Abstract
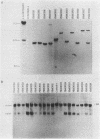
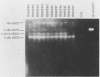
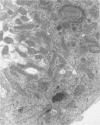
Superoxide dismutases (SOD) play a major role in the intracellular defense against oxygen radical damage to aerobic cells. In eucaryotes, the cytoplasmic form of the enzyme is a 32-kDa dimer containing two copper and two zinc atoms (CuZn SOD) that catalyzes the dismutation of the superoxide anion (O2-) to H2O2 and O2. Superoxide-mediated damage has been implicated in a number of biological processes, including aging and cancer; however, it is not certain whether endogenously elevated levels of SOD will reduce the pathological events resulting from such damage. To understand the in vivo relationship between an efficient dismutation of O2- and oxidative injury to biological structures, we generated transgenic strains of Drosophila melanogaster overproducing CuZn SOD. This was achieved by microinjecting Drosophila embryos with P-elements containing bovine CuZn SOD cDNA under the control of the Drosophila actin 5c gene promoter. Adult flies of the resulting transformed lines which expressed both mammalian and Drosophila CuZn SOD were then used as a novel model for evaluating the role of oxygen radicals in aging. Our data show that expression of enzymatically active bovine SOD in Drosophila flies confers resistance to paraquat, an O2(-)-generating compound. This is consistent with data on adult mortality, because there was a slight but significant increase in the mean lifespan of several of the transgenic lines. The highest level of expression of the active enzyme in adults was 1.60 times the normal value. Higher levels may have led to the formation of toxic levels of H2O2 during development, since flies that died during the process of eclosion showed an unusual accumulation of lipofuscin (age pigment) in some of their cells. In conclusion, our data show that free-radical detoxification has a minor by positive effect on mean longevity for several strains.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagley A. C., Krall J., Lynch R. E. Superoxide mediates the toxicity of paraquat for Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3189–3193. doi: 10.1073/pnas.83.10.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bloch C. A., Ausubel F. M. Paraquat-mediated selection for mutations in the manganese-superoxide dismutase gene sodA. J Bacteriol. 1986 Nov;168(2):795–798. doi: 10.1128/jb.168.2.795-798.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O., Bernstein Y., Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986 Mar;5(3):615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein C. J., Avraham K. B., Lovett M., Smith S., Elroy-Stein O., Rotman G., Bry C., Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. E., Miquel J., Cottrell S. F., Yengoyan L. S., Economos A. C. Is cell aging caused by respiration-dependent injury to the mitochondrial genome? Gerontology. 1982;28(1):44–53. doi: 10.1159/000212510. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Gruber M. Y., Glick B. R., Thompson J. E. Cloned manganese superoxide dismutase reduces oxidative stress in Escherichia coli and Anacystis nidulans. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2608–2612. doi: 10.1073/pnas.87.7.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARMAN D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956 Jul;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- Hassan H. M., Moody C. S. Superoxide dismutase protects against paraquat-mediated dioxygen toxicity and mutagenicity: studies in Salmonella typhimurium. Can J Physiol Pharmacol. 1982 Nov;60(11):1367–1373. doi: 10.1139/y82-204. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Weber U., Gehring W. J. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 1987 May 26;15(10):3947–3959. doi: 10.1093/nar/15.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall J., Bagley A. C., Mullenbach G. T., Hallewell R. A., Lynch R. E. Superoxide mediates the toxicity of paraquat for cultured mammalian cells. J Biol Chem. 1988 Feb 5;263(4):1910–1914. [PubMed] [Google Scholar]
- Latter G. I., Burbeck S., Fleming J., Leavitt J. Identification of polypeptides on two-dimensional electrophoresis gels by amino acid composition. Clin Chem. 1984 Dec;30(12 Pt 1):1925–1932. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Peng T. X., Moya A., Ayala F. J. Irradiation-resistance conferred by superoxide dismutase: possible adaptive role of a natural polymorphism in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1986 Feb;83(3):684–687. doi: 10.1073/pnas.83.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. D., Meshnick S. R., Eaton J. W. Superoxide dismutase-rich bacteria. Paradoxical increase in oxidant toxicity. J Biol Chem. 1987 Mar 15;262(8):3640–3645. [PubMed] [Google Scholar]
- Sinet P. M., Lejeune J., Jerome H. Trisomy 21 (Down's syndrome). Glutathione peroxidase, hexose monophoshate shunt and I.Q. Life Sci. 1979 Jan 1;24(1):29–33. doi: 10.1016/0024-3205(79)90276-5. [DOI] [PubMed] [Google Scholar]
- Siwecki G., Brown O. R. Overproduction of superoxide dismutase does not protect Escherichia coli from stringency-induced growth inhibition by 1mM paraquat. Biochem Int. 1990;20(1):191–199. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Xiao H., Lis J. T. Heat shock and developmental regulation of the Drosophila melanogaster hsp83 gene. Mol Cell Biol. 1989 Apr;9(4):1746–1753. doi: 10.1128/mcb.9.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]