Abstract
Oxidized phospholipids are found in the vasculature of animal models of atherosclerosis, in human atherosclerotic lesions, and in other inflammatory diseases. Oxidized phospholipids cause vascular and nonvascular cells to initiate an inflammatory reaction. Metabolites of arachidonic acid, such as 12-hydroxyeicosatetraenoic acid, can mimic some of the inflammatory properties of oxidized phospholipids. In vitro and in vivo normal high-density lipoprotein (HDL), normal apolipoprotein A-I, and apolipoprotein A-I mimetic peptides, each likely acting in a different manner, prevent the inflammatory reaction characteristic of atherosclerosis, and this is associated with decreased levels of oxidized lipids in tissues and cells. HDL from animal models of atherosclerosis or from humans with atherosclerosis or from humans or animals with other chronic inflammatory diseases does not prevent the inflammatory reaction characteristic of atherosclerosis and may even enhance the inflammatory reaction. In mice and perhaps humans, ≈30% of the steady-state plasma HDL-cholesterol pool is derived from the small intestine. The metabolism of phospholipids by gut bacteria has been recently implicated in atherosclerosis in both mice and humans. Studies with apolipoprotein A-I mimetic peptides suggest that the small intestine is a major tissue regulating systemic inflammation in mouse models of atherosclerosis and may be important for determining the functionality of HDL.
Keywords: apolipoprotein A-I, apolipoprotein A-I mimetic peptides, high-density lipoprotein, hydroxyeicosatetraenoic acid, hydroxyoctadecadienoic acid, lipoproteins, oxidized lipids, small intestine
Much work in the field has suggested that oxidation of low-density lipoprotein (LDL) was a key event in atherogenesis as it led to enhanced uptake and foam cell formation. However, it was seen almost at the same time that the many oxidized lipids in oxidized-LDL could be responsible for the proinflammatory effects of oxidized-LDL. Studies to define these oxidized lipids led to the discovery of oxidized phospholipids (Ox-PL) as a major class of proinflammatory lipids.1 During this period, studies to explain how highdensity lipoprotein (HDL) influences atherogenesis led to the realization that it had a major role in providing antioxidant properties to dampen the proinflammatory properties of oxidized-LDL.2 In turn, this led to the development of apolipoprotein (apo)A-I mimetic peptides such as 4F and others.3 Subsequent studies showed that not only was 4F peptide able to inhibit atherosclerosis, but it potently inhibited inflammation in a surprisingly wide variety of animal models of disease.3
In preliminary studies in humans,4 4F peptide was administered orally at doses from 0.43 to 7.14 mg/kg. The plasma levels achieved were very low (Cmax 15.9±6.5 ng/mL). However, peptide administration at doses of 4.3 and 7.14 mg/ kg significantly improved the HDL inflammatory index, whereas doses of 0.43 and 1.43 mg/kg did not.4 In a second clinical trial5 it was decided to achieve high plasma levels of peptide by using low doses (0.042–1.43 mg/kg) of peptide administered intravenously or subcutaneously. Despite achieving very high plasma levels of peptide (Cmax 3255±640 ng/ mL), there was no improvement in HDL inflammatory index.5 This led to a reappraisal of why the peptide was so effective in mice and led to the surprising discovery that a major site of action for the 4F peptide may be in the intestine, even when it is administered subcutaneously.6
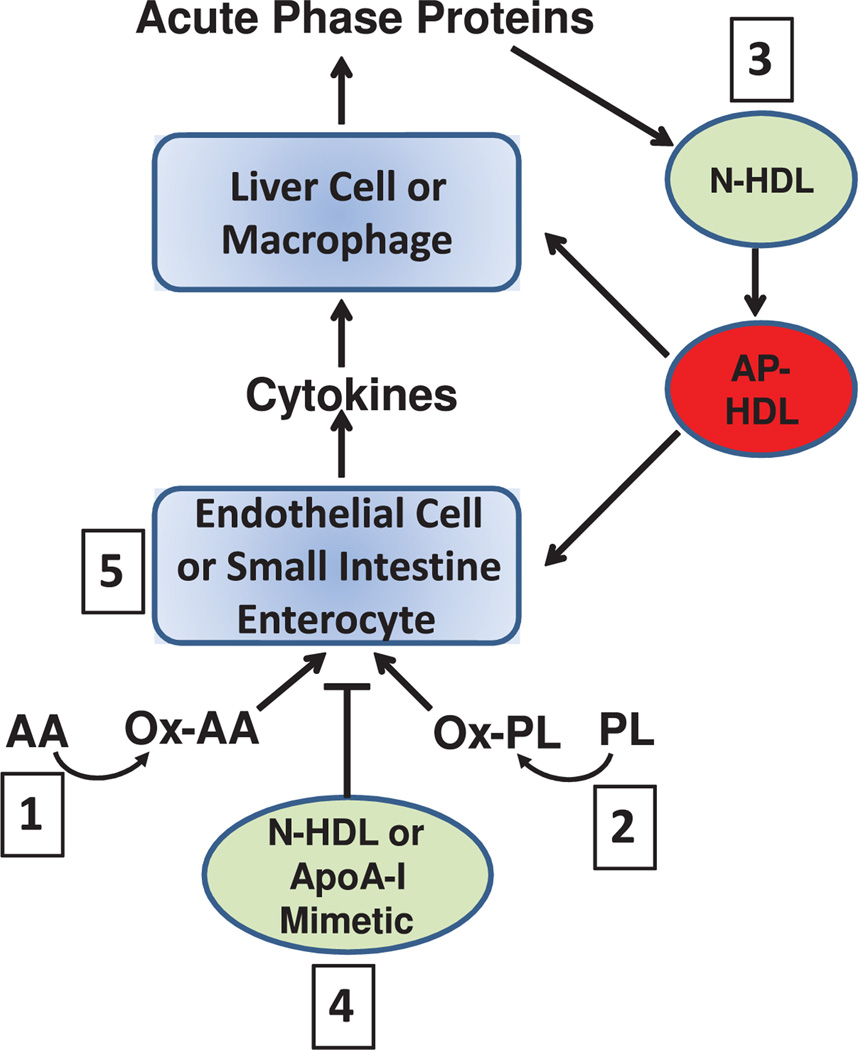
This pathway of discovery has led us to the following 5 hypotheses, which are depicted in the Figure. Hypothesis 1 is that the oxidation of normal lipids by metabolic pathways or by nonenzymatic means produces oxidized lipids, which trigger an inflammatory response in many tissues including the small intestine and vasculature. Hypothesis 2 is that metabolites of arachidonic acid, such as 12-hydroxyeicosatetraenoic acid (HETE), can act similar to Ox-PL to induce inflammation. Hypothesis 3 is that HDL contains proteins and enzymes that can inactivate or remove these proinflammatory lipids, but in some circumstances, such as a systemic acute phase response, the proteins and enzymes associated with HDL are altered so that the inflammatory response is either not inhibited or is enhanced. Hypothesis 4 is that apoA-I mimetic peptides, such as 4F, reduce inflammation by binding and removing oxidized lipids from tissues. Hypothesis 5 is that oxidized lipids in the small intestine are important in modulating systemic inflammation and the intestine is a major site for the action of apoA-I mimetic peptides, such as 4F, which bind these oxidized lipids.
Figure.
Free arachidonic acid (AA) is metabolized to an oxidized fatty acid (Ox-AA), such as 12-hydroxyeicosatetraenoic acid. Phospholipids (PL) are also oxidized (Ox-PL). These oxidized lipids act on endothelial cells or on enterocytes in the small intestine. The cells respond by producing cytokines that act on liver cells or macrophages, leading to the production of acute phase proteins. Normal high-density lipoprotein (N-HDL) or apolipoprotein A-I (apoA-I) mimetic peptides (apoA-I mimetic) block this sequence of events. However, if the acute phase reaction is established the normal HDL is converted to acute phase HDL (AP-HDL), which loses its ability to inhibit this process or may even interact with the cells to amplify the acute phase reaction. The numbers shown in the figure relate to each of the 5 hypotheses discussed in the text of this review.
In this article, we will review the many lines of evidence garnered from many laboratories that have led us to construct these 5 novel hypotheses of how HDL and apoA-I mimetic peptides, such as 4F, may influence oxidized lipid metabolism in the intestine, thus providing systemic anti-inflammatory and antiatherosclerotic properties.
Oxidized Lipids Are Found in the Vasculature of Animal Models of Atherosclerosis, in Human Atherosclerotic Lesions, and in Other Inflammatory Diseases
Phospholipids are an integral component of all mammalian membranes and are the source of substrates for multiple enzymatic pathways. The role of phospholipid oxidation products in atherosclerosis has recently been reviewed.1 Watson et al7 and Subbanagounder et al8 demonstrated by liquid chromatographyelectrospray ionization/multi-stage mass spectrometry that Ox-PL were present in fatty streaks from cholesterol-fed rabbits7,8 and in lesions of apolipoprotein E–null mice.7 Subbanagounder et al8 also demonstrated that the group at the sn-2 position of Ox-PL determines the specific bioactivity and that the substitution of stearoyl for palmitoyl at the sn-1 position or ethanolamine for choline at the sn-3 position of the phospholipid did not alter bioactivity. Subbanagounder et al8 further showed that all parts of the phospholipid molecules are required for these bioactivities.
The binding of oxidized-LDL to the scavenger receptor CD36 in mice was demonstrated to be attributable to Ox-PL that were associated with both lipid and protein moieties of the lipoprotein.9 Podrez et al10 demonstrated that a variety of Ox-PL beyond those described by Watson et al7 and Subbagounder et al8 are present in lesions and that they interact with CD36 in the mouse as first suggested by Boullier et al.9 A simple phospholipid, such as 1-palmitoyl-2- arachidonoyl-sn-glycero-3-phosphorylcholine, when air oxidized produces hundreds of compounds,7 and hence it is not surprising that there are a myriad of Ox-PL found in nature.
Oxidized lipids can initiate an inflammatory response and are also formed in an inflammatory reaction. To understand the sequence of events, Napoli et al11 followed the time course of the appearance of Ox-PL and monocytes in aortas of human fetuses. They found that the presence of Ox-PL preceded the appearance of the monocytes.11 The findings of Nishi et al12 highlight the importance of Ox-PL to human disease. They found that the vulnerability of plaques was related to the amount of LDL containing oxidized phosphatidylcholine in the lesion. Tsimikas et al13 reported that after percutaneous angioplasty there was a dramatic increase in plasma levels of Ox-PL confirming the presence of Ox-PL in clinically important lesions in humans. Cardiolipin is found in bacteria, in the inner membrane of mitochondria, and in LDL. Tuominen et al14 reported that a natural antibody to oxidized cardiolipin bound to oxidized-LDL, apoptotic cells, and atherosclerotic lesions but this antibody did not recognize native cardiolipin or native LDL, confirming that the oxidation of phospholipids occurs in inflammatory conditions in vivo in rabbits and humans.
In monkey and rabbit models of atherosclerosis, Tsimikas et al15 demonstrated that during regression of lesions Ox-PL increased in plasma and decreased in lesions, consistent with the findings of this group in humans.13 Interestingly, Tsimikas et al16 found that Ox-PL in human plasma is largely associated with Lp(a) lipoprotein and is strongly associated with angiographically documented coronary artery disease (CAD), particularly in patients 60 years of age or younger. Leibundgut et al17 found that Ox-PL are also present in plasminogen, which is homologous to Lp(a), and affects fibrinolysis.
Although it is not known precisely how diet-induced inflammation produces Ox-PL, the process seems to be wide-spread in nature as Fang et al18,19 demonstrated that Ox-PL accumulated in lesions induced by cholesterol feeding zebra fish larvae.
The presence of Ox-PL at sites of inflammation is not restricted to atherosclerosis. For example, Imai et al20 found Ox-PL in lungs of human and animals infected with severe acute respiratory syndrome (SAARS), anthrax, or H5N1. Pulmonary challenge with inactivated H5N1 avian influenza virus rapidly induced acute lung injury and Ox-PL formation in mice.20 Consistent with these findings, Crowe et al21 found that interleukin-17RA–null mice had markedly better survival and less Ox-PL formation in the lungs after influenza infection. Ox-PL has also been found in the mucosa of the small intestine of mice that are genetically prone to polyp formation and colon cancer.22 In humans, Ox-PL has been found in brain lesions of patients with multiple sclerosis23 and in skin lesions of patients with leprosy.24 Ox-PL have also been found in nonalcoholic fatty liver disease, and Ox-PL levels correlated with disease severity in humans.25 In a mouse model of this disease, administration of an apoAI mimetic peptide known to bind Ox-PL with extraordinary high affinity26 (the 4F peptide) significantly reduced hepatic fibrosis.27 However, the authors did not measure Ox-PL levels in their study.27 The presence of Ox-PL in human eyes was seen to increase with age and was increased in eyes from patients with age-related macular degeneration.28 In a mouse model of scleroderma, the hearts contained higher levels of antibody to Ox-PL than controls and the tissue levels of these antibodies decreased29 with administration of the 4F peptide.
The presence of Ox-PL in a wide range of inflammatory conditions in species ranging from zebra fish to humans is consistent with hypothesis 1 proposed in this review. Some of the studies cited in this section are also consistent with hypothesis 4 proposed in this review.
Oxidized Lipids Cause Vascular and Nonvascular Cells to Initiate an Inflammatory Reaction
In vitro, a mixture of Ox-PL made by air oxidation of 1-palmitoyl- 2-arachidonoyl-sn-glycero-3-phosphorylcholine causes human aortic endothelial cells (ECs) to bind monocytes (but not neutrophils) and causes the cells to secrete cytokines, including the potent monocyte chemoattractant factor monocyte chemotactic protein 1.1 Individual components of air oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine have different effects in vitro. For example, 1- palmitoyl- 2-glutaroyl-sn-glycero-3-phosphorylcholine stimulates ECs to bind both neutrophils and monocytes, whereas 1-palmitoyl- 2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine only stimulates monocyte binding and strongly inhibits lipopolysaccharide- mediated induction of neutrophil binding and expression of E-selectin protein and mRNA.30 In rabbits, vascular inflammation was seen to tightly track with the content of Ox-PL, oxidized fatty acids, and malondialdehyde in lesions.31 Interestingly, the relative levels of oxidized fatty acids derived from arachidonic acid, linoleic acid, and oleic acid in plasma also tracked well with levels in lesions.31
Furnkranz et al32 directly applied Ox-PL to carotid arteries in mice and found that the arteries responded by inducing a set of atherosclerosis-related genes including monocyte chemotactic protein 1, keratinocyte-derived chemokine, tissue factor, interleukin 6, heme oxygenase 1, and early growth response 1. In isolated perfused carotid arteries, Ox-PL triggered rolling and firm adhesion of monocytes in a P-selectin and keratinocyte- derived chemokine-dependent manner.32 Monocyte adhesion to ECs induced by Ox-PL seems to involve activation of cytosolic phospholipase A2 and 12-lipoxygenase; indeed, 12-HETE mimicked the effects of Ox-PL.33 Honda et al34 found that the 12-lipoxygenase pathway was critical for mildly oxidized-LDL (which contains Ox-PL) to induce monocyte binding to human aortic EC. Similar to Ox-PL, 12-HETE induces monocyte but not neutrophil binding to human aortic EC.35 The number of compounds that can be generated by enzymatic pathways, such as the lipoxygenase pathways, is dramatically increased by the crossover of multiple pathways and the ability of the lipids that are formed to undergo nonenzymatic rearrangements.36 The complexity is further increased by the ability of some enzyme systems to oxidize fatty acids to biologically active molecules while they are still esterified to cholesterol or phospholipids.37 Thus, the biologic activity of 12-HETE used in the examples mentioned earlier is just that, an example. The number of biologically active oxidized lipids present in nature is very large.
The mechanism(s) by which Ox-PL induce cells to initiate an inflammatory response is complex.38–47 Ox-PL seems to have oxidation-specific epitopes, which are recognized as danger-associated molecular patterns by pattern recognition receptors involved in innate immunity.48 Antioxidant enzymes can modulate the stress response induced by Ox-PL.49 Ox-PL have also been shown to significantly alter the phenotype of macrophages accounting for some of the characteristics of macrophages that have been noted in atherosclerotic lesions.50
Inflammatory response of vascular cells in vitro and in vivo to a variety of oxidized lipids and the striking similarity between the response to Ox-PL and to a metabolite of arachidonic acid, such as 12-HETE, is consistent with both hypothesis 1 and hypothesis 2 of this review.
In Vitro and In Vivo Normal HDL, Normal ApoA-I, and ApoA-I Mimetic Peptides Prevent Inflammatory Reaction Characteristic of Atherosclerosis and this Is Associated With Decreased Levels of Oxidized Lipids In Vitro and In Vivo
The role of normal HDL and mimetics of HDL in preventing inflammatory reaction initiated by oxidized lipids involves multiple components of HDL.51,52 In vitro studies demonstrated that an apoA-I mimetic peptide (4F) prevented the formation and secretion of Ox-PL in response to influenza A infection.53 In vivo, this peptide prevented the trafficking of macrophages into the aorta in response to influenza A infection in mice.54 In other in vitro studies, normal human HDL modulated the proinflammatory response of human aortic EC to Ox-PL to a signaling cascade that was anti-inflammatory.55
An HDL-associated enzyme, paraoxonase 1 (PON1), which inhibited the response of ECs to Ox-PL in vitro when delivered into arteries in vivo inhibited the response to balloon injury.56 Another component of normal HDL, apoM, has been reported to bind Ox-PL and increase the antioxidant effect of HDL.57
Feeding LDL receptor–null mice (LDLR−/−) a Western diet (WD) induced the formation of Ox-PL in their kidneys, which was accompanied by inflammatory changes similar to those in the arteries of these mice.58 Treating the mice with the 4F peptide significantly reduced tissue levels of Ox-PL and significantly reduced the inflammation in the tissues without changing plasma lipid levels.58
As noted earlier,31 the plasma and lesion content of oxidized fatty acids seem to parallel one another. Administration of the 4F peptide to mouse models of atherosclerosis reduced the plasma levels of oxidized fatty acids and resulted in more anti-inflammatory HDL.59 Another line of evidence suggesting that the mechanism by which apoA-I mimetic peptides work involves binding and removing Ox-PL comes from the finding that their use induces natural antibodies that recognize Ox-PL,60,61 which would be consistent with the plasma increase in Ox-PL that has been seen in animal models of regression.15
The studies in this section are consistent with hypothesis 3 and hypothesis 4 as proposed in this review.
HDL From Animal Models of Atherosclerosis or From Humans With Atherosclerosis or From Mammals With Other Chronic Inflammatory Diseases or HDL with ApoA-I Modified by Products Found in Inflammatory Reactions Does Not Prevent Inflammatory Reaction Characteristic of Atherosclerosis and May Even Enhance Inflammatory Reaction
Van Lenten et al62 were the first to report that during an acute phase response anti-inflammatory HDL becomes proinflammatory in rabbits and humans. Subsequently, Navab et al63 reported that injection of Ox-PL into mice genetically susceptible to diet-induced atherosclerosis (C57BL/6J mice), but not in mice resistant to diet-induced atherosclerosis (C3H/ HeJ mice), induced an acute phase response with elevations of apoJ and decreased PON1 activity.
Hedrick et al64 reported that feeding an atherogenic diet to LDLR−/− mice for 3 days did not decrease hepatic PON1 mRNA but caused a dramatic decrease in plasma PON1 activity and mass. The decreased activity and mass were temporally related to an increase in the lipid hydroperoxide content of HDL with a decrease in HDL-cholesterol, native apoA-I and apoA-II levels. As the native apoA-I disappeared from the circulation, higher molecular weight forms of apoA-I appeared, some of which contained epitopes recognized by an antibody that recognizes Ox-PL (EO6).64 At present there are no studies that present specific evidence of the levels of various components of Ox-PL in the vessel wall and as function of HDL mass and functionality.
The ability of HDL to prevent the formation of Ox-PL was found to be reduced in mice after exposure of the mice to second-hand cigarette smoke.65 The role of Ox-PL in modulating HDL function has been previously reviewed.2,66 The genetic control of the anti-inflammatory properties of HDL in mice and the inverse relationship of these anti-inflammatory properties in humans with the ability of HDL to promote cholesterol efflux from cholesterol loaded macrophages was reported by Navab et al.66
Vaisar et al67 reported that HDL from patients with coronary heart disease (CAD) is associated with many acute phase proteins, supporting the proposal that HDL is changed in the presence of chronic inflammation. The effects of chronic inflammation on HDL were favorably modified in humans with a regimen of combined statin and niacin treatment.68
Abnormalities in HDL have been identified in a variety of inflammatory states including patients with systemic lupus erythematousus,69 rheumatoid arthritis,70 and diabetes mellitus. 71,72 Kontush and Chapman have emphasized the importance of HDL subpopulations.73 Bhattacharyya et al74 reported that in humans there is a strong relationship between the activity of the HDL-associated enzyme PON1, systemic oxidative stress, and cardiovascular risk. Moreover, the levels of metabolites of arachidonic acid in these patients strongly tracked with PON1 activity and risk for cardiovascular events.74
The work of Undurti et al75 showed that modification of HDL by an enzyme released from macrophages and neutrophils during inflammation (myeloperoxidase) generates a proinflammatory HDL particle. The levels of hemoglobin (a potent oxidant when freed from red blood cells) and haptoglobin (an acute phase reactant) that were associated with HDL in CAD patients significantly predicted the inflammatory properties and function of their HDL.76 HDL from patients with another chronic inflammatory condition (rheumatoid arthritis) was associated with acute phase proteins and complement factors77 similar to that reported in patients with CAD.67
Modifying apoA-I with malondialdehyde (but not other reactive carbonyls) blocked cholesterol efflux by the ATP binding cassette A1 pathway and HDL from atherosclerotic lesions contained more malondialdehyde than normal HDL.78 Besler et al79 reported that HDL from patients with stable CAD or an acute coronary syndrome did not have anti-inflammatory properties when presented to ECs in vitro and did not stimulate EC repair because it failed to induce EC NO production. They found that HDL from these subjects (1) activated endothelial lectin-like oxidized- LDL receptor, (2) this triggered endothelial protein kinase C βII activation, which (3) inhibited EC NO-activating pathways and NO production. They identified reduced PON1 activity as a molecular mechanism leading to generation of HDL with protein kinase C βII-activating properties, which was in part attributable to increased formation of malondialdehyde in HDL.79
The importance of HDL in promoting cholesterol efflux independent of HDL-cholesterol levels was demonstrated by the studies of Khera et al,80 and it was suggested by Heinecke81 to be a possible therapeutic target. Cavigiolio et al82 reported that oxidative damage to apoA-I inhibited the disassociation of apoA-I from HDL to the lipid poor form of apoA-I that is critical for promoting cholesterol efflux via ATP binding cassette A1.
Thus, (1) HDL and its associated proteins are highly susceptible to modification by lipid oxidation products and enzymes produced or released at sites of inflammation; (2) the proteins and enzymes associated with HDL are significantly changed to a proinflammatory phenotype during an acute phase response; (3) the ability of HDL to promote cholesterol efflux and to be anti-inflammatory is dramatically reduced during a chronic acute phase reaction such as seen in CAD patients.
The studies in this section are consistent with hypothesis 3 proposed in this review.
Small Intestine Is Important in Modulating Systemic Inflammation
In mice, ≈30% of the steady-state plasma HDL-cholesterol pool is derived from the small intestine.83 Studies in chyluric humans suggest that a similar fraction of HDL in humans comes from the small intestine.84 The metabolism of phospholipids by gut bacteria has been recently implicated in atherosclerosis in both mice and humans.85 The level of oxidized lipids, phospholipids, and fatty acids in germ-free animals or animals treated with broad spectrum antibiotics are currently unknown.
Studies with the 4F peptide suggest that the small intestine is a major tissue-regulating systemic inflammation in mouse models of atherosclerosis and may be an important site for determining the functionality of HDL.86 To test this hypothesis Navab et al86 administered the 4F peptide at equal doses orally or by subcutaneous injection (subcutaneous) to LDLR−/− mice on a WD. Plasma and liver peptide levels were 298-fold and 96-fold higher, respectively, after subcutaneous administration, whereas peptide levels in the intestine only varied by 1.66±0.33-fold. Levels of metabolites of arachidonic and linoleic acids known to bind with high affinity to the peptide were significantly reduced in intestine, liver, and hepatic bile to a similar degree whether the peptide was administered subcutaneously or orally. However, levels of 20-HETE, which is known to bind to the 4F peptide with low affinity, were unchanged. Peptide treatment reduced serum amyloid A and triglyceride levels and increased HDL-cholesterol levels similarly after subcutaneous or oral administration. Plasma levels of metabolites of arachidonic and linoleic acids significantly correlated with serum amyloid A levels. Feeding metabolites of arachidonic acid (eg, 12- or 15-HETE) in mouse chow without the WD significantly increased plasma serum amyloid A and triglyceride levels and decreased HDL-cholesterol and PON1 activity, all of which were significantly ameliorated by subcutaneous administration of the 4F peptide.86 In these studies, 86 it was noted that feeding LDLR−/− mice a WD resulted in increased levels of free arachidonic acid, suggesting that the WD increased phospholipase activity. Treatment with the 4F peptide reduced enterocyte and hepatic levels of free arachidonic acid consistent with a reduction in phospholipase activity. 86 Interestingly, the levels of free arachidonic acid were similar in enterocytes from the small intestine and in the liver of these mice.86 However, the levels of free metabolites of arachidonic and linoleic acids (except for 20-HETE) were many fold greater in the enterocytes of the small intestine compared with the liver.86
The importance of phospholipase activity in the intestine in regulating the metabolism of arachidonic acid was shown by studies of patients with an inherited cytosolic phospholipase A2-α deficiency who were found to have impaired eicosanoid biosynthesis and small intestine ulceration.87 Montrose et al88 reported that cytosolic phospholipase A2 is protective against cyclooxygenase inhibitor-induced intestinal damage. Huber et al33 reported that cytosolic phospholipase A2 and 12-lipoxygenase mediate monocyte adhesion to ECs in response to Ox-PL. Watanabe et al89 reported that endothelial specific deletion of cyclooxygenase-2 in mice results in intestinal inflammation similar to Crohn disease. van Leuven et al90 reported that patients with Crohn disease have evidence of systemic inflammation, increased risk for cardiovascular events, and altered ability of HDL to act on Ox-PL. Ox-PL have been implicated in the regulation of biofilm formation in bacteria.91 Moreover, Ox-PL were found to be increased at an early stage of intestinal polyp formation in a mouse model of familial adenomatous polyposis.22 Treatment of these mice with the 4F peptide resulted in decreased polyp formation and reduced colon cancer. 92 Treatment of these mice with the 4F peptide also significantly decreased plasma levels of lyosphosphatidic acid.92 It was shown that lysophosphatidic acid binds to the 4F peptide with an affinity of 0.000523 nmol/L, which is of 2.5-millionfold higher affinity than the binding of lysophosphatidic acid to human apoA-I.93 These studies suggest that binding and removal of proinflammatory lipids is a potential mechanism for the inhibition of tumor development in these mouse models.92,93 These studies also suggest that therapies targeted to reduce inflammation in the small intestine may benefit a number of important human illnesses including atherosclerosis.
The studies cited in this section are consistent with hypothesis 4 and hypothesis 5 proposed in this review.
Summary
The studies cited in this review are consistent with hypothesis 1 that the oxidation of normal lipids by metabolic pathways or by nonenzymatic means produces oxidized lipids which trigger an inflammatory response in many tissues including the vasculature. The cited studies are also consistent with hypothesis 2 that metabolites of arachidonic acid, such as 12-HETE (and likely many other oxidized fatty acids including those esterified to cholesterol or phospholipids), can act similar to Ox-PL to induce inflammation. Although some of the biologic activities of metabolites of arachidonic acid, such as 12-HETE and Ox-PL, are similar (eg, both induce binding of monocytes but not neutrophils to ECs), the pathways by which they exert their biologic activity may not be similar and remain to be defined by future research. The studies described in this review are consistent with hypothesis 3 that HDL contains proteins and enzymes that can inactivate or remove these proinflammatory lipids, but in some circumstances, such as a systemic acute phase response, the proteins and enzymes associated with HDL are altered so that the inflammatory response is either not inhibited or is enhanced. The studies reviewed here are also consistent with hypothesis 4 that apoA-I mimetic peptides, such as 4F, reduce inflammation by binding and removing oxidized lipids from tissues. And finally, the studies cited in this review are consistent with hypothesis 5 that oxidized lipids in the small intestine are important in modulating systemic inflammation, and the intestine is a major site for the action of apoA-I mimetic peptides, such as 4F, which bind these oxidized lipids. Although these studies are consistent with each of these 5 hypotheses, definitive proof of each of these hypotheses is lacking and will require extensive future research by many laboratories.
Acknowledgments
Sources of Funding
This work was supported in part by United States Public Health Service grant HL30568 and a Leducq Network grant.
Footnotes
Disclosures
A.M.F., M.N., and S.T.R. are principals in Bruin Pharma, and A.M.F. is an officer in Bruin Pharma. The other authors have no conflicts to report.
References
- 1.Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA. Role of phospholipid oxidation products in atherosclerosis. Circ Res. 2012;111:778–799. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navab M, Berliner JA, Subbanagounder G, Hama S, Lusis AJ, Castellani LW, Reddy S, Shih D, Shi W, Watson AD, Van Lenten BJ, Vora D, Fogelman AM. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21:481–488. doi: 10.1161/01.atv.21.4.481. [DOI] [PubMed] [Google Scholar]
- 3.Navab M, Shechter I, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM. Structure and function of HDL mimetics. Arterioscler Thromb Vasc Biol. 2010;30:164–168. doi: 10.1161/ATVBAHA.109.187518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson CE, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, Navab M, Hama S, Hough G, Reddy ST, Soffer D, Rader DJ, Fogelman AM, Schecter A. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J Lipid Res. 2011;52:361–373. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navab M, Reddy ST, Anantharamaiah GM, Imaizumi S, Hough G, Hama S, Fogelman AM. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. J Lipid Res. 2011;52:1200–1210. doi: 10.1194/jlr.M013144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson AD, Leitinger N, Navab M, Faull KF, Hörkkö S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 8.Subbanagounder G, Leitinger N, Schwenke DC, Wong JW, Lee H, Rizza C, Watson AD, Faull KF, Fogelman AM, Berliner JA. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler Thromb Vasc Biol. 2000;20:2248–2254. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 9.Boullier A, Gillotte KL, Hörkkö S, Green SR, Friedman P, Dennis EA, Witztum JL, Steinberg D, Quehenberger O. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J Biol Chem. 2000;275:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 10.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, Hajjar DP, Silverstein RL, Hoff HF, Salomon RG, Hazen SL. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 11.Napoli C, D’Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishi K, Itabe H, Uno M, Kitazato KT, Horiguchi H, Shinno K, Nagahiro S. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol. 2002;22:1649–1654. doi: 10.1161/01.atv.0000033829.14012.18. [DOI] [PubMed] [Google Scholar]
- 13.Tsimikas S, Lau HK, Han KR, Shortal B, Miller ER, Segev A, Curtiss LK, Witztum JL, Strauss BH. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): shortterm and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109:3164–3170. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- 14.Tuominen A, Miller YI, Hansen LF, Kesäniemi YA, Witztum JL, Hörkkö S. A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2006;26:2096–2102. doi: 10.1161/01.ATV.0000233333.07991.4a. [DOI] [PubMed] [Google Scholar]
- 15.Tsimikas S, Aikawa M, Miller FJ, Jr, Miller ER, Torzewski M, Lentz SR, Bergmark C, Heistad DD, Libby P, Witztum JL. Increased plasma oxidized phospholipid:apolipoprotein B-100 ratio with concomitant depletion of oxidized phospholipids from atherosclerotic lesions after dietary lipid-lowering: a potential biomarker of early atherosclerosis regression. Arterioscler Thromb Vasc Biol. 2007;27:175–181. doi: 10.1161/01.ATV.0000251501.86410.03. [DOI] [PubMed] [Google Scholar]
- 16.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 17.Leibundgut G, Arai K, Orsoni A, Yin H, Scipione C, Miller ER, Koschinsky ML, Chapman MJ, Witztum JL, Tsimikas S. Oxidized phospholipids are present on plasminogen, affect fibrinolysis, and increase following acute myocardial infarction. J Am Coll Cardiol. 2012;59:1426–1437. doi: 10.1016/j.jacc.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang L, Harkewicz R, Hartvigsen K, Wiesner P, Choi SH, Almazan F, Pattison J, Deer E, Sayaphupha T, Dennis EA, Witztum JL, Tsimikas S, Miller YI. Oxidized cholesteryl esters and phospholipids in zebrafish larvae fed a high cholesterol diet: macrophage binding and activation. J Biol Chem. 2010;285:32343–32351. doi: 10.1074/jbc.M110.137257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang L, Green SR, Baek JS, Lee SH, Ellett F, Deer E, Lieschke GJ, Witztum JL, Tsimikas S, Miller YI. In vivo visualization and attenuation of oxidized lipid accumulation in hypercholesterolemic zebrafish. J Clin Invest. 2011;121:4861–4869. doi: 10.1172/JCI57755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda K, Mutoh M, Teraoka N, Nakanishi H, Wakabayashi K, Taguchi R. Increase of oxidant-related triglycerides and phosphatidylcholines in serum and small intestinal mucosa during development of intestinal polyp formation in Min mice. Cancer Sci. 2011;102:79–87. doi: 10.1111/j.1349-7006.2010.01754.x. [DOI] [PubMed] [Google Scholar]
- 23.Haider L, Fischer MT, Frischer JM, Bauer J, Höftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134(Pt 7):1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz D, Watson AD, Miller CS, Montoya D, Ochoa MT, Sieling PA, Gutierrez MA, Navab M, Reddy ST, Witztum JL, Fogelman AM, Rea TH, Eisenberg D, Berliner J, Modlin RL. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest. 2008;118:2917–2928. doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, Inoue T, Naruko T, Ehara S, Kawada N, Arakawa T, Ueda M. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43:506–514. doi: 10.1002/hep.21070. [DOI] [PubMed] [Google Scholar]
- 26.Van Lenten BJ, Wagner AC, Jung CL, Ruchala P, Waring AJ, Lehrer RI, Watson AD, Hama S, Navab M, Anantharamaiah GM, Fogelman AM. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res. 2008;49:2302–2311. doi: 10.1194/jlr.M800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLeve LD, Wang X, Kanel GC, Atkinson RD, McCuskey RS. Prevention of hepatic fibrosis in a murine model of metabolic syndrome with nonalcoholic steatohepatitis. Am J Pathol. 2008;173:993–1001. doi: 10.2353/ajpath.2008.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki M, Kamei M, Itabe H, Yoneda K, Bando H, Kume N, Tano Y. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Mol Vis. 2007;13:772–778. [PMC free article] [PubMed] [Google Scholar]
- 29.Weihrauch D, Xu H, Shi Y, Wang J, Brien J, Jones DW, Kaul S, Komorowski RA, Csuka ME, Oldham KT, Pritchard KA. Effects of D-4F on vasodilation, oxidative stress, angiostatin, myocardial inflammation, and angiogenic potential in tight-skin mice. Am J Physiol Heart Circ Physiol. 2007;293:H1432–H1441. doi: 10.1152/ajpheart.00038.2007. [DOI] [PubMed] [Google Scholar]
- 30.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, Berliner JA, Vora DK. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci USA. 1999;96:12010–12015. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napoli C, Witztum JL, Calara F, de Nigris F, Palinski W. Maternal hypercholesterolemia enhances atherogenesis in normocholesterolemic rabbits, which is inhibited by antioxidant or lipid-lowering intervention during pregnancy: an experimental model of atherogenic mechanisms in human fetuses. Circ Res. 2000;87:946–952. doi: 10.1161/01.res.87.10.946. [DOI] [PubMed] [Google Scholar]
- 32.Furnkranz A, Schober A, Bochkov VN, Bashtrykov P, Kronke G, Kadl A, Binder BR, Weber C, Leitinger N. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler Thromb Vasc Biol. 2005;25:633–638. doi: 10.1161/01.ATV.0000153106.03644.a0. [DOI] [PubMed] [Google Scholar]
- 33.Huber J, Fürnkranz A, Bochkov VN, Patricia MK, Lee H, Hedrick CC, Berliner JA, Binder BR, Leitinger N. Specific monocyte adhesion to endothelial cells induced by oxidized phospholipids involves activation of cPLA2 and lipoxygenase. J Lipid Res. 2006;47:1054–1062. doi: 10.1194/jlr.M500555-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Honda HM, Leitinger N, Frankel M, Goldhaber JI, Natarajan R, Nadler JL, Weiss JN, Berliner JA. Induction of monocyte binding to endothelial cells by MM-LDL: role of lipoxygenase metabolites. Arterioscler Thromb Vasc Biol. 1999;19:680–686. doi: 10.1161/01.atv.19.3.680. [DOI] [PubMed] [Google Scholar]
- 35.Patricia MK, Kim JA, Harper CM, Shih PT, Berliner JA, Natarajan R, Nadler JL, Hedrick CC. Lipoxygenase products increase monocyte adhesion to human aortic endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:2615–2622. doi: 10.1161/01.atv.19.11.2615. [DOI] [PubMed] [Google Scholar]
- 36.Griesser M, Suzuki T, Tejera N, Mont S, Boeglin WE, Pozzi A, Schneider C. Biosynthesis of hemiketal eicosanoids by cross-over of the 5-lipoxygenase and cyclooxygenase-2 pathways. Proc Natl Acad Sci USA. 2011;108:6945–6950. doi: 10.1073/pnas.1019473108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized low density lipoprotein. J Biol Chem. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berliner JA, Gharavi NM. Endothelial cell regulation by phospholipid oxidation products. Free Radic Biol Med. 2008;45:119–123. doi: 10.1016/j.freeradbiomed.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 2009;50(Suppl):S207–S212. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, Gharavi NM, Honda H, Chang I, Kim B, Jen N, Li R, Zimman A, Berliner JA. A role for NADPH oxidase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free Radic Biol Med. 2009;47:145–151. doi: 10.1016/j.freeradbiomed.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimman A, Chen SS, Komisopoulou E, Titz B, Martínez-Pinna R, Kafi A, Berliner JA, Graeber TG. Activation of aortic endothelial cells by oxidized phospholipids: a phosphoproteomic analysis. J Proteome Res. 2010;9:2812–2824. doi: 10.1021/pr901194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romanoski CE, Che N, Yin F, Mai N, Pouldar D, Civelek M, Pan C, Lee S, Vakili L, Yang WP, Kayne P, Mungrue IN, Araujo JA, Berliner JA, Lusis AJ. Network for activation of human endothelial cells by oxidized phospholipids: a critical role of heme oxygenase 1. Circ Res. 2011;109:e27–e41. doi: 10.1161/CIRCRESAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadl A, Sharma PR, Chen W, Agrawal R, Meher AK, Rudraiah S, Grubbs N, Sharma R, Leitinger N. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radic Biol Med. 2011;51:1903–1909. doi: 10.1016/j.freeradbiomed.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birukova AA, Lee S, Starosta V, Wu T, Ho T, Kim J, Berliner JA, Birukov KG. A role for VEGFR2 activation in endothelial responses caused by barrier disruptive OxPAPC concentrations. PLoS ONE. 2012;7:e30957. doi: 10.1371/journal.pone.0030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starosta V, Wu T, Zimman A, Pham D, Tian X, Oskolkova O, Bochkov V, Berliner JA, Birukova AA, Birukov KG. Differential regulation of endothelial cell permeability by high and low doses of oxidized 1-palmitoyl- 2-arachidonyl-sn-glycero-3-phosphocholine. Am J Respir Cell Mol Biol. 2012;46:331–341. doi: 10.1165/rcmb.2011-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S, Springstead JR, Parks BW, Romanoski CE, Palvolgyi R, Ho T, Nguyen P, Lusis AJ, Berliner JA. Metalloproteinase processing of HBEGF is a proximal event in the response of human aortic endothelial cells to oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2012;32:1246–1254. doi: 10.1161/ATVBAHA.111.241257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Springstead JR, Gugiu BG, Lee S, Cha S, Watson AD, Berliner JA. Evidence for the importance of OxPAPC interaction with cysteines in regulating endothelial cell function. J Lipid Res. 2012;53:1304–1315. doi: 10.1194/jlr.M025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JB, Xia YR, Romanoski CE, Lee S, Meng Y, Shi YS, Bourquard N, Gong KW, Port Z, Grijalva V, Reddy ST, Berliner JA, Lusis AJ, Shih DM. Paraoxonase-2 modulates stress response of endothelial cells to oxidized phospholipids and a bacterial quorum-sensing molecule. Arterioscler Thromb Vasc Biol. 2011;31:2624–2633. doi: 10.1161/ATVBAHA.111.232827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, Reddy ST, Sevanian A, Fonarow GC, Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41:1495–1508. [PubMed] [Google Scholar]
- 52.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP, Fogelman AM. D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation. 2004;110:3252–3258. doi: 10.1161/01.CIR.0000147232.75456.B3. [DOI] [PubMed] [Google Scholar]
- 54.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Garber DW, Fishbein MC, Adhikary L, Nayak DP, Hama S, Navab M, Fogelman AM. Influenza infection promotes macrophage traffic into arteries of mice that is prevented by D-4F, an apolipoprotein A-I mimetic peptide. Circulation. 2002;106:1127–1132. doi: 10.1161/01.cir.0000030182.35880.3e. [DOI] [PubMed] [Google Scholar]
- 55.Gharavi NM, Gargalovic PS, Chang I, Araujo JA, Clark MJ, Szeto WL, Watson AD, Lusis AJ, Berliner JA. High-density lipoprotein modulates oxidized phospholipid signaling in human endothelial cells from proinflammatory to anti-inflammatory. Arterioscler Thromb Vasc Biol. 2007;27:1346–1353. doi: 10.1161/ATVBAHA.107.141283. [DOI] [PubMed] [Google Scholar]
- 56.Miyoshi M, Nakano Y, Sakaguchi T, Ogi H, Oda N, Suenari K, Kiyotani K, Ozono R, Oshima T, Yoshida T, Chayama K. Gene delivery of paraoxonase-1 inhibits neointimal hyperplasia after arterial balloon-injury in rabbits fed a high-fat diet. Hypertens Res. 2007;30:85–91. doi: 10.1291/hypres.30.85. [DOI] [PubMed] [Google Scholar]
- 57.Elsøe S, Ahnström J, Christoffersen C, Hoofnagle AN, Plomgaard P, Heinecke JW, Binder CJ, Björkbacka H, Dahlbäck B, Nielsen LB. Apolipoprotein M binds oxidized phospholipids and increases the antioxidant effect of HDL. Atherosclerosis. 2012;221:91–97. doi: 10.1016/j.atherosclerosis.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 58.Buga GM, Frank JS, Mottino GA, Hakhamian A, Narasimha A, Watson AD, Yekta B, Navab M, Reddy ST, Anantharamaiah GM, Fogelman AM. D-4F reduces EO6 immunoreactivity, SREBP-1c mRNA levels, and renal inflammation in LDL receptor-null mice fed a Western diet. J Lipid Res. 2008;49:192–205. doi: 10.1194/jlr.M700433-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Imaizumi S, Grijalva V, Navab M, Van Lenten BJ, Wagner AC, Anantharamiah GM, Fogelman AM, Reddy ST. L-4F differentially alters plasma levels of oxidized fatty acids resulting in more anti-inflammatory HDL in mice. Drug Metab Lett. 2010;4:139–148. doi: 10.2174/187231210791698438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wool GD, Cabana VG, Lukens J, Shaw PX, Binder CJ, Witztum JL, Reardon CA, Getz GS. 4F Peptide reduces nascent atherosclerosis and induces natural antibody production in apolipoprotein E-null mice. FASEB J. 2011;25:290–300. doi: 10.1096/fj.10-165670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Getz GS, Reardon CA. Apolipoprotein A-I and A-I mimetic peptides: a role in atherosclerosis. J Inflamm Res. 2011;4:83–92. doi: 10.2147/JIR.S12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–2767. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Navab M, Hama-Levy S, Van Lenten BJ, Fonarow GC, Cardinez CJ, Castellani LW, Brennan ML, Lusis AJ, Fogelman AM, La Du BN. Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J Clin Invest. 1997;99:2005–2019. doi: 10.1172/JCI119369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hedrick CC, Hassan K, Hough GP, Yoo JH, Simzar S, Quinto CR, Kim SM, Dooley A, Langi S, Hama SY, Navab M, Witztum JL, Fogelman AM. Short-term feeding of atherogenic diet to mice results in reduction of HDL and paraoxonase that may be mediated by an immune mechanism. Arterioscler Thromb Vasc Biol. 2000;20:1946–1952. doi: 10.1161/01.atv.20.8.1946. [DOI] [PubMed] [Google Scholar]
- 65.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res. 2001;42:1308–1317. [PubMed] [Google Scholar]
- 66.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Hama S, Hough G, Bachini E, Grijalva VR, Wagner AC, Shaposhnik Z, Fogelman AM. The double jeopardy of HDL. Ann Med. 2005;37:173–178. doi: 10.1080/07853890510007322. [DOI] [PubMed] [Google Scholar]
- 67.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Green PS, Vaisar T, Pennathur S, Kulstad JJ, Moore AB, Marcovina S, Brunzell J, Knopp RH, Zhao XQ, Heinecke JW. Combined statin and niacin therapy remodels the high-density lipoprotein proteome. Circulation. 2008;118:1259–1267. doi: 10.1161/CIRCULATIONAHA.108.770669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McMahon M, Skaggs BJ, Sahakian L, Grossman J, FitzGerald J, Ragavendra N, Charles-Schoeman C, Chernishof M, Gorn A, Witztum JL, Wong WK, Weisman M, Wallace DJ, La Cava A, Hahn BH. High plasma leptin levels confer increased risk of atherosclerosis in women with systemic lupus erythematosus, and are associated with inflammatory oxidised lipids. Ann Rheum Dis. 2011;70:1619–1624. doi: 10.1136/ard.2010.142737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, Taylor M, McMahon M, Paulus HE, Reddy ST. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157–1162. doi: 10.1136/annrheumdis-2011-200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mastorikou M, Mackness M, Mackness B. Defective metabolism of oxidized phospholipid by HDL from people with type 2 diabetes. Diabetes. 2006;55:3099–3103. doi: 10.2337/db06-0723. [DOI] [PubMed] [Google Scholar]
- 72.Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM, Ferrannini E, Reddy ST. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes. 2011;60:2617–2623. doi: 10.2337/db11-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kontush A, Chapman MJ. Antiatherogenic function of HDL particle subpopulations: focus on antioxidative activities. Curr Opin Lipidol. 2010;21:312–318. doi: 10.1097/MOL.0b013e32833bcdc1. [DOI] [PubMed] [Google Scholar]
- 74.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem. 2009;284:30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watanabe J, Grijalva V, Hama S, Barbour K, Berger FG, Navab M, Fogelman AM, Reddy ST. Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J Biol Chem. 2009;284:18292–18301. doi: 10.1074/jbc.M109.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watanabe J, Charles-Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G, Lee TD, Reddy ST. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum. 2012;64:1828–1837. doi: 10.1002/art.34363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shao B, Pennathur S, Pagani I, Oda MN, Witztum JL, Oram JF, Heinecke JW. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J Biol Chem. 2010;285:18473–18484. doi: 10.1074/jbc.M110.118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Besler C, Heinrich K, Rohrer L, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121:2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heinecke J. HDL and cardiovascular-disease risk–time for a new approach? N Engl J Med. 2011;364:170–171. doi: 10.1056/NEJMe1012520. [DOI] [PubMed] [Google Scholar]
- 82.Cavigiolio G, Geier EG, Shao B, Heinecke JW, Oda MN. Exchange of apolipoprotein A-I between lipid-associated and lipid-free states: a potential target for oxidative generation of dysfunctional high density lipoproteins. J Biol Chem. 2010;285:18847–18857. doi: 10.1074/jbc.M109.098434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Green PH, Glickman RM, Saudek CD, Blum CB, Tall AR. Human intestinal lipoproteins. Studies in chyluric subjects. J Clin Invest. 1979;64:233–242. doi: 10.1172/JCI109444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Navab M, Reddy ST, Anantharamaiah GM, Hough G, Buga GM, Danciger J, Fogelman AM. D-4F-mediated reduction in metabolites of arachidonic and linoleic acids in the small intestine is associated with decreased inflammation in low-density lipoprotein receptor-null mice. J Lipid Res. 2012;53:437–445. doi: 10.1194/jlr.M023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adler DH, Cogan JD, Phillips JA 3rd, Schnetz-Boutaud N, Milne GL, Iverson T, Stein JA, Brenner DA, Morrow JD, Boutaud O, Oates JA. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest. 2008;118:2121–2131. doi: 10.1172/JCI30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Montrose DC, Kadaveru K, Ilsley JN, Root SH, Rajan TV, Ramesh M, Nichols FC, Liang BT, Sonin D, Hand AR, Zarini S, Murphy RC, Belinsky GS, Nakanishi M, Rosenberg DW. cPLA2 is protective against COX inhibitor-induced intestinal damage. Toxicol Sci. 2010;117:122–132. doi: 10.1093/toxsci/kfq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watanabe J, Lin JA, Narasimha AJ, Shahbazian A, Ishikawa TO, Martin MG, Herschman HR, Reddy ST. Novel anti-inflammatory functions for endothelial and myeloid cyclooxygenase-2 in a new mouse model of Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2010;298:G842–G850. doi: 10.1152/ajpgi.00468.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Leuven SI, Hezemans R, Levels JH, Snoek S, Stokkers PC, Hovingh GK, Kastelein JJ, Stroes ES, de Groot E, Hommes DW. Enhanced atherogenesis and altered high density lipoprotein in patients with Crohn’s disease. J Lipid Res. 2007;48:2640–2646. doi: 10.1194/jlr.M700176-JLR200. [DOI] [PubMed] [Google Scholar]
- 91.Rao J, DiGiandomenico A, Artamonov M, Leitinger N, Amin AR, Goldberg JB. Host derived inflammatory phospholipids regulate rahU (PA0122) gene, protein, and biofilm formation in Pseudomonas aeruginosa. Cell Immunol. 2011;270:95–102. doi: 10.1016/j.cellimm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su F, Grijalva V, Navab K, Ganapathy E, Meriwether D, Imaizumi S, Navab M, Fogelman AM, Reddy ST, Farias-Eisner R. HDL mimetics inhibit tumor development in both induced and spontaneous mouse models of colon cancer. Mol Cancer Ther. 2012;11:1311–1319. doi: 10.1158/1535-7163.MCT-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Su F, Kozak KR, Imaizumi S, et al. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc Natl Acad Sci USA. 2010;107:19997–20002. doi: 10.1073/pnas.1009010107. [DOI] [PMC free article] [PubMed] [Google Scholar]