this method for SMA gene therapy in SMNdelta7 mice.
First, we showed that a single administration of scAAV9 into the GA muscle mediated an unexpectedly widespread transduction of MNs throughout the entire spinal cord and in peripheral organs, whereas other AAV serotypes (or other viral vectors) were previously reported to mediate the specific transduction of MNs connected to the injected muscle. These findings show that, in addition to axonal retrograde transport to the connected MN cell bodies, the vector was released into the bloodstream, either directly, through leakage at the time of injection, or via the secretion of AAVs-associated microvesicules termed vector-exosomes (vexosomes).29 These vexosomes were recently described in culture media of AAV1 and AAV2 producer cells and were shown to be capable of enhancing gene transfer in cultured cells as compared with conventionally purified vectors. However, such secretion mechanisms of AAV vectors remain to be further investigated in vivo. The transduction of the entire spinal cord following a single unilateral injection of scAAV9 into the GA was particularly unexpected in light of previous findings suggesting that retrograde axonal transport was the only mechanism of viral dissemination in CNS after i.m. injection of viral vectors,23,30,31 and that particularly low efficiencies of MN gene transfer was found after i.m. delivery of AAV9 or other AAV serotypes.23,30,31 Our findings therefore indicate, for the first time, that a single i.m. injection of scAAV9 transduces MNs in a nonsegmented way, and can be used for widespread gene transfer to MNs in adult mice.
Of note, the rates of MN transduction mediated by i.m. scAAV9-GFP injection to adult mice appeared higher than those previously reported after i.v. injection of a similar vector.12 Although the viral stocks used in these two independent studies were carefully titer-matched and purified according to the same procedure, this unexpected finding most probably results from variations in the production quality or the titration of the viral batches. First, although quantitative PCR only measures the number of DNA-containing particles, the variable efficiency of recombinant AAV encapsidation generates a high proportion of vector particles which are devoid of DNA in a recombinant AAV preparation.32 Different proportions of these contaminants (empty capsids) in the i.v. versus i.m. AAV9-GFP preparations may imply a differential biological activity, thereby explaining the apparent superiority of the i.m. delivery route. The viral preparations could also be contaminated by defective particles containing only fragmented viral genomes, an information that is not provided by common quantitative PCR assays. However, the discrepancy appears modest with WT serotype capsid and short viral genomes, and has been mainly demonstrated with capsid mutant or large genome vectors.33 Similarly, the titer-matched AAV9-GFP preparations used in our i.v. and i.m. studies could also contain variable proportions of transgene protein contaminants capable of inducing artifactual gene expression called “pseudotransduction”.34 Finally, the determination of infectious recombinant AAV titers by quantitative PCR has been recently reported as not completely reliable to provide information about the level of vector infectivity.35 Specifically, systemic errors have been reported in quantitative PCR titration of scAAV vectors, resulting from inhibition of amplification due to covalently closed hairpins in the self-complementary genome.35
Alternatively, capsid-interacting blood factors could have promoted or inhibited AAV9 transduction depending on the delivery route of the vector and its concentration in the bloodstream. Indeed, serum containing factors, such as the C-reactive protein in mice (or the galectin 3 binding protein in humans and dogs) have been recently reported to interact with AAVs in the bloodstream.36 A further side-by-side comparative study of the efficacy of i.m. and i.v. scAAV9 injections to mediate spinal cord gene transfer in adult mice (using the same batch of vector) will be necessary to definitely ascertain the respective efficacy of each delivery route. In neonates, widespread MN transduction has also been observed in the whole spinal cord after i.m. AAV9 injection (Figure 3a,b) albeit to a smaller degree than in adults (Figure 3b). We do not know the exact cause for this difference, but one explanation could be an age-dependent interaction of the vectors with serum proteins. These interacting factors could vary with age, differentially scavenging the AAV particles in the bloodstream, and thereby modifying cell transduction efficacy. Alternatively, the weaker MN transduction observed in the neonate spinal cord, in particular, at the lumbar segment, might also result from the immaturity of the neuromuscular junctions. Further studies will be necessary to definitely conclude about the origin of these differences.
The therapeutic relevance of i.m. AAV9 delivery to MNs was confirmed by subsequent experiments in which i.m. scAAV9 injection was used for the global delivery of SMN to neonatal SMNdelta7 mice. This study showed indeed that injection of SMN-encoding scAAV9 vectors into a limited number of muscles significantly increased the life expectancy of SMNdelta7 mice (median survival of 163 days versus 12 days in noninjected mice) and improved behavioral performance. This treatment also greatly improved the body weight loss phenotype, although the treated mice remained smaller than WT controls, probably due to early impairment of the growth hormone–insulin-like growth factor 1 axis, as recently reported.37 None of the treated mice showed overt signs of motor dysfunction, and all could run and climb normally in their cages. However, the monitoring of spontaneous activity showed a deficiency of rearing behavior but not of other behavioral parameters. Similar results, previously reported by Hua and colleagues,37 were interpreted as being due to residual hind-limb weakness. The therapeutic benefits of i.m. scAAV9-SMNopti delivery were significantly greater than those of multiple i.m. injections of a pseudotyped equine lentiviral vector expressing SMN in the tongue, facial, diaphragm, intercostal, and GA muscles of SMAdelta7 mice.25 In this previous study, i.m. injection of EIAV-SMN extended survival only slightly (3–days), despite reportedly highly efficient axonal retrograde transport of the vector (up to 70% of all MNs were transduced).25
Following i.m. scAAV9 injection, the transgene was widely expressed in the CNS, the peripheral nervous system, and nonnervous system organs, suggesting a potential role of both neurons and other cell types like muscular, cardiac, and pancreatic cells, in the rescue of SMA mice.38,39,40 Indeed, it has been suggested that SMA is a global neuromuscular disease not restricted to MNs.38,39,40,41,42,43,44,45,46 Notably, the recent study by Hua and colleagues,37 elegantly demonstrated a role of peripheral organs in SMA, through a comparative analysis of intracerebroventricular (i.c.v.) and subcutaneous (s.c.) injections of antisense oligonucleotides designed to redirect SMN2 splicing. The authors reported a high therapeutic effect of systemically injected antisense oligonucleotides on the survival of mice with severe SMA (SMN2+/−, Smn−/−), with a combination of i.c.v. and s.c. delivery of antisense oligonucleotides providing the greatest benefit (up to 500 days of survival versus 30 days after a single i.c.v. injection). Our study, together with previous studies reporting a high efficiency of global SMN delivery via i.v. AAV9-SMN injection,18,19,25 suggests that SMN expression on both sides of the blood–brain barrier could be required for therapeutic efficacy. Additional systemic injection of scAAV9 vectors from which SMN is expressed under the control of various tissue-specific promoters should make it possible to specify the potential contribution of each organ to the therapeutic effect.
In summary, we have showed for the first time that a single i.m. injection of scAAV9 vectors allowed widespread gene transfer to MNs and peripheral organs in both neonatal and adult mice, strongly suggesting the leakage of the vector into the bloodstream and subsequent entry into the CNS. Of note, we further showed that i.m. injection of scAAV9 expressing SMN significantly alleviated the pathology of SMA mice providing the first demonstration for the therapeutic efficacy of this delivery route in an SMA mouse model. This simple and practical method for transferring genes of interest to MNs may have potential for use in the treatment of MN degeneration in many diseases in children and adults.
Materials and Methods
AAV vectors. Pseudotyped AAV9 vectors encoding GFP or codon-optimized SMN1 (SMNopti) were prepared by the helper virus-free three-plasmid transfection of HEK293T cells, as previously described.12 Briefly, cells transfected with the adenovirus helper plasmid, the AAV packaging plasmid carrying the rep2 and cap9 genes (p5E18-VD2/9), and the AAV2 plasmid encoding either SMNopti under control of the PGK promoter or GFP under control of the cytomegalovirus promoter. The recombinant vectors were purified by ultracentrifugation on an iodixanol density gradient.47 The viral preparation was desalted and concentrated with Amicon Ultra—Ultra cell 100K filter units (Millipore, Molsheim, France). Aliquots were stored at −80 °C until use. Vector titers were determined by real-time PCR and are expressed as viral genomes per ml (vg/ml).
Animals. SMNdelta7 founder mice (JACKSON no. SN 5025) were purchased from Jackson Laboratories (Bar Harbor, ME). SMNdelta7 mice were (SMN2+/+, SMNdelta7+/+, Smn−/−), WT mice were (SMN2+/+, SMNdelta7+/+, Smn+/+), and heterozygous (carrier) mice were (SMN2+/+, SMNdelta7+/+, Smn+/−). The mice were maintained under controlled conditions (22 ± 1 °C, 60 ± 10% relative humidity, 12 hour light/12 hour dark cycle, food and water ad libitum). All animal experiments were carried out according to European guidelines for the care and use of experimental animals.
In vivo AAV injections.
scAAV9-GFP. Eight-weeks-old WT mice were injected into the right GA in one site with scAAV9-GFP (9 × 1010 vg in 100 µl, n = 6 or in 50 µl, n = 3). At birth, neonatal mice received injections of scAAV9-GFP (9 × 109 vg in 10 µl, one site, n = 6) either into the right GA or into both the TRI and GA (8 × 1010 vg in 40 µl, 10 µl per muscle, n = 3). All animals were killed for analyses of GFP expression 44 days after the injection.
scAAV9-SMNopti. Neonatal SMNdelta7 mice were bilaterally injected with scAAV9-SMNopti into the GA muscles at birth and at P1 to maximize the amount of injected vector (7 × 1010 vg total in 40 µl, 10 µl per GA at P0 and at P1) (n = 7), or into both the TRI and GA muscles (7 × 1010 vg to 1.6 × 1011 total in 80 µl, 10 µl per muscle at P0 and at P1) (n = 5).
Spontaneous activity. scAAV9-SMNopti-injected SMNdelta7 mice (n = 4) were compared with age-matched heterozygous mice (n = 4) for spontaneous activity at ~100 days of age using an actimeter system (Activmeter; Bioseb, Vitrolles, France). This apparatus uses vibrations within the cage to measure locomotion and infrared photocell detectors to record the number of rearing movements. The distance covered (cm), the average speed (cm/s), and number of rearing movements were measured over a 60-minute period.
Western blotting. Protein extracts were prepared from mouse tissues 44 and 14 days after the injection of scAAV9-GFP and scAAV9-SMNopti, respectively. Brain, spinal cord, and liver extracts were prepared with the Qproteome FFPE tissue kit (Qiagen, Courtaboeuf, France). The muscles were lysed in RIPAE buffer (150 mmol/l NaCl, 50 mmol/l Tris–HCl, 0.5% sodium deoxycholate, 1% NP40, 1% sodium dodecyl sulfate) with a protease inhibitor cocktail (Complete Mini, Roche Diagnostics, Meylan, France). Fifty µg of total protein extracts were run on sodium dodecyl sulfate 10% polyacrylamide gel, and transferred to Immobilon-p membrane (Millipore, Billerica, MA). The membranes were incubated successively with a mouse anti-SMN antibody (1:1,000; BD Bioscience, San Jose, CA) or a rabbit anti-GFP antibody (1:1,000; Abcam, Cambridge, UK) and a mouse anti-α tubulin (1:10,000; Sigma-Aldrich, St Louis, MO) diluted in tris-buffered saline supplemented with 0.2% tween 20 and 5% nonfat dry milk blocking buffer. Membranes were washed three times in tris-buffered saline supplemented with 0.2% Tween 20 and 5% nonfat dry milk and incubated with peroxidase-conjugated swine antimouse or antirabbit antibody (1:10,000; GE Healthcare, Amersham Pharmacia Biotech, Uppsala, Sweden) in blocking buffer. Membranes were then further processed by incubation with the Super Signal Ultra chemiluminescence reagent (Thermo Scientific, Rockford, IL).
Histology. Forty-four days after vector injection, the mice were lethally anesthetized with a mix of Ketamin (100 mg/kg) and xylazine (10 mg/kg) and perfused transcardially with 4% paraformaldehyde. The tissues were removed, postfixed in the same solution, and cryoprotected by incubation overnight in 30% phosphate buffered saline (PBS)-sucrose solution. Tissue samples were frozen in cold isopentane (−50 °C) and serial 14 µm sections were cut on a cryostat. For GFP, GFAP, and NeuN immunohistochemistry, the sections were first incubated for 1 hour at room temperature in a PBS buffer containing 5% bovine serum albumin (Sigma), 3% donkey serum (Millipore), and 0.4 % Triton X-100. They were then incubated overnight at 4 °C in the same buffer supplemented with the primary rabbit anti-GFP antibody (ab6556, ½,000, Abcam). Sections were washed in PBS and incubated for 1 hour at room temperature with Alexa 488-conjugated donkey antirabbit (1/500; Life technologies, Carlsbad, CA) antibodies. The sections were then washed in PBS and blocked with the Mouse On Mouse kit (Vector Laboratories, Nanterre, France), according to the manufacturer's protocol. Primary mouse anti-NeuN (Chemicon International, St Quentin en Yvelines, France 1/300) and rabbit anti-GFAP antibodies (1/1,000; Dako) were diluted in Mouse On Mouse diluent and incubated with the sections overnight at 4 °C. Sections were washed with fluoromount-G (Calbiochem, Fontenay sous Bois, France), observed by confocal microscopy (Axio Imager Z1; Zeiss, Le Pecq, France) and images were analyzed with AxioVision 4.7 software (Zeiss, Oberkochen, Germany).
For GFP immunohistochemistry, the sections were washed in 0.1% Triton X-100 in PBS and incubated in a PBS solution containing hydrogen peroxide 1%, methanol 20%, and Triton X-100 0.1% for 20 minutes to block endogenous peroxidase activity. Sections were blocked by incubation in PBS buffer with 4% goat serum (Dako, Trappes, France), 4% bovine serum albumin (Sigma), and 0.1% Triton X-100 for 1 hour at room temperature. Sections were then incubated overnight with a rabbit polyclonal anti-GFP antibody (1/5,000; Abcam), rinsed with PBS, and incubated with a horseradish peroxidase–conjugated biotinylated antirabbit antibody (1/250; Vectastain, Vector Laboratories) for 2 hours and then with an avidin-biotin complex (Vectastain Elite ABC kit, Vector Laboratories) for 30 minutes. The sections were rinsed with 0.1% Triton X-100 in PBS and dark brown staining was detected with the 3,3′-diaminobenzidine substrate kit for peroxidase (Vector Laboratories). The reaction was stopped by adding distilled water, and the sections were dehydrated and mounted in Eukitt.
Motor neuron and astrocyte quantification. The GFP-positive cells were scored in four WT mice and four scAAV9-GFP-treated mice, by confocal microscopy (green, laser emission: 488 nm), on 20 sections (14-µm thick) per mouse spanning the cervical enlargement. These sections were double-stained for NeuN and GFAP. The GFP/NeuN-expressing cells, which had a morphology typical of MNs, were counted in the ventral horn (large cells (>20 µm), polygonal profile and with a visible nucleus), to estimate the percentage of GFP-expressing MNs relative to the total number of NeuN-stained MNs. The number of GFP-expressing astrocytes (GFAP/GFP coimmunolabelled astrocytes) was also estimated by manual counting and the MN/astrocyte transduction ratio was determined (number of GFP-positive MN versus GFP-positive astrocytes relative to the total number of GFP-positive cells).
Statistical analysis. Statistical analysis was performed with Graph Pad Prism software (San Diego, CA). Kaplan–Meier survival curves were compared using a χ2 test. Differences in transduction efficiency between neurons versus astrocytes, in MNs transduction efficiency according to the volume, and in the different spontaneous activity parameters between groups were analyzed using the unpaired Student's t test (two-tailed). Differences in weight between the groups were analyzed using a two-way analysis of variance with a post hoc Bonferroni test. Results are expressed as means ± SEM with a P threshold of 0.05.
Acknowledgments
We thank GENETHON for providing us with the adenovirus helper plasmid (pXX6) and the pAAV packaging plasmid (p5E18-VD2/9) and Yannick Tanguy for statistical analyses and helpful comments. This work was supported by SMA-Europe, Association Française contre les Myopathies (France) and Famiglie SMA (Italy), the Université Pierre et Marie Curie (UPMC), the Institut National de la Santé et de la Recherche Médicale (INSERM), the Centre National de la Recherche Scientifique (CNRS), and the Association Institut de Myologie (A.I.M). The authors declare no conflict of interest.
REFERENCES
- Azzouz M, Le T, Ralph GS, Walmsley L, Monani UR, Lee DC.et al. (2004Lentivector-mediated SMN replacement in a mouse model of spinal muscular atrophy J Clin Invest 1141726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM.et al. (2004VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model Nature 429413–417. [DOI] [PubMed] [Google Scholar]
- Baumgartner BJ., and, Shine HD. Neuroprotection of spinal motoneurons following targeted transduction with an adenoviral vector carrying the gene for glial cell line-derived neurotrophic factor. Exp Neurol. 1998;153:102–112. doi: 10.1006/exnr.1998.6878. [DOI] [PubMed] [Google Scholar]
- Gimenez y Ribotta M, Revah F, Pradier L, Loquet I, Mallet J, Privat A. Prevention of motoneuron death by adenovirus-mediated neurotrophic factors. J Neurosci Res. 1997;48:281–285. doi: 10.1002/(sici)1097-4547(19970501)48:3<281::aid-jnr11>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Lladó J, Sherkat N, Rothstein JD., and, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Lu YY, Wang LJ, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T.et al. (2003Intramuscular injection of AAV-GDNF results in sustained expression of transgenic GDNF, and its delivery to spinal motoneurons by retrograde transport Neurosci Res 4533–40. [DOI] [PubMed] [Google Scholar]
- Finiels F, Gimenez y Ribotta M, Barkats M, Samolyk ML, Robert JJ, Privat A.et al. (1995Specific and efficient gene transfer strategy offers new potentialities for the treatment of motor neurone diseases Neuroreport 7373–378. [PubMed] [Google Scholar]
- Kelkar S, De BP, Gao G, Wilson JM, Crystal RG., and, Leopold PL. A common mechanism for cytoplasmic dynein-dependent microtubule binding shared among adeno-associated virus and adenovirus serotypes. J Virol. 2006;80:7781–7785. doi: 10.1128/JVI.00481-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL.et al. (2001Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery Hum Mol Genet 102109–2121. [DOI] [PubMed] [Google Scholar]
- Towne C, Schneider BL, Kieran D, Redmond DE., Jr, and, Aebischer P. Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther. 2010;17:141–146. doi: 10.1038/gt.2009.119. [DOI] [PubMed] [Google Scholar]
- Barkats M.2007Widespread gene delivery to motor neurons using peripheral injection of AAV vectors PCT/EP2008/063297 GENETHON/CNRS(Priority date: 5 October 2007).
- Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM.et al. (2009Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons Mol Ther 171187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM., and, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L.et al. (2011Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders Mol Ther 191971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR., and, Samulski RJ. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L.et al. (1995Identification and characterization of a spinal muscular atrophy-determining gene Cell 80155–165. [DOI] [PubMed] [Google Scholar]
- Le TT, Pham LT, Butchbach ME, Zhang HL, Monani UR, Coovert DD.et al. (2005SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN Hum Mol Genet 14845–857. [DOI] [PubMed] [Google Scholar]
- Dominguez E, Marais T, Chatauret N, Benkhelifa-Ziyyat S, Duque S, Ravassard P.et al. (2011Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice Hum Mol Genet 20681–693. [DOI] [PubMed] [Google Scholar]
- Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM.et al. (2010Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN Nat Biotechnol 28271–274. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Passini MA., and, Cheng SH. Prospects for the gene therapy of spinal muscular atrophy. Trends Mol Med. 2011;17:259–265. doi: 10.1016/j.molmed.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Brandon EF, Hermsen HP, van Eijkeren JC., and, Tiesjema B. Effect of administration route on the biodistribution and shedding of replication-deficient AAV2: a qualitative modelling approach. Curr Gene Ther. 2010;10:91–106. doi: 10.2174/156652310791111047. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J.et al. (2005Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart Nat Biotechnol 23321–328. [DOI] [PubMed] [Google Scholar]
- Zheng H, Qiao C, Wang CH, Li J, Li J, Yuan Z.et al. (2010Efficient retrograde transport of adeno-associated virus type 8 to spinal cord and dorsal root ganglion after vector delivery in muscle Hum Gene Ther 2187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Kadoya K, Hirsch M, Samulski RJ., and, Tuszynski MH. Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol Ther. 2008;16:296–301. doi: 10.1038/sj.mt.6300367. [DOI] [PubMed] [Google Scholar]
- Valori CF, Ning K, Wyles M, Mead RJ, Grierson AJ, Shaw PJ.et al. (2010Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy Sci Transl Med 235ra42. [DOI] [PubMed] [Google Scholar]
- Hsieh-Li HM, Chang JG, Jong YJ, Wu MH, Wang NM, Tsai CH.et al. (2000A mouse model for spinal muscular atrophy Nat Genet 2466–70. [DOI] [PubMed] [Google Scholar]
- Narver HL, Kong L, Burnett BG, Choe DW, Bosch-Marcé M, Taye AA.et al. (2008Sustained improvement of spinal muscular atrophy mice treated with trichostatin A plus nutrition Ann Neurol 64465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LK, Chen YC, Cheng WC, Ting CH, Dodge JC, Hwu WL.et al. (2012IGF-1 delivery to CNS attenuates motor neuron cell death but does not improve motor function in type III SMA mice Neurobiol Dis 45272–279. [DOI] [PubMed] [Google Scholar]
- Maguire CA, Balaj L, Sivaraman S, Crommentuijn MH, Ericsson M, Mincheva-Nilsson L.et al. (2012Microvesicle-associated AAV vector as a novel gene delivery system Mol Ther 20960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Tuszynski MH. Neurotrophins: potential therapeutic tools for the treatment of spinal cord injury. Neurotherapeutics. 2011;8:694–703. doi: 10.1007/s13311-011-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinov VN, Sefland I, Walaas SI, Lømo T, Njå A., and, Hoover F. Targeting functional subtypes of spinal motoneurons and skeletal muscle fibers in vivo by intramuscular injection of adenoviral and adeno-associated viral vectors. Anat Embryol. 2002;205:215–221. doi: 10.1007/s00429-002-0233-1. [DOI] [PubMed] [Google Scholar]
- Grimm D, Kern A, Pawlita M, Ferrari F, Samulski R., and, Kleinschmidt J. Titration of AAV-2 particles via a novel capsid ELISA: packaging of genomes can limit production of recombinant AAV-2. Gene Ther. 1999;6:1322–1330. doi: 10.1038/sj.gt.3300946. [DOI] [PubMed] [Google Scholar]
- Wang Y.et al. (2012Limitations of encapsidation of recombinant scAAV2 genomes in different serotype capsids and their quantitation Hum Gene Ther Methods 23225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander IE, Russell DW., and, Miller AD. Transfer of contaminants in adeno-associated virus vector stocks can mimic transduction and lead to artifactual results. Hum Gene Ther. 1997;8:1911–1920. doi: 10.1089/hum.1997.8.16-1911. [DOI] [PubMed] [Google Scholar]
- Fagone P, Wright JF, Nathwani AC, Nienhuis AW, Davidoff AM., and, Gray JT. Systemic errors in quantitative polymerase chain reaction titration of self-complementary adeno-associated viral vectors and improved alternative methods. Hum Gene Ther Methods. 2012;23:1–7. doi: 10.1089/hgtb.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denard J, Beley C, Kotin R, Lai-Kuen R, Blot S, Leh H.et al. (2012Human galectin 3 binding protein interacts with recombinant adeno-associated virus type 6 J Virol 866620–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Sahashi K, Rigo F, Hung G, Horev G, Bennett CF.et al. (2011Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model Nature 478123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman M, Swoboda KJ, Michalski JP, Wang GS, Reeks C, Beauvais A.et al. (2012Glucose metabolism and pancreatic defects in spinal muscular atrophy Ann Neurol 72256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaers CA, Wishart TM, Lamont DJ, Riessland M, Schreml J, Comley LH.et al. (2011Reversible molecular pathology of skeletal muscle in spinal muscular atrophy Hum Mol Genet 204334–4344. [DOI] [PubMed] [Google Scholar]
- Shababi M, Habibi J, Yang HT, Vale SM, Sewell WA., and, Lorson CL. Cardiac defects contribute to the pathology of spinal muscular atrophy models. Hum Mol Genet. 2010;19:4059–4071. doi: 10.1093/hmg/ddq329. [DOI] [PubMed] [Google Scholar]
- Bevan AK, Hutchinson KR, Foust KD, Braun L, McGovern VL, Schmelzer L.et al. (2010Early heart failure in the SMNDelta7 model of spinal muscular atrophy and correction by postnatal scAAV9-SMN delivery Hum Mol Genet 193895–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo-Miralles V, Cardona-Rossinyol A, Garcera A, Villalonga P, Soler RM, Olmos G.et al. (2012SMN deficiency attenuates migration of U87MG astroglioma cells through the activation of RhoA Mol Cell Neurosci 49282–289. [DOI] [PubMed] [Google Scholar]
- Dupuis L., and, Echaniz-Laguna A. Skeletal muscle in motor neuron diseases: therapeutic target and delivery route for potential treatments. Curr Drug Targets. 2010;11:1250–1261. doi: 10.2174/1389450111007011250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier CR, Satta R, Lutz C., and, DiDonato CJ. Arrhythmia and cardiac defects are a feature of spinal muscular atrophy model mice. Hum Mol Genet. 2010;19:3906–3918. doi: 10.1093/hmg/ddq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling KK, Lin MY, Zingg B, Feng Z., and, Ko CP. Synaptic defects in the spinal and neuromuscular circuitry in a mouse model of spinal muscular atrophy. PLoS ONE. 2010;5:e15457. doi: 10.1371/journal.pone.0015457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Blivis D, Liu W, Drobac E, Crowder ME, Kong L.et al. (2011Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy Neuron 69453–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens WT, ter Brake O, Dijkhuizen PA, Sonnemans MA, Grimm D, Kleinschmidt JA.et al. (1999Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous system Hum Gene Ther 101885–1891. [DOI] [PubMed] [Google Scholar]