Abstract
Rationale
Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) is caused by mutations in cardiac ryanodine receptor (RyR2) or calsequestrin (Casq2) genes. Sinoatrial node dysfunction associated with CPVT may increase the risk for ventricular arrhythmia.
Objective
To test the hypothesis that CPVT is suppressed by supraventricular overdrive stimulation.
Methods and Results
Using CPVT mouse models (Casq2−/− and RyR2R4496C+/− mice), the effect of increasing sinus heart rate was tested by pretreatment with atropine and by atrial overdrive pacing. Increasing intrinsic sinus rate with atropine before catecholamine challenge suppressed ventricular tachycardia (VT) in 86% of Casq2−/− mice (6/7) and significantly reduced the ventricular arrhythmia (VA) score (atropine: 0.6±0.2 vs. vehicle: 1.7±0.3, p<0.05). Atrial overdrive pacing completely prevented VA in 16/19 (84%) Casq2−/− and in 7/8 (88%) RyR2R4496C+/− mice and significantly reduced ventricular premature beats in both CPVT models (p<0.05). Rapid pacing also prevented spontaneous calcium waves and triggered beats in isolated CPVT myocytes. In humans, heart-rate dependence of CPVT was evaluated by screening a CPVT patient registry for antiarrhythmic drug-naïve individuals that reached >85% of their maximum predicted heart rate during exercise testing. All 18 CPVT patients who fulfilled the inclusion criteria exhibited VA before reaching 87% of maximum heart rate. In six CPVT patients (33%), VA were paradoxically suppressed as sinus heart rates increased further with continued exercise.
Conclusions
Accelerated supraventricular rates suppress VAs in two CPVT mouse models and in a subset of CPVT patients. Hypothetically, atrial overdrive pacing may be a therapy for preventing exercise-induced VT in treatment-refractory CPVT patients.
Keywords: Catecholaminergic polymorphic ventricular tachycardia, arrhythmia suppression, parasympathetic tone
INTRODUCTION
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited arrhythmogenic disorder characterized by stress-induced ventricular tachyarrhythmias in the absence of any structural heart disease.1 CPVT patients usually present with normal sinus rhythm at rest, except for sinus bradycardia in some of the patients2. The underlying defect in CPVT is impaired homeostasis of intracellular Ca due to mutations in the genes encoding for the cardiac ryanodine receptor (RyR2)3 or the RyR2 binding partners calsequestrin (Casq2)4, 5 and triadin.6 Experimental work in CPVT mouse models suggests that CPVT is the result of catecholamine-induced pathological triggered activity in Purkinje cells7, 8 and/or ventricular myocardium9, 10: stress-induced β-adrenergic stimulation increases Ca load in the sarcoplasmic reticulum (SR), which results in premature spontaneous SR Ca release during diastole.11 Such abnormal Ca releases (i.e., spontaneous Ca waves [SCW]) can activate the Na/Ca exchanger in the plasma membrane and induce an inward Na current. The resulting Na/Ca exchange current will depolarize the plasma membrane and generate a delayed after depolarization (DAD) with high arrhythmogenic potential.12
Although β-adrenergic-dependent sinus tachycardia is observed during physical or emotional stress, the catecholamine-induced SR Ca loading plays a main role in promoting DADs and ventricular tachyarrhythmias.11, 13 At the same time, CPVT patients sometimes exhibit resting bradycardia,1, 12 which is also found in CPVT mouse models,9, 10, 14, 15 and may reflect a primary sinoatrial (SA) node dysfunction.16, 17 While the underlying cause for the SA node dysfunction remains to be determined, any inappropriately slow supraventricular rates will favour the emergence of ventricular ectopy.18 Thus, SA node dysfunction may contribute to the ventricular arrhythmia in CPVT patients. Furthermore, since ectopic activity originating from the specialized conduction system can be suppressed by overdrive pacing or by administration of atropine,19, 20 we hypothesized that accelerated sinus rates may be able to reset ventricular foci and thereby prevent premature ventricular focal activity. To test this hypothesis, we used vagolytic therapy with atropine and atrial pacing in two CPVT mouse models to investigate the anti-arrhythmic potential of a catecholamine-independent heart rate (HR) acceleration preceding a β-adrenergic stress. To further evaluate the HR dependence of CPVT in humans, we screened a CPVT patient registry for individuals that (1) were off antiarrhythmic drug therapy and (2) reached >85% of their maximum predicted HR during exercise testing.
METHODS
Detailed methods are provided in the online supplement
Animal studies
Animal use was in accordance with the National Institutes of Health guidelines and approved by the institutional animal care and use committee. Casq2−/− and RyR2R4496C+/− mice generation and in vivo characterization have been previously described.10, 21
Atropine treatment prior to ISO challenge and surface ECG recording
Experiments were carried out in a randomised crossover design. Surface ECGs were recorded from Casq2−/− mice (n=15) and wild-type (WT) littermates (n=11), challenged with 3mg/kg of isoproterenol (ISO) 8 minutes after injection of either vehicle (distilled water) or atropine (0.5mg/Kg). Arrhythmia incidence and arrhythmia score were determined in the atropine/ISO group and compared to the incidence and score in the same mice when treated with vehicle/ISO.
Atrial pacing in the presence of ISO challenge
Supraventricular overdrive stimulation was performed in anesthetized Casq2−/− (N=19) and RyR2R4496C+/− mice (n=9) using transesophageal atrial pacing. Atrial stimulation rates were selected to exceed the maximum intrinsic sinus rate achieved during catecholamine challenge (i.e., 600 b/min for Casq2−/− mice and 660 b/min for RyR2R4496C+/− mice). The atrial overdrive pacing was maintained for the entire period the CPVT mice develop ventricular ectopy in response to the catecholamine challenge. Arrhythmia incidence and arrhythmia score were compared in the same mice challenged with ISO/caffeine in the presence or absence of atrial pacing.
Single myocyte studies
Ventricular myocytes were isolated from Casq2−/− mice and loaded with Fura-2 AM for intracellular Ca measurements as previously described.10 Myocytes were field-stimulated at increasing pacing rates (0.5 – 4Hz) after adding ISO (1µM) to induce SCW. Ca fluorescence ratio (Fratio) was recorded for 30s at each pacing rate and incidences of SCW and triggered beats quantified.
CPVT patient data
To evaluate the HR dependence of CPVT in humans, we screened the entire registry of RyR2-mutation positive CPVT patients (N=162) of the Academic Medical Center (Amsterdam, the Netherlands) for exercise tests that were done before antiarrhythmic drug therapy was started. A total of 42 drug-naïve patients with VA were identified. Exercise testing was performed on a treadmill (standard or modified Bruce protocol) or a bicycle ergometer with a gradually increasing workload protocol. Patients exercised until exhaustion. In order to test our hypothesis that a fast supraventricular rhythm can overdrive ventricular arrhythmias, only exercise tests from patients who reached at least 85% of their estimated maximum HR were used for the analysis (21 out of 42 patients tested). Of these 21 patients, 3 developed ventricular arrhythmias only at peak exercise and were excluded because the stress test was stopped before the suppression of arrhythmias could potentially occur by achieving a higher sinus rate. Thus, a total of 18 patients exercised beyond the onset of ventricular arrhythmias and were included in the analysis.
Statistical analysis
Data are presented as mean ± SEM. Arrhythmia scores and number of VPBs recorded were compared by means of the Mann-Whitney U test. Incidence of VA in the different groups was compared by Fisher exact test. A 2-tailed p value <0.05 was considered statistically significant.
RESULTS
Vagolytic therapy reduces the incidence of ventricular arrhythmias in a CPVT mouse model
Injection of atropine i.p. induced a 15% increase of the intrinsic sinus rate in Casq2−/− anaesthetized mice compared to control injection of vehicle in the same mice (atropine 430±7 bpm vs. vehicle 374±16 bpm, n=15, p<0.05, Fig. 1A). Eight minutes after atropine or vehicle injection, a catecholaminergic challenge with ISO was performed. As shown in Fig. 1B, ISO induced a robust HR increase in both groups. Peak heart rate was higher in the atropine/ISO group (604±12 in atropine/ISO vs. 565±19 in vehicle/ISO, n=15, p=0.09), whereas the magnitude of heart rate increase was comparable to that of the vehicle/ISO group.
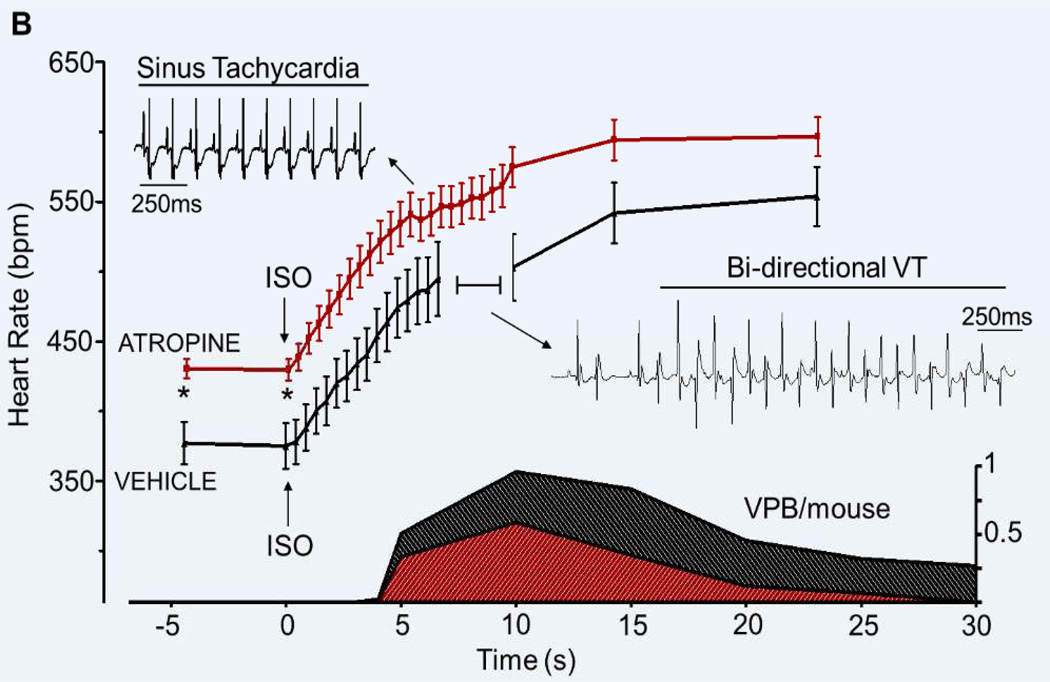
Figure 1. Effect of vagolytic therapy on heart rate and ISO-induced arrhythmia in Casq2−/− mice.
A) Eight minutes after atropine injection Casq2−/− mice show a significant increase in intrinsic sinus rate compared to vehicle controls. When ISO is administered, both groups respond with a steep HR increase. The area in the blue rectangle is magnified in panel B. B) HR response and corresponding arrhythmia burden (VPB/mouse, shaded area, bottom graph) in response to ISO injection. Note that the HR increase after ISO injection is similar in both groups but it is significantly shifted upwards in the atropine treated mice. In both groups, the number of VPB/mouse/s peaks while the HR is still increasing. By the time the maximum HR is reached the arrhythmias are almost completely suppressed. *p<0.05, n= 15 mice.
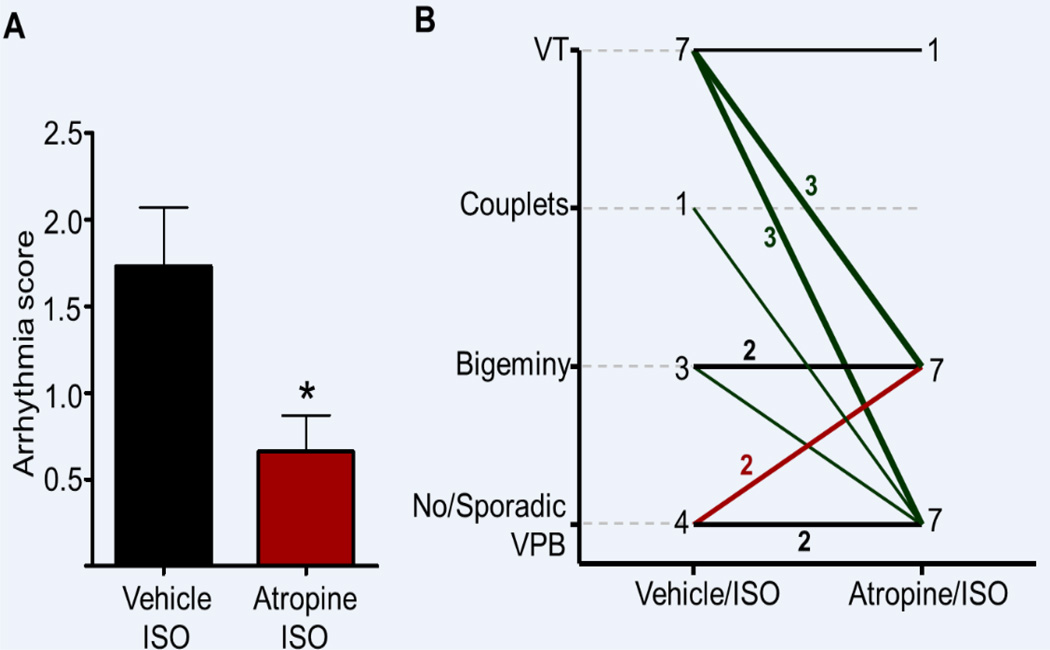
Atropine pretreatment significantly reduced the ventricular arrhythmia score compared to vehicle/ISO controls (0.66±0.21 in atropine/ISO vs. 1.73±0.34 in vehicle/ISO, p<0.05, Fig. 2A). Of the 11 mice with significant ventricular ectopy during vehicle/ISO treatment, 8 (73%) showed an improvement of their ventricular arrhythmia score, with 5 mice having complete ventricular arrhythmia suppression. Of the 7 mice with ISO–induced VT, 6 (86%) were free of VT after pre-treatment with atropine (Fig. 2B). Only 2 out of 15 mice tested had a worsening of their ventricular arrhythmia score from 0 (no/sporadic VPBs) to 1 (frequent/bigeminal VPBs) with atropine/ISO (Fig. 2B).
Figure 2. Vagolytic therapy suppresses CPVT in Casq2−/− mice.
A) The cumulative arrhythmia score is significantly reduced by atropine pretreatment compared to vehicle. B) Most of the mice pretreated with atropine display an improved arrhythmic phenotype compared to vehicle. *P<0.05, vehicle/ISO vs. atropine/ISO). N = 15 mice. Line thickness indicates the number of mice.
We then quantified the ventricular arrhythmia burden (VPBs/mouse). Mice pretreated with atropine displayed a reduction of ISO-induced VPBs compared to vehicle-treated mice (20±7 in atropine/ISO vs. 51±17 in vehicle/ISO, N=15, Fig. 1B). Furthermore, the incidence of VPBs peaked before the peak of sinus HR was reached (Fig. 1B). In other words, VPBs were suppressed as the sinus HR rose further (Fig. 1B). Interestingly, the same dose of atropine (0.5 mg/kg) did not significantly alter the sinus HR in anaesthetized WT mice (427±10 bpm at baseline vs. 432±16 bpm 8 minutes after atropine injection, p=0.7). Together with the observation that Casq2−/− mice displayed a slower basal sinus HR compared to WT mice (388±8 bpm in Casq2−/− vs. 427±10 bpm in WT, p<0.05), this result indicates that vagal stimulation contributed to the sinus bradycardia of Casq2−/− mice.
Atrial pacing prevents ventricular arrhythmias in Casq2−/− mice
To control for possible confounding effects by atropine (e.g., direct pharmacological effects in the specialized conduction system and/or the ventricular myocardium), we next tested the effect of increasing supraventricular HR by transesophageal overdrive pacing in Casq2−/− mice. Atria were paced at 10Hz (600 bpm) to match the peak sinus rates obtained with a catecholaminergic challenge. Electrical capture of the atrium and appropriate AV node conduction was verified on ECG (Fig. 3A). In the absence of catecholamine challenge, rapid atrial pacing (10 Hz, 30 s) induced ventricular ectopy in only 1 out of 19 Casq2−/− mice tested. Intermittent second degree AV block was observed in 3/19 mice during initial control pacing.
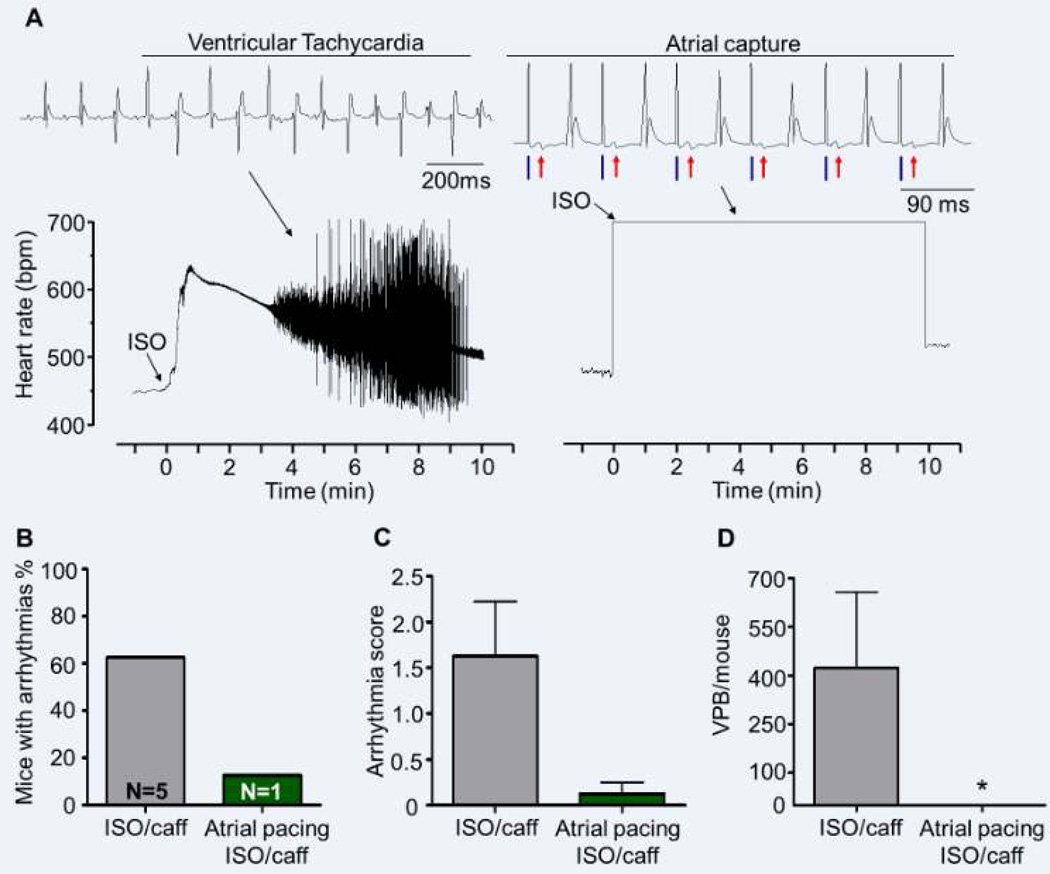
Figure 3. Atrial overdrive pacing prevents catecholamine-induced ventricular arrhythmias in Casq2−/− mice.
A) Representative example of ECG records (top panels) and heart rate (bottom panels) in Casq2−/− mice. In the absence of atrial pacing (left side), ISO injection (black arrow) induces a rapid increase in the heart rate and arrhythmias that last for a few seconds before reverting back to sinus rhythm. When ISO is injected during atrial overdrive pacing, ISO-induced arrhythmias are prevented (blue dashes underneath the ECG trace indicate pacing artefacts, red arrows indicate the paced p waves). Arrhythmia incidence (B), cumulative arrhythmia score (C) and number of VPB/mouse (D) are significantly reduced by atrial overdrive pacing during ISO. N = 19 mice, *P<0.05 and **P<0.01 vs no pacing.
In response to ISO challenge, 11/19 (58%) Casq2−/− mice developed ventricular ectopy (Fig. 3B). Atrial overdrive pacing completely prevented ventricular arrhythmias in 8 out of those 11 mice (73%), with only 3/19 (15%) mice overall developing ventricular arrhythmias (p<0.05, Fig. 3B). The ventricular arrhythmia score in the atrial pacing/ISO group was significantly reduced compared to ISO only control group (from 1.42±0.28 in ISO to 0.47±0.25 in atrial pacing/ISO, p<0.05, Fig. 3C). Overall, atrial overdrive pacing resulted in a 10-fold reduction in the number of ISO-induced VPBs (from 58±16 to 7±4 VPB/mouse, p<0.01, Fig. 3D).
Prolonged atrial pacing prevents ventricular arrhythmias in RyR2R4496C+/− mice
Since heterozygous mutations in the RyR2 gene are the most common cause of CPVT in patients, we next tested our hypothesis in a RyR2-linked CPVT mouse model, RyR2R4496C+/− mice. 21 Compared to Casq2−/−, RyR2R4496C+/− mice displayed both a higher heart rate at baseline (384±13 in Casq2−/− vs. 472±12 in RyR2R4496C+/−, p<0.001), and a higher peak sinus rate after ISO challenge (555±17 in Casq2−/− vs. 653±10 in RyR2R4496C+/−, p<0.001). Unlike Casq2−/− mice, RyR2R4496C+/− did not display ventricular ectopy in response to ISO only (3 VPBs in 1/7 mice tested),. Hence, we used a combination of caffeine (120 mg/kg) and ISO (3mg/kg) as catecholamine challenge to induce arrhythmias.
Injection of ISO/caffeine induced a rapid HR increase in all mice (n=9) and ventricular arrhythmias in 66% of them (Fig. 4A–B). However, in contrast to Casq2−/− mice, where ectopy appeared during the first few seconds of the HR upstroke, RyR2R4496C+/− developed ventricular arrhythmias on average 3.5±0.8 min after injection and the episodes lasted longer (7.1 ±0.8 min until ventricular arrhythmias stopped). Interestingly, at the onset of ventricular ectopy the HR in RyR2R4496C+/− mice had already slowed down to 68.5±6.7% of the maximum HR reached (Fig. 4A). Prolonged overdriving pacing (660bpm for 10min) successfully prevented ventricular arrhythmias in all but one mouse and reduced the ventricular arrhythmia score (from 1.8±0.58 in ISO/caffeine to 0.11±0.11 in atrial pacing-ISO/caffeine, p<0.05) and the number of VPBs recorded (from 543±239 VPBs in ISO/caffeine to 0.55±0.55 VPBs in atrial pacing-ISO/caffeine, p<0.05) compared to the same mice treated with ISO/caffeine in the absence of atrial pacing (Fig. 4B–C–D).
Figure 4. Prolonged atrial overdrive pacing prevents catecholamine-induced ventricular arrhythmias in RyR2R4496C+/− mice.
A) Representative example of ECG records (top panels) and heart rate (bottom panels) in RyR2R4496C+/− mice. ISO/caffeine injection (arrow) caused a rapid rise in heart rate, but ventricular ectopic activity occurred with a delay and lasted several minutes (panel A, left side). Hence, a longer protocol of 10 min overdrive pacing was used to suppress arrhythmias in RyR2R4496C+/− mice (panel A, right side). Atrial capture was monitored throughout the 10 min pacing (blue dashes = pacing artefacts; red arrows = paced p wave). Arrhythmia incidence (B), cumulative arrhythmia score (C) and the number of VPB/mouse (D) are significantly reduced when the mice are subjected to atrial overdrive pacing during ISO/caffeine challenge. N = 9 mice, *P<0.05 vs no pacing.
Overdrive pacing supresses ISO-induced SCWs in Casq2−/− myocytes
To further investigate the underlying mechanism of arrhythmia suppression at fast heart rates, we next tested the effects of progressively shorter diastolic interval in single myocytes of Casq2−/− mice. In presence of ISO (1µM), myocytes were field-stimulated at 0.5, 1, 2, 3, and 4 Hz. SCWs were present in approximately 40% of Casq2−/− paced at 0.5 and 1 Hz. Reduction of the diastolic interval by higher pacing frequencies progressively reduced the incidence of SCWs (Fig. 5). At 3Hz only 8% of Casq2−/− myocytes developed SCWs and complete prevention of SCWs was achieved with overdriving pacing at 4Hz (Fig. 5). Taken together, consistent with our in vivo experiment, overdrive pacing prevents ISO-induced SCWs in CPVT myocytes.
Figure 5. Fast pacing suppresses ISO-induced SCWs in Casq2−/− myocytes.
A) Example of Ca transients from an isolated Casq2−/− myocyte paced at 0.5Hz and 4Hz in the presence of ISO (1µM). Arrows indicate SCW. SCW and triggered beats (TB) that are present at 0.5Hz are completely prevented with faster pacing at 4Hz. B) Percentage of myocytes that exhibit either SCW or TB at different pacing frequencies. N=10–13 cells per group. *P<0.05 vs. 0.5 Hz.
Sinus tachycardia paradoxically suppresses arrhythmias in a subpopulation of CPVT patients
The exercise tests of 18 CPVT patients carrying a mutation in the RyR2 gene met our inclusion criteria and were therefore used for the analysis. Basal HR was 76±4 bpm. As expected, at the beginning of the stress test patients did not develop ventricular ectopy. With increasing exercise, all 18 patients exhibited ventricular ectopy. Three of them displayed couplets or VT as their first ventricular arrhythmia, the other 15 patients had frequent VPBs and bigeminal VPBs first. In 86% of the latter group, ventricular arrhythmia complexity increased leading to couplets (13/15, 86%) and VT (6/15, 43%). HR at the onset of ectopic activity was variable ranging from 95 to 150 bpm (mean 128±4 bpm). The first ventricular arrhythmia appeared at 65% of peak HR as shown in Fig. 6A. Upon reaching 87% of their maximum HR peak, all the patients had developed ventricular arrhythmias. However, in 6 out of 18 patients (33%), ventricular arrhythmias subsided as their sinus HR further increased up to 89%, 91%, 93% (in 2 patients), 95% and 99% of their peak HR (Fig. 6A). The most severe arrhythmic phenotype displayed in these 6 patients before sinus tachycardia took over was bigeminal VPBs in one patient, couplets in 4, and VT in one (Fig. 6B). Taken together, we find VA suppression with increasing sinus tachycardia in a subset of CPVT patients. We next compared the HR response in “responders” (=VA suppression by exercise) and “non-responders” (=No VA suppression). As shown in table 1, resting HR, HR at the beginning of arrhythmia and HR increase during exercise were not significantly different in the two groups. There was a trend that responders exercised for a longer period of time and at a higher intensity (MET values) compared to non-responders.
Figure 6. Fast sinus rhythm suppresses ventricular arrhythmias in a subpopulation of CPVT patients.
Cumulative arrhythmia incidence as a function of sinus HR. HR is expressed as a percentage of the maximum HR reached during the exercise test by each patient. When a patient experienced a ventricular arrhythmia, he or she “entered” the Kaplan Meier curve. All patients developed arrhythmias during exercise; however, in 6 patients ventricular arrhythmias were suppressed when their sinus heart rate further increased nearing peak exercise (downward steps in the graph). B) Example of ECG recorded during a stress test. This patient displays the first arrhythmias at a HR of 102 bpm (VPBs are marked by black arrows). With the progression of the exercise arrhythmia complexity increases up to VT (ECG panel 3). When his HR reaches 148 bpm (95% of his maximum HR) arrhythmias are suppressed for the remaining time of the exercise test (ECG panels 4 and 5).
Table 1.
Characteristics of CPVT patients grouped by whether or not VA suppression occurred during exercise stress test. MET = Metabolic Equivalent of Task
| Patient group | Age (years) |
Resting HR (bpm) |
HR at VA induction (bpm) |
Peak HR (bpm) |
HR increase (bpm) |
Exercise duration (s) |
METs achieved |
|---|---|---|---|---|---|---|---|
| VA suppression at peak exercise (n=6) | 37.8±5.2 | 75.5±4.7 | 130±9 | 171±5.4 | 95.0±4.1 | 663±70 | 13.9±1.5 |
| No VA suppression (n=12) | 43.9±2.8 | 76.4±4.9 | 127±4.7 | 169±4.8 | 92.9±6.0 | 507±72 | 10.3±0.9 |
| P-value | 0.48 | 0.85 | 0.88 | 1 | 0.81 | 0.054 | 0.061 |
DISCUSSION
Main findings
Sinus bradycardia1, 2, 9, 10, 12, 14, 15 and sinatrial node dysfunction16, 17, 22, 23 can be found in CPVT patients and CPVT mouse models. Here, we tested the hypothesis that augmenting supraventricular heart rates prevents CPVT in vivo. We find that both vagolytic therapy (Figs. 1 & 2) and atrial overdrive pacing (Fig. 3 & 4) can suppress catecholamine-induced ventricular arrhythmia in Casq2−/− and RyR2R4496C+/− mice. Our in vitro studies confirm that reduction of diastolic interval suppresses ISO induced-SCW in isolated Casq2−/− myocytes, thereby reducing the risk of DADs and triggered activity (Fig. 5). In addition, we observed that in a subset of CPVT patients, exercise-induced ventricular arrhythmias were paradoxically suppressed as sinus heart rates increased further with continued exercise (Fig. 6).
Antiarrhythmic effect of vagolytic treatment in a CPVT mouse model
Pretreatment with atropine, which blocks muscarinic receptors24 and increases SA node firing rates,25 not only significantly reduced the overall ventricular arrhythmia score and the number of VPBs compared to controls, but also prevented the most severe triggered events (couplets and VTs). We hypothesize that the atropine-induced acceleration of the supraventricular pacemaker caused an upward shift of the entire HR response curve to ISO (Fig. 1). As a result, the diastolic intervals in the ventricular tissue is shorter at every heart rate, thereby reducing the likelihood that spontaneous Ca waves and DADs reach threshold and triggered beats. It could be argued that during CPVT, mice can reach higher ventricular rates26 than the fastest sinus rate obtained in atropine treated mice (Fig. 1). However, when ventricular arrhythmias start, usually with sporadic VPBs and bigeminy, the HR increase is still moderate (Fig. 1). It is only as ventricular arrhythmia complexity worsens that the coupling time of ectopic beats progressively reduces until fast VT is triggered. We hypothesize that atropine induced accelerated sinus rhythm may be sufficient to interrupt simple arrhythmias such as sporadic VPBs and bigeminy, arresting the likely progression of these events into ventricular tachycardia.
Moreover, it will take at least a few seconds for the ISO-induced signal transduction events to occur that promote SR Ca overload and triggered arrhythmias.27 By the time these effects have taken place, vehicle treated mice develop complex arrhythmias (8 seconds after ISO injection), whereas atropine treated mice are protected possibly due to their higher HR and hence shorter diastolic interval. Our in vitro studies (Fig. 5) confirm that a reduction of diastolic interval suppresses ISO induced-SCR in isolated Casq2−/− myocytes, thereby reducing the risk of DADs and triggered activity. We speculate that the reduction of diastolic interval might be particularly effective in the Purkinje cells (PC) of the conduction system. In fact, it has recently been shown that PC are more prone to developing DADs than working ventricular myocytes28 because of intracellular characteristics that increase the Ca handling dysregulation in these cells.29
The data from the RyR2R4496C+/− CPVT mouse model reported here also support our hypothesis: compared to Casq2−/− mice, RyR2R4496C+/− mice have significantly higher resting heart rates, and a significantly bigger response to ISO (compare Fig. 3 and 4), similar to Casq2−/− mice pretreated with atropine, which are resistant to ISO challenge (Fig. 1). In RyR2R4496C+/−, ventricular arrhythmias can only be induced after sensitizing the RyR2 channels with caffeine, and even then the ventricular ectopy occurs only when the intrinsic sinus rate drops below 70% of its peak value (Fig. 4). Other groups have also reported that unlike Casq2−/− mice, RyR2R4496C+/− do not exhibit spontaneous or exercise-induced ventricular arrhythmias15. Pharmacological challenges that include the use of catecholamines and/or caffeine are always required to elicit arrhythmias in this model.15, 30. Our data demonstrate that overdrive pacing was effective also against drug-induced ventricular arrhythmias in RyR2R4496C+/− mice.
Interestingly, atropine injection increased the sinus HR to a much greater extent in Casq2−/− compared to WT mice. This result suggests that vagal tone contributes to the sinus bradycardia observed in Casq2−/− mice, either due to increased vagal tone, or due to increased responsiveness of the SA node to vagal stimulation. Since sinus bradycardia1, 2, 12 and SA node dysfunction16, 17, 22 are also observed in CPVT patients, it is conceivable that an impaired sinus rate response during exercise contributes to the exercise-induced ventricular ectopy in CPVT patients. Consistent with this hypothesis, we observed complete ventricular arrhythmia suppression as the HR further increased approaching its peak in a subgroup of patients with significant ventricular ectopy in the early stages of the exercise (Fig. 5). This occurred despite the fact that catecholamine-induced HR increase initially is accompanied by progression of ventricular arrhythmia complexity.2 Although it is well recognized that ectopic ventricular automaticity can be reset by overdrive pacing,31–33 it is still unexpected that sinus tachycardia could provide sufficient protection against clinical CPVT. A possible explanation is that the positive chronotropic effect of β adrenergic stimulation during stress could paradoxically counterbalance the triggered activity simultaneously promoted by β-adrenergically induced SR Ca overload. However, possibly due to the small number of patients and the heterogeneity of exercise test protocols used, we could not detect a significant difference in the rate of HR increase in the subset of patients with arrhythmia suppression compared to the rest (table 1). A prospective study on a larger population would be needed to clarify the relationship between HR increase and arrhythmia suppression in CPVT patients.
Antiarrhythmic effect of atrial overdrive pacing in CPVT
As important proof of principle, we find that atrial overdrive pacing almost completely prevented catecholamine-induced ventricular arrhythmia in both Casq2−/− and RyR2R4496C+/− mice (Fig. 3 & 4). Finding alternative treatment approaches is an important issue for CPVT patients, since β-blockers, which are the current gold standard in CPVT treatment, are not fully protective in up to 30% of the patients.34, 35 Based on our experimental results reported here, one might speculate that the negative chronotropic effect of β-blockers might contribute to treatment failure. In fact, by slowing SA node activity, β-blockers may suppress an intrinsic antiarrhythmic mechanism of the heart rate increase. In support of this hypothesis, recent work has shown that while β-blockers protect most CPVT patients from severe exercise-induced arrhythmias, they reduce the sinus HR threshold at which milder ventricular arrhythmias start.36 On the other hand, molecules that regulate the RyR2 open probability (such as flecainide, propafenone and RyR2 inhibitors S107, JTV519) reduce the frequency of diastolic SR Ca waves and triggered beats.13, 37–39 Consistent with this mechanism, flecainide increased the sinus HR threshold at which ventricular arrhythmias were triggered clinically, while at the same time reducing the peak HR reached during exercise.40
Clinical implications and caveats
While vagolytic therapy would be difficult to carry out in CPVT patients because of systemic side effects,41 overdrive atrial pacing could be performed in patients with dual chamber pacemakers or ICDs. In support of this concept is a recent clinical case report of the temporary overdrive suppression of bidirectional VT by a rapid atrial tachycardia in a young CPVT patient.42 One concern is that increasing the HR will independently load the SR with Ca43 and may promote the spontaneous Ca release events that trigger CPVT. We tested this hypothesis experimentally, and only found one instance where rapid atrial pacing induced ventricular ectopy. Our data in CPVT patients further demonstrates that at least 30% of CPVT tolerated very fast sinus rates with paradoxical suppression of ventricular arrhythmia. To what extent this finding is applicable to other CPVT patients remains to be determined. For example, the incidence of atrial tachyarrhythmia is increased in CPVT patients1, 17 and atrial fibrillation has been associated with triggering CPVT clinically.44 Another important consideration is the site of overdrive pacing. Given that electrical stimulation by the pacing lead may trigger the release of norepinephrine from sympathetic nerve terminals in the ventricle,45 which would further exacerbate the ventricular automaticity, atrial pacing likely will be the preferable approach. In either case, atrial overdrive pacing will have to be tested in humans with CPVT to evaluate its safety and efficacy. If rapid atrial pacing is shown to prevent exercise-induced VT in CPVT patients, it may be feasible to automatically program high atrial rates during exercise using the rate-response feature.46
CONCLUSIONS
We report that increasing supraventricular rates, either by vagolytic therapy or by atrial overdrive pacing, protected against catecholamine-induced VT in two different mouse models of CPVT. Likewise, clinically, in a substantial portion of CPVT patients ventricular arrhythmias were suppressed at peak exercise. Taken together, these results suggest that overdrive atrial pacing should be explored as a possible therapy for preventing exercise-induced VT in treatment-refractory CPVT patients.
Supplementary Material
Novelty and Significance.
What Is Known?
Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) is caused by mutations in cardiac ryanodine receptor (RyR2) or calsequestrin (Casq2) genes.
The ventricular tachycardia is caused by spontaneous calcium release that triggers premature activation of the ventricular conduction system and/or working myocardium.
CPVT is often associated with sinoatrial node (SAN) dysfunction and slow sinus rhythm..
What New Information Does This Article Contribute?
Rapid pacing prevents premature spontaneous calcium release and triggered beats in CPVT ventricular myocytes.
Accelerating supraventricular heart rates suppresses catecholamine-induced ventricular arrhythmia in two CPVT mouse models and in a subset of CPVT patients.
CPVT is an inherited arrhythmic disorder characterized by stress-induced ventricular tachyarrhythmias in the absence of structural heart disease. CPVT patients also exhibit slow sinus rhythm, but it is not known whether SAN dysfunction contributes to the ventricular arrhythmia risk of CPVT patients. Experimental evidence indicates that CPVT is caused by premature activity in the ventricular conduction system and/or working myocardium. Since ectopic activity originating from the specialized conduction system is suppressed by overdrive pacing, we hypothesized that accelerated supraventricular heart rates (HR) will reset ventricular foci and thereby prevent premature ventricular focal activity. Consistent with this hypothesis, we found that catecholamine-independent HR acceleration preceding the β-adrenergic stress (vagolytic therapy or atrial pacing) suppressed ventricular arrhythmias in two CPVT mouse models. In humans, a subset of CPVT patients who reached >85% of their maximum-predicted HR during exercise testing reverted to sinus rhythm as HR increased further with continued exercise. These findings support the notion that SAN dysfunction contributes to the risk of arrhythmias in CPVT patients and suggest that atrial overdrive pacing could be a new therapeutic approach for preventing exercise-induced VT in patients with CPVT.
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by the US National Institutes of Health grants HL88635 and HL71670 (to BCK), by the American Heart Association Established Investigator Award 0840071N (to BCK), by a Heart Rhythm Society Fellowship Award (to MF) and by ZorgOnderzoek Nederland Medische Wetenschappen (ZonMW, grant 120610013 to CW and AAMW).
Non-standard Abbreviations and Acronyms
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- RyR2
cardiac ryanodine receptor Ca2+ release channel
- Casq2
cardiac calsequestrin
- VA
ventricular arrhythmias
- VPB
ventricular premature beat
- VT
ventricular tachycardia
- DAD
delayed after depolarization
- ISO
isoproterenol
- HR
heart rate
- WT
wild type
- ICD
implantable cardioverter-defibrillator
- SA
sinoatrial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 2.Postma AV, Denjoy I, Kamblock J, Alders M, Lupoglazoff JM, Vaksmann G, Dubosq-Bidot L, Sebillon P, Mannens MM, Guicheney P, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia: Ryr2 mutations, bradycardia, and follow up of the patients. J Med Genet. 2005;42:863–870. doi: 10.1136/jmg.2004.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swan H, Piippo K, Viitasalo M, Heikkila P, Paavonen T, Kainulainen K, Kere J, Keto P, Kontula K, Toivonen L. Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol. 1999;34:2035–2042. doi: 10.1016/s0735-1097(99)00461-1. [DOI] [PubMed] [Google Scholar]
- 4.Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of casq2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in bedouin families from israel. Am J Hum Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, Mannens MM, Wilde AA, Guicheney P. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:e21–e26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- 6.Roux-Buisson N, Cacheux M, Fourest-Lieuvin A, Fauconnier J, Brocard J, Denjoy I, Durand P, Guicheney P, Kyndt F, Leenhardt A, Le Marec H, Lucet V, Mabo P, Probst V, Monnier N, Ray PF, Santoni E, Tremeaux P, Lacampagne A, Faure J, Lunardi J, Marty I. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum Mol Genet. 2012;21:2759–2767. doi: 10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang G, Giovannone SF, Liu N, Liu FY, Zhang J, Priori SG, Fishman GI. Purkinje cells from ryr2 mutant mice are highly arrhythmogenic but responsive to targeted therapy. Circ Res. 2010;107:512–519. doi: 10.1161/CIRCRESAHA.110.221481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuyvers BD, Dun W, Matkovich S, Sorrentino V, Boyden PA, ter Keurs HE. Ca2+ sparks and waves in canine purkinje cells: A triple layered system of ca2+ activation. Circ Res. 2005;97:35–43. doi: 10.1161/01.RES.0000173375.26489.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, Bicciato S, Arcelli D, Spedito A, Scelsi M, Villani L, Esposito G, Boncompagni S, Protasi F, Volpe P, Priori SG. Unexpected structural and functional consequences of the r33q homozygous mutation in cardiac calsequestrin: A complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008;103:298–306. doi: 10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- 10.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashimura TBS, Trafford AW, Napolitano C, Priori SG, Eisner DA, Venetucci LA. In the ryr2(r4496c) mouse model of cpvt, β-adrenergic stimulation induces ca waves by increasing sr ca content and not by decreasing the threshold for ca waves. Circ Res. 2010;107:1483–1489. doi: 10.1161/CIRCRESAHA.110.227744. [DOI] [PubMed] [Google Scholar]
- 12.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 13.Sedej S, Heinzel FR, Walther S, Dybkova N, Wakula P, Groborz J, Gronau P, Maier LS, Vos MA, Lai FA, Napolitano C, Priori SG, Kockskamper J, Pieske B. Na+-dependent sr ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human cpvt mutation. Cardiovasc Res. 2010;87:50–59. doi: 10.1093/cvr/cvq007. [DOI] [PubMed] [Google Scholar]
- 14.Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG. Calsequestrin 2 (casq2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2007;117:1814–1823. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, Napolitano C, Priori SG. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 16.Joshi P, Saxena A, Kaul U, Mansoor AH. Catecholaminergic polymorphic ventricular tachycardia with associated sinus node dysfunction. Indian Heart J. 2010;62:84–86. [PubMed] [Google Scholar]
- 17.Sumitomo N, Sakurada H, Taniguchi K, Matsumura M, Abe O, Miyashita M, Kanamaru H, Karasawa K, Ayusawa M, Fukamizu S, Nagaoka I, Horie M, Harada K, Hiraoka M. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J. 2007;71:1606–1609. doi: 10.1253/circj.71.1606. [DOI] [PubMed] [Google Scholar]
- 18.Han J, DeTraglia J, Millet D, Moe GK. Incidence of ectopic beats as a function of basic rate in the ventricle. American heart journal. 1966;72:632–639. doi: 10.1016/0002-8703(66)90346-2. [DOI] [PubMed] [Google Scholar]
- 19.Zipes DP. The clinical significance of bradycardic rhythms in acute myocardial infarction. The American journal of cardiology. 1969;24:814–825. doi: 10.1016/0002-9149(69)90470-6. [DOI] [PubMed] [Google Scholar]
- 20.Goel BG, Han J. Atrial ectopic activity associated with sinus bradycardia. Circulation. 1970;42:853–858. doi: 10.1161/01.cir.42.5.853. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, Smith CD, Xie C, Chen W, Zhang J, Tian X, Jones PP, Zhong X, Guo A, Chen H, Zhang L, Zhu W, Yang D, Li X, Chen J, Gillis AM, Duff HJ, Cheng H, Feldman AM, Song LS, Fill M, Back TG, Chen SR. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced ca(2+) release. Nat Med. 2011 doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, Alders M, Postma AV, van Langen I, Mannens MM, Wilde AA. Expanding spectrum of human ryr2-related disease: New electrocardiographic, structural, and genetic features. Circulation. 2007;116:1569–1576. doi: 10.1161/CIRCULATIONAHA.107.711606. [DOI] [PubMed] [Google Scholar]
- 23.van der Werf C, Nederend I, Hofman N, van Geloven N, Ebink C, Frohn-Mulder IM, Alings AM, Bosker HA, Bracke FA, van den Heuvel F, Waalewijn RA, Bikker H, van Tintelen JP, Bhuiyan ZA, van den Berg MP, Wilde AA. Familial evaluation in catecholaminergic polymorphic ventricular tachycardia: Disease penetrance and expression in cardiac ryanodine receptor mutation-carrying relatives. Circ Arrhythm Electrophysiol. 2012;5:748–756. doi: 10.1161/CIRCEP.112.970517. [DOI] [PubMed] [Google Scholar]
- 24.Goodman LS, Brunton LL, Chabner B, Knollmann BC. Goodman & gilman's pharmacological basis of therapeutics. New York: McGraw-Hill; [Google Scholar]
- 25.Dhingra RC, Amat YLF, Wyndham C, Denes P, Wu D, Pouget JM, Rosen KM. Electrophysiologic effects of atropine on human sinus node and atrium. Am J Cardiol. 1976;38:429–434. doi: 10.1016/0002-9149(76)90458-6. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herron TJ, Milstein ML, Anumonwo J, Priori SG, Jalife J. Purkinje cell calcium dysregulation is the cellular mechanism that underlies catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2010;7:1122–1128. doi: 10.1016/j.hrthm.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li P, Rudy Y. A model of canine purkinje cell electrophysiology and ca(2+) cycling: Rate dependence, triggered activity, and comparison to ventricular myocytes. Circ Res. 2011;109:71–79. doi: 10.1161/CIRCRESAHA.111.246512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri S, Imbriani M, Napolitano C, Lai FA, Priori SG. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: Insights from a ryr2 r4496c knock-in mouse model. Circ Res. 2006;99:292–298. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- 31.Bennett MA, Pentecost BL. Suppression of ventricular tachyarrhythmias by transvenous intracardiac pacing after acute myocardial infarction. Br Med J. 1970;4:468–470. doi: 10.1136/bmj.4.5733.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naito S, Tada H, Kaneko T, Oshima S, Taniguchi K. Biatrial epicardial pacing prevents atrial fibrillation and confers hemodynamic benefits after coronary artery bypass surgery. Pacing Clin Electrophysiol. 2005;28(Suppl 1):S146–S149. doi: 10.1111/j.1540-8159.2005.00075.x. [DOI] [PubMed] [Google Scholar]
- 33.Mont L, Ruiz-Granell R, Martinez JG, Carmona JR, Fidalgo M, Cobo E, Riera M, Navarro X. Impact of anti-tachycardia pacing on atrial fibrillation burden when added on top of preventive pacing algorithms: Results of the prevention or termination (pot) trial. Europace. 2008;10:28–34. doi: 10.1093/europace/eum268. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, Klug D, Takatsuki S, Villain E, Kamblock J, Messali A, Guicheney P, Lunardi J, Leenhardt A. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 35.van der Werf C, Zwinderman AH, Wilde AA. Therapeutic approach for patients with catecholaminergic polymorphic ventricular tachycardia: State of the art and future developments. Europace. 2012;14:175–183. doi: 10.1093/europace/eur277. [DOI] [PubMed] [Google Scholar]
- 36.Haugaa KH, Leren IS, Berge KE, Bathen J, Loennechen JP, Anfinsen OG, Fruh A, Edvardsen T, Kongsgard E, Leren TP, Amlie JP. High prevalence of exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia mutation-positive family members diagnosed by cascade genetic screening. Europace. 2010;12:417–423. doi: 10.1093/europace/eup448. [DOI] [PubMed] [Google Scholar]
- 37.Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, Piston DW, Huke S, Knollmann BC. Flecainide inhibits arrhythmogenic ca2+ waves by open state block of ryanodine receptor ca2+ release channels and reduction of ca2+ spark mass. J Mol Cell Cardiol. 2010;48:293–301. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehnart SE, Wehrens XH, Marks AR. Calstabin deficiency, ryanodine receptors, and sudden cardiac death. Biochem Biophys Res Commun. 2004;322:1267–1279. doi: 10.1016/j.bbrc.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 39.Hwang HS, Hasdemir C, Laver D, Mehra D, Turhan K, Faggioni M, Yin H, Knollmann BC. Inhibition of cardiac ca2+ release channels (ryr2) determines efficacy of class i antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011;4:128–135. doi: 10.1161/CIRCEP.110.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt A, Shimizu W, Sumitomo N, Fish FA, Bhuiyan ZA, Willems AR, van der Veen MJ, Watanabe H, Laborderie J, Haissaguerre M, Knollmann BC, Wilde AA. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011;57:2244–2254. doi: 10.1016/j.jacc.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson ME, Lee GK, Chandra A, Kane GC. Central anticholinergic syndrome following dobutamine-atropine stress echocardiography. Echocardiography. 2011;28:E205–E206. doi: 10.1111/j.1540-8175.2011.01509.x. [DOI] [PubMed] [Google Scholar]
- 42.Richter S, Gebauer R, Hindricks G, Brugada P. A classic electrocardiographic manifestation of catecholaminergic polymorphic ventricular tachycardia. J Cardiovasc Electrophysiol. 2012;23:560. doi: 10.1111/j.1540-8167.2011.02138.x. [DOI] [PubMed] [Google Scholar]
- 43.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 44.Sumitomo N, Nakamura T, Fukuhara J, Nakai T, Watanabe I, Mugishima H, Hiraoka M. Clinical effectiveness of pulmonary vein isolation for arrhythmic events in a patient with catecholaminergic polymorphic ventricular tachycardia. Heart Vessels. 2010;25:448–452. doi: 10.1007/s00380-009-1214-6. [DOI] [PubMed] [Google Scholar]
- 45.Blinks JR. Field stimulation as a means of effecting the graded release of autonomic transmitters in isolated heart muscle. J Pharmacol Exp Ther. 1966;151:221–235. [PubMed] [Google Scholar]
- 46.Thomas VC, Law IH, Evans WN. Cardioversion of intraatrial reentrant tachycardia using rate response. Pacing Clin Electrophysiol. 2012 doi: 10.1111/j.1540-8159.2012.03387.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.