Abstract
Photodynamic therapy (PDT) is an alternative treatment for infections that can kill drug resistant bacteria without damaging host-tissue. In this study we used bioluminescent methicillin-resistant Staphylococcus aureus, in a mouse skin abrasion model, to investigate the effect of PDT on bacterial inactivation and wound healing. RLP068/Cl, a tetracationic Zn(II)phthalocyanine derivative and toluidine blue (TBO) were used. The light-dose response of PDT to kill bacteria in vivo and the possible recurrence in the days post-treatment were monitored by real-time bioluminescence imaging, and wound healing by digital photography. The results showed PDT with RLP068/Cl (but not TBO) was able to kill bacteria, to inhibit bacterial re-growth after the treatment and to significantly accelerate the wound healing process.
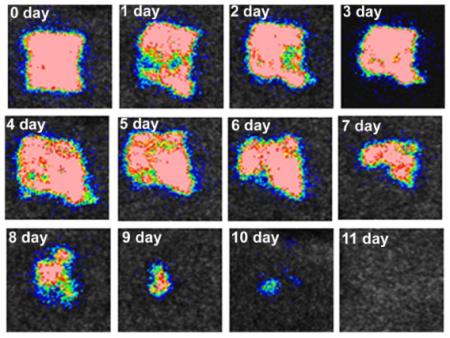
Successive bioluminescence images of a representative mouse skin scratch model infected with 108 CFU MRSA.
Keywords: Antimicrobial photodynamic therapy, methicillin resistant Staphylococcus aureus, skin and soft tissue infection, wound healing, cationic zinc phthalocyanine bioluminescence imaging, skin abrasion
1. Introduction
Skin is the first line of defense against pathogens and acts as a physical barrier against microbial invasion. In normal uninjured skin, the colonization and growth of pathogenic organisms is inhibited by low surface pH, sebum, and fatty acids [1]. The wound healing process has been well characterized histologically; the various stages of wound healing have been widely investigated over the years and the orchestrated events associated with normal healing have been discussed in the Refs. [2–4]. The initial stages of wound healing involve the formation of a blood clot and inflammation. The inflammatory response is followed by proliferation and migration of dermal and epidermal cells, and then by matrix synthesis, in order to fill the wound gap and re-establish the skin barrier [5, 6]. Finally, tissue remodeling and differentiation enable full recovery of the skin tissue and restoration of skin aesthetics [5, 7]. The consensus in the literature is that the stepwise process of wound healing first strives toward immediate filling of the gap, followed by re-epithelialization and re-establishment of the skin barrier function [8]. Wound healing is therefore an essential physiological process important for tissue homeostasis leading to restoration of skin function. Many factors such as location of the wound, its etiology, size, and the presence of concomitant diseases are important in governing the rate and quality of the wound healing process. Bacteria seek to establish themselves in ecologically suitable niches such as open wounds to ensure their own survival. Infection is a common problem in cutaneous wounds and it is one of the key reasons why wound healing may stall, leading to increased risks of patient morbidity and even mortality. The discovery of antibiotics revolutionized the treatment of infections but the recent increase in antibiotic resistance among pathogenic bacteria has led to a growing interest in researching alternative treatments for localized infections. Photodynamic therapy (PDT) is currently applied as a treatment for various kinds of cancers [9–11], and has been proposed for the treatment of microbial infections. PDT involves the topical application of a non-toxic dye, named photosensitizer (PS), into infected tissue, followed by illumination preferentially with red or near infrared light that is able to penetrate the tissues [12, 13]. In the presence of oxygen, light induces the formation of ROS by energy or electron transfer from the PS excited state to ambient oxygen, and these ROS are able to oxidize bio-molecules and thereby kill cells. The aim of this study was to investigate the effect of PDT on bacterial inactivation and on the wound healing process, using RLP068/Cl, a recently developed tetracationic Zn(II)phthalocyanine photosensitizer [14, 15] in a mouse cutaneous scratch model infected with a bioluminescent methicillin resistant Staphylococcus aureus (MRSA) strain. To evaluate the efficacy of PDT with RLP068/Cl, toluidine blue O (TBO), an established antibacterial PS, was also tested in vivo under the same conditions. The inactivation of bacteria and any bacterial re-growth in the days post infection was followed by non-invasive, real-time bioluminescence imaging. Wound healing was assessed by daily observation and digital photography until healing of the wound.
2. Materials and methods
2.1 Bacteria
The bioluminescent mouse pathogenic MRSA strain 8325-4 (XEN 8.1) was created by introducing a modified Photorhabdus luminescens lux operon using the gram-positive lux transposon plasmid pAUL-Atn4001 luxABCDE Kmr 27 and was provided by Caliper Corp (Princeton, NJ). Bacteria were grown overnight in brain heart infusion (BHI) at 37 °C with 100 rpm orbital shaking. The bacteria growth were assessed with an Evolution 300 UV–Vis Spectrophotometer (Thermo Scientific, Waltham, MA), when optical density (OD) at 600 nm reached 0.8, that corresponds to a bacterial cell density of 108 CFU/ml, cells were washed and re-suspended in PBS (Dulbecco) and used at a density of 108 CFU/mL for the in vitro and in vivo experiments.
2.2 Photosensitizers
The PS RLP068/Cl, a tetracationic Zn(II) phthalocyanine chloride [14], was supplied by Molteni Therapeutics (Siena, Italy) as a 7.5 mM solution in 100% DMSO. Mice were treated locally with 40 μL of solution 75 μM in 5% dextrose (Hospira, Inc., Lake Forest, IL USA). The treated lesions were illuminated with a 690 nm wavelength light providing a 100 mW/cm2 for 14 min corresponding to a total dose of light of 84 J/cm2. Toluidine blue O (TBO) was purchased from Sigma-Aldrich (Allentown, PA). The stock solution was dissolved at 7.5 mM in 100% DMSO. The animals were treated topically with 40 μL of 75 mM TBO solution in 5% dextrose. The illumination was performed at 635 nm wavelength, giving 100 mW/cm2 for 14 min corresponding to a total dose of 84 J/cm2. The chemical structures of the two PS together with their absorption spectra and the corresponding emission spectra of the light sources are shown in Figure 1. It is evident that there is very good overlap between the PS absorption and the lamp emission wavelength in both cases.
Figure 1.

(a) Structure of the phenothiazinium salt photosensitizer TBO (b) structure of the tetracationic Zn(II) phthalocyanine chloride (RLP068/Cl) photosensitizer. (c) Visible absorption spectra of TBO and RLP068/Cl and emission spectra of the 630+/−15 nm and 690+/−15 nm light sources.
2.3 Light sources
A Lumacare lamp (Newport Beach, CA) fitted with a light guide and two different bandpass filters was used. For RLP068/Cl we used 690+/−15 nm and for TBO we used 630+/−15 nm. See Figure 1 for absorption spectra of the PS and emission spectra of the light sources. Light guides were adjusted to give a uniform spot with an irradiance of 100 mW/cm2 for in vivo treatments. The spot had a diameter of 2.5 cm that covered the whole abrasion. Light power was measured with a power meter (model DMM 199 with 201 standard head, Coherent, Santa Clara, CA).
2.4 Photodynamic inactivation of bacteria in vitro
Suspensions of bacteria (108 CFU/mL) in PBS were incubated in the dark at room temperature for 30 min with different concentration (1–300 nM) of the RLP068/Cl. Then, 100 μL aliquots of the cell suspensions were placed in 96 well plates. The wells were illuminated from the top of the plates by use of red light 690+/−15 nm and fluences ranged from 0 to 10 J/cm2. At times during the illumination when the requisite fluences had been delivered, aliquots were taken from each well. To determine CFU, the aliquot was serially diluted, streaked on nutrient agar, and incubated in the dark for 18 h at 37 °C. Experiments were carried out in triplicate for each condition. Controls, the cells without light (dark control), and with light alone (light alone), were performed for all experimental conditions. The correlation between colony forming units counts and bacterial bioluminescence was evaluated in all plates.
2.5 Animal model
All animal experiments were approved by Subcommittee on Research Animal Care (IACUC) of Massachusetts General Hospital and met National Institutes of Health (NIH) guidelines. Female BALB/c mice 6–8 week-old and weighing 17–21 g were used. At days 4 and 1 before the infection, mice were administered two doses of cyclophosphamide (CY). The first dose, 150 mg/kg mouse body weight was injected I.P. followed by the second dose of 100 mg/kg, to reduce peripheral blood neutrophils and to promote an environment prone to infection. Skin abrasion wounds were made in anesthetized mice (i.p. injections of ketamine/xylazine cocktail) on the shaved dorsal surface, using a 28-gauge needle by creating 6 × 6 orthogonally crossed scratch lines in a 1 × 1 cm2 area [16]. The scratches damaged the stratum corneum and upper-layer of the epidermis but not the dermis. Five minutes after wounding, an aliquot of 40 μL suspension containing 108 CFU of bioluminescent MRSA in PBS was inoculated on each area of scratches with a pipette tip. Bioluminescence images were taken just after the inoculation of bacteria and 30 min after inoculation.
The animals were divided into 6 groups as shown in Table 1. The animals of group A (n = 6) were absolute controls that received no treatment; group B (n = 6) was treated with light alone; group C (n = 6) was dark control that received RLP068/Cl but no light; group D (n = 6) received PDT with RLP068/Cl and 690 nm light; group E (n = 6) was dark control for TBO (no light); group F (n = 6) received PDT with TBO + light.
Table 1.
Experimental plan of animal experiments.
| Group | Number of animals | Treatment |
|---|---|---|
| Absolute control | 6 | No treatment |
| Light control | 6 | Light alone (12, 36, 84 J/cm2; wavelength 690 nm) |
| Dark control for RLP068/Cl | 6 | RLP068/C1 (40 μL of 75 μM) |
| PDT using RLP068/Cl | 6 | RLP068/Cl + Light (12, 36, 84 J/cm2; wavelength 690 nm) |
| Dark control for TBO | 6 | TBO (40 μL of 75 μM) |
| PDT using TBO | 6 | TBO + Light (12, 36, 84 J/cm2; wavelength 630 nm) |
2.6 Photodynamic therapy
PS (40 μL of 75 μM RLP068/Cl or 75 μM TBO solution) were added 30 minutes after infection, allowing the bacteria time to bind to the host tissue. Fifteen minutes after the addition of PS the mice were again imaged to quantify any dark toxicity of the PS. Mice were illuminated with light from a non-coherent light source as described above. Mice were given total light doses of 84 J/cm2 delivered in aliquots in periods of 2, 4 and 8 min for a total of 14 min. Bioluminescence imaging was carried out after each dose of light (12, 36, 84 J/cm2). To compare the results obtained with PDT treatment, dark controls (animals that received PS without light treatment) were assessed at the same times correspondent to the light delivery in the PDT treatment. To test any possible effect of the light alone a group of animals received light and no PS.
2.7 Bioluminescent imaging
The bioluminescence imaging system (Hamamatsu Photonics KK, Bridgewater, NJ) has been described before [17]. Briefly, it consisted of an intensified charge coupled device camera mounted in a light-tight specimen chamber fitted with a light-emitting diode, a setup that allowed for a background grayscale image of the entire mouse to be captured. In the photon-counting mode, the light emitted from the bacteria was captured by using an integration time of 2 min at a maximum setting on the image-intensifier control module. By use of ARGUS software (Hamamatsu), the luminescence image was presented as a false-color image superimposed on top of the grayscale reference image. The image-processing component of the software calculated the total pixel values (relative luminescence units RLU) from the luminescence images of the infected wound area. The wounds were observed daily and photographs captured every other day until the wounds had healed, using a Nikon Coolpix L20 camera. Wound area analysis was performed by ImageJ software.
2.8 Statistical analysis
Values are shown as means +/− standard error of the mean (SEM). The area-under-the-curve data, which represent the time courses of the wound healing, were calculated by the use of numerical integration [18]. Differences in time course of bacterial luminescent and the differences in the areas under the curves between the control and the treatment groups and between different treatments groups were compared for statistical significance by a t test or one way ANOVA followed by Tukey's post-hoc test. P values of <0.05 were considered significant.
3. Results
3.1 Photodynamic inactivation of bacteria in vitro
The results for photodynamic inactivation of MRSA at a cell density 108 CFU/ml using RLP068/Cl is shown in Figure 2a and b. We measured the loss of colony forming ability as well as the loss of bioluminescence. In Figure 2a we show the PS concentration dependence of the photoinactivation at a constant fluence of 10 J/cm2, while in Figure 2b we show the fluence dependence at a constant concentration of 100 nM. In both cases the loss of CFU closely paralleled the loss of bioluminescence and total killing was achieved at 100 nM (Figure 2a) and at 5 J/cm2 (Figure 2b). We also correlated the number of MRSA CFU and MRSA bioluminescence (RLU) in intact cells and in PDT damaged cells. (Figure 2c) show a linear correlation in intact control cells (R2 = 0.9292) and in Figure 2d we show the linear correlation in cells after treatment with PDT (R2 = 0.9413) showing that the PDT loss of colony forming ability closely parallels the PDT-induced loss of bioluminescence.
Figure 2.

(a) Dose-dependent photodynamic inactivation of MRSA in vitro using 1, 3, 10, 30, 100, 300 nM of RLP068/Cl. (b) Light dose dependent inactivation of MRSA in vitro using 100 nM of RLP068/Cl. (c) Linear correlation between bacterial bioluminescence and coloni forming units in dark control and (d) after treatment with PDT.
3.2 Mouse model of infection in scratch wound
The inoculation of 108 CFU of MRSA into scratch wounds, on the shaved backs of immunosuppressed female BALB/c mice, led to development of a stable infection as characterized by bioluminescence imaging. After bacterial inoculation, the luminescence intensity remained strong and stable until day 6 and detectable until day 10. Figure 3 shows a series of bioluminescence images of a representative mouse infected with luminescent MRSA. The body weight and the good general health conditions of the animals were checked during the whole period of the treatment to detect possible development of systemic infection.
Figure 3.

Successive bioluminescence images of a representative mouse skin scratch model infected with 108 CFU MRSA.
3.3 Response of MRSA infection in scratch wound to PDT
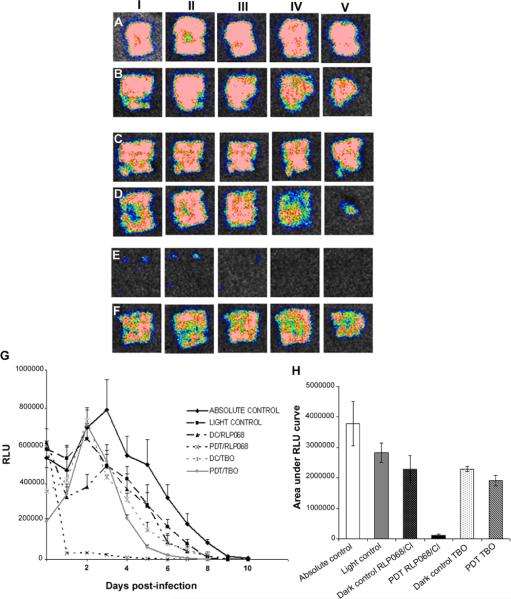
Figure 4 shows a set of 5 representative time courses from infected mouse scratch wounds (each time course from a single mouse in each of the 5 groups) infected with MRSA. The bacterial bioluminescence was largely preserved during the treatment with light alone (Figure 4A) and in dark controls of RLP068/Cl (Figure 4D) and TBO (Figure 4E) (the picture were captured after 2, 6 and 14 minutes from time 0, the minutes correspondent to light delivery time) (Figure 4D, E). In contrast, PDT gave a light dose-dependent reduction of bacterial bioluminescence from mice wounds treated with RLP068/Cl (Figure 4B) or TBO (Figure 4C) during PDT treatment. We assigned the bioluminescent signal at 10 min after inoculation of PS as time 0 (immediately before light delivery). Interestingly at 10 minutes after TBO application there was a drop in bioluminescence (0.5–0.6 log unit reduction) which was almost 10 times higher than other groups. It could be either due to a toxic effect of TBO on the bacteria or to an optical quenching effect. In Figure 4F we show the dose response curves of all groups. Each curve represents the average SEM of bacterial luminescence in each group (n = 6). PDT using RLP068/Cl induced a decrease of 2.9 log unit in bacterial bioluminescence while the RLP068/Cl alone only induced a 0.85 log unit reduction during the same time. PDT treatment with TBO induced approximately 1.0 log unit reduction in bacterial bioluminescence compared to negligible reduction with TBO dark control. The follow up monitoring of the bioluminescence signal (time courses over 5 days) after PDT in representative mice from all the treatment groups is depicted in Figure 5. Significant differences were observed in bacterial re-growth between PDT performed by using RLP068/Cl (Figure 5E) and the other groups (Figure 5A, B, C, D). In first case the re-growth is minimal, 4 fold less than control group and bioluminescence was not detectable after 3 days. The time courses (from day 0 to day 10) of the mean bacterial bioluminescence signal of infected wounds in the different groups of mice are shown in Figure 5G. The areas under the curve (Figure 5H) were compared and PDT with RLP068/Cl (group E) was significantly lower than the other groups (p < 0.001).
Figure 4.
(A–E) Successive bioluminescence images of a representative mouse skin scratch model infected with 108 CFU MRSA treated with: (A) Light alone; (B) PDT using RLP068/Cl (40 μL of 75 μM solution) or (C) PDT using TBO (40 μL of 75 μM solution) at 30 minutes after bacterial inoculation +15 min from PS application. PDT was carried out with a combination of 40 μL of 75 μM PS and 84 J/cm2 red light. (D) RLP068/Cl or (E) TBO alone 30 minutes after bacterial inoculation +15 minutes after application of PS. Animals were imaged at 2, 6 and 14 min, without to be exposed to light. (F) Dose-response of mean bacterial bioluminescent of mouse scratch abrasion infected with MRSA after treatment with light alone, RLP068/Cl and TBO dark controls and PDT using RLP068/Cl or TBO assuming as TIME 0 the end of 10 minutes.
Figure 5.
(A–F) Successive bioluminescence images of the follow up (from 1 to 5 days) of a representative mouse scratch infected wound with MRSA and (A) not treated; (B) treated with light alone; (C) treated with RLP068/Cl alone; (D); treated with TBO alone; (E) treated with PDT using RLP068/Cl; (F) treated with PDT using TBO. (G) Plots of quantified mean bacterial bioluminescence values from all the mice in the different groups +/− SEM. (H) Mean areas under the curve determined from Figure 4G +/− SEM.
3.4 Wound healing
PDT treatment using RLP068/Cl (Figure 6E) improved the wound healing process, as shown in Figure 6. In this group of mice, only minimal scab formation occurred at day 4, much earlier than the other groups, in which the scab was observed at day 7 (Figure 6B, C) or day 8 (Figure 6A, D). Interestingly, the area where scab formation occurred within the 1 cm2 of the wound corresponded exactly to the area where bacterial re-growth was observed. Moreover, PDT with RLP068/Cl induced a faster healing process. As shown in Figure 6 in mice treated with PDT using TBO (Figure 6F) the scab formation started at 5 day however the healing process was slower probably due to presence of bacteria re-growth after PDT treatment. The time-course (from day 4 to 9 day) of the remaining unhealed area of the wound is shown in Figure 7A. The analysis of the area under the curve, reported in Figure 7B showed that in the group of mice treated by PDT with RLP068/Cl, the unhealed area was only 22%, against 100% for control group, 98% for light control group, 78% for RLP068/Cl dark control group, 76% for PDT with TBO and 89% for TBO dark control.
Figure 6.
Macroscopic assessment of wound healing from the 5th until 9th day of different groups (a) control group, (b) light control, (c) Dark control for RLP068/Cl, (d) Dark control for TBO, (e) PDT using RLP068/Cl, (f) PDT using TBO.
Figure 7.
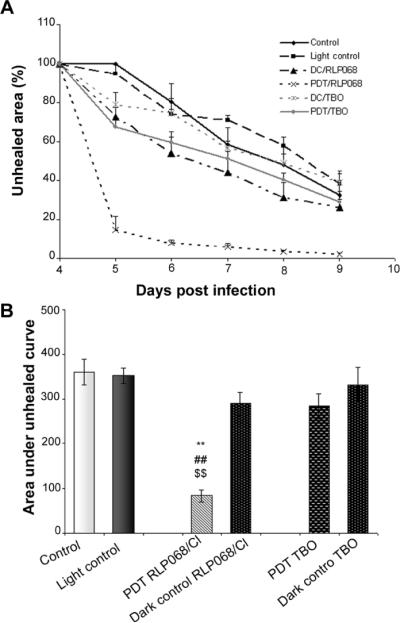
(A) Time course of wound healing measured by mean unhealed areas of mice in different treatment groups +/− SEM. (B) Means of area under the curve of wound healing (in two dimensional coordinate system from Figure 6A) representing integrated time course of unhealed area +/− SEM.
4. Discussion
In recent years several in vivo models have been developed for the purpose of testing new products claimed to promote the wounding healing process. The intent of using animal models of disease is to establish a wound and/or infection that mimics the process seen in humans. A key to developing an animal model is the selection of an animal whose physiology, reaction to an infection, and the nature of the infection itself all mirror as closely as possible the situation in humans, so that the data from animal models provide a means of indicating the potential of a treatment [19, 20]. In this study we used a scratch wound made on the dorsal surface of mice by creating crossed scratch lines within a defined area. The scratches only damaged the stratum corneum and upper-layer of the epidermis but not the dermis and the skin abrasion could be inoculated by bacterial cells [21]. Temporary immunosuppression to reduce circulating neutrophils was necessary to allow the infection to become established at the skin level. This model was used to define the dose-response relationship of antimicrobial PDT using a recently introduced PS RLP068/Cl. The possible recurrence of the infection and the influence of PDT on the wound healing process and time were also determined. The results obtained in the present study, demonstrated the ability of PDT using RLP068/Cl to inactivate MRSA in scratch wounds. Following the treatment, we observed significant improvement in the wound healing process. The choice of PS is a key factor in optimizing the efficacy of the treatment with PDT. Some features of the ideal antimicrobial PS are a high affinity for microbial cells, a lower risk to induce damage to host cells, and the ability to prevent the re-growth of bacteria after treatment with PDT [22]. It has been demonstrated that some antimicrobial agents exert their effect after penetration into the cytoplasm of the bacteria; these processes can require transport mechanisms that could induce microbial cells to develop resistance mechanisms [23]. On the other hand drugs with high molecular weight could be excluded from penetrating into microbial cells. Cationic PS endowed with relative high molecular mass, such as RLP068/Cl (a tetracationic Zn(II) phthalocyanine chloride), could offer an advantage to act without penetration into microbial cells, retaining their efficacy as the main targets for these kind of compounds are cell walls and membranes [24–26]. RLP068/Cl has been tested in an in vitro PDT inactivation study [14]. Its efficacy on bacterial inactivation after illumination was tested against three important human pathogens, S. aureus, P. aeruginosa and C. albicans. The authors demonstrated that RLP068/Cl and PDT treatment was able to kill more than 3 log10 for all microbial strains. Moreover they demonstrated a low propensity to induce resistance mechanisms after light activation of RLP068/Cl confirming what it is considered an unlikely event during PDT microbial inactivation. In another previous study, Simonetti et al. [15] used RLP068/Cl in an undisclosed proprietary gel formulation in a mouse model of a full-thickness excisional wound, infected with MRSA. They demonstrated that RLP068/Cl gave a dose related reduction of bacterial counts; the analysis was performed by bacterial counts immediately and at 7 day after treatment with PDT. Moreover, RLP068/Cl accelerated the wound healing process [15]. The results, in agreement with previous results [15] demonstrated that RLP068/Cl significantly reduced the bacterial burden in the wound. In this study we used a bioluminescent strain of lux-MRSA allowed a real-time non invasive monitoring of the extent of infection in mice through bioluminescence imaging. The real-time images captured during the PDT treatment using RLP068/Cl showed a dose response to the light and the bacteria were killed after administration of 84 J/cm2 at 690 nm wavelength by using the photosensitizer at 75 μM concentration. In the day post-PDT treatment, minimal bacterial re-growth was observed in the lesions and at 2 days post infection the bioluminescence was not detectable. At the same time a decrease of bacterial bioluminescence in dark controls with RLP068/Cl treated but not illuminated lesions was not observed. We also compared the effect of RLP068/Cl PDT with PDT mediated by TBO. TBO is a known antimicrobial PS that has been used in many studies to evaluate the efficacy of PDT in cutaneous and dental infections, caused by different bacterial strains [27–29]. To compare the two PS we used the same conditions and concentrations for both PS, but used light with different wavelengths according to their absorption maxima which for TBO is reported to be 635 nm [29]. The concentration of TBO was 75 μM diluted in the same solution used for RLP068/Cl (1%DMSO in 5% dextrose). Under these conditions we observed a light dose-response in the group of animals treated with PDT using TBO however a bacterial re-growth on the following days was also observed. In other studies performed with this photosensitizer [27, 29] the concentration of TBO used was higher and light fluencies lower compared to the present values. We assume the relatively low concentration of TBO and low dose of the light were not able to completely kill all the bacteria in this type of lesion, and this could explain the bacterial regrowth observed in the days post-PDT. The photosensitizer RLP068/Clmediated PDT treatment was also able to improve the wound healing process. At least in part, this was due to the bacterial inactivation within the lesion as the presence of bacteria are known to inhibit the wound healing process [30]. In the pictures (Figure 6E) it can be seen that the formation of the scab in the group of mice treated with RLP068/Cl occurred earlier and correlated with minimal bacterial re-growth. In animals treated with TBO mediated PDT we observed a slower wound-healing process, and at the same time bacteria were not completely inactivated by PDT and the occurred re-growth further interfered with the healing process. No effect on wound healing was observed in animal groups treated with RLP068/Cl or TBO alone or treated with light alone. These results demonstrate the efficacy of PDT using RLP068/Cl as in our model both parameters evaluated: bacterial inactivation and improved wound healing were positively impacted. Future studies should be performed (1) to confirm the efficacy of PDT using RLP068/Cl in other animal models of wound infection; (2) to clarify the mechanisms of action of RPL068/Cl to photoinactivate bacteria and also to how PDT improves the wound healing process.
Acknowledgements
This work was supported by Fondazione MPS by Alta Formazione Internazionale. Fellowship to D. Vecchio, T. Dai was supported by an Airlift Research Foundation Extremity Trauma Research Grant 109421. M. R. Hamblin was supported by US NIH R01AI050875. L. Fantetti and G. Roncucci were supported by POR CREO FESR 2007–2013 Orione Toscana. The funding agencies had no involvement in planning the study or writing the paper.
Biographies

Daniela Vecchio, Ph.D., is currently a postdoctoral fellow in Dr. Hamblin's laboratory. Her research interests include the antimicrobial effect of photodynamic therapy in vivo and in vitro studies and wound healing.

Tianhong Dai, Ph.D., is currently an instructor in Dr. Hamblin's laboratory. His research interest is focused on the use of light-based antimicrobial approaches for skin and soft tissue infections.

Liyi Huang, MD, Ph.D., was a postdoctoral fellow at Dr. Hamblin's laboratory. At present she is a Professor at the Department of Infectious Diseases, First Affiliated College & Hospital, Guangxi Medical University, China.

Gabrio Roncucci is an experienced organic and bioorganic chemist, leading the Molteni Therapeutic group focused on research from drug discovery to clinical trials of novel, functional metallophthalocyanines. This PDT work has led to fifteen families of granted patents. Dr. Roncucci graduated in Organic Chemistry at the University of Florence, and the University of Siena. He joined the Molteni company where he has been the Research Director since 1993.

Lia Fantetti has worked for Molteni Farmaceutici R&D since 1991 and is involved in the screening and toxicological development of new compounds with anti-inflammatory activity. She has been responsible for the full pharmacological and toxicological development program of the new photosensitizers with powerful antimicrobial properties. She is currently involved in preclinical studies on PDT that focused on antimicrobial and wound healing effects.

Michael R. Hamblin, Ph.D., is a Principal Investigator at the Wellman Center for Photomedicine at Massachusetts General Hospital, an Associate Professor of Dermatology at Harvard Medical School and the Harvard-MIT Division of Health Science and Technology. His research interests are in photodynamic therapy and in low-level light therapy. In 2011 Dr Hamblin was honored by election as a Fellow of SPIE.
Footnotes
Conflict of interest L. Fantetti and G. Roncucci are employees of Molteni Therapeutics and are inventors on a patent for RLP068.
References
- [1].Landis SJ. Adv. Skin Wound Care. 2008;21:531–540. doi: 10.1097/01.ASW.0000323578.87700.a5. quiz 541–542. [DOI] [PubMed] [Google Scholar]
- [2].Braiman-Wiksman L, Solomonik I, Spira R, Tennenbaum T. Toxicol. Pathol. 2007;35:767–779. doi: 10.1080/01926230701584189. [DOI] [PubMed] [Google Scholar]
- [3].Watsky MA, Weber KT, Sun Y, Post-lethwaite A. Int. Rev. Cell Mol. Biol. 2010;282:165–192. doi: 10.1016/S1937-6448(10)82004-0. [DOI] [PubMed] [Google Scholar]
- [4].Shaw TJ, Martin P. J. Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hackam DJ, Ford HR. Surg. Infect. (Larchmt) 2002;3(Suppl. 1):S23–S35. doi: 10.1089/sur.2002.3.s1-23. [DOI] [PubMed] [Google Scholar]
- [6].Harding KG, Moore K, Phillips TJ. Int. Wound J. 2005;2:364–368. doi: 10.1111/j.1742-4801.2005.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Diegelmann RF, Evans MC. Front. Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- [8].Yamaguchi Y, Yoshikawa K. J. Dermatol. 2001;28:521–534. doi: 10.1111/j.1346-8138.2001.tb00025.x. [DOI] [PubMed] [Google Scholar]
- [9].Guyon L, Ascencio M, Collinet P, Mordon S. Photodiagnosis Photodyn. Ther. 2012;9:16–31. doi: 10.1016/j.pdpdt.2011.08.003. [DOI] [PubMed] [Google Scholar]
- [10].Biel MA. Methods Mol. Biol. 2010;635:281–293. doi: 10.1007/978-1-60761-697-9_18. [DOI] [PubMed] [Google Scholar]
- [11].Braathen LR, Morton CA, Basset-Seguin N, Bissonnette R, Gerritsen MJ, Gilaberte Y, Calzavara-Pinton P, Sidoroff A, Wulf HC, Szeimies RM. J. Eur. Acad. Dermatol. Venereol. 2012;26:1063–1066. doi: 10.1111/j.1468-3083.2011.04432.x. [DOI] [PubMed] [Google Scholar]
- [12].Demidova TN, Hamblin MR. Int. J. Immunopathol. Pharmacol. 2004;17:245–254. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hamblin MR, Hasan T. Photochem. Photobiol. Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Giuliani F, Martinelli M, Cocchi A, Arbia D, Fantetti L, Roncucci G. Antimicrob. Agents Chemother. 2010;54:637–642. doi: 10.1128/AAC.00603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Simonetti O, Cirioni O, Orlando F, Alongi C, Lucarini G, Silvestri C, Zizzi A, Fantetti L, Roncucci G, Giacometti A, Offidani A, Provinciali M. Br. J. Dermatol. 2011;164:987–995. doi: 10.1111/j.1365-2133.2011.10232.x. [DOI] [PubMed] [Google Scholar]
- [16].Dai T, Kharkwal GB, Tanaka M, Huang YY, Bil de Arce VJ, Hamblin MR. Virulence. 2011;2:296–315. doi: 10.4161/viru.2.4.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hamblin MR, O'Donnell DA, Murthy N, Contag CH, Hasan T. Photochem. Photobiol. 2002;75:51–57. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [18].Davis PJ, Rabinowitz P. Academic Press; New York: 1975. p. 149. [Google Scholar]
- [19].Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. J. Photochem. Photobiol. B. 2005;81:15–25. doi: 10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sharma SK, Dai T, Kharkwal GB, Huang YY, Huang L, De Arce VJ, Tegos GP, Hamblin MR. Curr. Pharm. Des. 2011;17:1303–1319. doi: 10.2174/138161211795703735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. Lasers Surg. Med. 2010;42:38–44. doi: 10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G. Lasers Surg. Med. 2006;38:468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- [23].Nikaido H. Microbiol. Mol. Biol. Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Demidova TN, Hamblin MR. Antimicrob. Agents Chemother. 2005;49:2329–2335. doi: 10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lambrechts SA, Aalders MC, Van Marle J. Antimicrob. Agents Chemother. 2005;49:2026–2034. doi: 10.1128/AAC.49.5.2026-2034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mantareva V, Kussovski V, Angelov I, Borisova E, Avramov L, Schnurpfeil G, Wohrle D. Bioorg. Med. Chem. 2007;15:4829–4835. doi: 10.1016/j.bmc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- [27].Garcia VG, de Lima MA, Okamoto T, Milanezi LA, Junior EC, Fernandes LA, de Almeida JM, Theodoro LH. Lasers Med. Sci. 2010;25:221–228. doi: 10.1007/s10103-009-0694-z. [DOI] [PubMed] [Google Scholar]
- [28].Dai T, Bil de Arce VJ, Tegos GP, Hamblin MR. Antimicrob. Agents Chemother. 2011;55:5710–5717. doi: 10.1128/AAC.05404-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lin J, Bi LJ, Zhang ZG, Fu YM, Dong TT. Lasers Med. Sci. 2010;25:233–238. doi: 10.1007/s10103-009-0700-5. [DOI] [PubMed] [Google Scholar]
- [30].Bowler PG, Duerden BI, Armstrong DG. Clin. Microbiol. Rev. 2001;14:244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]